ABSTRACT
Background: We previously reported that dietary lipid quality during early life can have long-lasting effects on metabolic health and adiposity. Exposure to a postnatal diet with low dietary omega-6 (n−6) or high omega-3 (n−3) fatty acid (FA) content resulted in reduced body fat accumulation when challenged with a moderate Western-style diet (WSD) beginning in adolescence.
Objective: We determined whether this programming effect is accompanied by changes in hypothalamic neural projections or modifications in the postnatal leptin surge, which would indicate the altered development of hypothalamic circuits that control energy balance.
Design: Neonatal mice were subjected to a control diet (CTR) or experimental diet with altered relative n−6 and n−3 FA contents [ie, a diet with a relative reduction in n−6 fatty acid (LOW n−6) or a diet with a relative increase in n−3 fatty acid (HIGH n−3) compared with the CTR from postnatal day (PN) 2 to 42].
Results: Compared with CTR mice, mice fed a LOW n−6 or HIGH n−3 during postnatal life showed significant reductions in the density of both orexigenic and anorexigenic neural projections to the paraventricular nucleus of the hypothalamus at PN 28. These impairments persisted into adulthood and were still apparent after the WSD challenge between PNs 42 and 98. However, the neuroanatomical changes were not associated with changes in the postnatal leptin surge.
Conclusion: Although the exact mechanism remains to be elucidated, our data indicate that the quality of dietary FA during postnatal life affects the development of the central regulatory circuits that control energy balance and may do so through a leptin-independent mechanism.
INTRODUCTION
The incidence of obesity and type 2 diabetes has been rising over the past decades and occurs increasingly in younger children (1–3). A predisposition to obesity and metabolic disease can be influenced by the perinatal environment, which is a concept known as developmental programming (4, 5). Both perinatal overnutrition and undernutrition are closely related to the development of obesity and adult metabolic disease. Rodent studies have indicated that underlying mechanisms may include the altered formation of hypothalamic circuits that regulate energy balance. Neurons in the arcuate nucleus of the hypothalamus (ARH)4 projecting to various target sites in the hypothalamus including the paraventricular nucleus (PVH) are key components of this circuitry. The secretion of orexigenic neuropeptides including Agouti-related peptide (AgRP) at these target sites increases food intake and stimulates energy expenditure, whereas the secretion of anorexigenic neuropeptides such as α-melanocyte stimulating hormone (α-MSH) has opposite effects (6).
Axonal projections from ARH to PVH develop during the first postnatal weeks in rodents, and this process requires a surge in the adipocyte-derived hormone leptin (7). The timing and amplitude of the postnatal leptin surge are critical for ARH connectivity and functionality; the development of ARH projections is altered by increased leptin concentrations (8) or when leptin signaling is absent (7, 9). Such changes lead to the altered hypothalamic control of energy balance and predispose animals to obesity (10, 11). Postnatal leptin concentrations are enhanced by a perinatal high-fat diet (12) and blunted by undernutrition (13), providing mechanisms by which nutrition may influence hypothalamic development and neural control of energy metabolism. Both mechanisms are accompanied by altered ARH development and increased obesity risk later in life (12, 13).
Although the link between perinatal undernutrition and overnutrition and hypothalamic function has been clearly shown, little is known about the impact of nutrient quality rather than quantity on these variables. It has been postulated that the quality of dietary lipids [ie, omega-6 (n−6) and omega-3 (n−3) PUFAs] is a determinant of later metabolic health. n−6 PUFAs stimulate adipogenesis during critical periods of development and promote body fat accumulation (14), whereas n−3 PUFAs have opposite effects (15). The increased use of vegetable oils in the human diet has led to a higher n−6 PUFA intake, in particular of linoleic acid (LA), and decreased n−3 PUFA intake over the past decades (16). This shift may play a considerable role in the increased prevalence of (childhood) obesity (14).
With the use of a mouse model of nutritional programming, we showed previously that postnatal diets with altered relative n−6 and n−3 PUFA contents, either by decreasing the n−6 content or increasing the n−3 content, protect against excessive body fat accumulation after a moderate Western-style diet (WSD) challenge during adulthood without affecting caloric intake (17, 18). In the current study, we investigated the effects of these postnatal diets on the development of ARH neural projections to PVH and the postnatal leptin surge.
MATERIALS AND METHODS
Animals and study design
Experimental groups were obtained from C57Bl/6J dams and housed in a controlled environment (12-h/12-h light/dark cycle; 21± 2°C) with ad libitum access to food (AIN93-G; Research Diet Services). At postnatal day (PN) 2, litters were culled to 6 pups/dam and randomly assigned to reduce between-litter variability and avoid litter effects, respectively, because of the genetic similarity of full siblings (male:female ratio in litter: 1:1 or 2:1; the male offspring in each litter was derived from different dams) (19) and assigned to either control or experimental diets. Diets were provided to the lactating dam with a litter from PN 2 onward. The fatty acid (FA) composition of the maternal diet rapidly translates in the FA composition of the milk (20, 21), which was confirmed for these experimental diets in a previous study (18). At PNs 5, 10, 12, 14, 16, and 21, male pups were killed by decapitation. Trunk blood was collected and centrifuged, and plasma leptin was measured by using an ELISA (Millipore). Part of the remaining blood sample obtained at PNs 16 and 21 was immediately treated with protease inhibitors (Pefabloc SC; Merck Millipore) before centrifugation, and plasma was acidified with HCl for the analysis of acylated ghrelin according to the manufacturers protocol (Millipore). In a separate cohort, male pups were weaned at PN 21 onto their respective intervention diets. One group was perfused at PN 28, whereas the other group was challenged with a moderate obesogenic WSD from PN 42 to 98 (18). The perfusion at PN 98 of animals from the latter group was designed as a pilot test to explore whether effects of early diet on fiber density were permanent. All experimental procedures were evaluated by the Animal Experimental Committee (Dierexperimentencommissie–Consult), and complied with the principles of good laboratory animal care.
Experimental diets
All semisynthetic diets (Research Diet Services) (Table 1) had a macronutrient and micronutrient composition according to American Institute of Nutrition formulation of AIN93-G purified diets for laboratory rodent (22) and fulfilled the defined needs for essential FAs in the diet. All diets consisted of 60 wt:wt% carbohydrates, 20 wt:wt% proteins, 10 wt:wt% lipids, and 5 wt:wt% fibers. The 3 experimental diets were isocaloric and differed only in FA composition. The control diet (CTR) contained 14.8% LA. In the diet with a relative increase in n−3 fatty acid (HIGH n−3), the relative amount of n−3 long-chain PUFAs, mainly DHA and EPA, was increased compared with in the CTR, which resulted in a n−6:n−3 FA ratio of 1.54, but the LA content was comparable. In the diet with a relative reduction in n−6 fatty acid (LOW n−6), the LA content was reduced to 6.4% compared with in the CTR (Table 1). The moderate WSD contained 10% lipids, of which a 50% lard and 50% vegetable oil blend that resulted in a high saturated FA content. This WSD is a nonhyperphagic diet that was previously shown to induce increased adiposity development without altering body weight (18). The FA composition of erythrocytes of the pups was analyzed at weaning as an index for the effect of early diet on the tissue of the offspring (23, 24).
TABLE 1.
Fatty acid composition (g/100 g lipids) of diets (CTR, HIGH n−3, LOW n−6, and WSD)1
| Fatty acid | Name | CTR | HIGH n−3 | LOW n−6 | WSD |
| 4:0 | Butyric acid | 0.00 | 0.00 | 1.05 | 0.00 |
| 6:0 | Caproic acid | 0.11 | 0.07 | 0.81 | 0.06 |
| 8:0 | Caprylic acid | 1.70 | 1.07 | 2.09 | 0.85 |
| 10:0 | Capric acid | 1.36 | 0.86 | 2.17 | 0.68 |
| 12:0 | Lauric acid | 10.53 | 6.69 | 11.42 | 5.27 |
| 14:0 | Myristic acid | 4.38 | 3.62 | 7.24 | 2.69 |
| 16:0 | Palmitic acid | 17.14 | 19.38 | 12.40 | 23.07 |
| 16:1n−7 | Palmitoleic acid | 0.13 | 1.20 | 0.78 | 1.56 |
| 17:0 | Margaric acid | 0.00 | 0.37 | 0.00 | 0.00 |
| 18:0 | Stearic acid | 3.07 | 3.70 | 5.12 | 9.03 |
| 18:1n−9 | Oleic acid | 37.94 | 35.27 | 40.79 | 40.47 |
| 18:2n−6 | Linoleic acid | 14.80 | 11.89 | 6.38 | 11.90 |
| 18:3n−3 | α-Linolenic acid | 2.61 | 1.07 | 1.57 | 1.30 |
| 18:4n−3 | Stearidonic acid | 0.00 | 0.19 | 0.00 | 0.00 |
| 20:0 | Arachidic acid | 0.34 | 0.26 | 0.20 | 0.17 |
| 20:1n−9 | Eicosaenoic acid | 0.41 | 0.15 | 0.22 | 0.21 |
| 20:4n−3 | Eicosatetraenoic acid | 0.00 | 0.07 | 0.00 | 0.00 |
| 20:4n−6 | Arachidonic acid | 0.00 | 0.28 | 0.00 | 0.00 |
| 20:5n−3 | EPA | 0.00 | 1.20 | 0.00 | 0.00 |
| 22:0 | Behenic acid | 0.23 | 0.24 | 0.33 | 0.11 |
| 22:1n−9 | Erucic acid | 0.14 | 0.05 | 0.08 | 0.07 |
| 22:5n−3 | Docosapentaenoic acid | 0.00 | 0.37 | 0.00 | 0.00 |
| 22:6n−3 | DHA | 0.00 | 5.00 | 0.00 | 0.00 |
| 24:0 | Lignoceric acid | 0.02 | 0.02 | 0.00 | 0.01 |
| SFA (%) | — | 38.88 | 36.28 | 42.84 | 41.94 |
| MUFA (%) | — | 38.62 | 36.68 | 41.86 | 42.31 |
| PUFA % | — | 17.41 | 20.07 | 7.96 | 13.20 |
| Σ n−6 | — | 14.80 | 12.17 | 6.38 | 11.90 |
| Σ n−3 | — | 2.61 | 7.90 | 1.57 | 1.30 |
| n−6:n−3 | — | 5.67 | 1.54 | 4.05 | 9.12 |
| Linoleic acid:α-linolenic acid | — | 5.67 | 11.10 | 4.05 | 9.12 |
CTR, control diet; HIGH n−3, diet with a relative increase in n−3 fatty acid; LOW n−6, diet with relative reduction in n−6 fatty acid; WSD, Western-style diet.
Immunochemistry of brain tissue
Mice were anesthetized with pentobarbital and perfused transcardially with a 4% paraformaldehyde solution. Brains were carefully dissected and postfixed for 4 h in 4% paraformaldehyde solution that contained 20% sucrose. Brains were frozen on powdered dry ice and stored at −80°C. Series of consecutive 30-μm-thick frozen sections were collected through the hypothalamus of each brain and subsequently processed for multiple immunofluorescence detection as previously described (25). Briefly, sections were incubated for 72 h with rabbit anti-AgRP (Phoenix Pharmaceuticals), sheep anti–α-MSH (Millipore), and mouse anti-human neuronal protein HuC and HuD (Invitrogen Life Technologies), which is a marker of neuronal cells. Sections were incubated with the appropriate secondary antibodies conjugated to Alexa-Fluor dyes (488, 568, and 647; Invitrogen Life Technologies) for 1 h at room temperature to localize the primary antibodies. Immunostained sections through the PVH were examined on a laser-scanning confocal microscope (Zeiss LSM710; Carl Zeiss), and image analyses were performed with Volocity quantitation software version 6.2 (Improvision; PerkinElmer), as previously described (25).
Statistics
Statistical analyses were performed with SAS version 9.2, Enterprise Guide 4.1 software (SAS Institute Inc). Effects of diets on body weight and plasma hormones during early dietary intervention (PNs 5–28) were analyzed by using mixed-effects regression models, including diet, age, and a diet-by-age interaction as fixed effects. Effects of diets on the erythrocyte FA composition at weaning, hypothalamic fiber density at PN 28, and body weight after the WSD challenge at PN 98 were analyzed by using a mixed-effects regression model with the diet as a fixed effect. In all regression models, litters were used as experimental units as opposed to individual pups, and the correlation in pups within a litter was accounted for by their sharing a common random effect. Pairwise comparisons were adjusted for multiple comparisons by using a Tukey-Kramer approach. Because we restricted PN 98 perfusions to offspring within one litter in both LOW n−6 and HIGH n−3 groups, the fiber density at PN 98 could not be included in the mixed-effects regression model and was, therefore, analyzed by using a less-stringent statistical method (1-factor ANOVA followed by pairwise Tukey-Kramer test) with the use of individual pups as experimental units. Differences were considered significant at P < 0.05.
RESULTS
Unaltered body weight and plasma leptin surge in mice fed a postnatal HIGH n−3 or LOW n−6
To explore the consequences of dietary FA quality on postnatal leptin, we exposed mice to experimental diets that differed only in FA composition during the postnatal period as previously described (18).
There was no significant difference in postnatal growth between experimental groups during the period of dietary intervention (Table 2). These observations were consistent with those in our previous studies in which body weight was similar between experimental groups at the start of adolescence PN 42 (17, 18). At PN 98, after the WSD, body weight was comparable between experimental groups (Table 2). Differences in body weight at PN 98 were not expected because we previously reported that these programming diets affect body composition but not body weight at PN 98 after the moderate WSD challenge (17, 18).
TABLE 2.
Body weight and plasma hormones of pups in CTR, HIGH n−3, and LOW n−6 groups at PNs 5, 10, 12, 14, 16, 21, 28, and 981
| CTR | HIGH n−3 | LOW n−6 | ||||
| Mean ± SEM | n [litters (pups)] | Mean ± SEM | n [litters (pups)] | Mean ± SEM | n [litters (pups)] | |
| Body weight (g) | ||||||
| PN | ||||||
| 5 | 2.8 ± 0.3 | 3 (9) | 2.8 ± 0.3 | 3 (8) | 2.7 ± 0.3 | 3 (10) |
| 10 | 4.9 ± 0.3 | 3 (9) | 5.3 ± 0.3 | 3 (9) | 5.1 ± 0.3 | 3 (9) |
| 12 | 6.4 ± 0.3 | 3 (9) | 6.8 ± 0.3 | 3 (9) | 6.1 ± 0.3 | 3 (9) |
| 14 | 6.8 ± 0.3 | 3 (9) | 7.6 ± 0.3 | 3 (8) | 7.0 ± 0.3 | 3 (9) |
| 16 | 8.0 ± 0.3 | 3 (8) | 7.8 ± 0.3 | 3 (9) | 7.1 ± 0.3 | 3 (9) |
| 21 | 8.6 ± 0.3 | 3 (9) | 10.2 ± 0.3 | 3 (9) | 9.1 ± 0.3 | 3 (9) |
| 28 | 15.9 ± 0.4 | 4 (14) | 16.1 ± 0.5 | 3 (12) | 16.6 ± 0.5 | 3 (11) |
| 98 | 32.7 ± 0.8 | 3 (12) | 32.3 ± 0.8 | 3 (12) | 32.5 ± 0.8 | 3 (11) |
| Leptin (ng/mL) | ||||||
| PN | ||||||
| 5 | 4.9 ± 1.5 | 3 (9) | 5.3 ± 1.6 | 3 (7) | 3.6 ± 1.4 | 3 (10) |
| 10 | 10.4 ± 1.5 | 3 (9) | 11.4 ± 1.5 | 3 (8) | 9.4 ± 1.5 | 3 (8) |
| 12 | 12.7 ± 1.5 | 3 (8) | 13.4 ± 1.5 | 3 (9) | 14.9 ± 1.5 | 3 (9) |
| 14 | 8.1 ± 1.5 | 3 (9) | 7.4 ± 1.5 | 3 (8) | 8.5 ± 1.5 | 3 (9) |
| 16 | 5.9 ± 1.5 | 3 (8) | 6.0 ± 1.5 | 3 (9) | 7.4 ± 1.5 | 3 (9) |
| 21 | 3.5 ± 1.5 | 3 (9) | 3.2 ± 1.5 | 3 (8) | 4.4 ± 1.5 | 3 (8) |
| Ghrelin (ng/mL) | ||||||
| PN | ||||||
| 16 | 0.43 ± 0.07 | 3 (8) | 0.36 ± 0.07 | 3 (8) | 0.30 ± 0.07 | 3 (7) |
| 21 | 0.21 ± 0.07 | 3 (8) | 0.22 ± 0.07 | 3 (7) | 0.21 ± 0.07 | 3 (8) |
Data from each sample time represent a different set of animals. Significances between groups were determined by using a mixed-effects regression model followed by Tukey-Kramer post hoc tests. There were no significant differences between groups in body weight and plasma hormones. CTR, control diet; HIGH n−3, diet with a relative increase in n−3 fatty acid; LOW n−6, diet with relative reduction in n−6 fatty acid; PN, postnatal day.
On the basis of a study by Korotkova et al (26), which showed that the postnatal leptin surge was altered by the perinatal dietary FA composition, we hypothesized that the postnatal dietary intervention may affect the secretion pattern of leptin. A distinctive surge in circulating plasma leptin concentrations was observed during the suckling period (P < 0.001). However, the amplitude, duration, and overall concentrations were comparable between groups (Table 2).
Because recent unpublished observations have suggested a role for ghrelin in the organization of central feeding circuits (SG Bouret, unpublished observations, 2010), we measured plasma ghrelin concentrations in the remaining plasma collected at PNs 16 and 21. Plasma acylated ghrelin concentrations decreased between PNs 16 and 21 (P = 0.02) and were not different between groups (Table 2). These results suggest that the postnatal LOW n−6 and HIGH n−3 does not affect circulating leptin or ghrelin plasma concentrations during postnatal life.
The analysis of the erythrocyte FA composition in pups at weaning confirmed a change in the FA supply during lactation. Compared with the CTR (n = 9 pups; 3 litters), the HIGH n−3 (n = 9 pups; 3 litters) resulted in a higher relative amount of EPA (2.31 ± 0.05% in the HIGH n−3; 0.34 ± 0.05% in the CTR; P < 0.001) and DHA (10.7 ± 0.2% in the HIGH n−3; 4.4 ± 0.2% in the CTR; P < 0.01), whereas the LOW n−6 (n = 8 pups; 3 litters) resulted in a lower concentration of LA (6.7 ± 0.5% in the LOW n−6; 10.5 ± 0.5% in the CTR; P < 0.001) in erythrocytes.
Disrupted organization of hypothalamic neural projections in mice fed with HIGH n−3 or LOW n−6
To determine whether the postnatal dietary FA quality influences the organization of hypothalamic circuits involved in energy balance, we performed immunofluorescence labeling of AgRP and α-MSH containing neural projections in brain sections at PN 28 (Figure 1A). The density of AgRP-immunoreactive fibers in the PVH was reduced by ∼40% in LOW n−6 and in HIGH n−3 mice compared with CTR mice (Figure 1B). Similarly, a 30% reduction in the density of α-MSH–immunoreactive fibers was observed in both diet groups compared with the CTR group (Figure 1C). However, the overall distribution pattern of AgRP and α-MSH–immunoreactive fibers in the PVH was similar between groups, suggesting that the dietary FA composition altered the density but not the distribution of AgRP- and α-MSH–immunoreactive fibers in the PVH.
FIGURE 1.
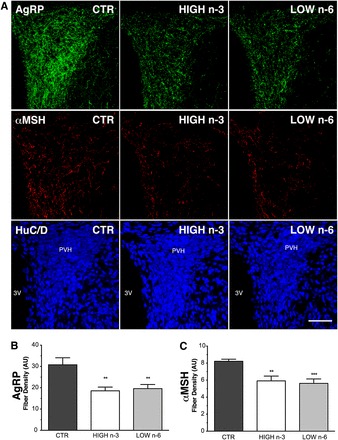
Altered AgRP and αMSH neural projections in postnatal day 28 male mice fed postnatally with a HIGH n−3 or LOW n−6. A: Representative confocal images through the PVH delineated by using antisera against HuC/D (blue channel), illustrating AgRP (green channel) and αMSH (red channel) immunoreactivity in the PVH of postnatal day 28 male mice. Scale bar: 100 μm. Quantitative comparison of the density of AgRP-immunoreactive fibers (B) and αMSH–immunoreactive fibers (C) innervating the PVH in HIGH n−3 (n = 8 pups; 3 litters), LOW n−6 (n = 8 pups; 3 litters), and CTR groups (n = 10 pups; 4 litters). Values represent means (±SEMs) of fiber length per set of volume sampled. Significances between groups were determined by using a mixed-effects regression model followed by Tukey-Kramer post hoc tests. **P = 0.01, ***P = 0.001 between HIGH n−3 or LOW n−6 and CTR groups. AgRP, Agouti-related peptide; AU, arbitrary units; CTR, control diet; HIGH n−3, diet with a relative increase in n−3 FA; HuC/D, human neuronal protein HuC and HuD; LOW n−6, diet with relative reduction in n−6 FA; PVH, paraventricular nucleus of the hypothalamus; 3V, third ventricle; αMSH, α-melanocyte stimulating hormone.
At PN 98, the density of AgRP- and α-MSH–immunoreactive fibers in the PVH was largely comparable to that observed at PN 28. These data could not be included in the mixed-effects regression model because of the restricted sample size in both HIGH n−3 (n = 4 pups; 1 litter) and LOW n−6 (n = 3 pups; 1 litter) group. However, a less-stringent statistical analysis (1-factor ANOVA followed by a Tukey-Kramer post hoc comparison) indicated that, compared with the CTR (n = 6 pups; 2 litters), the average density of AgRP-immunoreactive fibers was reduced by 40% and 30% in mice fed LOW n−6 and HIGH n−3, respectively, during postnatal life [3176.99 ± 35.78 arbitrary units (AU) in HIGH n−3, 4002.2 ± 44.48 AU in LOW n−6, and 5495.51 ± 56.74 AU in CTR groups; P < 0.001 between HIGH n−3 or LOW n−6 and CTR groups], and the average density of α-MSH–immunoreactive fibers was reduced by 70% and 30%, respectively (1565.86 ± 84.85 AU in HIGH n−3, 2372.88 ± 72.43 AU in LOW n−6, and 3641.38 ± 72.22 AU in CTR groups; P < 0.001 between HIGH n−3 or LOW n−6 and CTR groups). Together, these observations suggest that relatively modest alterations in the dietary FA composition during early development lead to substantial and permanent changes in peptidergic innervation of neurons in the PVH.
DISCUSSION
The current study clearly showed that a postnatal diet with a difference in n−6 and n−3 FA contents, either by increasing the relative contribution of n−3 or decreasing n−6 PUFAs within the total lipid fraction, affects the density of AgRP- and α-MSH–immunoreactive fibers innervating the PVH. These structural modifications appear to be permanent because similar differences were observed after a WSD challenge in adulthood. Moreover, these alterations appear to be leptin independent. Changes in the hypothalamic structure associated with the modification of the perinatal nutritional environment have been reported previously, but in the current study, we showed, for the first time to our knowledge, that relatively mild dietary manipulations in lipid quality, but not quantity, restricted to the postnatal period are capable of causing profound changes in the development of hypothalamic neural connections.
It was not entirely surprising that the development of ARH projections was affected by the FA composition of the postnatal diet, but the direction of the observed changes was quite unexpected. Various studies have shown that the density of AgRP- and α-MSH–immunoreactive fibers was reduced in animals prone to adult obesity (9, 27, 28) or in rats that were undergoing an intrauterine and postnatal growth restriction (29). These alterations were associated with changes in the timing and amplitude of the leptin surge (8, 13). Moreover, in rats selectively bred to develop diet-induced obesity, large litter rearing has been sufficient to rescue fiber density by correcting the postnatal leptin surge and alleviating the adult obese phenotype (30). Surprisingly, our findings showed that, although mice fed a HIGH n−3 or LOW n−6 exhibited significant reductions in the density of AgRP- and α-MSH–immunoreactive fibers in the PVH, their body weights remained unaffected by the dietary manipulation. Furthermore, in a previous study, we showed that these mice exhibited reduced fat deposition and reduced plasma leptin concentration when subsequently exposed to a mild WSD in adulthood (17, 18). Although different mechanisms may be involved in the current study compared with studies with obesity-prone animals, our results suggested that the relation between the density of AgRP and α-MSH fibers in the PVH and expression of particular components of obesity was complex and context dependent. Although it seems clear that diet affects projections from the ARH to the PVH, the impact on other terminal fields remains to be determined. In leptin-deficient mice, the density of peptidergic fibers is reduced in all ARH targets (31), and leptin is required for normal axonal targeting of ARH projections to functionally distinct components of the PVH (25). Thus, in future studies, it will be valuable to define diet-induced alterations in postsynaptic targeting. A reduction in AgRP or α-MSH–immunoreactive fibers in the PVH may also reflect reduced peptide synthesis or alterations in the transport to fiber terminals. Alternatively, reductions in fiber staining may also reflect an increased release of the peptide. The use of genetically targeted axonal markers will be required to distinguish between diet-induced alterations in peptide processing and changes in axonal targeting.
In contrast to the results obtained in rats by Korotkova et al (26), we did not detect significant changes in leptin concentrations in postnatal mice exposed to diets with altered relative n−6 and n−3 FA contents. This discrepancy may have been attributable to species differences but may also have been the result of having restricted our dietary intervention to the lactation period.
Although the impaired development of ARH fibers has been attributed to insufficient leptin signaling during postnatal life (9, 13), our data indicated that structural changes in peptidergic projections from ARH to PVH may occur independent of alterations in postnatal leptin kinetics. Other mechanisms may affect the development of ARH projections to the PVH, including diet-dependent changes in axon guidance or impaired leptin signaling in ARH neurons that blocks leptin’s neurotrophic effects (12, 27). Abnormalities in postnatal leptin receptor expression or function have been associated with leptin resistance later in life (10, 11), but the underlying mechanisms remain ill defined. However, the reduced fat mass and lower concentrations of circulating leptin observed in adulthood during our previous study (17, 18) argued against the development of classical leptin resistance in the current study.
Although leptin’s developmental actions are widely appreciated, other circulating hormones may also alter the development and activity of feeding circuits. For example, insulin has long been associated with brain development, and an injection of insulin to the region of the mediobasal hypothalamus in the early postnatal period generates metabolic and structural abnormalities similar to the one shown in pups born to insulin-deficient dams (32, 33). More recently, unpublished observations suggest that the gut hormone ghrelin may inhibit the formation of ARH neural projections during development (SG Bouret, unpublished results, 2010). Plasma ghrelin concentrations are responsive to dietary FAs (34, 35), and in our study, ghrelin concentrations tended to be slightly higher at PN 16 in the mice fed a LOW n−6 or HIGH n−3, but this result did not reach significance. Because ghrelin concentrations peaked during the second postnatal week, and the amount of blood available from these young mice was limited, we were not able to analyze ghrelin before PN 16 in this study. Whether exposure to altered n−6 and n−3 FA diets increases circulating ghrelin during early development, thereby contributing to the altered brain phenotype, remains to be investigated.
In addition to influencing hormone concentrations, FA can modulate hypothalamic neuronal activity and neuropeptide expression directly (36, 37). This effect suggests that, during early development, FA could have an additional role in ARH neurodevelopment by modulating the activity of hypothalamic neurons. However, to our knowledge, there have been no reports about direct effects of n−6 and n−3 long-chain PUFAs on activity-dependent mechanisms affecting the development of hypothalamic circuits.
The timing of hypothalamic maturation in rodents appears to differ from that of primates, including humans. Although rodent development of ARH projections takes place during postnatal life, this process commences during the third trimester of pregnancy in nonhuman primates (38). Thus, in utero exposure to increased n−3 or reduced n−6 FAs may shape the formation of neuronal networks that regulated energy balance in the developing human fetus in a similar way to that shown in the current study for rodents during postnatal life. Maternal dietary FAs are known to transfer across the placenta (39) and are secreted in breast milk during early postnatal life (40). Therefore, understanding the role of specific dietary components such as FA quality in the programming of energy-balance regulation will enable a better understanding of how early nutrition affects metabolic health and susceptibility to obesity later in life.
Acknowledgments
The authors’ responsibilities were as follows—LS, AO, EMvdB, and RBS: designed the research; LS and KB: conducted the research and analyzed data; LS: wrote the first draft of the manuscript; and all authors: critically revised the manuscript for content and approved the final version of the manuscript. This study was funded by Nutricia Research, and LS, AO and EMvdB are employed by Nutricia Research (formerly known as Danone Research – Centre for Specialised Nutrition). Because of the participation of these employees in the study, Nutricia Research contributed to the study design, conduct of the study, analysis of samples and data, interpretation of the findings, and preparation of the manuscript. KB and RBS had no conflicts of interest.
ABBREVIATIONS
- AgRP
Agouti-related peptide
- ARH
arcuate nucleus of the hypothalamus
- AU
arbitrary units
- CTR
control diet
- FA
fatty acid
- HIGH n−3
diet with a relative increase in n−3 fatty acid
- LA
linoleic acid
- LOW n−6
diet with a relative reduction in n−6 fatty acid
- PN
postnatal day
- PVH
paraventricular nucleus of the hypothalamus
- WSD
Western-style diet
- α-MSH
α-melanocyte stimulating hormone
FOOTNOTES
Supported by Nutricia Research – Danone Nutricia Early Life Nutrition.
REFERENCES
- 1. Wang Y, Lobstein T.. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 2006;1:11–25. [DOI] [PubMed] [Google Scholar]
- 2. Lobstein T, Baur L, Uauy R.. Obesity in children and young people: a crisis in public health. Obes Rev 2004;5(suppl 1):4–104. [DOI] [PubMed] [Google Scholar]
- 3. Low S, Chin MC, Deurenberg-Yap M.. Review on epidemic of obesity. Ann Acad Med Singapore 2009;38:57–9. [PubMed] [Google Scholar]
- 4. Taylor PD, Poston L.. Developmental programming of obesity in mammals. Exp Physiol 2007;92:287–98. [DOI] [PubMed] [Google Scholar]
- 5. Hales CN, Barker DJ.. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG.. Central nervous system control of food intake. Nature 2000;404:661–71. [DOI] [PubMed] [Google Scholar]
- 7. Bouret SG, Draper SJ, Simerly RB.. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 2004;24:2797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, et al. . Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 2005;1:371–8. [DOI] [PubMed] [Google Scholar]
- 9. Bouret SG, Simerly RB.. Development of leptin-sensitive circuits. J Neuroendocrinol 2007;19:575–82. [DOI] [PubMed] [Google Scholar]
- 10. Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J.. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 2008;32:1153–60. [DOI] [PubMed] [Google Scholar]
- 11. Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC.. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr 2006;95:830–7. [DOI] [PubMed] [Google Scholar]
- 12. Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW.. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE 2009;4:e5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D.. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 2008;149:470–5. [DOI] [PubMed] [Google Scholar]
- 14. Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P.. Temporal changes in dietary fats: role of n−6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res 2006;45:203–36. [DOI] [PubMed] [Google Scholar]
- 15. Muhlhausler BS, Gibson RA, Makrides M.. The effect of maternal omega-3 long-chain polyunsaturated fatty acid (n−3 LCPUFA) supplementation during pregnancy and/or lactation on body fat mass in the offspring: a systematic review of animal studies. Prostaglandins Leukot Essent Fatty Acids 2011;85:83–8. [DOI] [PubMed] [Google Scholar]
- 16. Simopoulos AP.. Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999;70(suppl):560S–9S. [DOI] [PubMed] [Google Scholar]
- 17. Oosting A, Kegler D, van de Heijning BJ, van der Beek EM.. Reduced n−6 polyunsaturated fatty acids during postnatal development affect white adipose tissue development and prevent excessive fat deposition in adulthood (abstract PIII-399). J Dev Orig Health Dis 2011;2(suppl S2):S35. [Google Scholar]
- 18. Oosting A, Kegler D, Boehm G, Jansen HT, van de Heijning BJ, van der Beek EM.. n−3 long-chain polyunsaturated fatty acids prevent excessive fat deposition in adulthood in a mouse model of postnatal nutritional programming. Pediatr Res 2010;68:494–9. [DOI] [PubMed] [Google Scholar]
- 19. Festing MF.. Design and statistical methods in studies using animal models of development. ILAR J 2006;47:5–14. [DOI] [PubMed] [Google Scholar]
- 20. Koletzko B, Rodriguez-Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T.. Physiological aspects of human milk lipids. Early Hum Dev 2001;65(suppl):S3–18. [DOI] [PubMed] [Google Scholar]
- 21. Francois CA, Connor SL, Wander RC, Connor WE.. Acute effects of dietary fatty acids on the fatty acids of human milk. Am J Clin Nutr 1998;67:301–8. [DOI] [PubMed] [Google Scholar]
- 22. Reeves PG, Nielsen FH, Fahey GC Jr.. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 23. Levant B, Ozias MK, Carlson SE.. Diet (n−3) polyunsaturated fatty acid content and parity affect liver and erythrocyte phospholipid fatty acid composition in female rats. J Nutr 2007;137:2425–30. [DOI] [PubMed] [Google Scholar]
- 24. Baur LA, O’Connor J, Pan DA, Wu BJ, O’Connor MJ, Storlien LH.. Relationships between the fatty acid composition of muscle and erythrocyte membrane phospholipid in young children and the effect of type of infant feeding. Lipids 2000;35:77–82. [DOI] [PubMed] [Google Scholar]
- 25. Bouyer K, Simerly RB.. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J Neurosci 2013;33:840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korotkova M, Gabrielsson B, Lonn M, Hanson LA, Strandvik B.. Leptin levels in rat offspring are modified by the ratio of linoleic to alpha-linolenic acid in the maternal diet. J Lipid Res 2002;43:1743–9. [DOI] [PubMed] [Google Scholar]
- 27. Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB.. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 2008;7:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steculorum SM, Bouret SG.. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology 2011;152:4171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coupé B, Amarger V, Grit I, Benani A, Parnet P.. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 2010;151:702–13. [DOI] [PubMed] [Google Scholar]
- 30. Patterson CM, Bouret SG, Park S, Irani BG, Dunn-Meynell AA, Levin BE.. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology 2010;151:4270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bouret SG, Draper SJ, Simerly RB.. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004;304:108–10. [DOI] [PubMed] [Google Scholar]
- 32. Plagemann A, Harder T, Janert U, Rake M, Rittel F, Rohde W, Dorner G.. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev Neurosci 1999;21:58–67. [DOI] [PubMed] [Google Scholar]
- 33. Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G.. Lifelong enhanced diabetes susceptibility and obesity after temporary intrahypothalamic hyperinsulinism during brain organization. Exp Clin Endocrinol 1992;99:91–5. [DOI] [PubMed] [Google Scholar]
- 34. Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M.. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology 2005;146:2255–64. [DOI] [PubMed] [Google Scholar]
- 35. Burghardt PR, Kemmerer ES, Buck BJ, Osetek AJ, Yan C, Koch LG, Britton SL, Evans SJ.. Dietary n−3:n−6 fatty acid ratios differentially influence hormonal signature in a rodent model of metabolic syndrome relative to healthy controls. Nutr Metab (Lond) 2010;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dziedzic B, Szemraj J, Bartkowiak J, Walczewska A.. Various dietary fats differentially change the gene expression of neuropeptides involved in body weight regulation in rats. J Neuroendocrinol 2007;19:364–73. [DOI] [PubMed] [Google Scholar]
- 37. Migrenne S, Magnan C, Cruciani-Guglielmacci C.. Fatty acid sensing and nervous control of energy homeostasis. Diabetes Metab 2007;33:177–82. [DOI] [PubMed] [Google Scholar]
- 38. Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL.. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience 2006;143:975–86. [DOI] [PubMed] [Google Scholar]
- 39. Herrera E.. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development–a review. Placenta 2002;23(suppl A):S9–19. [DOI] [PubMed] [Google Scholar]
- 40. Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM.. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 2007;85:1457–64. [DOI] [PubMed] [Google Scholar]


