Abstract
Background
Information regarding comparison of the environmental prevalence of avian influenza virus (AIVs), before and after massive poultry vaccinations, is limited. Our study aimed to detect differences in the prevalence of AIVs type A and subtypes H5, H7, and H9 before and after the September 2017 massive poultry vaccination, across different sampling places and types.
Methods
We collected 55 130 environmental samples from 11 cities in Zhejiang Province (China) between March 2013 and December 2018. Multivariate logistic regression analyses were conducted to determine the prevalence of AIV type A and subtypes H5, H7, and H9 across different sampling places and types, before and after massive poultry vaccination.
Results
After the vaccination, contamination risk of AIV type A (adjusted odds ratio [aOR] = 1.08; 95% confidence interval [CI], 1.03–1.14) and subtype H9 (aOR = 1.58; 95% CI, 1.48–1.68) increased, and that of subtype H7 (aOR = 0.12; 95% CI, 0.10–0.14) decreased. Statistically significant decreased risk for H7 subtype contamination and increased risk for H9 subtype contamination were observed in backyard poultry flocks, live poultry markets, and slaughtering/processing plants. Swabs from poultry cages and slaughtering tables showed a statistically significant increased risk for H5 subtype contamination. The prevalence of H7 subtype decreased statistically significantly, whereas that of H9 subtype increased across the 5 sample types (poultry cages swabs, slaughtering table swabs, poultry feces, poultry drinking water, and poultry sewage).
Conclusions
Despite the sharp decrease in H7 subtype prevalence, reduction measures for AIV circulation are still imperative, given the high type A prevalence and the increase in H9 subtype contamination across different sampling places and types.
Keywords: avian influenza virus, environment contamination, poultry vaccination
Our study revealed differences in the prevalence of avian influenza virus type A and subtypes H5, H7, and H9, before and after massive poultry vaccination.
Avian influenza viruses (AIVs) have become a major threat to human and livestock health in the last 2 decades. With new strains widely circulating in China, human infections with new subtypes of AIVs, including H7N9, H5N6, H10N8, and H7N4, are being reported in the recent years [1–4]. Specifically, more human infections with H9N2 have been reported since 2014 [5, 6]. The potential pandemic caused by these viral subtypes requires preventive measures such as surveillance and poultry vaccination [7, 8].
The sudden increase in the number of H7N9 cases during the 2016/2017 epidemic season raised concerns that a next wave of the pandemic might occur in China [9]. Moreover, the H7N9 virus evolved into a highly pathogenic avian influenza virus during winter 2016 to 2017, capable of disseminating systematically and causing high mortality rates in chicken [10–12]. Responding to the serious threat posed by H7N9, a bivalent H5/H7-inactivated influenza vaccine was introduced by the Chinese government to be used in domestic poultry [13].
The Zhejiang province in southeast China has the largest proportion of H7N9 cases among other Chinese provinces [14]. To control the H7N9 epidemic, the Zhejiang government implemented bivalent H5/H7-inactivated influenza poultry vaccination, according to a national strategy, in September 2017. Previous studies have suggested the effectiveness of massive poultry vaccination in controlling H7N9 infection [13, 15, 16]. By conducting postvaccination surveillance, Zeng et al [13] showed that H7N9 virus isolation rate decreased by 93.3% after chickens were inoculated with the new H5/H7 vaccine. Another study from Guangdong province showed that the vaccine was associated with a 92% reduction in H7 subtype positivity rates among poultry [15]. However, AIVs contamination across different sampling places and sample types was not studied and, hence, specific controlling measures could not be adopted [13, 15]. Our study was aimed at detecting significant differences in the prevalence of AIVs type A and subtypes H5, H7, and H9 before and after massive poultry vaccination, particularly across different sampling places and sample types.
METHODS
Avian Influenza Environmental Surveillance Program
For the routine surveillance program, samples were collected in all 90 counties of the 11 prefectures in Zhejiang province, after March 2013. In each prefecture, one third of the counties were surveyed per quarter, to cover all the counties in the year of 2013. In 2014, the survey was done every month, with a quarterly coverage requirement for all of the counties in each prefecture. Approximately 10 environmental specimens (1–2 for each sample type) were collected from each sampling site. Overall, each prefecture was asked to collect 15–30 specimens from April to September and at least 30 specimens from October to March of the following year [17]. Due to the high incidence of H7N9 in early 2015, an avian influenza environmental surveillance program was strengthened and implemented in March 2015 with different sampling frequency and sample number. Accordingly, during the AIVs epidemic period from October to March of the following year, each prefecture was required to collect samples semimonthly, taking at least 30 samples each time, covering all the counties. Villages or towns were selected by turns in each county, and at least 2 sampling sites were randomly selected from each village or town. Emergency environmental surveillance programs were implemented in areas with confirmed cases of H7N9 infection in April 2013 [18].
Sources and Types of Environmental Sample
As introduced in previous studies [17–20], the sampling places included live poultry markets (LPMs), backyard poultry flocks, poultry-rearing farms, slaughtering and processing plants, wild bird habitats, and others. Six different sampling types were collected: swabs from poultry cages, swabs from tables for slaughtering or holding poultry, poultry feces, drinking water for poultry, sewage from cleaning poultry, and others. Each prefecture was not required to collect all 6 sampling types from each sampling place every month, but each one was recommended to collect as many as possible.
Sample Collection, Transportation, Storage, and Laboratory Testing
The samples were stored at 4°C, sent to local network laboratories within 48 hours, divided into 3 equal potions, and stored in 2-mL screw cap microtubes: 1 part was tested by local network laboratories; 1 was used for validation by Zhejiang provincial Center for Disease Control and Prevention (CDC); and 1 was transported to China CDC to be stored as backup sample. Each portion was at least 1.5 mL, and sample numbers were marked on the screw caps of the microtubes. Within 1 week after sampling, sample-related information was entered into the information management system, and the remaining portions, stored at −70°C, were sent to Zhejiang CDC by the network laboratories [19].
Viral ribonucleic acid was extracted from specimens using QIAGEN RNeasy Mini Kits (Hilden, Germany). Quantitative real-time reverse transcription polymerase chain reaction assays were used to detect influenza A virus, using primers FluA-Forward (5’-GAC CRA TCC TGT CAC CTC TGA C-3’) and FluA-Reverse (5’-GGG CAT TYT GGA CAA AKC GTC TAC G-3’) and a specific probe, FluA-Probe (5’-TGC AGT CCT CGC TCA CTG GGC ACG-3’) [18]. If the sample was positive for AIV type A, it was typed further for subtypes H5, H7, and H9 by using specific polymerase chain reaction primers/probes [19]. In the present study, if specimens were positive for influenza A while negative for subtypes H5, H7, and H9 at the same type, the test result was defined as “Other”. After laboratory tests were performed, the local CDC uploaded the laboratory results, matched to the corresponding specimen, to the online reporting system.
Definition of Period and Season
Based on a national study [9], we divided the study period into 7 periods with reference to the start of H7N9 epidemic. Each period started in September, except the first period. The first period was from March 1, 2013 to August 31, 2013, followed by the second period from September 1, 2013 to August 31, 2014, the third period from September 1, 2014 to August 31, 2015, and the fourth, fifth, and sixth periods of the same time range. The seventh period started from September 1, 2018 to December 31, 2018, when the current study was initiated. Spring, summer, autumn, and winter seasons were defined to be from March to May, June to August, September to November, and December to February of the next year, respectively [21].
Statistical Analysis
Data obtained from the online system were reported monthly or semimonthly by the laboratory staff. Percentages were calculated for categorical variables. To describe the spatiotemporal pattern of the prevalence of AIVs, we used the ring map toolbox in ArcGIS 10.2 (Esri Inc, Redlands, CA). Multivariable logistic regression analysis was used to analyze the risk of contamination by AIVs A and subtypes H5, H7, and H9 after the introduction of bivalent H5/H7-inactivated influenza vaccine compared with that before September 1, 2017, across different sampling places and sampling types. The criterion for variables included for multivariable analysis was P < .1. Stepwise variable selection was performed for logistic regression analysis. Two-sided P < .05 were considered statistically significant. SAS 9.2 (SAS Institute, Cary, NC) was used for analyses.
Ethical Consideration
The National Health Commission ruled that the collection of data for surveillance of avian influenza environmental contamination was part of a continuing public health response to prevent and control avian influenza. Therefore, the study was exempted from institutional review board assessment.
RESULTS
The Prevalence of Avian Influenza Viruses in Zhejiang Province From 2013 to 2018
From March 2013 to December 2018, a total of 55 130 specimens were collected and tested. The highest overall prevalence rate for the subtypes of AIV type A was for H9 subtype (10.73%) and the lowest was for H5 subtype (2.77%). The sample positive rate for H7 subtype gradually increased from period 1 to 4, dropped from 9.88% in period 5 to 1.16% in period 6, and to 0.07% in period 7. Except for H7 subtype, the sample positive rate did not show a remarkable decline for AIV type A and subtypes H5, H9, and other from period 5 to 7 (Table 1).
Table 1.
The Prevalence of Avian Influenza Viruses in Zhejiang Province From 2013 to 2018
| Period | Total | A (%) | H5 (%) | H7 (%) | H9 (%) | Other (%) |
|---|---|---|---|---|---|---|
| Overall | 55 130 | 13 802 (25.04) | 1529 (2.77) | 3912 (7.1) | 5915 (10.73) | 4780 (8.67) |
| 1 | 1340 | 87 (6.49) | 1 (0.07) | 18 (1.34) | 55 (4.1) | 14 (1.04) |
| 2 | 7382 | 1596 (21.62) | 92 (1.25) | 633 (8.57) | 430 (5.82) | 574 (7.78) |
| 3 | 9036 | 2650 (29.33) | 297 (3.29) | 929 (10.28) | 1040 (11.51) | 925 (10.24) |
| 4 | 10 548 | 3374 (31.99) | 586 (5.56) | 1058 (10.03) | 1655 (15.69) | 1050 (9.95) |
| 5 | 11 590 | 2883 (24.87) | 195 (1.68) | 1145 (9.88) | 990 (8.54) | 1016 (8.77) |
| 6 | 10 847 | 2293 (21.14) | 224 (2.07) | 126 (1.16) | 1140 (10.51) | 943 (8.69) |
| 7 | 4387 | 919 (20.95) | 134 (3.05) | 3 (0.07) | 605 (13.79) | 258 (5.88) |
Temporal Trend of the Prevalence of Avian Influenza Viruses in Zhejiang Province From 2013 to 2018
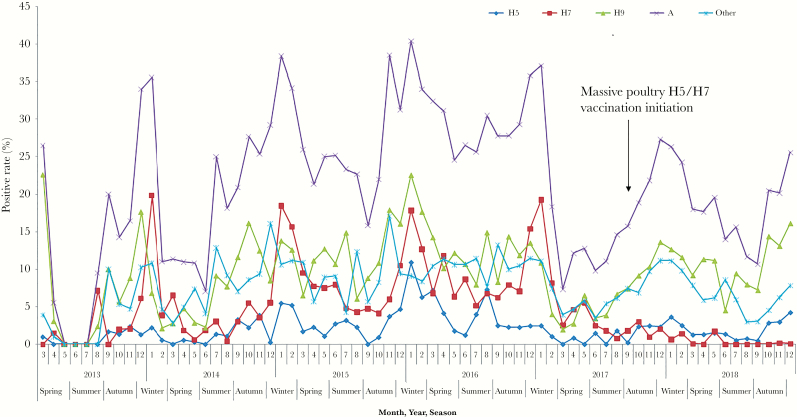
Before introduction of the poultry vaccine in September 2017, the observed seasonal pattern of AIV type A and subtypes H5, H7, and H9 showed autumn-winter peaks. After poultry vaccine introduction, the monthly positive rates of AIV type A, subtype H9, and other showed no obvious changes. However, the positives rate of H7 subtype was low and stayed at zero for several months, since September 2017. In contrast to H7, the positive rate of H5 subtype was low since September 2017 and showed an increasing trend after October 2018 (Figure 1).
Figure 1.
Temporal trend of the prevalence of avian influenza viruses in Zhejiang Province from 2013 to 2018.
Geographical Distribution of the Prevalence of Avian Influenza Viruses in Zhejiang Province From 2013 to 2018
Environmental contamination by AIV type A was detected in all the cities in Zhejiang province and was consistent with heavy pollution during periods 1 to 7 (Figure 2A). The prevalence of H5 subtype was almost stable from periods 5 to 7, with only the Taizhou city showing a declining trend (Figure 2B). Most cities showed increasing contamination by H7 subtype from periods 1 to 3, which remained stable during periods 4 to 5 and from period 6, began displaying a decreasing trend in all cities, and no positive sample was detected during periods 6 or 7 in half of the cities (Figure 2C). For the H9 subtype, the sample positive rate either remained steady or increased from period 5 to 7 in most cities (Figure 2D).
Figure 2.
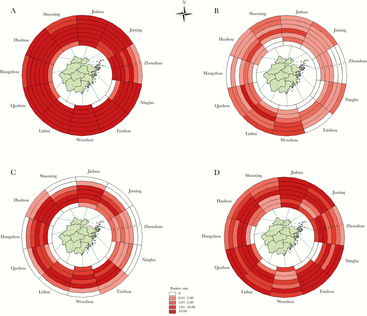
Geographical distribution of the prevalence of avian influenza viruses in Zhejiang Province from 2013 to 2018.
Overall Odds Ratio for the Prevalence of Avian Influenza Viruses in Zhejiang Province From 2013 to 2018
Overall, after adjusting for the sampling types, sampling places, season, and sampling cities, there was an increased risk of contamination by AIV A (adjusted odds ratio [aOR] = 1.08; 95% confidence interval [CI], 1.03–1.14) and H9 subtype (aOR = 1.58; 95% CI, 1.48–1.68) after September 2017, compared with that before September 2017. However, the risk for contamination by H7 subtype decreased (aOR = 0.12; 95% CI, 0.10–0.14), and there was no significant change for the H5 subtype (aOR = 1.10; 95% CI, 0.97–1.25).
Odds Ratio for the Prevalence of Avian Influenza Viruses Among Different Sampling Places in Zhejiang Province From 2013 to 2018
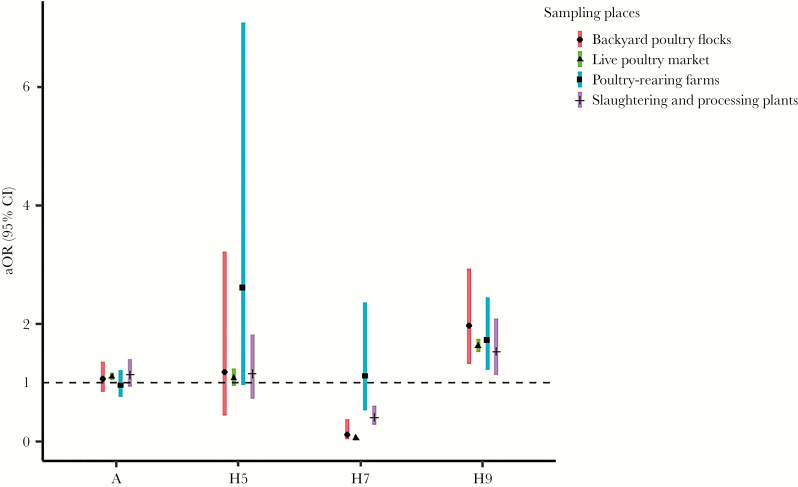
After adjusting for the sampling types, season, and sampling cities, a significantly increased risk was observed in the LPMs for the prevalence of AIV A (aOR = 1.10; 95% CI, 1.03–1.17). For H5 subtype, the change in prevalence across different sample places was not significant. However, in terms of the prevalence of H7 subtype, a statistically significant decreased risk of contamination was observed in backyard poultry flocks (aOR = 0.11; 95% CI, 0.03–0.38), LPMs (aOR = 0.06; 95% CI, 0.04–0.07), and slaughtering and processing plants (aOR = 0.41; 95% CI, 0.28–0.61). In contrast to H7 subtype, the risk of H9 subtype prevalence in all the 4 sampling places increased: backyard poultry flocks (aOR = 1.96; 95% CI, 1.31–2.93), LPMs (aOR = 1.62; 95% CI, 1.51–1.74), poultry-rearing farms (aOR = 1.72; 95% CI, 1.21–2.44), and slaughtering and processing plants (aOR = 1.53; 95% CI, 1.12–2.08) (Figure 3).
Figure 3.
Adjusted odds ratio (aOR) for the prevalence of avian influenza viruses among different sampling places in Zhejiang province from 2013 to 2018. CI, confidence interval.
Odds Ratio for the Prevalence of Avian Influenza Viruses Among Different Sampling Types in Zhejiang Province From 2013 to 2018
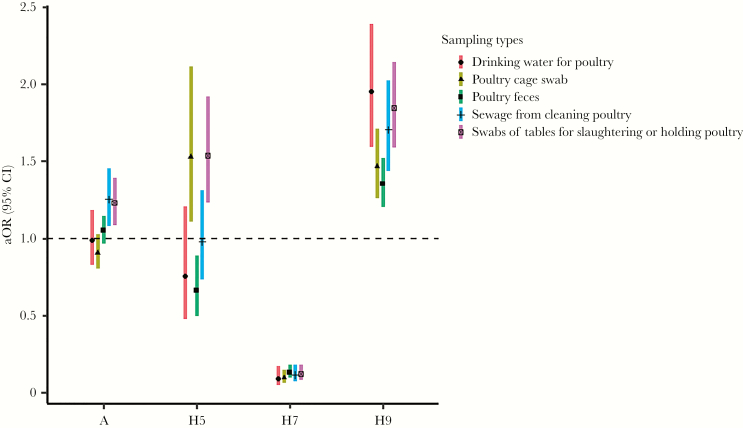
After adjusting for the sampling places, season, and sampling cities, there was an increased risk in the sewage from cleaning poultry (aOR = 1.25; 95% CI, 1.08–1.45) and swabs from tables for slaughtering or holding poultry (aOR = 1.23; 95% CI, 1.08–1.39) for the prevalence of AIV type A. In terms of the prevalence of H5 subtype, the risk increased significantly in the swabs from poultry cages (aOR = 1.53; 95% CI, 1.11–2.11) and swabs from tables for slaughtering or holding poultry (aOR = 1.54; 95% CI, 1.23–1.92) but decreased in poultry feces (aOR = 0.66; 95% CI, 0.50–0.89). When a comparison was made between subtypes H7 and H9, an inverse relation was observed in the 5 sampling types. The positive rate of H7 subtype consistently decreased in poultry feces (aOR = 0.13; 95% CI, 0.10–0.18), poultry cage swabs (aOR = 0.10; 95% CI, 0.06–0.15), drinking water for poultry (aOR = 0.09; 95% CI, 0.05–0.17), sewage from cleaning poultry (aOR = 0.12; 95% CI, 0.07–0.18), and swabs from tables for slaughtering or holding poultry (aOR = 0.12; 95% CI, 0.08–0.18), whereas that of the H9 subtype consistently increased, with the highest increased risk in drinking water for poultry (aOR = 1.95; 95% CI, 1.59–2.39) and the lowest in poultry feces (aOR = 1.35; 95% CI, 1.20–1.52) (Figure 4).
Figure 4.
Adjusted odds ratio (aOR) for the prevalence of avian influenza viruses among different sampling types in Zhejiang province from 2013 to 2018. CI, confidence interval.
DISCUSSION
In this study, based on 5 years of avian influenza environmental surveillance, we found changes in the prevalence of AIV type A and subtypes H5, H7, and H9 before and after massive H5/H7 poultry vaccination in September 2017. Since September 2017, the prevalence of H7 subtype had decreased across different areas, sampling places, and sampling types. In contrast to H7 subtype, the prevalence of H5 did not have a significant change in sampling places and the prevalence of AIV A and subtype H9 showed an increasing trend in some areas, sampling places, and sampling types. Overall, measures for reducing the AIVs circulation are still imperative, given the high contamination with AIVs A and the increasing trend in contamination by H9 subtype across different sampling places and sample types.
In agreement with previous studies [18, 22], our study demonstrated that the poultry-related environment was contaminated with AIV type A across different areas, sampling places, and sampling types. The significant increased risk of contamination by AIV type A occurring in LPMs, before and after massive poultry vaccination in the present study, indicated that LPMs have to be managed strictly. Previous studies had demonstrated that LPMs played an important role in promoting local transmission of multiple virus subtypes and generating opportunities for reassortment [23, 24]. Although human infection with H7N9 had decreased remarkably, and massive closures of LPMs had a substantial economic impact on the poultry industry, we recommend that the July 2014 policy of permanent closure of LPMs in urban areas in Zhejiang province should be kept. In addition, due to incomplete LPMs closures, effective poultry vaccines should also be actively developed and implemented to further reduce different types of AIVs contamination [7, 8].
Consistent with previous studies, our study showed that the H7 subtype contamination risk with LPMs decreased sharply after the introduction of H5/H7 bivalent-inactivated vaccine [13, 15]. Moreover, although previous studies showed that contamination by H7N9 has already expanded from LPMs to backyard poultry during the fourth and fifth epidemic seasons [20, 25], which made controlling difficult, our study showed that contamination by H7 subtype in backyard poultry was also significantly decreased. In addition, decreased contamination by H7 subtype in different sampling types is crucial for the reduction in human infections with avian influenza subtype H7, because people are prone to be exposed to these sample types when they are raising, killing, carrying, or selling poultry.
Similar to the recent report from the Guangdong province [15], the present study also showed that the prevalence of H5 subtype did not change significantly after the introduction of H5/H7 vaccine. The H5 vaccination program began in 2005 [13] and has successfully controlled H5 subtype to a low prevalence level. Although the new H5/H7 bivalent-inactivated vaccine could effectively reduce the prevalence of H7 subtype, it could not further reduce contamination by H5 subtype, due to 3 possible reasons. First, H5/H7 massive poultry vaccination was mainly implemented in chickens, and a previous study had indicated that H5 subtype detected in LPMs was mainly from ducks [13]. Therefore, the prevalence of H5 subtype in the environment could not be influenced by the new H5/H7 vaccine. Second, in terms of the vaccine effect of H5, evidence demonstrating the superiority of the new H5/H7 vaccine over the previous single H5 vaccine was not available. Finally, an immune competition between H5 and H7 subtypes is possible, as implied by a study which showed that competition between related subtypes could regulate influenza A virus subtype population dynamics [26], which necessitates further study. Nevertheless, in the present study, it could be concluded that the overall prevalence of H5 subtype did not increase after the implementation of the new poultry vaccine. Moreover, the significant increase in contamination by H5 subtype in the swabs from tables for slaughtering or holding poultry may indicate that the internal organs of poultry still contain high viral loads, as suggested by previous studies [23, 27, 28]. Therefore, to limit virus spread, segregating poultry-related slaughtering and chopping activities into zones are still recommended strongly [27]. In addition, the significant increase in contamination of H5 subtype in the poultry cage swab revealed that timely cleaning and disinfection are required to reduce the risk of infection.
According to the literature [29, 30], H9 subtype has significance for public health, because not only can it directly infect humans, but it can also donate partial or even a whole cassette of internal genes to generate novel human-lethal reassortants like H5N1, H7N9, H10N8, and H5N6 viruses. Our study showed that the environment was heavily contaminated with the H9 subtype, in agreement with previous reports [5, 29]. Moreover, the present study also showed that there is a significant increase in contamination by H9 subtype across different sample places and types. However, whether this increased contamination is related to the introduction of H5/H7 vaccine could not be answered and has to be explored further. Nevertheless, urgent controlling measures should be adopted, given the public health significance of H9 subtype. Besides LPMs closures, other effective interventions such as timely cleaning and disinfection of poultry contaminated areas, wearing protective mask, and washing hands frequently should also be encouraged [19, 20, 23].
This study has several limitations. First, although we revealed differences in the prevalence of AIV type A and subtypes H5, H7, and H9, before and after September 2017, it cannot be concluded that these changes were caused by the introduction of H5/H7 bivalent-inactivated vaccine, because observational study cannot prove causality. However, our results were comparable to those of studies done in other Chinese districts [13, 15]. Second, during routine surveillance, due to limited resources, we tested hemagglutinin but not neuraminidase. This might affect the clear evaluation of the risk of human infection by H7N9, H5N1, H5N6, and H9N2 viruses. However, the association between H7 subtype positive rate and the number of H7N9 cases has previously been proved [18]. Third, other potential factors caused by sampling method could result in a noncomparable AIVs prevalence before and after the vaccination. For example, our previous study found that the duration of sales per day, types of live poultry, LPM location, and the number of live poultry were the main risk factors for environmental contamination in LPMs [19]. If a public health physician collected samples from sites more likely to have risk factors for contamination before poultry vaccination, this could bias our results. Finally, the viability of AIVs in positive samples was uncertain, because the isolates were not cultured for confirmation.
CONCLUSIONS
In conclusion, our study revealed differences in the prevalence of AIV type A and subtypes H5, H7, and H9, before and after massive poultry vaccination. Although there was a sharp decrease in the positive rate of H7 subtype, measures for reducing AIV circulation are still imperative, given the high contamination by AIV type A and the increasing trend in contamination by H9 subtype across different sampling places and sample types. In addition, surveillance for different types of AIVs needs to be strengthened because early detection of the evolution and spread of these viruses is crucial. Regular monitoring of vaccination effectiveness should be conducted in the future to prevent potential human avian influenza transmissions.
Notes
Acknowledgments. We thank public health physicians and staff from county and city level of Centers for Disease Control and Prevention across Zhejiang province for their invaluable assistance with sample collection, laboratory testing and data reporting.
Author contributions. W. C., K. C. C., and E. C. conceived and designed the study. W. C. analyzed the data. X. W., S. Y.-F. L., Z. Y., and J. P. contributed reagents, materials, and/or analysis tools. W. C. wrote the paper. K. C. C., S. Y.-F. L., and M. W. reviewed the manuscript. All authors read and approved the final manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Division of Biostatistics, JC School of Public Health and Primary Care, The Chinese University of Hong Kong.
Financial support. This work was financially supported by grants from Zhejiang Province, Medical Research Programme (Grant numbers 2016RCA008 and 2017KY033) and the Natural Science Foundation (Grant number Y17H260003).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Gao R, Cao B, Hu Y, et al. . Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 2013; 368:1888–97. [DOI] [PubMed] [Google Scholar]
- 2. Pan M, Gao R, Lv Q, et al. . Human infection with a novel, highly pathogenic avian influenza A (H5N6) virus: virological and clinical findings. J Infect 2016; 72:52–9. [DOI] [PubMed] [Google Scholar]
- 3. Chen H, Yuan H, Gao R, et al. . Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet 2014; 383:714–21. [DOI] [PubMed] [Google Scholar]
- 4. Tong XC, Weng SS, Xue F, et al. . First human infection by a novel avian influenza A(H7N4) virus. J Infect 2018; 77:249–57. [DOI] [PubMed] [Google Scholar]
- 5. Gu M, Xu L, Wang X, Liu X. Current situation of H9N2 subtype avian influenza in China. Vet Res 2017; 48:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma MJ, Zhao T, Chen SH, et al. . Avian influenza a virus infection among workers at live poultry markets, China, 2013–2016. Emerg Infect Dis 2018; 24:1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuk SS, Erdene-Ochir TO, Kwon JH, et al. . Efficacy of clade 2.3.2 H5 commercial vaccines in protecting chickens from clade 2.3.4.4 H5N8 highly pathogenic avian influenza infection. Vaccine 2017; 35:1316–22. [DOI] [PubMed] [Google Scholar]
- 8. Yang W, Yin X, Guan L, et al. . A live attenuated vaccine prevents replication and transmission of H7N9 highly pathogenic influenza viruses in mammals. Emerg Microbes Infect 2018; 7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou L, Ren R, Yang L, et al. . Sudden increase in human infection with avian influenza A(H7N9) virus in China, September-December 2016. Western Pac Surveill Response J 2017; 8:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi J, Deng G, Ma S, et al. . Rapid evolution of H7N9 highly pathogenic viruses that emerged in China in 2017. Cell Host Microbe 2018; 24: 558–568.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou L, Tan Y, Kang M, et al. . Preliminary epidemiology of human infections with highly pathogenic avian influenza A(H7N9) virus, China, 2017. Emerg Infect Dis 2017; 23:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma MJ, Yang Y, Fang LQ. Highly pathogenic avian H7N9 influenza viruses: recent challenges. Trends Microbiol 2019; 27:93–5. [DOI] [PubMed] [Google Scholar]
- 13. Zeng X, Tian G, Shi J, et al. . Vaccination of poultry successfully eliminated human infection with H7N9 virus in China. Sci China Life Sci 2018; 61:1465–73. [DOI] [PubMed] [Google Scholar]
- 14. Cheng W, Wang X, Shen Y, et al. . Comparison of the three waves of avian influenza A(H7N9) virus circulation since live poultry markets were permanently closed in the main urban areas in Zhejiang Province, July 2014-June 2017. Influenza Other Respir Viruses 2018; 12: 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu J, Ke C, Lau EHY, et al. . Influenza H5/H7 virus vaccination in poultry and reduction of zoonotic infections, Guangdong Province, China, 2017-18. Emerg Infect Dis 2019; 25:116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Luo T, Shen Y. Deciphering the sharp decrease in H7N9 human infections. Trends Microbiol 2018; 26:971–3. [DOI] [PubMed] [Google Scholar]
- 17. He F, Chen EF, Li FD, et al. . Human infection and environmental contamination with avian influenza A (H7N9) virus in Zhejiang Province, China: risk trend across the three waves of infection. BMC Public Health 2015; 15:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Liu S, Mao H, et al. . Surveillance of avian H7N9 virus in various environments of Zhejiang Province, China before and after live poultry markets were closed in 2013–2014. PLoS One 2015; 10:e0135718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Wang Q, Cheng W, et al. . Risk factors for avian influenza virus contamination of live poultry markets in Zhejiang, China during the 2015–2016 human influenza season. Sci Rep 2017; 7: 42722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang XX, Cheng W, Yu Z, et al. . Risk factors for avian influenza virus in backyard poultry flocks and environments in Zhejiang Province, China: a cross-sectional study. Infect Dis Poverty 2018; 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu Z, Wu L, Gao D, Fan G. Method for four seasons division in Zhejiang Province. Meteorological Science and Technology 2014; 42: 474–81. [Google Scholar]
- 22. Zhou J, Wu J, Zeng X, et al. . Isolation of H5N6, H7N9 and H9N2 avian influenza A viruses from air sampled at live poultry markets in China, 2014 and 2015. Euro Surveill 2016; 21. doi:10.2807/1560-7917.ES.2016.21.35.30331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y, Biswas PK, Giasuddin M, et al. . Prevalence of avian influenza A(H5) and A(H9) viruses in live bird markets, Bangladesh. Emerg Infect Dis 2018; 24:2309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu B, Havers F, Chen E, et al. . Risk factors for influenza A(H7N9) disease--China, 2013. Clin Infect Dis 2014; 59: 787–94. [DOI] [PubMed] [Google Scholar]
- 25. Zhou L, Ren R, Ou J, et al. . Risk factors for Influenza A(H7N9) disease in China, a matched case control study, October 2014 to April 2015. Open Forum Infect Dis 2016; 3:ofw182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Latorre-Margalef N, Brown JD, Fojtik A, et al. . Competition between influenza A virus subtypes through heterosubtypic immunity modulates re-infection and antibody dynamics in the mallard duck. PLoS Pathog 2017; 13:e1006419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Indriani R, Samaan G, Gultom A, et al. . Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerg Infect Dis 2010; 16:1889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertran K, Balzli C, Kwon YK, et al. . Airborne transmission of highly pathogenic influenza virus during processing of infected poultry. Emerg Infect Dis 2017; 23:1806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang W, Liu S, Hou G, et al. . Chinese and global distribution of H9 subtype avian influenza viruses. PLoS One 2012; 7:e52671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li C, Wang S, Bing G, et al. . Genetic evolution of influenza H9N2 viruses isolated from various hosts in China from 1994 to 2013. Emerg Microbes Infect 2017; 6:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]