ABSTRACT
Background: Overfeeding can lead to multiple metabolic and clinical complications and has been associated with increased mortality in the critically ill. Continuous venovenous hemofiltration (CVVH) represents a potential source of calories that is poorly recognized and may contribute to overfeeding complications.
Objective: We aimed to quantify the systemic caloric contribution of acid-citrate-dextrose regional anticoagulation and dextrose-containing replacement fluids in the CVVH circuit.
Design: This was a prospective study in 10 critically ill adult patients who received CVVH from April 2014 to June 2014. Serial pre- and postfilter blood samples (n = 4 each) were drawn and analyzed for glucose and citrate concentrations on each of 2 consecutive days.
Results: Participants included 5 men and 5 women with a mean ± SEM age of 61 ± 4 y (range: 42–84 y) and body mass index (in kg/m2) of 28 ± 2 (range: 18.3–36.2). There was generally good agreement between data on the 2 study days (CV: 7–11%). Mean ± SEM pre- and postfilter venous plasma glucose concentrations in the aggregate group were 152 ± 10 and 178 ± 9 mg/dL, respectively. Net glucose uptake from the CVVH circuit was 54 ± 5 mg/min and provided 295 ± 28 kcal/d. Prefilter plasma glucose concentrations were higher in patients with diabetes (n = 5) than in those without diabetes (168 ± 12 compared with 140 ± 14 mg/dL; P < 0.05); however, net glucose uptake was similar (46 ± 8 compared with 61 ± 6 mg/min; P = 0.15). Mean ± SEM pre- and postfilter venous plasma citrate concentrations were 1 ± 0.1 and 3.1 ± 0.2 mmol/L, respectively. Net citrate uptake from the CVVH circuit was 60 ± 2 mg/min and provided 218 ± 8 kcal/d.
Conclusions: During CVVH there was a substantial net uptake of both glucose and citrate that delivered exogenous energy and provided ∼512 kcal/d. Failure to account for this source of calories in critically ill patients receiving nutrition on CVVH may result in overfeeding.
Keywords: nutrition, CRRT, CVVH, citrate, anticoagulation, caloric uptake
INTRODUCTION
Overfeeding is associated with multiple complications in critically ill patients, including hypercapnia, hepatic dysfunction, azotemia, altered immune function, and hyperglycemia (1). It is also associated with higher mortality in nonseptic mechanically ventilated patients in intensive care units (ICUs)8 (2–4). The role of the counterregulatory response to stress and injury as a cause of hyperglycemia has been emphasized for many years, but there has been less emphasis on exogenous energy supply as a contributor to increased glucose concentrations, although hyperglycemia, which itself is associated with increased mortality, is a known consequence of overfeeding (5–7). In patients who require nutritional support, there is a strong relation between the amount of feeding administered and both hyperglycemia and insulin requirements (8). Continuous renal replacement therapy (CRRT) is now used universally in hemodynamically unstable individuals with acute and chronic kidney impairment, and it has been recognized as a potential source for macronutrient losses as well as macronutrient uptake, depending on the composition of the fluids used (9–12). However, to our knowledge, the possibility for net macronutrient uptake has not been mentioned in published guidelines from nephrology and nutrition societies (13–15). We aimed to quantify the energy supply from CRRT with the use of contemporary guideline-recommended low-lactate replacement fluids and citrate anticoagulation in continuous venovenous hemofiltration (CVVH) (14).
METHODS
Participants
After approval of the protocol by the Mayo Clinic Institutional Review Board, patients who received CVVH from April 2014 to June 2014 were identified from a daily drug utilization report. Individuals aged ≥18 y admitted to an ICU and receiving CVVH with the use of regional citrate anticoagulation with acid-citrate-dextrose formula A (ACD-A) solution (2.2% sodium chloride citrate and 2.45% dextrose) (Baxter) were considered eligible. Patients were excluded if they were pregnant, were expected to receive <24 h CVVH therapy, were on concomitant extracorporeal membrane oxygenation therapy, had a BMI (in kg/m2) >40, or had reversal of port connections on the dialysis catheter and dialysis circuit lines. A graphic depiction of the participant selection process is shown in Supplemental Figure 1. Written informed consent was obtained from the patients or legally authorized representatives.
Protocol
The clinical care of patients was managed by the ICU service, generally with the use of standard guidelines for initiating nutritional support (15). The participants were studied twice on consecutive days. CVVH therapy was initiated and managed per institutional CVVH protocol and per physician discretion. All patients were treated with CVVH with the use of the Prismaflex system (Gambro) and high-efficiency polyarylethersulfone HF 1400 disposable filter set (blood volume = 186; surface area = 1.4 m2). Standard blood flow rates were 205 mL/min, ultrafiltration rates were ∼30 · mL−1 · kg−1 actual body weight, and ACD-A rates were 300 mL/h (5 mL/min), unless a calcium gap was present that required reducing the ACD-A infusion rate. Pre- and postfilter hemofiltration replacement fluids were administered as low- or high-bicarbonate solutions with minimal lactate (Table 1). Fifty percent of the replacement fluid was administered prefilter, and 50% was administered postfilter.
TABLE 1.
Content of replacement fluids
| Component | Low-bicarbonate solution PrismaSate B22GK4/01 | High-bicarbonate solution PrismaSate BGK2/01 |
| Sodium, mEq/L | 140 | 140 |
| Potassium, mEq/L | 4 | 2 |
| Chloride, mEq/L | 120.5 | 120.5 |
| Sodium bicarbonate, mEq/L | 22 | 32 |
| Magnesium, mEq/L | 1.5 | 1.5 |
| Dextrose, mg/dL | 110 | 110 |
| Lactate, mEq/L | 3 | 3 |
Gambro.
Between 12 and 36 h after the start of CVVH (T1), the first set of 4 samples, each drawn 10 min apart, was obtained simultaneously from the pre- and postfilter sampling sites of the CVVH circuit (Figure 1) for measuring hematocrit, glucose concentration, and citrate concentration. The second set of samples was obtained ≥12 h later (T2), 36–60 h after CVVH initiation.
FIGURE 1.
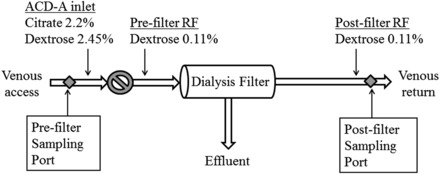
A diagram of the CVVH circuit and sampling sites used for obtaining glucose and citrate concentrations. The circle with a diagonal line represents the blood flow pump. ACD-A, acid-citrate-dextrose formula A; CVVH, continuous venovenous hemofiltration; RF, replacement fluid.
Data collection and analysis
Baseline demographic data, including age, sex, race, weight, BMI, history of diabetes mellitus or hepatic cirrhosis, admitting diagnosis, and etiology of renal failure were recorded for each patient. Hospital and ICU admission and discharge dates and CVVH characteristics were also documented. Glucose concentrations were determined with the use of a glucose oxidase method (GM9 Analyzer; Analox Corp). Plasma citrate concentration was determined with the use of liquid chromatography–tandem mass spectrometry.
Calculations
Net citrate and glucose uptake were calculated with the use of the concentrations and flow rates at each sampling site. Plasma flow [blood flow × (1 − hematocrit)] was used in citrate calculations because citrate does not distribute within erythrocytes; blood flow was used in glucose calculations (16). Flow delivered at pre- and postfilter ports were calculated with Equations 1 and 2:
where Qb is the blood flow rate, Qcitrate is the flow of ACD-A solution, and Qremoval is the net fluid removal rate.
Equations 3 and 4 were used to determine citrate and glucose uptake per hour:
where Cpost is the concentration of the citrate postfilter sample site, Cpre is the concentration of citrate at the prefilter sample site, Gpost is the concentration of the glucose postfilter sample site, Gpre is the concentration of glucose at the prefilter sample site, Hctpre is the prefilter hematocrit, Hctpost is the postfilter hematocrit, Qpost is the blood flow at the postfilter sample site, and Qpre is the blood flow at the prefilter sample site. The sieving coefficient of glucose is 1; therefore, postfilter glucose concentrations were not adjusted for the ultrafiltration rate (17). Mean values for glucose and citrate concentrations for the 4 blood samples were used in all calculations. Daily energy uptake from CVVH-derived glucose was determined by multiplying glucose uptake (grams per day) by 3.8 kcal/g. Energy uptake from CVVH-derived citrate was determined by multiplying citrate uptake (grams per day) by 2.5 kcal/g (18).
Statistical analysis
Statistical analysis was performed with a t test with the use of Microsoft Excel 2010. Categorical data are expressed as frequencies and percentages. Continuous data are shown as means ± SEMs. P < 0.05 was considered significant.
RESULTS
Ten patients were studied. Thirty-six patients were excluded or did not provide consent (Supplemental Figure 1). Patient characteristics are summarized in Table 2. Most patients were postsurgical, including individuals who had undergone cardiovascular (n = 5) and abdominal (n = 2) procedures. Most patients (n = 7) started CVVH within 48 h of admission to the ICU. Five patients were on an insulin infusion before CVVH initiation; 6 patients were on an insulin infusion during CVVH initiation, including during sample collection. Estimated basal energy requirements equal to estimated basal energy expenditure (Harris-Benedict equation) were 1543 ± 81 kcal/d. CVVH characteristics are provided in Table 3. One patient was studied only on day 1 because CVVH was discontinued on day 2. Two patients received a lower ACD-A rate of 200 mL/h, 1 patient on both days and 1 patient only on the second day; the other 8 patients received ACD-A at 300 mL/h. There were no substantial interruptions in CVVH therapy between sampling times.
TABLE 2.
Patient characteristics1
| Characteristic | Value |
| Age, y | 61 ± 4 |
| Men, n | 5 |
| Weight, kg | 79 ± 5 |
| BMI, kg/m2 | 28 ± 2 |
| Surgical, n | 7 |
| Comorbidities, n | |
| Diabetes mellitus | 5 |
| Cirrhosis | 2 |
| Etiology of AKI, n | |
| ATN | 7 |
| Multifactorial | 3 |
| Nutrition administration, n | |
| Oral diet or enteral | 5 |
| Parenteral | 1 |
| None | 4 |
| SOFA score | 12.6 ± 1 |
Values are means ± SEMs unless otherwise indicated. n = 10. AKI, acute kidney injury; ATN, acute tubular necrosis; SOFA, Sequential Organ Failure Assessment.
TABLE 3.
CVVH characteristics1
| Characteristic | Value |
| Q c i t ra te, mL/min | 4.6 ± 0.2 |
| Ultrafiltration rate, mL · kg−1 · h−1 | 30 ± 2 |
| Q removal, mL/min | 3.2 ± 0.7 |
| Transmembrane pressure, mm Hg | 92 ± 10.3 |
All values are means ± SEMs. n = 10. CVVH, continuous venovenous hemofiltration; Qcitrate, flow rate of acid citrate dextrose solution; Qremoval, net volume removal rate.
Plasma glucose and citrate concentrations on day 1 are depicted in Figure 2, which shows that steady-state conditions were achieved for both at both sampling sites. Mean glucose and citrate concentrations on days 1 and 2 are shown in Figure 3; results from 8 patients are shown because 1 patient was studied only on day 1 and another had different ACD-A flow rates on the 2 days. Results of the 2 study days in those 8 patients were reproducible, with CVs of 7–11% (data not shown). Postfilter citrate concentrations were not corrected for the sieving coefficient, which is <1, because the error that this introduces is negligible (19). Prefilter plasma glucose concentrations were higher in patients with diabetes (n = 5) (168 ± 12 compared with 140 ± 14 mg/dL; P < 0.05), but net glucose uptake was not different in patients with and without diabetes (46 ± 8 compared with 61 ± 6 mg/min; P = 0.15). Because of this, results in the diabetic and nondiabetic groups were combined for analysis. Mean pre- and postfilter glucose concentrations in the 10 patients were 152 ± 10 and 178 ± 9 mg/dL, respectively. Mean pre- and postfilter citrate concentrations were 1.0 ± 0.1 and 3.1 ± 0.2 mmol/L, respectively. Net glucose and citrate uptake from CVVH fluids are shown in Table 4. As can be seen, net energy uptake from glucose and citrate averaged 295 and 218 kcal/d, respectively, for a total of 512 kcal/d or 33% of the estimated basal energy expenditure.
FIGURE 2.
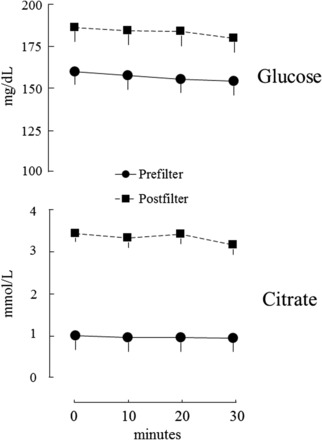
Mean glucose and citrate concentrations on day 1 (n = 10).
FIGURE 3.
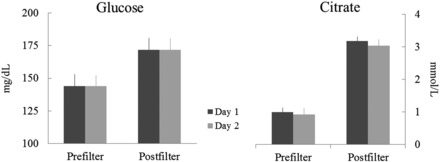
Comparison of glucose and citrate concentrations on days 1 and 2 (n = 8).
TABLE 4.
Net macronutrient uptake1
| Macronutrient | Uptake, mg/min | Energy gained, kcal/d |
| Glucose | 54 ± 5 | 295 ± 28 |
| Citrate | 60 ± 2 | 218 ± 8 |
| Total | 512 ± 32 |
Data are presented as means ± SEMs. n = 10.
DISCUSSION
We undertook this study to assess the role of CVVH as a source of exogenous energy in critically ill patients. We found that CVVH contributes substantially to total calorie intake primarily because of the uptake of citrate and dextrose from the ACD-A solution when low lactate replacement fluids are used (∼512 kcal/d or ∼30% of basal energy requirements). The caloric contribution of citrate and dextrose were roughly equivalent (218 and 295 kcal, respectively). Although glucose concentrations were higher in patients with underlying diabetes mellitus, total glucose uptake did not differ between individuals with and without diabetes.
Citrate is now widely used as a regional anticoagulant in CVVH to prevent hemofilter clotting caused by prolonged exposure of blood to foreign surfaces of the extracorporeal circuit (20). It is well tolerated and has been shown to reduce bleeding risk and improve circuit life compared with traditional systemic heparin anticoagulation (14, 21). Citrate, which exerts its anticoagulant effect by chelating calcium, is subsequently removed either through the dialysis filter or via rapid metabolism in various tissues, predominantly the liver (22).
The potential for energy exchange across the CRRT circuit has been recognized for many years. Monaghan et al. (12) reported the uptake of ≤300 g glucose/d from replacement fluids with a high glucose content (83 mmol/L). CRRT can potentially lead to both energy loss and gain depending on the fluids used (9). In this study, energy gain (∼500 kcal/d) was caused almost exclusively by the uptake of dextrose and citrate from ACD-A. The contribution of replacement fluids was negligible because of their low lactate content. In the absence of high lactate delivery, a substantial gain or loss of energy would not be expected from the replacement fluids because of the small difference in glucose concentrations between these fluids and blood. The only previous demonstration of energy gain with the use of citrate for anticoagulation to our knowledge is that from Balik et al. (10, 11), who reported that when an acid-citrate-dextrose solution is used with high lactate replacement fluids, CRRT can provide ≤1300 kcal/d. Our results are more relevant to the most recently published Improving Global Outcomes Acute Kidney Injury Work Group acute kidney injury guidelines (14), which recommend lactate-free replacement fluids.
Despite the potential for net energy accrual during CRRT, most authors have emphasized nutrient loss, including loss of glucose, micronutrients, and especially protein (23–26). The International Society of Nephrology clinical practice guideline for acute kidney injury emphasizes increased protein needs, partly because of the extracorporeal loss of amino acids, in CRRT patients (14). Similarly, the American Society for Parenteral and Enteral Nutrition guidelines for nutrition support in acute renal failure describe profound losses of calcium and magnesium in the dialysate effluent and emphasize higher protein requirements in CRRT but do not explicitly mention macronutrient losses (15). Net uptake of calories is not mentioned in either document.
To our knowledge, this study is the first to measure energy uptake from CVVH when low lactate replacement fluids are used. It should be emphasized that the content and amount of replacement fluids, the ACD solution used, and dialysis devices and filters used during CRRT differ among institutions. In the case of citrate anticoagulation fluids or replacement fluids that contain little or no dextrose, for example, the energy uptake would be much less. For this reason, our results should be generalized with caution.
These findings have important implications in the care of patients on CRRT who are receiving nutritional support. Energy requirements in the critically ill are not as high as once was thought, and overfeeding, especially with parenteral nutrition, is commonplace (7, 27). Our results indicate that CRRT, if not recognized as a source of calories, can increase total energy supply by ∼33% in patients receiving full estimated basal energy requirements (which are similar to resting energy expenditure in many patients) via artificial nutrition (27). In fact, there is a role for underfeeding selected patients with obesity and diabetes because this approach results in less hyperglycemia (15, 27). Nonnutritional sources of energy such as dextrose-containing fluids used to treat hypernatremia or administered with intravenous medications, propofol, and the dialysis modalities used in the critically ill have received relatively little attention as sources of energy (28, 29). Our CRRT patients were not overfed because most of them were not receiving nutrition support. However, we did show a potential for overfeeding by demonstrating that the use of CRRT, if not taken into account, could dramatically increase total energy supply. Overfeeding, especially with carbohydrates, contributes to complications, such as hypercarbia, which may prolong mechanical ventilation, and hepatic steatosis (15, 27, 30, 31). Excessive calories may also increase the risk of refeeding and electrolyte abnormalities in severely malnourished patients (1). Overfeeding patients in the ICU has been associated with increased mortality, morbidity, and length of stay (4, 30, 32, 33).
In this study, net energy uptake was measured in only 10 patients on CVVH; however, the study design was robust, including sequential sampling to improve precision and measurements on separate days to affirm reproducibility. Our findings are consistent with previous studies that have shown a net caloric gain from CRRT fluids and, as explained previously, relevant to current guidelines for CRRT use. The results should be interpreted in the context of local practices with regard to CRRT fluids and anticoagulation, infusion rates, etc. Additional studies are needed to clarify the impact of alternative CRRT protocols on energy balance.
In conclusion, CRRT is an important potential source of unrecognized exogenous calories. We estimated that the daily metabolic contribution of citrate and glucose from CVVH in critically ill patients is substantial at ∼512 kcal/d. Given this important contribution, practitioners should consider the additional energy provided by CRRT when designing enteral or parenteral nutrition regimens in the context of institutional CRRT practices.
Supplementary Material
Acknowledgments
We thank M Persson and A Smailovic for assistance with citrate and glucose analyses, respectively.
The authors’ responsibilities were as follows—AMN and EMN: conducted the study enrollment and the research; AMN and JMM: analyzed the data; AMN, EMN, and JMM: wrote the manuscript and had primary responsibility for the final content; and all authors: designed the research, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
ABBREVIATIONS
- ACD-A
acid-citrate-dextrose formula A
- CRRT
continuous renal replacement therapy
- CVVH
continuous venovenous hemofiltration
- ICU
intensive care unit
FOOTNOTES
Supported by the Mayo Clinic Pharmacy Services Discretionary Fund, US Public Health Service grant HL67933 (JMM), National Center for Advancing Translational Sciences grant UL1 TR000135, and the Earl and Annette R. McDonough Professorship (JMM).
REFERENCES
- 1. Ziegler TR.. Parenteral nutrition in the critically ill patient. N Engl J Med 2009;361:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casaer MP, Van den Berghe G.. Nutrition in the acute phase of critical illness. N Engl J Med 2014;370:1227–36. [DOI] [PubMed] [Google Scholar]
- 3. Fraipont V, Preiser JC.. Energy estimation and measurement in critically ill patients. JPEN J Parenter Enteral Nutr 2013;37:705–13. [DOI] [PubMed] [Google Scholar]
- 4. Weijs PJ, Looijaard WG, Beishuizen A, Girbes AR, Oudemans-van Straaten HM.. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care 2014;18:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beal AL, Cerra FB.. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. JAMA 1994;271:226–33. [PubMed] [Google Scholar]
- 6. Krinsley JS.. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003;78:1471–8. [DOI] [PubMed] [Google Scholar]
- 7. Miles JM, McMahon MM, Isley WL.. No, the glycaemic target in the critically ill should not be < or = 6.1 mmol/l. Diabetologia 2008;51:916–20. [DOI] [PubMed] [Google Scholar]
- 8. Muthusamy KMJ, Miles JM.. Hyperglycemia should be avoided in critical illness and the postoperative period In: Vella A, Rizza R, editors. Clinical dilemmas in diabetes. Chichester (United Kingdom): Wiley-Blackwell; 2011. p. 134–44. [Google Scholar]
- 9. Druml W.. Metabolic aspects of continuous renal replacement therapies. Kidney Int Suppl 1999;72:S56–61. [PubMed] [Google Scholar]
- 10. Balik M, Zakharchenko M, Leden P, Otahal M, Hruby J, Polak F, Rusinova K, Stach Z, Tokarik M, Vavrova J, et al. Bioenergetic gain of citrate anticoagulated continuous hemodiafiltration—a comparison between 2 citrate modalities and unfractionated heparin. J Crit Care 2013;28:87–95. [DOI] [PubMed] [Google Scholar]
- 11. Balik M, Zakharchenko M, Otahal M, Hruby J, Polak F, Rusinova K, Stach Z, Vavrova J, Jabor A.. Quantification of systemic delivery of substrates for intermediate metabolism during citrate anticoagulation of continuous renal replacement therapy. Blood Purif 2012;33:80–7. [DOI] [PubMed] [Google Scholar]
- 12. Monaghan R, Watters JM, Clancey SM, Moulton SB, Rabin EZ.. Uptake of glucose during continuous arteriovenous hemofiltration. Crit Care Med 1993;21:1159–63. [DOI] [PubMed] [Google Scholar]
- 13. Brown RO, Compher C.. A.S.P.E.N. clinical guidelines: nutrition support in adult acute and chronic renal failure. JPEN J Parenter Enteral Nutr 2010;34:366–77. [DOI] [PubMed] [Google Scholar]
- 14. Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney inter 2012;2(Suppl 1):95–100. [Google Scholar]
- 15. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159–211. [DOI] [PubMed] [Google Scholar]
- 16. Whitfield LR, Levy G.. Permeability of human and rat red blood cells to citrate. Thromb Res 1981;21:681–4. [DOI] [PubMed] [Google Scholar]
- 17. Henderson LW.. Biophysics of ultrafiltration and hemofiltration In: Drukker W, Parsons FM, Maher JF, editors. Replacement of renal function by dialysis: a textbook of dialysis. Dordrecht (Netherlands): Martinus Nifhoff Publishers; 1983. p. 242–64. [Google Scholar]
- 18. Merrill AL, Watt BK.. Energy value of foods: basis and derivation. Agriculture handbook no. 74. Washington (DC): USDA; 1973. [Google Scholar]
- 19. Swartz R, Pasko D, O’Toole J, Starmann B.. Improving the delivery of continuous renal replacement therapy using regional citrate anticoagulation. Clin Nephrol 2004;61:134–43. [DOI] [PubMed] [Google Scholar]
- 20. Schetz M.. Anticoagulation in continuous renal replacement therapy. Contrib Nephrol 2001;132:283–303. [DOI] [PubMed] [Google Scholar]
- 21. Bai M, Zhou M, He L, Ma F, Li Y, Yu Y, Wang P, Li L, Jing R, Zhao L, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med 2015;41:2098–110. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen TT, Thomsen PE.. Leg and splanchnic arteriovenous differences of plasma citrate in exercising man. J Appl Physiol 1979;46:120–7. [DOI] [PubMed] [Google Scholar]
- 23. Casaer MP, Mesotten D, Schetz MR.. Bench-to-bedside review: metabolism and nutrition. Crit Care 2008;12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frankenfield DC, Reynolds HN, Badellino MM, Wiles CE III.. Glucose dynamics during continuous hemodiafiltration and total parenteral nutrition. Intensive Care Med 1995;21:1016–22. [DOI] [PubMed] [Google Scholar]
- 25. Berger MM, Shenkin A, Revelly JP, Roberts E, Cayeux MC, Baines M, Chiolero RL.. Copper, selenium, zinc, and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. Am J Clin Nutr 2004;80:410–6. [DOI] [PubMed] [Google Scholar]
- 26. Frankenfield DC, Badellino MM, Reynolds HN, Wiles CE III, Siegel JH, Goodarzi S.. Amino acid loss and plasma concentration during continuous hemodiafiltration. JPEN J Parenter Enteral Nutr 1993;17:551–61. [DOI] [PubMed] [Google Scholar]
- 27. Miles JM.. Energy expenditure in hospitalized patients: implications for nutritional support. Mayo Clin Proc 2006;81:809–16. [DOI] [PubMed] [Google Scholar]
- 28. Sterns RH.. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015;372:55–65. [DOI] [PubMed] [Google Scholar]
- 29. Bousie E, van Blokland D, Lammers HJ, van Zanten AR.. Relevance of non-nutritional calories in mechanically ventilated critically ill patients. Eur J Clin Nutr 2016;70:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klein CJ, Stanek GS, Wiles CE III.. Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc 1998;98:795–806. [DOI] [PubMed] [Google Scholar]
- 31. Talpers SS, Romberger DJ, Bunce SB, Pingleton SK.. Nutritionally associated increased carbon dioxide production. Excess total calories vs high proportion of carbohydrate calories. Chest 1992;102:551–5. [DOI] [PubMed] [Google Scholar]
- 32. Btaiche IF, Khalidi N.. Metabolic complications of parenteral nutrition in adults, part 1. Am J Health Syst Pharm 2004;61:1938–49. [DOI] [PubMed] [Google Scholar]
- 33. Fiaccadori E, Regolisti G, Maggiore U.. Specialized nutritional support interventions in critically ill patients on renal replacement therapy. Curr Opin Clin Nutr Metab Care 2013;16:217–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


