ABSTRACT
Background: The WHO estimates that 190 million preschool children have vitamin A deficiency (VAD). Serum retinol (SR) concentration is a common indicator of vitamin A (VA) status, but SR is homeostatically controlled and suppressed during inflammation, which may lead to misdiagnosis.
Objective: The sensitivity and specificity of SR compared with VA total liver reserves (TLRs) were evaluated for VAD in children from Thailand (n = 37) and Zambia (n = 128). SR was adjusted for inflammation in the Zambian children.
Design: Each child was classified as VA-deficient or not based on cutoffs of <0.1 μmol VA/g liver with the use of retinol isotope dilution and <0.7 μmol/L for SR concentrations. Four categories of infection status in the Zambian children were based on elevated C-reactive protein (CRP) and α1-acid glycoprotein (AGP). Sensitivity and specificity were calculated with the use of unadjusted and inflammation marker–adjusted SR cutoffs.
Results: VAD was 65% and 0% according to TLRs and SR, respectively, in Thai children and 0% and 17%, respectively, in Zambian children. No true positive VAD cases occurred; thus, sensitivity was 0% and indeterminable, respectively; specificity was 100% and 82.8%, respectively. CRP was elevated in 26.6% of Zambian children, whereas 97.7% had elevated AGP, categorizing them as having no infection (2.3%) or in early (26.6%) or late (58.6%) convalescence. With the use of marker-adjusted SR cutoffs of 0.6 μmol/L for late convalescence and 0.5 μmol/L for early convalescence, the adjusted prevalence of SR deficiency was 2.3%, increasing specificity to 97.3%.
Conclusions: No cases of VAD were identified by both TLRs and SR (true positives) in Thai or Zambian children. Specificity of SR to evaluate VAD was high, but additional research is needed to investigate sensitivity. Adjusting SR cutoffs for inflammation improved specificity by reducing false positives. SR as a VAD indicator may depend on infection rates, which should be taken into consideration. These studies were registered at clinicaltrials.gov as NCT01061307 (for Thailand) and NCT01814891 (for Zambia).
Keywords: school-age children, stable isotope dilution, Thailand, vitamin A deficiency, Zambia
INTRODUCTION
Vitamin A (VA)8 is a fat-soluble vitamin that supports growth, reproduction, vision, and immune function. The WHO estimates that 190 million preschool children have VA deficiency (VAD), which is implicated in childhood morbidity, mortality, and blindness (1). Accurate indicators of VA status are needed to determine population prevalence of VAD and evaluate the efficacy and effectiveness of interventions aimed at reducing VAD. Serum retinol (SR) concentration is a common indicator of VA status and is recommended by the WHO when used with other indicators (2). The WHO defines the population prevalence of VAD as ≥20% of children with an SR concentration of <0.7 μmol/L as a severe public health problem (2). However, correction for inflammation or infection status has not been evaluated as part of WHO recommendations.
Studies in which children are supplemented with VA have found that SR does not always respond to the intervention because SR concentration is homeostatically controlled and therefore not always associated with changes in liver VA reserves (3). Furthermore, SR is suppressed by 25–69% during infection, independent of liver VA stores (3–7). A study in Ghanaian children found that elevated concentrations of the acute-phase protein α1-acid glycoprotein (AGP) were associated with a 24% decrease in mean SR (4), whereas a study in Zambia found that SR decreased by 20–30% during early convalescence (7). Nevertheless, the cutoff for SR concentration to define VAD was selected irrespective of infection prevalence of a population, with the justification that populations with high infection rates are more likely to be affected by VAD (8). However, this may lead to overdiagnosed VAD, wasted resources, and even potential risk that oversupplementation or excessive fortification may lead to hypervitaminosis A (3).
Thurnham and McCabe (9) proposed correction factors that can be applied to SR cutoffs to account for the suppression of SR concentrations during infection, inflammation, or trauma. The SR-adjusted cutoffs are applied based on measurement of 2 acute-phase proteins, C-reactive protein (CRP) and AGP, which can be used to identify stages of infection: incubation (elevated CRP only), early convalescence (elevated CRP and AGP), and late convalescence (elevated AGP only) (9).
Recently, 2 VA intervention studies were undertaken in Thai and Zambian children (10, 11). These studies uniquely included measures of SR concentrations and VA total body stores (TBSs) to estimate VA total liver reserves (TLRs) with the use of stable retinol isotope dilution (RID) technology (12). This article is novel in that it compares SR with TLRs in a large number of children with and without adjustment for inflammation markers. Sensitivity and specificity evaluate the utility of clinical tests to determine the presence of a disease (13). Sensitivity is the ability of a test to correctly identify whether someone has the disease, whereas specificity is the ability of a test to correctly identify whether someone does not have the disease (Supplemental Table 1). The first objective was to compare VAD prevalence according to the 2 indicators and calculate sensitivity and specificity of SR concentrations to determine VAD in children from Thailand and Zambia; the second objective was to examine the effect of inflammation by correcting for infection status on the sensitivity and specificity of SR to determine VAD in the Zambian children.
METHODS
We used preintervention data from 2 studies conducted in groups of children from Thailand in 2010 (10) and Zambia in 2012 (11) to determine specificity and sensitivity. Children needed to be free from febrile illness and in general good health at the time of enrollment. The Thai study enrolled children from one elementary school (n = 50), and the Zambian study recruited children from 4 rural village sites (n = 143). The current analysis included only children who had both SR and TLR measurements available. The study protocol in Thailand was approved by the ethics committees of ETH Zurich, Switzerland, and Mahidol University, Thailand (NCT01061307). All procedures in Zambia were approved by Tropical Diseases Research Centre’s Ethics Review Committee and University of Wisconsin-Madison’s Health Sciences Human Subjects Institutional Review Board (NCT01814891). Informed consent procedures were followed and written forms were obtained from all parents or caregivers. No adverse events were reported in either study from the interventions.
TLRs were used as the reference standard, estimated with the use of TBSs, which were determined similarly in both studies by a master’s-level technician who remained blinded during the integration of sample outputs, with the use of the 13C-RID test and applying the mass balance equation with specific assumptions for each group regarding inflammation and dose absorption, storage, and metabolism (10–12). Briefly, 1 μmol 13C2-retinyl acetate dissolved in soybean oil was delivered orally to each child with the use of a positive displacement pipette and immediately followed with a fat-containing snack to facilitate absorption. After a 14 d mixing period, a blood sample was taken and the 13C:total C ratio in SR was determined by gas chromatography/combustion/isotope ratio mass spectrometry. Together with the baseline natural abundance 13C:total C ratio of SR from a group mean of nonintervened children to minimize blood draws per child (Thailand) or each child (Zambia), TBSs were calculated and TLRs were estimated.
Mean TLR and SR concentrations in each group were compared with the use of t tests, with P < 0.05 indicating significance. Each child was determined to be VA-deficient or not deficient based on the cutoffs of <0.1 μmol/g liver for estimated TLR (3) and <0.7 μmol/L for SR concentration (2). Comparisons were made between presence of VAD in each child as defined by TLRs and SR to calculate the rate of true positives, false positives, true negatives, and false negatives (Supplemental Table 1) (13).
Correlations between the indicators of inflammation, i.e., AGP and CRP, and SR and estimated TLRs were then examined with the use of Pearson’s correlation. Infection status in the Zambian children was calculated based on the categories proposed by Thurnham and McCabe (9) with the use of cutoffs of CRP >5 mg/L and AGP >1 g/L: children with neither acute-phase protein elevated were considered to be the healthy reference group, those with only elevated CRP were in the incubation stage, those with elevated CRP and AGP were in the early convalescent stage, and children with only raised AGP were in the late convalescent stage.
Thurnham and McCabe (9) proposed an infection correction factor applied to the 0.7-μmol/L SR concentration cutoff used to define deficiency rather than correcting individual results. For incubation or late convalescence (elevated CRP or AGP), the adjusted SR cutoff for VAD would be 0.6 μmol/L, and for early convalescence (elevated CRP and AGP), it would be 0.5 μmol/L. These correction factors were used to calculate adjusted VAD rates based on infection status, and sensitivity and specificity of SR concentrations to determine VAD were again calculated in the Zambian children after correction for presence of inflammation. Statistical analysis and calculations were performed in Stata/SE 13.1 (StataCorp).
RESULTS
Out of the original study sample sizes, 37 had both SR and TLRs in the Thai study and 128 children had both SR and TLRs in the Zambian study. Baseline characteristics are shown in Table 1. Whereas mean SR concentrations were higher in the Thai children, their TLRs were much lower than those of the Zambian children; TLR distribution was very different between the populations in comparison with SR (Figure 1). The mean ± SD values of estimated TLRs were 0.09 ± 0.05 μmol/g in the Thai children and 1.14 ± 0.41 μmol/g in the Zambian children (Table 1); the mean and variance of these distributions were both significantly different between the countries (P < 0.001). Mean SR values were 1.19 ± 0.22 μmol/L and 0.97 ± 0.27 μmol/L in the Thai and Zambian children, respectively; mean SR concentrations between the countries were significantly different (P < 0.001), but variance was not. In the Thai children, 10.8% had elevated CRP; AGP was not measured in this group, so infection status could not be determined. Among the Zambian children, elevated CRP (>5 mg/L) was present in 34 children (26.6%), and AGP was elevated (>1 g/L) in 125 (97.7%) of the children, which categorized them as having no infection (2.3%), or in either early (26.6%) or late (58.6%) convalescence, with no children in the incubation stage. Sixteen Zambian children were missing CRP values, and therefore their infection status could not be determined.
TABLE 1.
Baseline anthropometric characteristics, vitamin A indicators, and markers of infection in the Thai and Zambian study participants with both SR concentrations and TLR values, as well as infection stage in the Zambian study participants1
| Thai children (n = 37) | Zambian children (n = 128) | |
| Population characteristics of children with both SR and TLR values | ||
| Age, y | 9.1 ± 1.3 | 5.9 ± 0.57 |
| Weight, kg | 24.4 ± 5.2 | 17.3 ± 2.0 |
| Height, m | 1.26 ± 0.09 | 1.08 ± 0.06 |
| BMI-for-age z score | −0.67 ± 0.97 | −0.34 ± 0.73 |
| Vitamin A indicators | ||
| Vitamin A TLR, μmol/g | 0.09 ± 0.05* | 1.14 ± 0.41 |
| SR, μmol/L | 1.19 ± 0.22* | 0.97 ± 0.27 |
| Markers of infection | ||
| CRP >5 mg/L | 4 (10.8) | 34 (26.6) (16 missing) |
| AGP >1 g/L | No data | 125 (97.7) |
| Infection stage2 | ||
| No infection (neither CRP nor AGP elevated) | 3 (2.3) | |
| Incubation (only CRP elevated) | 0 (0) | |
| Early convalescence (both CRP and AGP elevated) | 34 (26.6) | |
| Late convalescence (only AGP elevated) | 75 (58.6) | |
| Cannot be determined (missing CRP) | 16 (12.5) |
Values are means ± SDs or n (%). *P < 0.001 for difference between Thai and Zambian mean values with the use of t tests. AGP, α1-acid glycoprotein; CRP, C-reactive protein; SR, serum retinol; TLR, total liver reserve.
Could not be determined for Thai participants because of lack of AGP data.
FIGURE 1.
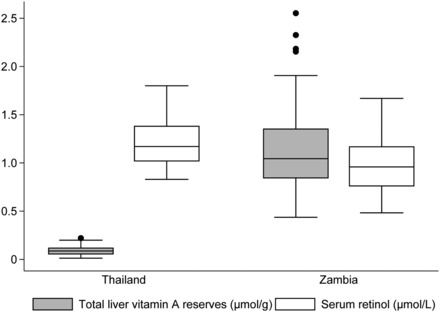
Comparison of total liver vitamin A reserves and serum retinol concentration between the Thai (n = 37) and Zambian (n = 128) study populations. Lines within boxes represent medians, boxes represent IQRs, and lines outside boxes represent upper and lower adjacent values with circles indicating outside values.
Overall, SR concentrations and TLRs were poorly correlated in both populations. The Pearson correlation and r2 between calculated TLRs and SR were 0.08 and 0.01, respectively (P = 0.63), in the Thai data, and 0.15 and 0.02, respectively (P = 0.09), in the Zambian data (Figure 2). In the Thai children, the correlation between SR and CRP was −0.04 (P = 0.81), and the correlation between TLRs and CRP was −0.13 (P = 0.46). In the Zambian children, SR concentrations were negatively correlated with AGP (−0.30, P = 0.001) and CRP (−0.30, P = 0.001). The correlation between TLRs and CRP was −0.15 (P = 0.11), and that between TLRs and AGP was −0.12 (P = 0.20), but neither was significant.
FIGURE 2.
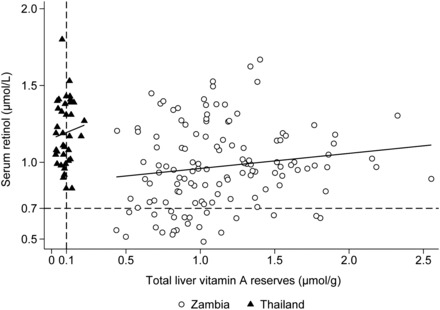
Association between serum retinol concentration and TLRs in children from Thai (n = 37, r = 0.15, P = 0.63 with Pearson’s correlation) and Zambian (n = 128, r = 0.08, P = 0.09) studies. The horizontal dashed black line represents the cutoff for VAD defined by serum retinol concentration at 0.7 μmol/L; the vertical dashed black line represents the cutoff for VAD defined by TLRs at 0.1 μmol/g. TLR, total liver reserve; VAD, vitamin A deficiency.
The prevalence of VAD as determined by TLR or SR concentrations between the 2 populations was opposite: the Thai children had no cases of VAD according to SR concentrations, but there was a 65% prevalence of VAD according to TLR; in contrast, in Zambia, there were no children found deficient with the use of TLR, whereas 17.2% were found deficient with the use of SR (Table 2). It is also of note that 74 (57.8%) of the Zambian children had calculated TLR >1 μmol/g, which is considered to be hypervitaminosis A (3).
TABLE 2.
Prevalence of VA deficiency calculated with the use of VA TLR and SR concentrations and specificity of SR as an indicator of VA deficiency in Thai and Zambian children, as well as in Zambian children adjusted for infection status1
| Thai children (n = 37) | Zambian children (n = 128) | Zambian children, adjusted for infection (n = 128) | |
| Prevalence of VA deficiency, by indicator | |||
| VA TLR | 24 (65) | 0 | 0 |
| Serum retinol | 0 | 22 (17.2) | 3 (2.3) |
| Cannot be determined | 0 | 0 | 16 (12.5)2 |
| True positives (TLR+, SR+) | 0 | 0 | 0 |
| False positives (TLR−, SR+) | 0 | 22 (17.2) | 3 (2.3) |
| False negatives (TLR+, SR−) | 24 (65) | 0 | 0 |
| True negatives (TLR−, SR−) | 13 (35) | 106 (82.8) | 109 (85.2) |
| Cannot be determined | 0 | 0 | 16 (12.5) |
| Sensitivity3 | 0 | NC | NC |
| Specificity4 | 100 | 82.8 | 97.3 |
Values are percentages or n (%). Both TLR+ and SR+: positive for VAD according to that indicator. Both TLR– and SR−: negative for VAD according to that indicator. CRP, C-reactive protein; NC, not calculable (divided by zero); SR, serum retinol; TLR, total liver reserve; VA, vitamin A; VAD, vitamin A deficiency.
Could not be adjusted for infection because of missing CRP values.
Calculated as true positives/(true positives + false negatives).
Calculated as true negatives/(true negatives + false positives).
In the Thai population, there were 24 false negative and 13 true negative cases, with no true positive or false positive cases. Sensitivity was thus 0% [0/(0 + 24)], meaning that of the “true” VAD cases identified by TLRs, none were detected by SR (false negatives). Specificity was 100% [13/(13 + 0)], meaning that all Thai children without VAD according to TLRs were correctly identified by SR (true negatives). In the Zambian population, there were 22 false positive and 106 true negative cases of VAD, with no true positives or false negatives; accordingly, sensitivity could not be calculated [0/(0 + 0)]. Specificity was 82.8% [106/(106 + 22)], meaning that although all children were negative for VAD according to TLRs, 82.8% were also found to be negative by SR (true negatives), and 17% were found to have VAD by SR (false positives).
The kernel density estimate of SR concentration in the Zambian children by infection stage is illustrated in Figure 3. The estimated smoothed distribution of SR concentration in the late convalescent infection stage is shifted to the left of “healthy” children with no inflammation, whereas the distribution of SR concentrations in children in the early convalescent stage is shifted further left, projecting an overall decrease in concentration. In the Zambian children, with the use of the marker-adjusted SR cutoffs of 0.6 μmol/L for late convalescence and 0.5 μmol/L for early convalescence, the adjusted prevalence of SR deficiency was 2.3%, which decreased false positive cases from 22 to 3, thus increasing specificity to 97.3% (Table 2). A scatterplot of TLR vs. SR concentrations for Zambian children shows how the lowered marker-adjusted SR cutoffs decreased the number of false positive cases of VAD (Figure 4).
FIGURE 3.
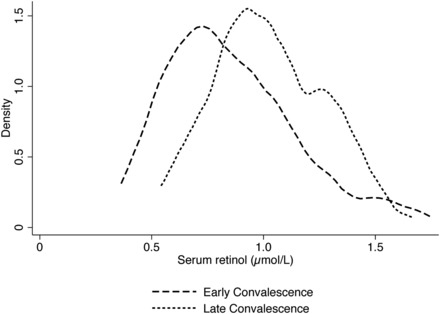
Kernel density estimation visually depicts the trend in serum retinol concentration shifts (μmol/L) in Zambian children in early or late convalescence stage of infection (n = 34, early convalescence; n = 75, late convalescence; 16 excluded because of missing CRP data). There were not enough children in the incubation stage (n = 3) or with no inflammation (n = 0) to produce a kernel density estimation, which produces a smoothed distribution curve of the data. CRP, C-reactive protein.
FIGURE 4.
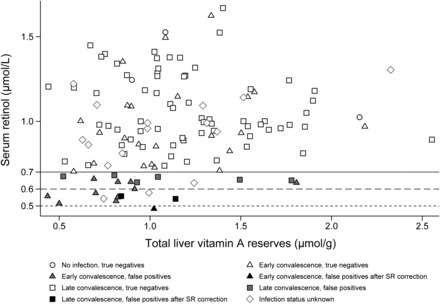
Comparison of the association between liver VA and serum retinol concentration by infection status in Zambian children. The standard cutoff for VAD defined by serum retinol concentration is 0.7 μmol/L. Gray shaded markers below that line indicate false positives; black shaded markers are the false positives identified after adjustment of the SR cutoffs to 0.6 μmol/L for children in the late convalescent infection stage (square markers) and 0.5 μmol/L for children in the early convalescent stage (triangle markers). n = 3 (no infection), n = 0 (incubation), n = 34 (early convalescence), n = 75 (late convalescence), and n = 16 (infection status unknown because of missing CRP data). CRP, C-reactive protein; SR, serum retinol; VA, vitamin A; VAD, vitamin A deficiency.
DISCUSSION
The specificity and sensitivity of SR concentration to determine VAD was examined between 2 groups of children in Thailand and Zambia and found to be divergent. The Thai and Zambian children had very different TLRs (in both magnitude and variance) determined in the same laboratory. Among the Thai children, specificity of SR was 100%, indicating that all children without VAD (as determined by estimated TLRs) were correctly identified as not having VAD. Sensitivity was 0%, meaning that no cases of VAD were determined by SR, despite high VAD having been determined by TLR, resulting in a high number of false negatives. In Zambian children, specificity was 82.8%, indicating that in this context, SR concentration was able to correctly identify 82.8% of the population without VAD, but 17% were found to have VAD when they did not by SR (i.e., false positives), which the WHO would consider to be a moderate public health problem (2). By adjusting the SR cutoffs for defining VAD based on infection status in the Zambian children, false positives were reduced, increasing specificity to 97.3%. In the case of Zambia, there were no cases of VAD because of relatively high TLRs, and sensitivity was indeterminable (divided by zero). There were no cases of VAD identified by both SR and TLRs (i.e., true positives) in either the Thai or Zambian groups, an interesting but limiting finding of our study.
The finding of higher SR in the Thai children despite very low TLRs could be explained by the low prevalence of elevated CRP, which likely keeps SR concentrations “healthy” even in light of deficient TLRs. Animal studies consistently show that TLRs can be exhausted even when SR concentrations are still maintained at >1 μmol/L (reviewed in reference 14). Furthermore, the contrasting magnitude of TLR and SR concentrations in these children may indicate that the Thai population was recycling more of the retinol–retinol binding protein complex because of low liver stores (15), resulting in normal SR concentrations, whereas the Zambian population was sequestering VA in the liver because this group was replete and on the verge of hypervitaminosis A (16). Although the degree to which prior food insecurity in humans affects VA metabolism is not known, there are possible epigenetic factors at play that may result in extra storage of VA because of risk of long-term VA deficits in this population (17, 18), before the introduction of widespread fortification (11, 14). Other studies have shown that different groups within a population have very different distributions of SR concentrations (19). A study in Nicaragua found a greater improvement in TLRs among 21 children with the use of an RID method than in SR concentrations 1 y after sugar fortification with VA was initiated; in fact, some children actually had decreased plasma retinol concentrations even with simultaneous increases in hepatic VA (20).
Our results from Zambia are similar to a 2010 study in Zambian children in which the effect of infection stage on indicators of micronutrient status, including SR concentrations, was evaluated (7). Lower SR concentrations were found in children with inflammation than in healthy children, indicating higher rates of VAD with SR than liver reserves measured qualitatively with the modified relative dose response in children with active infection or recovering from infection. In addition, a high prevalence of elevated AGP was found, which resulted in few children being in the incubation stage of infection (elevated CRP only). We found no children in the incubation stage. A high prevalence of elevated AGP with a lower prevalence of high CRP, which were positively correlated (11), may indicate chronic inflammation that children in this environment experience daily, perhaps in part because of a mild subclinical environmental enteropathy (21). Chronic inflammation and the acute-phase response on nutrient biomarkers need further investigation (22).
There is a cyclical nature to nutrition and infection, with poor nutrition leading to increased risk of infection and infection negatively affecting nutritional status (23). Given the high rates of elevated acute-phase proteins in children in these and other similar studies in developing countries (5, 24), and given that infection affects micronutrient status (8, 9, 25, 26), it may be necessary to take infection into account when measuring VA and other micronutrient statuses. The shift in SR concentration distribution that we found in Zambian children by infection stage illustrates the suppression of SR in a dose response with stage of infection. This follows Thurnham and McCabe’s adjusted SR cutoffs (9), which are more suppressed during the stage of infection in which both CRP and AGP are elevated, during early convalescence, which corresponds to the SR distribution that was shifted furthest to the left. Applying the adjusted SR cutoffs to children in the identified stages of infection was a relatively simple addition to the data analysis methods and could be added to studies that use SR concentrations to identify VAD with the availability of CRP and AGP measurements. In the Thai study, we were not able to adjust for infection stage, because AGP measurements were not available, but if we could have, it would not have changed our specificity or sensitivity results, because there were no children found to have VAD by SR (false positives or true positives). The Thai children were slightly older than the Zambian children, and SR concentrations tend to increase with age. Some researchers have advocated for deficiency to be defined as 1 μmol/L (8). This may be considered for some population groups, but infection status should be included.
We used enrollment data, but there were 3 additional time points of SR data in the Zambian study. SR was affected by time (11), whereas 22 children (16.5%) had an SR <0.7 μmol/L at baseline, 44 children (33%) had a low SR value at any sampled time point. This reiterates the fluctuations of SR in children who are exposed to different infections over the course of several months, which is supported by the sustained high concentrations of AGP in this community (7, 11, 27).
Calculation of TLR with the use of the 13C-RID test is based on the assumption that in populations with low VA status, 50% of TBSs are stored in the liver, whereas in replete populations, 80% is stored in liver (12). In the Thai study, the figure of 50% was used to calculate TLRs, whereas in Zambia 80% was used, which was based on the large difference in TBSs. To support the current sensitivity and specificity calculations, we applied the 50% value to the Zambian TLR calculation. This yields a mean of 0.72 ± 0.25 μmol/g, with still no values <0.1 μmol/g (the cutoff used for VAD in this study); therefore, this difference in recommended assumptions (12) between studies does not change our conclusions based on VAD defined by TLRs.
Inflammation and infection may affect isotope tests through mechanisms of altered dose absorption, storage, and metabolism. Although these relations have not been fully investigated, clinical data indicate that infections reduce VA dose absorption in children (28). In the Zambian study (11), the factor for absorption of the dose was reduced from 0.9 to 0.8 if a subject had elevated CRP >10 mg/L at the time of dosing, indicating subclinical inflammation. The mathematical consequence is that for subjects with elevated CRP, their estimated TBSs and TLRs would be reduced by ∼11.1% [1 – (0.8/0.9)] as a result of this reduced factor for absorption (12). In the original analysis (11), 15.8% of values were adjusted at baseline because of elevated CRP. Therefore, the group means for TBSs and TLRs were reduced by ∼1.8% because of this correction. The Thai data were not corrected (10), but because of much lower TBSs and TLRs, the absolute change resulting from this correction would be minimal.
Specificity of SR concentration to determine VAD was 100% and 82.8% in Thai and Zambian children, respectively. Sensitivity was 0% in the Thai data, but it could not be calculated from Zambian data, meriting further investigation. Lack of true positive cases in either group restricted interpretation of results, and additional data are needed. The utility of SR as an indicator of actual VA status is questionable, considering that 65% of the Thai children had VAD, which was not captured by SR. On the other hand, the Zambian children’s SR values were not elevated even though estimated TLRs were quite high. This reiterates the homeostatic nature of SR, which is one of the reasons why the WHO advocates that it not be used alone as a biomarker (2, 29). Adjusting SR cutoffs with the use of Thurnham and McCabe’s correction factors for infection status improved specificity to 97.3% in the Zambian children by reducing false positives. Using SR as an indicator of VAD may result in a different accuracy in different populations, depending on TLRs and infection rate, which should be taken into consideration when SR is used as a biomarker.
Supplementary Material
Acknowledgments
We thank Sara Arscott, Samantha Schmaelzle, and Fabiana Moura for assistance in coordinating the study in Zambia. We also thank Christopher Davis for assisting in the analysis of both sample sets on the mass spectrometer.
The authors’ responsibilities were as follows—DJS: performed the statistical evaluations and calculations and produced the first draft of the manuscript (DJS was not involved in the field work and was hired to perform these analyses in part because of her credentials as a Master of Public Health with an emphasis in epidemiology and biostatistics and a Master of Science in nutrition); JPT: independently confirmed the retinol analyses and performed the preliminary statistical evaluations; BMG: analyzed the samples for retinol and was involved in the orchestration of the Zambian field work; SP: coordinated the work in Thailand; CK: was instrumental in the coordination of the Zambian study and communication among partners; JC: was the point of contact for the Zambian ethics committee, supervised the team from the Tropical Diseases Research Centre in the collection of blood samples, and prepared the paperwork related to releasing the samples from Zambia; SAT: designed the studies as principal investigator, including manuscript revision, and is the guarantor of this evaluation; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
ABBREVIATIONS
- AGP
α1-acid glycoprotein
- CRP
C-reactive protein
- RID
retinol isotope dilution
- SR
serum retinol
- TBS
total body store
- TLR
total liver reserve
- VA
vitamin A
- VAD
vitamin A deficiency.
FOOTNOTES
This work was presented at Experimental Biology 2015, Boston, MA, 31 March 2015 (DJS), and at Experimental Biology 2014, San Diego, CA, 26 April 2014 (JPT) as a poster finalist in the Community and Public Health Nutrition Research Interest Section of the American Society for Nutrition.
The field work was supported by the International Atomic Energy Agency for the work in Thailand and by HarvestPlus contract no. 8256 for the work in Zambia. HarvestPlus (www.harvestplus.org) is a global alliance of agriculture and nutrition research institutions working to increase the micronutrient density of staple food crops through biofortification. The views expressed do not necessarily reflect those of HarvestPlus. Neither of the organizations involved in supporting this work had a role in the analysis or interpretation of the data. The writing of the manuscript was funded by Global Health Funds at the University of Wisconsin–Madison.
REFERENCES
- 1. WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 2. WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. [Internet]. [cited 2014 Dec 2]. Geneva (Switzerland): World Health Organization; 2011. Available from: http://www.who.int/vmnis/indicators/retinol.pdf. [Google Scholar]
- 3. Tanumihardjo SA.. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94:658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filteau SM, Morris SS, Abbott RA, Tomkins AM, Kirkwood BR, Arthur P, Ross DA, Gyapong JO, Raynes JG.. Influence of morbidity on serum retinol of children in a community-based study in northern Ghana. Am J Clin Nutr 1993;58:192–7. [DOI] [PubMed] [Google Scholar]
- 5. Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P.. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 6. Mitra AK, Alvarez JO, Wahed MA, Fuchs GJ, Stephensen CB.. Predictors of serum retinol in children with shigellosis. Am J Clin Nutr 1998;68:1088–94. [DOI] [PubMed] [Google Scholar]
- 7. Bresnahan KA, Chileshe J, Arscott S, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA.. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J Nutr 2014;144:972–8. [DOI] [PubMed] [Google Scholar]
- 8. de Pee S, Dary O.. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 2002;132:2895S–901S. [DOI] [PubMed] [Google Scholar]
- 9. Thurnham DI, McCabe GP.. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. World Health Organization Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17September2010. [Internet]. [cited 2014November18]. Geneva (Switzerland); 2012. p. 63–80. Available from: http://www.who.int/nutrition/publications/micronutrients/background_paper4_report_assessment_vitAandIron_status.pdf. [Google Scholar]
- 10. Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA.. Triple-fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school-aged Thai children. J Nutr 2014;144:519–24. [DOI] [PubMed] [Google Scholar]
- 11. Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalaungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA.. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A. Am J Clin Nutr 2014;100:1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gannon BM, Tanumihardjo SA.. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr 2015;145:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lalkhen AG, McCluskey A.. Clinical tests: sensitivity and specificity. Contin Educ Anaesthesia, Crit Care Pain [Internet]. [cited 2014October30]. 2008;8:221–3. Available from: http://bjarev.oxfordjournals.org/cgi/doi/10.1093/bjaceaccp/mkn041. [Google Scholar]
- 14. Tanumihardjo SA.. Vitamin A fortification efforts require accurate monitoring of population vitamin A status to prevent excessive intakes. Procedia Chem 2015;14:398–407. [Google Scholar]
- 15. Green MH, Uhl L, Green JB.. A multicompartmental model of vitamin A kinetics in rats with marginal liver vitamin A stores. J Lipid Res 1985;26:806–18. [PubMed] [Google Scholar]
- 16. Wake K.. Development of vitamin A–rich lipid droplets in multivesicular bodies of rat liver stellate cells. J Cell Biol 1974;63:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barker DJ.. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 1997;13:807–13. [DOI] [PubMed] [Google Scholar]
- 18. Barker DJ, Martyn CN.. The maternal and fetal origins of cardiovascular disease. J Epidemiol Community Health 1992;46:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanumihardjo SA, Permaesih D, Dahro AM, Rustan E.. Muhilal, Karyadi D, Olson JA. Comparison of vitamin A status assessment techniques in children from two Indonesian villages. Am J Clin Nutr 1994;60:136–41. [DOI] [PubMed] [Google Scholar]
- 20. Ribaya-Mercado JD, Solomons NW, Medrano Y, Bulux J, Dolnikowski GG, Russell RM, Wallace CB.. Use of the deuterated-retinol-dilution technique to monitor the vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. Am J Clin Nutr 2004;80:1291–8. [DOI] [PubMed] [Google Scholar]
- 21. Korpe PS, Petri WA.. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med 2012;18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B,. the INSPIRE Consultative Group. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J Nutr 2015;145:1039S–1108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scrimshaw NS, SanGiovanni JP.. Synergism of nutrition, infection, and immunity: An overview. Am J Clin Nutr 1997;66:464S–77S. [DOI] [PubMed] [Google Scholar]
- 24. Grant FKE, Suchdev PS, Flores-Ayala R, Cole CR, Ramakrishnan U, Ruth LJ, Martorell R.. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J Nutr 2012;142:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louw JA, Werbeck A, Louw M, Kotze TJ, Cooper R, Labadarios D.. Blood vitamin concentrations during the acute phase response. Crit Care Med 1992;20:934–41. [DOI] [PubMed] [Google Scholar]
- 26. Bresnahan KA, Tanumihardjo SA.. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr 2014;5:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hotz C, Chileshe J, Siamusantu W, Palaniappan U, Kafwembe E.. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr 2012;15:1688–96. [DOI] [PubMed] [Google Scholar]
- 28. Sivakumar B, Reddy V.. Absorption of labelled vitamin A in children during infection. Br J Nutr 1972;27:299–304. [DOI] [PubMed] [Google Scholar]
- 29. WHO. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programs: report of a joint WHO/UNICEF consultation. Brown E, Akré J, editors. Geneva (Switzerland); 1996. WHO/NUT/96.10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


