ABSTRACT
Background: Although a high prevalence of anemia and related disease burden have been documented in China, limited evidence is available on the current population-level iron status and risk factors for iron imbalance.
Objective: We explored the associations of dietary, lifestyle, and sociodemographic factors with iron status in Chinese adults.
Design: Our study population consisted of 7672 adults aged 18–65 y from the 2009 China Health and Nutrition Survey. Diet was assessed with the use of 3 consecutive 24-h dietary recalls. Serum ferritin, serum transferrin receptor, and hemoglobin concentrations were measured.
Results: The geometric means ± SDs for ferritin concentrations were 135.9 ± 2.7 ng/mL in men and 42.7 ± 3.1 ng/mL in women. After adjustment for potential risk factors, including high-sensitivity C-reactive protein concentration, the association between age and ferritin concentration was inverse in men (P-trend < 0.001) and positive in women (P-trend < 0.001). We observed a positive association between body mass index (in kg/m2) and ferritin concentration in both men and women (both P-trends < 0.001). Dietary phytate intake was inversely associated with ferritin concentration in men (P-trend = 0.002) but not in women. Red meat consumption was positively associated with ferritin concentration both in men (P-trend = 0.002) and in older women (P-trend = 0.009). Lower intakes of grains and higher intakes of pork and poultry were associated with higher ferritin concentrations (all P-trends ≤ 0.05) in men but not in women. We observed variations in ferritin concentrations across different geographic regions (both P ≤ 0.01).
Conclusions: Serum ferritin concentrations varied across different sociodemographic, lifestyle, and dietary factors in this Chinese population. A higher intake of red meat was associated with higher ferritin concentrations in men and older women.
Keywords: China, anemia, diet, iron stores, lifestyle, ferritin
INTRODUCTION
Body iron stores are involved in multiple biological functions, including electron transfer reactions, gene regulation, binding and transport of oxygen, and regulation of cell growth and differentiation (1). Iron-deficient individuals may suffer from symptoms such as fatigue, dizziness, shortness of breath, and muscle weakness and have impaired work capacity and immune function (2). Serum ferritin concentration is the most specific biochemical test for measuring total body iron stores (2), although its usefulness in the presence of inflammation and iron depletion may be limited (3). The serum transferrin receptor:ferritin ratio (TfR:F)7 index has been proposed as an alternative to serum ferritin concentration for measuring iron stores because of its potential to offer additional information on functional iron quantified by serum transferrin receptor concentration (4). Multiple dietary, lifestyle, and sociodemographic factors have been related to body iron stores in Western populations. Dietary factors such as dietary heme and nonheme iron (5–9), alcohol consumption (5, 6), vitamin C intake (6, 10), consumption of meat (11–14) and fish (11), a dietary pattern featuring high vegetable and red meat intakes (14) and iron supplementation (5, 6) were positively associated with higher body iron stores, as measured by ferritin concentrations. Nondietary factors such as age (5, 6, 11), time since menopause (5), menopausal status (11), blood donation, BMI (5, 9), physical activity (5), aspirin use (5), gastrointestinal ulcer (5), sex (6, 9), race and ethnicity (15), menstrual blood loss (13), serum hepcidin (16), and genetic factors such as the hemochromatosis C282Y mutation (13) were also related to iron status.
In China, iron deficiency anemia still remains the most important nutritional disorder. According to the Global Burden of Disease Study 2010, iron deficiency anemia in China ranked as the top contributor to disease burden within the category of communicable, maternal, neonatal, and nutritional disorders (17). The most recent nationwide prevalence of anemia, as determined by measuring hemoglobin concentration, was reported by the 2002 China National Nutrition and Health Survey. The prevalence of anemia was 15.8% in men and 23.3% in women (18). Despite the high prevalence of anemia and its related disease burden, there are limited data on iron status, including hemoglobin concentrations and body iron stores, in Chinese adults. Although many dramatic changes have occurred in the food supply, dietary behaviors, and national economy in recent years, nationally representative estimates of iron status have not been updated for more than a decade (19). Furthermore, potential determinants of iron status have not been comprehensively investigated in China, although there is an imperative need to inform population-level strategies for iron-deficiency anemia prevention. Therefore, we examined the associations of dietary, lifestyle, and sociodemographic factors with body iron status in the 2009 China Health Nutrition Survey (CHNS). To characterize iron status in detail (2), we examined a series of biochemical markers, including the TfR:F index and serum ferritin, serum transferrin receptor, and hemoglobin concentrations.
METHODS
Study population
CHNS is a large-scale, longitudinal, household-based survey initiated in 1989 that consists of representative participants of varying economic status, health indicators, and geography from rural, urban, and suburban areas throughout China. CHNS geographically covers 9 provinces in China: Liaoning, Shandong, Heilongjiang, Henan, Jiangsu, Hubei, Hunan, Guizhou, and Guangxi. Approximately 56% of China’s total population lives in these 9 provinces. Although CHNS is not nationally representative, these provinces were selected to generally represent variations in geography, economic development, and health indicators of all provinces in the country (20). At baseline (1989), CHNS recruited study participants with the use of a multistage random-cluster design. Counties in the sampled provinces were stratified by 3 income levels. A weighted sampling scheme was used to randomly select 1 low-income county, 2 middle-income counties, and 1 high-income county in each sampled province. Probability proportional-to-size sampling was used to select the sample from these units. To further incorporate urban areas initially not within the county strata, the sample population later included the provincial capital and a low-income city from each province. Within each county, the township capital was selected, and 3 villages were randomly selected. Within each city, urban and suburban neighborhoods were randomly selected. For the selection of neighborhoods from townships and villages, the same random sampling method was applied. Participants were surveyed at baseline and every 2–3 y thereafter. Our study population consisted of 7672 (3318 men and 4354 women) adults aged 18–65 y from the 2009 CHNS. The study protocol and analysis were approved by the institutional review boards at the University of North Carolina at Chapel Hill, the Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, and the China-Japan Friendship Hospital.
Assessment of dietary variables
Dietary data were collected by trained interviewers with the use of 3 24-h dietary recalls taken on consecutive days. CHNS has made great efforts to interview participants in the same season in each survey cycle; most participants were interviewed within a short period in the fall (21). Nutrient intake, including intakes of iron, calcium, vitamin C, and total energy, was calculated based on dietary data and the Chinese Food Composition Table (22). Because the Chinese Food Composition Table does not include phytates, we calculated phytate intake based on the phytate content of foods commonly consumed in China, as measured by Ma et al. (23). We grouped food consumption into 10 food groups of interest, including red meat, grains, pork, vegetables, fish, poultry, eggs, tubers, fruits, and milk. Details of dietary information collection can be found elsewhere (24).
Assessment of nondietary variables
Trained interviewers administered questionnaires to collect information on sociodemographic and household variables. Participants’ physical activity was assessed with the use of a 7-d physical activity recall questionnaire that was previously validated in a Chinese population (25). Metabolic equivalent values were assigned to each physical activity according to the Compendium of Physical Activities, and time spent in moderate-to-vigorous physical activity was computed (25). Data on body height and weight were collected by trained health workers through a comprehensive physical examination conducted at a local clinic or at the respondent’s home if necessary. Body height was measured without shoes to the nearest 0.2 cm with the use of a portable stadiometer. Body weight was measured without shoes and in light clothing to the nearest 0.1 kg on a calibrated beam scale.
Biochemical analyses
Fasting blood samples were collected in neighborhood clinics or participants’ homes. Blood samples were collected by venipuncture (12 mL) after an overnight fast and centrifuged 30 min after blood collection, with 3000 × g for 15 min. Biochemical markers were measured at a national central laboratory in Beijing with strict quality control. Serum ferritin was measured via radioimmunoassay on an XH-6020 gamma counter (Beijing North Institute of Biotechnology). Hemoglobin concentration in whole blood was measured with the use of volume, conductivity, light-scatter technology on a Beckman Coulter LH750. Serum-soluble transferrin receptor concentration was measured via nephelometry on a Siemens B-type natriuretic peptide assay. Serum high-sensitivity C-reactive protein (hs-CRP) concentrations were measured with the use of a high-sensitivity immunoturbidmetric method (Hitachi 7600 automated analyzer) with Denka Seiken reagents. Because acute infection causes an artificially higher serum ferritin concentration, we multiplied ferritin values in participants with an hs-CRP ≥5 mg/L by 0.65 following WHO recommendations (26).
Statistical analysis
Because of sex differences in iron status as reported by previous studies (18, 27), we performed our analysis separately for men and women. To normalize skewed distributions in serum ferritin, we applied a logarithmic transformation. Values were back-transformed and presented as geometric means. We categorized age into 3 groups and moderate-to-vigorous physical activity into 4 groups. We also categorized participants into 4 BMI groups according to the cutoffs recommended by the Guidelines for Preventing and Controlling Overweight and Obesity in Chinese Adults (20). For dietary factors, we categorized variables into quartiles or arbitrary categories according to their population distribution. When examining the associations of dietary factors with iron stores, we excluded participants with missing dietary information (n = 719). We used general linear regression models to calculate multivariable-adjusted geometric means of various measures of iron status by categories of different dietary and nondietary factors, simultaneously adjusting for age, annual income level, moderate-to-vigorous physical activity, BMI (in kg/m2), alcohol intake, serum hs-CRP concentration, total energy intake, and dietary intakes of vitamin C, phytates, and calcium as continuous variables and educational status, place of residence, geographic region, and smoking status as categorical variables except the stratification variable. To quantify a linear trend, we assigned the median value within each category and modeled this variable continuously; the Wald test was used for testing statistical significance. Because accumulated menstrual blood loss has been shown to be a major contributor to the high prevalence of iron-deficiency anemia in premenopausal Chinese women (18), we hypothesized that the association between red meat, the major food source of heme iron, and body iron status might vary according to menopausal status in women. Therefore, we first categorized the study population into 2 subgroups based on a cutoff age of 50 y, which is close to the median age at menopause in Chinese women (28). We then examined the association between red meat consumption and ferritin concentration in each age group separately. Last, we added a multiplicative term between the binary variable of age and median intake of red meat to the multivariable models; the Wald test was used for testing the statistical significance of the interaction term. All analyses were performed with the use of SAS version 9.4 (SAS Institute) at a 2-tailed α of 0.05.
RESULTS
The geometric means for ferritin concentrations were 135.9 ng/mL (SD: 2.7 ng/mL) for men and 42.7 ng/mL (SD: 3.1 ng/mL) for women. Table 1 shows that most participants were aged 18–44 y, lived in rural areas, and had an annual household income <9000 renminbi (RMB) (1 RMB is equivalent to ∼0.12 US$). The study population covered wide regions of China.
TABLE 1.
Serum ferritin concentrations according to categories of sociodemographic and lifestyle factors1
| Men | Women | |||||
| n | Mean, ng/mL | Multivariable-adjusted mean (95% CI), ng/mL | n | Mean, ng/mL | Multivariable-adjusted mean (95% CI), ng/mL | |
| Age, y | ||||||
| 18–44 | 1457 | 132.8 | 137.4 (129.5, 145.9) | 2196 | 29.1 | 28.5 (26.9, 30.1) |
| 45–54 | 960 | 132.0 | 128.7 (119.7, 138.3) | 1114 | 39.3 | 40.1 (37.3, 43.1) |
| 55–65 | 901 | 117.4 | 118.7 (110.6, 127.4) | 1044 | 81.7 | 75.2 (69.8, 81.1) |
| P-trend | 0.009 | <0.001 | <0.001 | <0.001 | ||
| Place of residence | ||||||
| Urban | 918 | 122.4 | 120.7 (113.0, 128.9) | 1093 | 44.7 | 41.5 (39.0, 44.3) |
| Rural | 2400 | 131.1 | 133.4 (126.5, 140.7) | 3261 | 37.9 | 39.8 (37.7, 42.0) |
| P value | 0.06 | 0.008 | <0.001 | 0.24 | ||
| Annual income, RMB | ||||||
| <9000 | 1472 | 117.9 | 121.6 (113.3, 130.5) | 2660 | 39.7 | 39.2 (36.7, 41.8) |
| 9000–15,000 | 538 | 133.1 | 136.5 (125.3, 148.7) | 707 | 39.8 | 40.4 (37.3, 43.7) |
| >15,000–25,000 | 642 | 130.9 | 131.3 (121.6, 141.7) | 581 | 38.4 | 40.3 (37.3, 43.5) |
| >25,000 | 666 | 134.4 | 131.0 (122.5, 140.1) | 406 | 43.3 | 43.2 (40.2, 46.4) |
| P-trend | 0.01 | 0.28 | 0.07 | 0.03 | ||
| BMI, kg/m2 | ||||||
| <18.5 | 173 | 89.7 | 89.8 (77.3, 104.2) | 210 | 27.8 | 36.6 (31.8, 42.1) |
| 18.5–24 | 1712 | 113.4 | 114.2 (107.9, 120.9) | 2031 | 34.4 | 38.8 (36.7, 41.0) |
| >24–28 | 1063 | 152.0 | 153.4 (143.7, 163.8) | 1101 | 44.2 | 42.3 (39.4, 45.3) |
| >28 | 294 | 171.4 | 174.9 (155.8, 196.5) | 379 | 49.5 | 46.5 (41.7, 51.8) |
| P-trend | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Moderate-to-vigorous physical activity time, h/wk | ||||||
| <1 | 856 | 132.9 | 130.5 (121.7, 139.9) | 1152 | 35.1 | 40.5 (37.9, 43.2) |
| 1–3 | 688 | 134.6 | 137.0 (126.7, 148.2) | 890 | 39.7 | 40.6 (37.6, 43.7) |
| >3–6 | 793 | 124.9 | 127.1 (117.7, 137.3) | 930 | 40.7 | 40.4 (37.4, 43.5) |
| >6 | 905 | 121.4 | 124.1 (115.3, 133.6) | 749 | 37.5 | 39.9 (36.8, 43.4) |
| P-trend | 0.02 | 0.12 | 0.19 | 0.76 | ||
| Education | ||||||
| Illiterate | 349 | 121.7 | 131.6 (118.2, 146.4) | 1416 | 57.8 | 40.4 (37.6, 43.4) |
| Primary school | 582 | 119.1 | 124.1 (114.4, 134.7) | 792 | 39.8 | 36.4 (33.9, 39.2) |
| High school | 1906 | 130.9 | 128.6 (123.0, 134.4) | 1713 | 30.9 | 34.7 (33.0, 36.5) |
| Some college | 264 | 129.2 | 128.4 (113.9, 144.7) | 271 | 37.4 | 44.6 (39.4, 50.5) |
| ≥College | 217 | 140.4 | 133.7 (116.6, 153.2) | 162 | 33.9 | 47.0 (39.8, 55.5) |
| P-trend | 0.03 | 0.75 | <0.001 | 0.94 | ||
| Geographic region | ||||||
| South | 1040 | 124.9 | 119.4 (111.8, 127.6) | 1223 | 36.5 | 37.1 (34.8, 39.6) |
| Middle | 770 | 138.9 | 137.7 (127.7, 148.5) | 935 | 35.8 | 39.3 (36.5, 42.3) |
| North | 1508 | 125.3 | 131.2 (123.7, 139.2) | 2196 | 44.7 | 45.0 (42.3, 47.9) |
| P value | 0.04 | 0.01 | <0.001 | <0.001 | ||
| Tea consumption, times/wk | ||||||
| ≤1 | 1908 | 123.0 | 126.1 (119.5, 133.1) | 2891 | 37.4 | 40.7 (38.7, 42.8) |
| >1–6 | 302 | 134.7 | 133.2 (119.0, 149.1) | 236 | 40.2 | 41.2 (36.1, 47.0) |
| >6 | 1032 | 135.8 | 134.2 (125.3, 143.8) | 594 | 39.8 | 38.3 (35.0, 41.8) |
| P-trend | 0.009 | 0.11 | 0.19 | 0.19 | ||
| Smoking | ||||||
| No | 1308 | 124.3 | 124.3 (116.9, 132.2) | 4233 | 39.8 | 40.0 (38.2, 42.0) |
| Current or ever | 2010 | 130.8 | 132.3 (125.4, 139.7) | 121 | 62.5 | 51.2 (42.5, 61.7) |
| P value | 0.14 | 0.08 | <0.001 | 0.01 | ||
| Alcohol intake, g/d | ||||||
| 0 | 1339 | 121.1 | 124.8 (117.3, 132.7) | 3444 | 37.8 | 40.2 (38.3, 42.1) |
| >0–15 | 990 | 120.3 | 120.2 (112.4, 128.7) | 219 | 40.1 | 42.3 (37.0, 48.5) |
| >15 | 913 | 148.4 | 147.1 (137.0, 158.0) | 58 | 44.3 | 42.6 (32.8, 55.2) |
| P-trend | 0.87 | 0.41 | 0.45 | 0.46 | ||
| Iron intake | ||||||
| Quartile 1 | 795 | 134.2 | 133.4 (122.7, 145.1) | 906 | 36.6 | 38.1 (35.1, 41.4) |
| Quartile 2 | 818 | 125.3 | 126.2 (117.2, 136.0) | 1001 | 39.0 | 40.3 (37.4, 43.3) |
| Quartile 3 | 817 | 129.7 | 131.9 (122.5, 142.0) | 959 | 39.1 | 41.5 (38.6, 44.7) |
| Quartile 4 | 812 | 121.9 | 125.8 (115.7, 136.9) | 855 | 37.6 | 42.4 (39.0, 46.1) |
| P-trend | 0.09 | 0.50 | 0.85 | 0.11 | ||
| Phytate intake | ||||||
| Quartile 1 | 793 | 141.6 | 143.0 (132.1, 154.8) | 925 | 39.2 | 42.3 (39.1, 45.7) |
| Quartile 2 | 809 | 128.2 | 130.0 (120.6, 140.1) | 979 | 37.3 | 40.2 (37.4, 43.2) |
| Quartile 3 | 823 | 125.2 | 125.8 (116.9, 135.4) | 947 | 38.7 | 40.2 (37.4, 43.2) |
| Quartile 4 | 817 | 118.4 | 119.9 (110.7, 129.9) | 870 | 36.7 | 38.5 (35.4, 41.8) |
| P-trend | <0.001 | 0.002 | 0.30 | 0.11 | ||
| Vitamin C intake | ||||||
| Quartile 1 | 807 | 132.3 | 130.7 (121.4, 140.8) | 915 | 37.1 | 40.5 (37.6, 43.6) |
| Quartile 2 | 818 | 135.8 | 134.5 (125.1, 144.6) | 988 | 35.8 | 39.4 (36.7, 42.3) |
| Quartile 3 | 812 | 129.0 | 130.7 (121.3, 140.7) | 947 | 38.3 | 40.6 (37.8, 43.7) |
| Quartile 4 | 805 | 115.7 | 121.0 (112.0, 130.7) | 871 | 41.3 | 41.2 (38.1, 44.6) |
| P-trend | 0.002 | 0.07 | 0.01 | 0.57 | ||
| Calcium intake | ||||||
| Quartile 1 | 810 | 133.7 | 134.3 (124.2, 145.2) | 930 | 38.4 | 41.3 (38.1, 44.6) |
| Quartile 2 | 811 | 126.7 | 128.1 (119.0, 138.0) | 930 | 35.4 | 37.4 (34.8, 40.3) |
| Quartile 3 | 811 | 129.2 | 129.4 (120.1, 139.3) | 931 | 37.1 | 40.0 (37.2, 43.0) |
| Quartile 4 | 810 | 122.7 | 125.6 (116.2, 135.9) | 930 | 41.4 | 43.1 (39.8, 46.5) |
| P-trend | 0.12 | 0.30 | 0.03 | 0.13 | ||
All the mean values are the geometric mean of ferritin concentration. The medians for quartiles of nutrient intake (men/women) were as follows—iron: 15.0/13.0, 19.8/17.3, 24.9/21.8, and 35.8/31.7 mg/d; vitamin C: 37.3/33.6, 64.0/61.4, 90.6/88.6, and 137.4/137.0 mg/d; calcium: 230.4/198.8, 322.5/285.6, 431.2/381.8, and 640.7/578.6 mg/d; and phytates: 3.8/3.3, 9.7/8.4, 14.3/12.5, and 21.8/18.9 g/d. For categories of age, annual income, place of residence, geographic region, educational status, and smoking status, the general linear model (model 1) was simultaneously adjusted for age, annual income level, moderate-to-vigorous physical activity time, BMI, alcohol intake, tea consumption, and serum high-sensitivity C-reactive protein concentration as continuous variables and educational status, place of residence, geographic region, and smoking status as categorical variables except the stratification variable. For categories of moderate-to-vigorous physical activity time, BMI, alcohol intake, tea consumption, and dietary intakes of vitamin C, iron, phytates, and calcium, the general linear model was adjusted for total energy intake as continuous variables in addition to model 1 except the stratification variable. RMB, renminbi.
Geometric mean serum ferritin concentrations according to sociodemographic and lifestyle factors are shown in Table 1. After adjusting for potential risk factors, age was inversely associated with ferritin concentration in men but positively associated with ferritin concentration in women (both P-trends < 0.001). Serum ferritin concentration significantly varied across different geographic regions (P = 0.01 for men and P < 0.001 for women). Annual income (P-trend = 0.03) and smoking (P = 0.01) were positively associated with ferritin concentration in women but not in men. BMI was positively associated with ferritin concentration in both men and women (both P-trends < 0.001). Dietary intake of iron was not associated with ferritin concentration. Phytate intake was not associated with ferritin concentration in all participants; an inverse association between phytate intake and ferritin concentration only existed in men (P-trend = 0.002).
Table 2 shows serum ferritin concentrations according to categories of food intake. Red meat consumption was significantly associated with ferritin concentration in both men (P-trend = 0.002) and women (P-trend = 0.03). Figure 1 shows the joint associations of red meat consumption and age groups with serum ferritin concentrations in men and women. For men, serum ferritin was positively associated with red meat intake for both age groups. We observed a strong positive association between red meat intake and ferritin concentration (P-trend = 0.009) in women aged ≥50 y, whereas the association was not significant in women aged <50 y (P-trend = 0.44). Meanwhile, women aged ≥50 y had significantly higher serum ferritin concentrations than women aged <50 y. In men but not women, the consumption of pork (P-trend = 0.006) and poultry (P-trend < 0.001) was positively associated with ferritin concentration. Women who had a higher consumption of tubers such as sweet potatoes, Chinese yam, and taro had a higher concentration of ferritin (P-trend = 0.009).
TABLE 2.
Serum ferritin concentrations according to categories of food intake1
| Men | Women | |||||
| n | Mean, ng/mL | Multivariable-adjusted mean (95% CI), ng/mL | n | Mean, ng/mL | Multivariable-adjusted mean (95% CI), ng/mL | |
| Red meat | ||||||
| Quartile 1 | 801 | 115.4 | 116.2 (107.7, 125.3) | 876 | 38.0 | 39.6 (36.6, 42.8) |
| Quartile 2 | 850 | 127.5 | 129.0 (120.0, 138.6) | 1026 | 35.9 | 38.3 (35.7, 41.1) |
| Quartile 3 | 785 | 134.5 | 137.5 (127.5, 148.3) | 932 | 38.9 | 40.6 (37.7, 43.7) |
| Quartile 4 | 806 | 135.6 | 137.2 (127.0, 148.3) | 887 | 39.6 | 43.5 (40.2, 47.0) |
| P-trend | <0.001 | 0.002 | 0.17 | 0.03 | ||
| Grains | ||||||
| Quartile 1 | 793 | 136.1 | 137.0 (126.9, 147.9) | 966 | 36.6 | 39.6 (36.8, 42.7) |
| Quartile 2 | 815 | 129.8 | 131.9 (122.5, 141.9) | 1002 | 39.9 | 41.2 (38.4, 44.2) |
| Quartile 3 | 807 | 123.8 | 123.7 (114.8, 133.2) | 915 | 39.3 | 41.1 (38.2, 44.2) |
| Quartile 4 | 827 | 122.9 | 123.9 (114.5, 134.1) | 838 | 36.1 | 39.3 (36.1, 42.8) |
| P-trend | 0.03 | 0.05 | 0.52 | 0.77 | ||
| Pork | ||||||
| Quartile 1 | 828 | 117.9 | 119.0 (110.4, 128.2) | 921 | 37.8 | 39.8 (36.8, 42.9) |
| Quartile 2 | 783 | 124.8 | 125.6 (116.6, 135.2) | 980 | 36.4 | 39.3 (36.6, 42.2) |
| Quartile 3 | 822 | 135.8 | 139.0 (129.0, 149.7) | 904 | 38.2 | 40.3 (37.4, 43.4) |
| Quartile 4 | 809 | 134.2 | 136.4 (126.2, 147.4) | 916 | 39.8 | 42.4 (39.3, 45.8) |
| P-trend | 0.005 | 0.006 | 0.17 | 0.14 | ||
| Vegetables | ||||||
| Quartile 1 | 797 | 133.1 | 131.2 (121.8, 141.3) | 950 | 34.6 | 39.5 (36.7, 42.5) |
| Quartile 2 | 825 | 134.1 | 134.1 (124.7, 144.1) | 945 | 36.5 | 39.6 (36.8, 42.6) |
| Quartile 3 | 808 | 127.0 | 129.1 (119.8, 139.1) | 938 | 41.2 | 43.4 (40.4, 46.6) |
| Quartile 4 | 812 | 118.2 | 121.8 (112.7, 131.5) | 888 | 40.3 | 38.9 (36.0, 42.1) |
| P-trend | 0.006 | 0.09 | <0.001 | 0.97 | ||
| Fish, g/d | ||||||
| 0 | 1783 | 124.4 | 124.7 (118.0, 131.7) | 2133 | 37.6 | 39.9 (37.7, 42.1) |
| >0–60 | 511 | 134.3 | 136.3 (124.6, 149.1) | 694 | 36.8 | 40.2 (37.0, 43.6) |
| >60 | 948 | 131.5 | 134.6 (125.5, 144.4) | 894 | 40.0 | 41.6 (38.6, 44.8) |
| P-trend | 0.17 | 0.06 | 0.17 | 0.30 | ||
| Poultry, g/d | ||||||
| 0 | 2350 | 121.9 | 123.9 (117.8, 130.2) | 2772 | 38.9 | 40.7 (38.7, 42.9) |
| >0–15 | 26 | 169.2 | 171.3 (118.5, 247.7) | 37 | 32.6 | 37.0 (26.8, 51.1) |
| >15 | 866 | 144.7 | 143.7 (133.6, 154.5) | 912 | 35.5 | 39.6 (36.8, 42.6) |
| P-trend | <0.001 | <0.001 | 0.03 | 0.49 | ||
| Eggs, g/d | ||||||
| 0 | 1100 | 124.9 | 127.5 (119.1, 136.4) | 1242 | 38.8 | 39.1 (36.5, 41.9) |
| >0–40 | 1588 | 130.8 | 131.6 (124.4, 139.3) | 1919 | 37.2 | 40.2 (38.0, 42.5) |
| >40 | 554 | 126.1 | 125.8 (115.4, 137.2) | 560 | 38.9 | 43.2 (39.5, 47.2) |
| P-trend | 0.85 | 0.79 | 0.98 | 0.06 | ||
| Tubers, g/d | ||||||
| 0 | 1878 | 129.9 | 130.4 (123.3, 138.0) | 2152 | 38.0 | 39.2 (37.1, 41.5) |
| >0–60 | 524 | 133.6 | 135.7 (124.3, 148.1) | 680 | 36.1 | 39.7 (36.6, 43.1) |
| >60 | 840 | 120.5 | 122.4 (113.5, 132.0) | 889 | 39.6 | 44.0 (40.7, 47.6) |
| P-trend | 0.07 | 0.14 | 0.36 | 0.009 | ||
| Fruits, g/d | ||||||
| 0 | 2246 | 127.5 | 127.0 (120.5, 133.8) | 2297 | 37.9 | 39.6 (37.5, 41.9) |
| >0–80 | 290 | 150.4 | 154.4 (137.6, 173.4) | 346 | 41.9 | 44.3 (39.7, 49.5) |
| >80 | 706 | 121.3 | 126.9 (117.4, 137.3) | 1078 | 36.9 | 40.5 (37.8, 43.4) |
| P-trend | 0.29 | 0.96 | 0.49 | 0.61 | ||
| Milk, g/d | ||||||
| 0 | 3034 | 128.7 | 129.4 (123.4, 135.7) | 3410 | 37.8 | 40.3 (38.4, 42.4) |
| >0 | 208 | 118.0 | 127.4 (110.8, 146.4) | 311 | 39.9 | 40.7 (36.1, 45.9) |
| P-trend | 0.22 | 0.83 | 0.42 | 0.88 | ||
All the mean values are the geometric mean of ferritin concentration. The medians for quartiles of food intake (men/women) were as follows—red meat: 0/0, 50.0/35.0, 96.1/76.7, and 168.1/143.1 g/d; grains: 283.3/233.3, 383.3/316.7, 476.4/400.0, and 650.0/564.0 g/d; vegetables: 170.0/160.0, 283.3/266.7, 383.3/363.3, and 541.7/516.4 g/d; and pork: 0/0, 43.3/33.3, 83.3/70.0, and 160.0/133.3 g/d. The general linear model was adjusted for total energy intake, age, annual income level, moderate-to-vigorous physical activity time, BMI, alcohol intake, tea consumption, and serum high-sensitivity C-reactive protein concentration as continuous variables and educational status, place of residence, geographic region, and smoking status as categorical variables except the stratification variable.
FIGURE 1.
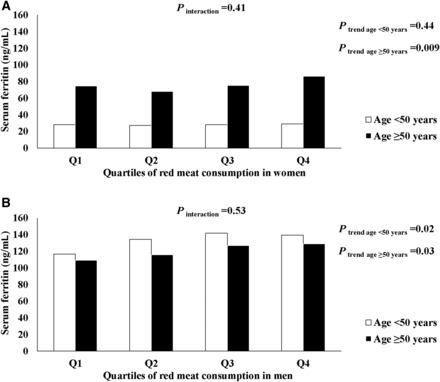
Joint associations of age and red meat consumption with serum ferritin concentration in men and women. Values are multivariable-adjusted mean ferritin concentrations in women (A) and men (B) estimated from a general linear regression model. Covariates included total energy intake, annual income level, moderate-to-vigorous physical activity time, BMI (in kg/m2), alcohol intake, and serum high-sensitivity C-reactive protein concentration as continuous variables and educational status, place of residence, geographic region, and smoking status as categorical variables. To quantify a linear trend between red meat consumption and serum ferritin concentration, we assigned the median value within each quartile and modeled this variable continuously; the Wald test was used for testing statistical significance. The interaction between age group and red meat consumption was tested by adding a multiplicative term into the multivariable model for each sex group. Q, quartile.
Table 3 presents the TfR:F index according to sociodemographic and lifestyle factors. The TfR:F index was not associated with age in men but was inversely associated with age in women (P-trend < 0.001). Educational status was inversely associated with the TfR:F index among all participants (P-trend < 0.05). Lower phytate intake was associated with a lower TfR:F index in women (P-trend = 0.05) but not men. Interestingly, we found a strong positive association between phytate intake and transferrin receptor concentration in both men and women (both P-trends < 0.001) (Supplemental Table 1). The consumption of foods from the major food groups was generally not associated with the TfR:F index or transferrin receptor concentration (Supplemental Tables 2 and 3).
TABLE 3.
Transferrin receptor and ferritin index according to categories of sociodemographic and lifestyle factors1
| Men | Women | |||||
| n | Mean | Multivariable-adjusted mean (95% CI) | n | Mean | Multivariable-adjusted mean (95% CI) | |
| Age, y | ||||||
| 18–44 | 1457 | 0.29 | 0.29 (0.24, 0.35) | 2196 | 0.55 | 0.55 (0.49, 0.60) |
| 45–54 | 960 | 0.35 | 0.36 (0.29, 0.43) | 1114 | 0.47 | 0.46 (0.39, 0.52) |
| 55–65 | 901 | 0.31 | 0.29 (0.22, 0.35) | 1044 | 0.36 | 0.34 (0.27, 0.41) |
| P-trend | 0.41 | 0.75 | <0.001 | <0.001 | ||
| Place of residence | ||||||
| Urban | 918 | 0.34 | 0.35 (0.29, 0.42) | 1093 | 0.45 | 0.46 (0.40, 0.53) |
| Rural | 2400 | 0.30 | 0.29 (0.23, 0.34) | 3261 | 0.50 | 0.47 (0.41, 0.52) |
| P value | 0.21 | 0.07 | 0.09 | 0.85 | ||
| Annual income, RMB | ||||||
| <9000 | 1472 | 0.36 | 0.35 (0.28, 0.42) | 2660 | 0.50 | 0.47 (0.41, 0.53) |
| 9000–15,000 | 538 | 0.29 | 0.29 (0.21, 0.37) | 707 | 0.50 | 0.49 (0.41, 0.56) |
| >15,000–25,000 | 642 | 0.29 | 0.30 (0.22, 0.37) | 581 | 0.48 | 0.46 (0.39, 0.54) |
| >25,000 | 666 | 0.28 | 0.29 (0.22, 0.35) | 406 | 0.45 | 0.44 (0.38, 0.51) |
| P-trend | 0.10 | 0.26 | 0.21 | 0.41 | ||
| BMI, kg/m2 | ||||||
| <18.5 | 173 | 0.34 | 0.32 (0.17, 0.46) | 210 | 0.50 | 0.45 (0.30, 0.59) |
| 18.5–24 | 1712 | 0.30 | 0.29 (0.24, 0.35) | 2031 | 0.52 | 0.49 (0.43, 0.55) |
| >24–28 | 1063 | 0.33 | 0.34 (0.27, 0.40) | 1101 | 0.47 | 0.46 (0.39, 0.53) |
| >28 | 294 | 0.27 | 0.28 (0.17, 0.39) | 379 | 0.38 | 0.36 (0.25, 0.48) |
| P-trend | 0.80 | 0.83 | 0.02 | 0.09 | ||
| Moderate-to-vigorous physical activity time, h/wk | ||||||
| <1 | 856 | 0.29 | 0.29 (0.22, 0.36) | 1152 | 0.50 | 0.48 (0.41, 0.55) |
| 1–3 | 688 | 0.30 | 0.30 (0.22, 0.37) | 890 | 0.49 | 0.48 (0.40, 0.55) |
| >3–6 | 793 | 0.36 | 0.35 (0.28, 0.43) | 930 | 0.48 | 0.47 (0.39, 0.54) |
| >6 | 905 | 0.29 | 0.30 (0.23, 0.37) | 749 | 0.48 | 0.43 (0.35, 0.52) |
| P-trend | 0.87 | 0.81 | 0.56 | 0.35 | ||
| Education | ||||||
| Illiterate | 349 | 0.44 | 0.44 (0.33, 0.54) | 1416 | 0.46 | 0.56 (0.49, 0.64) |
| Primary school | 582 | 0.31 | 0.31 (0.23, 0.39) | 792 | 0.51 | 0.52 (0.45, 0.60) |
| High school | 1906 | 0.29 | 0.29 (0.24, 0.33) | 1713 | 0.50 | 0.47 (0.42, 0.52) |
| Some college | 264 | 0.28 | 0.25 (0.13, 0.37) | 271 | 0.46 | 0.41 (0.28, 0.53) |
| >College | 217 | 0.29 | 0.25 (0.12, 0.38) | 162 | 0.46 | 0.37 (0.19, 0.54) |
| P-trend | 0.03 | 0.02 | 0.61 | 0.009 | ||
| Geographic region | ||||||
| South | 1040 | 0.30 | 0.31 (0.24, 0.37) | 1223 | 0.53 | 0.52 (0.46, 0.59) |
| Middle | 770 | 0.27 | 0.27 (0.19, 0.34) | 935 | 0.49 | 0.44 (0.37, 0.52) |
| North | 1508 | 0.34 | 0.35 (0.29, 0.41) | 2196 | 0.46 | 0.43 (0.36, 0.49) |
| P value | 0.18 | 0.16 | 0.13 | 0.05 | ||
| Tea consumption, times/wk | ||||||
| ≤1 | 1908 | 0.33 | 0.33 (0.27, 0.38) | 2891 | 0.50 | 0.47 (0.42, 0.52) |
| >1–6 | 302 | 0.29 | 0.30 (0.19, 0.41) | 236 | 0.42 | 0.41 (0.27, 0.54) |
| >6 | 1032 | 0.28 | 0.28 (0.21, 0.34) | 594 | 0.46 | 0.46 (0.37, 0.56) |
| P-trend | 0.23 | 0.17 | 0.29 | 0.83 | ||
| Smoking | ||||||
| No | 1308 | 0.35 | 0.35 (0.29, 0.41) | 4233 | 0.49 | 0.47 (0.42, 0.52) |
| Current or ever | 2010 | 0.28 | 0.28 (0.23, 0.34) | 121 | 0.37 | 0.35 (0.16, 0.55) |
| P value | 0.04 | 0.07 | 0.19 | 0.24 | ||
| Alcohol intake, g/d | ||||||
| 0 | 1339 | 0.35 | 0.34 (0.28, 0.40) | 3444 | 0.49 | 0.47 (0.42, 0.52) |
| >0–15 | 990 | 0.29 | 0.29 (0.22, 0.36) | 219 | 0.45 | 0.43 (0.29, 0.58) |
| >15 | 913 | 0.27 | 0.28 (0.21, 0.35) | 58 | 0.47 | 0.47 (0.20, 0.74) |
| P-trend | 0.16 | 0.34 | 0.55 | 0.66 | ||
| Iron intake | ||||||
| Quartile 1 | 795 | 0.29 | 0.29 (0.27, 0.30) | 906 | 0.52 | 0.49 (0.41, 0.58) |
| Quartile 2 | 818 | 0.29 | 0.29 (0.27, 0.30) | 1001 | 0.47 | 0.46 (0.38, 0.53) |
| Quartile 3 | 817 | 0.29 | 0.29 (0.27, 0.30) | 959 | 0.51 | 0.44 (0.37, 0.52) |
| Quartile 4 | 812 | 0.30 | 0.29 (0.27, 0.31) | 855 | 0.46 | 0.44 (0.36, 0.53) |
| P-trend | 0.65 | 0.73 | 0.85 | 0.50 | ||
| Phytate intake | ||||||
| Quartile 1 | 793 | 0.34 | 0.36 (0.28, 0.43) | 925 | 0.45 | 0.42 (0.34, 0.50) |
| Quartile 2 | 809 | 0.29 | 0.29 (0.22, 0.36) | 979 | 0.50 | 0.47 (0.39, 0.55) |
| Quartile 3 | 823 | 0.30 | 0.29 (0.22, 0.36) | 947 | 0.45 | 0.43 (0.36, 0.51) |
| Quartile 4 | 817 | 0.30 | 0.29 (0.21, 0.37) | 870 | 0.57 | 0.55 (0.47, 0.64) |
| P-trend | 0.47 | 0.28 | 0.04 | 0.05 | ||
| Vitamin C intake | ||||||
| Quartile 1 | 807 | 0.35 | 0.36 (0.29, 0.43) | 915 | 0.51 | 0.48 (0.40, 0.55) |
| Quartile 2 | 818 | 0.30 | 0.30 (0.23, 0.37) | 988 | 0.48 | 0.45 (0.37, 0.52) |
| Quartile 3 | 812 | 0.28 | 0.28 (0.21, 0.35) | 947 | 0.46 | 0.44 (0.36, 0.51) |
| Quartile 4 | 805 | 0.31 | 0.29 (0.21, 0.36) | 871 | 0.52 | 0.50 (0.42, 0.58) |
| P-trend | 0.42 | 0.17 | 0.78 | 0.55 | ||
| Calcium intake | ||||||
| Quartile 1 | 810 | 0.35 | 0.36 (0.28, 0.44) | 930 | 0.52 | 0.49 (0.41, 0.57) |
| Quartile 2 | 811 | 0.30 | 0.30 (0.23, 0.37) | 930 | 0.45 | 0.42 (0.35, 0.50) |
| Quartile 3 | 811 | 0.30 | 0.29 (0.22, 0.36) | 931 | 0.50 | 0.47 (0.39, 0.54) |
| Quartile 4 | 810 | 0.29 | 0.28 (0.21, 0.36) | 930 | 0.50 | 0.48 (0.40, 0.56) |
| P-trend | 0.29 | 0.24 | 0.89 | 0.86 | ||
The medians for quartiles of nutrient intake (men/women) were as follows—iron: 15.0/13.0, 19.8/17.3, 24.9/21.8, and 35.8/31.7 mg/d; vitamin C: 37.3/33.6, 64.0/61.4, 90.6/88.6, and 137.4/137.0 mg/d; calcium: 230.4/198.8, 322.5/285.6, 431.2/381.8, and 640.7/578.6 mg/d; and phytates: 3.8/3.3, 9.7/8.4, 14.3/12.5, and 21.8/18.9 g/d. For categories of age, annual income, place of residence, geographic region, and educational and smoking status, the general linear model (model 1) was simultaneously adjusted for age, annual income level, moderate-to-vigorous physical activity time, BMI, alcohol intake, tea consumption, and serum high-sensitivity C-reactive protein concentration as continuous variables and educational status, place of residence, geographic region, and smoking status as categorical variables except the stratification variable. For categories of moderate-to-vigorous physical activity time, BMI, alcohol intake, tea consumption, and dietary intakes of vitamin C, iron, phytates, and calcium, the general linear model was adjusted for total energy intake and dietary intakes of vitamin C, iron, phytates, and calcium as continuous variables in addition to model 1 except the stratification variable. RMB, renminbi.
A younger age and a higher BMI were associated with a higher hemoglobin concentration in all participants (Supplemental Table 4). Hemoglobin concentration varied significantly between urban and rural areas and across different geographic regions (all P < 0.001). In women, dietary iron intake was positively associated with hemoglobin concentration (P-trend = 0.004). Supplemental Table 5 shows that lower vegetable consumption and higher fish consumption were associated with higher hemoglobin concentration in men (both P-trends ≤ 0.01), whereas grain intake was positively associated with hemoglobin in women (P-trend = 0.05).
DISCUSSION
To our knowledge, this study is the first nationwide investigation on a wide range of factors related to iron status in a Chinese population. We found that certain sociodemographic, lifestyle, and dietary factors were associated with body iron status, especially iron stores measured by serum ferritin. Higher intake of red meat was associated with higher ferritin concentrations in both men and women, whereas this positive association was more pronounced among older women in this Chinese population. The observed sex differences may be explained by the low iron stores in younger Chinese women caused by menstrual blood loss. In addition, both serum ferritin and hemoglobin concentrations varied significantly across different geographic areas.
The geometric mean serum ferritin concentration in Chinese men (135.9 ng/mL) was comparable to that in Western men, whereas Chinese women (42.7 ng/mL), especially younger women, had a much lower geometric mean ferritin concentration than Western women. For example, the geometric mean ferritin concentrations were 152, 92, 93, and 58 ng/mL for African-American men, non-Hispanic white men, African-American women, and non-Hispanic white women, respectively, in NHANES II (29).
Our results on the association of higher ferritin concentrations with older age in women and younger age in men are consistent with previous studies (5, 6, 11, 14, 15). For instance, similar positive associations between age and serum ferritin concentration were observed in US women in the Framingham Heart Study (6) and the Nurses’ Health Study (5), which could be explained by accumulated menstrual blood loss in premenopausal women (5, 11, 14, 26). The inverse trend of ferritin concentration with age among men, similar to findings from NHANES, may be caused by the lower absorption rate of iron later in life. Annual income was positively associated with ferritin concentrations in women. Major food sources of heme iron, e.g., red meat, fish, and poultry, usually are relatively expensive and are considered luxuries in China. The high cost of foods rich in heme iron may be a barrier for low-income people who can only afford low-price plant-based diets that usually provide nonheme iron. Consistent with previous studies (5, 27, 30, 31), BMI was positively correlated to ferritin concentration. Because high BMI is an indicator of systematic inflammation, the positive association between BMI and ferritin concentration may be partly explained by inflammation. Interestingly, further adjusting for hs-CRP, a biomarker of systemic inflammation, did not attenuate the association between BMI and serum ferritin concentration, indicating other potential mechanisms for this association. For smokers, we observed a higher ferritin concentration in women, which is consistent with previous findings (32, 33). Dietary iron intake was not associated with various measures of iron stores, including ferritin concentration and the TfR:F index, which was possibly because dietary iron in the typical Chinese diet largely consists of nonheme iron with a low absorption rate. We also found that the dietary intake of phytates, which are major inhibitors of iron absorption, was inversely associated with ferritin and transferrin receptor concentrations, although the association between phytate intake and ferritin was not significant in women. This finding is consistent with a previous study (21) in a Chinese population that showed that the high phytate content of the Chinese diet contributed markedly to the prevalence of iron deficiency in China.
We observed a positive association between red meat consumption and serum ferritin concentration. Possibly because of their postmenopausal status, this association was more apparent among older women. We also found positive associations of pork and poultry consumption with serum ferritin concentration in men. Pork and poultry are both rich in heme iron, which is absorbed more completely than nonheme-iron foods (34). Meat, especially red meat, is the primary contributor of heme-iron absorption in the Chinese population (35). Our findings are consistent with previous studies that showed positive associations between heme-iron intake and iron status in Western populations (5, 6, 11, 13, 33, 36–40). Given the relatively high prevalence of iron-deficiency anemia and its related disease burden in China, the intake of heme-iron–rich foods (e.g., red meat), especially in women, still stands as an important dietary recommendation for the prevention of anemia. However, a higher risk of chronic diseases, including coronary artery disease, type 2 diabetes, and colorectal cancer, related to higher red meat intake, as reported in Western populations, should also be considered in dietary recommendations (41–44). We found that higher grain consumption was associated with lower ferritin concentration, possibly because grains are the largest contributor of phytates in the Chinese diet (23).
In addition to ferritin concentration, we examined the TfR:F index and found that the ratio was generally not associated with the dietary and nondietary factors. We confirmed that the associations of dietary and nondietary factors with iron stores varied by sex, possibly because of the small between-person variation in iron stores in women given their very low serum ferritin concentration. Younger Chinese women suffer from a double burden of iron depletion from menstruation and a low intake of foods rich in heme iron, such as red meat (45). In addition, 50% of our study population had an annual income <9000 RMB, and 66% of our study population was from rural areas, indicating low economic status. In China, low-income families from rural areas are more likely to give economic priorities to men rather than women, especially younger women, who have less opportunities to obtain animal-sourced foods, such as red meat, that are usually expensive. Chinese women, especially those from rural areas, have a substantially lower animal protein intake than women in Japan and the United Kingdom.
The strengths of this study include a relatively large updated sample population that geographically represents China, as well as comprehensive measurements of dietary, lifestyle, and sociodemographic variables. Our findings should be interpreted in the context of several limitations. First, because of the cross-sectional nature of this study design, we could not make casual inferences. However, CHNS comprehensively collected a wide range of dietary and nondietary information that allowed us to adjust for multiple risk and confounding factors, including circulating biomarkers for inflammation, and thus largely alleviated the possibility of confounding by inflammation and chronic diseases. Second, dietary information (especially iron intake) collected through three 24-h dietary recalls might not represent long-term usual intake (46). Measurement errors in 24-h dietary recalls tend to be random, which attenuates the association of dietary and nondietary factors with iron status. Third, menopausal status influences body iron stores in women. However, CHNS did not collect information related to menstrual status. Instead, we used median age at menopause in Chinese women as a surrogate of menopausal status. Fourth, our study did not collect information on several dietary and nondietary factors that may potentially be associated with iron status, such as serum hepcidin, genetic risk factors, blood donation, supplement use, and heme- and nonheme-iron intake.
Our study confirmed several dietary factors and nondietary factors that contribute to body iron status in Chinese adults. The observed associations varied by menopausal status in women; younger Chinese women are at a particularly high risk of low body iron stores. Given the high prevalence of anemia in China, our findings provide pivotal evidence for population-level intervention strategies such as dietary recommendations and food fortification for improving iron status in the Chinese population. In addition, fortification should consider both increasing the iron content and decreasing the phytate content of commonly consumed foods, such as grains, which would have a direct and profound influence on the iron status of the Chinese population. More comprehensive nationwide programs combining both dietary education for the general population and food fortification for the at-risk population, e.g., younger women, are imperatively needed to accelerate the improvement of iron status in the Chinese population. However, we should also be vigilant about potential chronic disease risks related to iron overload.
Supplementary Material
Acknowledgments
This research uses publicly available data from China Health and Nutrition Survey (CHNS). We thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Carolina Population Center, the University of North Carolina at Chapel Hill, the NIH, and the NIH Fogarty International Center for financial support for the CHNS data collection and analysis files from 1989 to 2015 and future surveys, and the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009, and Beijing Municipal Center for Disease Prevention and Control since 2011.
The authors’ responsibilities were as follows—PJH: drafted the manuscript; PJH and DDW: designed and conducted the analysis and interpreted the data; SHL, SNB, and YL: assisted in interpreting the data and edited the manuscript; DDW: had primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
ABBREVIATIONS
- CHNS
China Health Nutrition Survey
- hs-CRP
high-sensitivity C-reactive protein
- RMB
renminbi
- TfR:F
serum transferrin receptor:ferritin ratio
FOOTNOTES
This research did not receive any funding.
REFERENCES
- 1. Mackenzie EL, Iwasaki K, Tsuji Y.. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal 2008;10:997–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva (Switzerland): WHO; 2001. [Google Scholar]
- 3. Yang Z, Dewey KG, Lönnerdal B, Hernell O, Chaparro C, Adu-Afarwuah S, McLean ED, Cohen RJ, Domellöf M, Allen LH.. Comparison of plasma ferritin concentration with the ratio of plasma transferrin receptor to ferritin in estimating body iron stores: results of 4 intervention trials. Am J Clin Nutr 2008;87:1892–8. [DOI] [PubMed] [Google Scholar]
- 4. Punnonen K, Irjala K, Rajamäki A.. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997;89:1052–7. [PubMed] [Google Scholar]
- 5. Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J.. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr 2003;78:1160–7. [DOI] [PubMed] [Google Scholar]
- 6. Fleming DJ, Jacques PF, Dallal GE, Tucker KL, Wilson P, Wood RJ.. Dietary determinants of iron stores in a free-living elderly population: The Framingham Heart Study. Am J Clin Nutr 1998;67:722–33. [DOI] [PubMed] [Google Scholar]
- 7. Preziosi P, Hercberg S, Galan P, Devanlay M, Cherouvrier F, Dupin H.. Iron status of a healthy French population: factors determining biochemical markers. Ann Nutr Metab 1994;38:192–202. [DOI] [PubMed] [Google Scholar]
- 8. Armah SM, Boy E, Chen D, Candal P, Reddy MB.. Regular consumption of a high-phytate diet reduces the inhibitory effect of phytate on nonheme-iron absorption in women with suboptimal iron stores. J Nutr 2015;145:1735–9. [DOI] [PubMed] [Google Scholar]
- 9. Vandevijvere S, Michels N, Verstraete S, Ferrari M, Leclercq C, Cuenca-García M, Grammatikaki E, Manios Y, Gottrand F, Santamaría J.. Intake and dietary sources of haem and non-haem iron among European adolescents and their association with iron status and different lifestyle and socio-economic factors. Eur J Clin Nutr 2013;67:765–72. Erratum in: Eur J Clin Nutr 2013;67:1227. [DOI] [PubMed] [Google Scholar]
- 10. Payette H, Gray-Donald K.. Dietary intake and biochemical indices of nutritional status in an elderly population, with estimates of the precision of the 7-d food record. Am J Clin Nutr 1991;54:478–88. [DOI] [PubMed] [Google Scholar]
- 11. Galan P, Yoon H, Preziosi P, Viteri F, Valeix P, Fieux B, Briancon S, Malvy D, Roussel A, Favier A.. Determining factors in the iron status of adult women in the SU.VI.MAX study. Eur J Clin Nutr 1998;52:383–8. [DOI] [PubMed] [Google Scholar]
- 12. Yokoi K, Alcock NW, Sandstead HH.. Iron and zinc nutriture of premenopausal women: associations of diet with serum ferritin and plasma zinc disappearance and of serum ferritin with plasma zinc and plasma zinc disappearance. J Lab Clin Med 1994;124:852–61. [PubMed] [Google Scholar]
- 13. Blanco-Rojo R, Toxqui L, López-Parra AM, Baeza-Richer C, Pérez-Granados AM, Arroyo-Pardo E, Vaquero MP.. Influence of diet, menstruation and genetic factors on iron status: a cross-sectional study in Spanish women of childbearing age. Int J Mol Sci 2014;15:4077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beck KL, Kruger R, Conlon CA, Heath ALM, Matthys C, Coad J, Stonehouse W.. Suboptimal iron status and associated dietary patterns and practices in premenopausal women living in Auckland, New Zealand. Eur J Nutr 2013;52:467–76. [DOI] [PubMed] [Google Scholar]
- 15. Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM.. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J 2000;140:98–104. [DOI] [PubMed] [Google Scholar]
- 16. Means RT.. Hepcidin and anaemia. Blood Rev 2004;18:219–25. [DOI] [PubMed] [Google Scholar]
- 17. Finch CA, Cook J, Labbe R, Culala M.. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood 1977;50:441–7. [PubMed] [Google Scholar]
- 18. Piao JH, Lai JQ, Yin SA, Xu J, Xu QM, Yang XG.. Study on the anemia status of Chinese population. Acta Nutrimenta Sinica 2005;4:268–75. [Google Scholar]
- 19. Zhai F, Wang H, Du S, He Y, Wang Z, Ge K, Popkin BM.. Prospective study on nutrition transition in China. Nutr Rev 2009;67(Suppl 1):S56–61. [DOI] [PubMed] [Google Scholar]
- 20. Popkin BM, Paeratakul S, Ge K, Zhai F.. Body weight patterns among the Chinese: results from the 1989 and 1991 China Health and Nutrition Surveys. Am J Public Health 1995;85:690–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma G, Jin Y, Li Y, Zhai F, Kok FJ, Jacobsen E, Yang X.. Iron and zinc deficiencies in China: what is a feasible and cost-effective strategy? Public Health Nutr 2008;11:632–8. [DOI] [PubMed] [Google Scholar]
- 22. Yang Y.. Chinese food composition table 2004. Beijing (China): Peking University Medical Press; 2005. [Google Scholar]
- 23. Ma G, Jin Y, Piao J, Kok F, Guusje B, Jacobsen E.. Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in China. J Agric Food Chem 2005;53:10285–90. [DOI] [PubMed] [Google Scholar]
- 24. Yan S, Li J, Li S, Zhang B, Du S, Gordon‐Larsen P, Adair L, Popkin B.. The expanding burden of cardiometabolic risk in China: the China Health and Nutrition Survey. Obes Rev 2012;13:810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng SW, Norton EC, Popkin BM.. Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China Health and Nutrition Surveys. Soc Sci Med 2009;68:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harvey LJ, Armah CN, Dainty JR, Foxall RJ, Lewis DJ, Langford NJ, Fairweather-Tait SJ.. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br J Nutr 2005;94:557–64. [DOI] [PubMed] [Google Scholar]
- 27. Milman N, Byg KE, Ovesen L.. Iron status in Danes 1994. II: Prevalence of iron deficiency and iron overload in 1319 Danish women aged 40–70 years. Influence of blood donation, alcohol intake and iron supplementation. Ann Hematol 2000;79:612–21. [DOI] [PubMed] [Google Scholar]
- 28. Zhan Y, Chen R, Zheng W, Guo C, Lu L, Ji X, Chi Z, Yu J.. Association between serum magnesium and anemia: China health and nutrition survey. Biol Trace Elem Res 2014;159:39–45. [DOI] [PubMed] [Google Scholar]
- 29. Kato A, Tsuji T, Luo J, Sakao Y, Yasuda H, Hishida A.. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol 2008;28:115–21. [DOI] [PubMed] [Google Scholar]
- 30. Kim NH, Oh JH, Choi KM, Kim YH, Baik SH, Choi DS, Kim SJ.. Serum ferritin in healthy subjects and type 2 diabetic patients. Yonsei Med J 2000;41:387–92. [DOI] [PubMed] [Google Scholar]
- 31. Milman N, Kirchhoff M.. Relationship between serum ferritin and risk factors for ischaemic heart disease in 2235 Danes aged 30–60 years. J Intern Med 1999;245:423–33. [DOI] [PubMed] [Google Scholar]
- 32. Tamura T, Goldenberg RL, Johnston KE, Dubard MB.. Effect of smoking on plasma ferritin concentrations in pregnant women. Clin Chem 1995;41:1190–1. [PubMed] [Google Scholar]
- 33. Leggett BA, Brown NN, Bryant SJ, Duplock L, Powell LW, Halliday JW.. Factors affecting the concentrations of ferritin in serum in a healthy Australian population. Clin Chem 1990;36:1350–5. [PubMed] [Google Scholar]
- 34. Cook JD.. Adaptation in iron metabolism. Am J Clin Nutr 1990;51:301–8. [DOI] [PubMed] [Google Scholar]
- 35. Ortega DL, Holly Wang H, Eales JS.. Meat demand in China. China Agric Econ Rev 2009;1:410–9. [Google Scholar]
- 36. Gibson S, Ashwell M.. The association between red and processed meat consumption and iron intakes and status among British adults. Public Health Nutr 2003;6:341–50. [DOI] [PubMed] [Google Scholar]
- 37. Ortega R, Lopez-Sobaler A, Requejo A, Quintas M, Gaspar M, Andres P, Navia B.. The influence of meat consumption on dietary data, iron status and serum lipid parameters in young women. Int J Vitam Nutr Res 1997;68:255–62. [PubMed] [Google Scholar]
- 38. Brussaard JH, Brants HA, Bouman M, Löwik MR.. Iron intake and iron status among adults in the Netherlands. Eur J Clin Nutr 1997;51:S51–8. [PubMed] [Google Scholar]
- 39. Felipe A, Guadalupe E, Druso P, Carlos M, Pablo S, Oscar C, Luis V, Diego M, Jaime R, Inés U... Serum ferritin is associated with metabolic syndrome and red meat consumption. Oxid Med Cell Longev 2015;2015:769739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heath AL, Skeaff CM, Williams S, Gibson RS.. The role of blood loss and diet in the aetiology of mild iron deficiency in premenopausal adult New Zealand women. Public Health Nutr 2001;4:197–206. [DOI] [PubMed] [Google Scholar]
- 41. Pan A, Hu FB.. Can eating red meat increase the risk of developing Type 2 diabetes? Diabetes Manage 2014;4:1–4. [Google Scholar]
- 42. Norat T, Riboli E.. Meat consumption and colorectal cancer: a review of epidemiologic evidence. Nutr Rev 2001;59:37–47. [DOI] [PubMed] [Google Scholar]
- 43. Micha R, Wallace SK, Mozaffarian D.. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fernandez-Cao JC, Arija V, Aranda N, Bullo M, Basora J, Martínez-González MA, Díez-Espino J, Salas-Salvadó J.. Heme iron intake and risk of new-onset diabetes in a Mediterranean population at high risk of cardiovascular disease: an observational cohort analysis. BMC Public Health 2013;13:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Du S, Zhai F, Wang Y, Popkin BM.. Current methods for estimating dietary iron bioavailability do not work in China. J Nutr 2000;130:193–8. [DOI] [PubMed] [Google Scholar]
- 46. Gibson RS, Ferguson EL.. An interactive 24-hour recall for assessing the adequacy of iron and zinc intakes in developing countries. Washington (DC): ILSI Press; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


