Abstract
Background
Atrial fibrillation (AF) is associated with an increased risk of dementia. It is presently unknown to what extent AF contributes to dementia onset independently from prevalent and incident cerebrovascular accidents (CVAs)/transient ischaemic attacks (TIAs).
Methods
MEDLINE/PubMed and Embase databases were searched for prospective observational results, which produced risk estimates for dementia in AF patients, adjusted for prevalent and incident CVAs/TIAs.
Results
Five prospective observational studies were included, comprising 61 008 patients, having a median follow-up of 12.5 years. Meta-analysis of observational results indicates an increased risk of dementia in AF, adjusted for cerebrovascular clinical events (HR 1.28, 95% CI 1.17 to 1.41, I2=0%). Funnel plot analysis did not reveal a statistically significant asymmetry. Meta-regression analysis did not indicate statistically significant associations between baseline study-level covariates and risk estimates.
Conclusion
AF confers a nearly 30% increased risk of dementia, independently from CVAs/TIAs. Screening for AF and subsequent optimised management to lower risk of cranial injury could help in preventing dementia, a condition characterised by high social and healthcare costs.
Keywords: atrial fibrillation
Key questions.
What is already known about this subject?
Atrial fibrillation (AF) is associated with dementia independently from clinical cerebrovascular events (strokes/transient ischaemic attacks (TIAs)).
What does this study add?
Stroke/TIA-independent contribution of AF to dementia is more impactful then stroke/TIA-dependent effect.
How might this impact on clinical practice?
An aggressive approach towards AF, including early identification and optimised management could help in preventing progression of cognitive decline and dementia.
Introduction
Atrial fibrillation (AF) is the most common tachyarrhythmia, affecting 33.5 million people in the world in 2010, a number that is deemed to double by 2050. The relationship of AF with cerebrovascular events, such as cerebrovascular accident (CVA)/transient ischaemic attack (TIA) have been widely described since the last century. Recent data, however, suggest that AF can be associated with dementia independently from clinical cerebrovascular events, through multiple potential mechanisms,1 such as silent cerebral ischaemia (SCI), cerebral microbleeds, global cerebral hypoperfusion and transient extreme variability in perfusion in the distal cerebral circulation2 due to R-R interval inconsistency and loss of atrial systole that results in arteriolar hypotension, capillary hypertension and microvascular dysfunction. This field is becoming increasingly important among the medical scientific community prompting consensus recommendation to promote awareness and targeted approaches to lower incidence of cognitive decline in patients with cardiac arrhythmias.3 Previous systematic reviews and meta-analyses on this topic have focused on the analysis of the risk of incident dementia in AF patients, independent of prevalent stroke (ie, without a history of stroke at baseline), without analysing dementia risk in AF patients independently from both prevalent and incident (during follow-up) CVAs/TIAs. Evidence regarding the impact that AF has on cognitive function and dementia, not mediated by clinical events such as CVAs/TIAs, is presently lacking.
For this reason, this study was conducted to perform a systematic review and meta-analysis of prospective observational adjusted data, regarding the risk of dementia in AF patients as compared with the general population, in order to quantify AF-induced dementia not mediated by prevalent or incident clinical cerebrovascular events.
Methods
Search strategy and study selection
This work was conducted according to the most recent guidelines, including the Preferred Reporting Items for Systematic reviews and Meta-Analyses amendment to the Quality of Reporting of Meta-analyses statement and recommendations from The Cochrane Collaboration and Meta-analysis Of Observational Studies in Epidemiology.
PubMed/MEDLINE and Embase databases were screened for pertinent articles, using the following keywords: ((atrial fibrillation OR AF) AND (dementia OR cognitive)) NOT (review(pt) OR editorial(pt) OR letter(pt)). The search ended in April 2018.
Two independent reviewers (AS and MA) screened the retrieved citations through the title and/or abstract, and all disparities were resolved through consensus. If pertinent, those studies were described as complete reports, according to the following the selection criteria. Studies were included if they (1) described data from adjusted observational prospective studies regarding the risk of dementia in patients with AF, with respect to a control population, (2) presented risk estimates adjusted for prevalent and incident CVA/TIA. Studies including patients with previous or incident CVA/TIA were included in the analysis, provided that the risk estimates was adjusted for these variables. Duplicate reporting was an exclusion criterion.
Statistical analysis
Continuous variables and categorical variables were reported as numbers and percentages, respectively. Median (IQR) was used for the summary statistics. Meta-analysis of adjusted HR of dementia in AF patients versus controls was performed after logarithmic transformation using a random-effect model (inverse-variance weighting) and the results with the corresponding 95% CI reported on the logarithmic scale. Cochran I2 test was used to assess heterogeneity in the included studies. Funnel plot analysis and Egger’s test for funnel plot asymmetry were used to assess potential publication bias. Subgroup meta-analysis with a corresponding test for subgroup difference (random effect model) was implemented to assess the potential impact of AF presence at the beginning of follow-up (prevalent AF) with respect to AF occurrence only during the study period (incident AF). Using adjusted HR of dementia in AF patients with respect to controls as dependent variable, meta-regressions were performed to test whether an interaction with different baseline covariates was present. Statistical analyses were performed with STATA V.12 (Stata Corp) and R V.3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The initial search identified 864 potential studies: among these, nine were screened for possible inclusion, and full texts were carefully reviewed. Three studies were excluded due to the lack of accounting for incident stroke, while one study was excluded because it was not a prospective study (figure 1; see online supplementary material for the references of the excluded studies).
Figure 1.
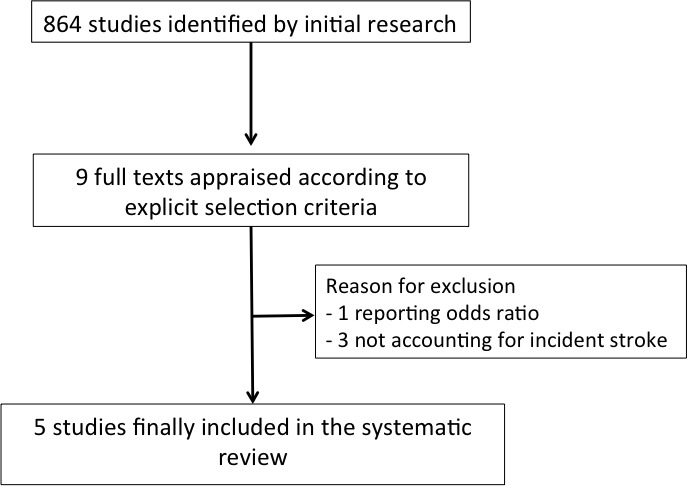
Flow diagram of selected studies.
openhrt-2018-000984supp001.docx (82.8KB, docx)
Finally, five studies were included in the present systematic review and meta-analysis,4–8 encompassing 61 008 patients (table 1). The median follow-up was 12.5 years (6.8–14.7). Table 2 summarises the pooled baseline clinical features of the included subjects. Their median age was 67 years (57–69), 56% (30%–59%) of them being female. Comorbidities, such as hypertension 32% (29%–32%), diabetes 10% (9%–15%), coronary heart disease 8% (5%–19%), heart failure 3% (0%–3%) and previous CVA/TIA 0% (0%–2%) were reported in table 2.
Table 1.
Characteristics of the studies included in the systematic review and meta-analysis
| Study | Year | Number of patients | Follow-up (years) | AF diagnosis | Dementia diagnosis | Study quality* |
| Dublin et al 4 | 2011 | 3045 | 6.8 | ICD codes | DSM-IV | High |
| Marzona et al 5 | 2012 | 31 506 | 4.7 | ECG, medical records | MMSE | High |
| de Bruijn et al 6 | 2015 | 6514 | 12.5 | ECG, medical records | DSM-III revised | High |
| Singh-Manoux et al 7 | 2017 | 7428 | 14.7 | ECG, ICD codes | ICD codes | High |
| Chen et al 8 | 2018 | 12 515 | 20.2 | ECG, ICD codes | National Institute on Aging–Alzheimer’s Association work groups, DSM-V | High |
*The items considered for assigning study quality were selection bias, detection bias and performance bias; high quality: no bias; moderate quality: one bias; low quality: two or more bias.
DSM, Diagnostic and Statistical Manual of Mental Disorders;ICD, International Classification of Diseases;MMSE, Mini-Mental State Exam.
Table 2.
Pooled baseline clinical features of the included studies (61 008 patients)
| Variables | Median value |
| Age (y) | 67 (57–69) |
| Female sex (%) | 56 (30–59) |
| Hypertension (%) | 32 (29–32) |
| Diabetes (%) | 10 (9–15) |
| Coronary heart disease (%) | 8 (5–19) |
| Heart failure (%) | 3 (0–3) |
| Previous stroke/TIA (%) | 0 (0–2) |
| Incident AF (%) | 76 (74–100) |
AF, atrial fibrillation; TIA, transient ischaemic attack.
More than three quarters of the pooled population had AF occurring during follow-up (incident AF), while the remaining had AF at the start of the follow-up (prevalent AF). Pooled analysis of adjusted observational results indicates an increased risk of dementia in AF (figure 2), with an estimated meta-analytic HR of 1.28 (95% CI 1.17 to 1.41). No apparent heterogeneity was present (I2=0%) and funnel plot analysis did not reveal a statistically significant asymmetry (p=0.06) (see online supplementary figure 1). No differences in the meta-analytic outcomes was found between the two subgroups (incident AF: HR 1.30, 95% CI 1.11 to 1.51; prevalent AF: HR 1.28, 95% CI 1.13 to 1.46; p for difference between subgroups=0.93) (figure 3).
Figure 2.
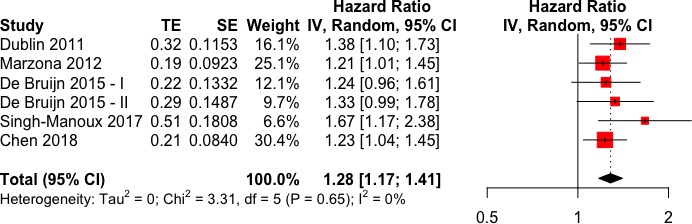
Forest plot for HR of dementia in atrial fibrillation (AF) patients with respect to non-AF patients.
Figure 3.
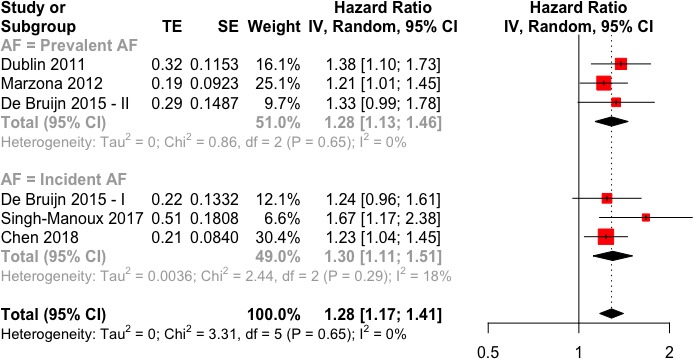
Forest plot for HR of dementia in atrial fibrillation (AF) patients with respect to non-AF patients, stratified by AF occurrence (prevalent vs incident).
Meta-regression analysis was performed to assess possible influence of baseline covariates in the included studies, on the estimated risk of dementia in AF. No significant association was found between the selected moderators (percentages of incident AF, age, female sex, hypertension, diabetes, coronary heart disease, heart failure and previous stroke/TIA) and the outcome (table 3).
Table 3.
Metaregression of different covariates related to the outcome “risk of dementia in AF”
| Variables | Beta | P value |
| Incident AF | −0.0002 | 0.91 |
| Age | 0.0007 | 0.93 |
| Female sex | −0.0001 | 0.97 |
| Hypertension | −0.0019 | 0.47 |
| Diabetes | −0.0044 | 0.28 |
| Coronary heart disease | −0.0016 | 0.55 |
| Heart failure | −0.0051 | 0.85 |
| Previous stroke/TIA | −0.0044 | 0.40 |
AF, atrial fibrillation; TIA, transient ischaemic attack.
Discussion
The main result of the present systematic review and meta-analysis is that AF can confer nearly a 30% excess risk of developing overt dementia independently of CVA/TIA. Compared with other meta-analytic estimates, the CVA/TIA-independent contribution of the arrhythmia to cognitive injury seems more impactful than that related to clinical events.
In the last decade, growing scientific evidence has accumulated regarding possible links between AF and cognitive decline/dementia,1 in addition to the well-known CVA/TIA-mediated cognitive damage in AF patients. Different hypotheses have been proposed to further explain mechanistic relationships between AF and the onset of cognitive dysfunction and dementia. As AF and dementia share multiple risk factors and presentation of cognitive decline is variable, there are likely multiple mechanisms that work in isolation and combination resulting in manifest end organ injury.9 As cognitive decline is often progressive, these mechanisms may have a prolonged period of subclinical influence before a tipping point is reach in which clinical symptoms of cognitive decline are manifest. First, subclinical and repetitive microembolic events in AF patients, manifesting as SCI, lesions at cerebral MRI scan, are potential contributors to the reduction in cognitive function.10 Similarly, it has been demonstrated that AF patients have an increased prevalence of cerebral microbleeds,11 whose connection to cognitive impairment have been established,12 which can be possibly related to suboptimal oral anticoagulation therapy (OAT).13–15 Another intriguing hypothesis is that AF can produce cerebral haemodynamic alteration due to R-R interval variability and loss of atrial systole, which can potentially lead to brain damage and atrophy.16 A recent work by Gardarsdottir et al 17 using phase-contrast MRI, showed that AF is associated with decreased total cerebral blood flow and brain perfusion; moreover, our group demonstrated through a computational model of cerebral circulation, that AF is associated with transient hypoperfusion and hypertensive events in the deep circle,2 possibly explaining the genesis of non-microembolic SCIs and non-OAT related microbleeds. Finally, since elevated levels of various inflammatory biomarkers have been associated with AF, the underlying prothrombotic state associated with inflammatory condition could represent another link between AF and dementia.1
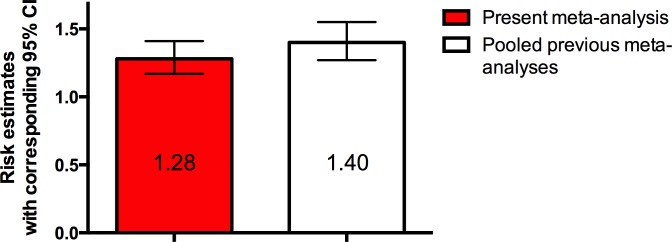
The comparison between the present results and those of previous studies,18–20 which did not investigate the impact of incident cerebrovascular accidents, is of clear interest, even though it is necessary to bear in mind that direct risk estimates comparisons are not permitted. Our results indicate a CVA/TIA independent impact of AF on dementia onset, estimated as a nearly 30% risk of developing dementia, while pooled risk data of the previous studies indicates a hybrid impact deriving both from CVA/TIA events and non-CVA/TIA events, quantifiable as a 40% risk (figure 4). Thus, the CVA/TIA independent contribution seems to emerge as the most impactful in the association between AF and dementia. The practical consequence of this is that managing AF with rate-control medications, even with a clinically effective anticoagulation, could not be sufficient to prevent cognitive decline and dementia. The pragmatic consideration then becomes the value of early screening for AF as a mean to lower risk of cognitive decline be targeting potential mechanisms of cranial risk.
Figure 4.
Column graph comparing risk estimate for the present meta-analysis compared with other meta-analyses not completely adjusted for incident strokes.
The fact that there is not a statistically significant difference in dementia risk between studies enrolling only patients with AF diagnosis during follow-up (incident AF) and studies enrolling also patients with AF at baseline (prevalent AF) can be explained in different ways. Since patients had normal cognitive function at baseline, prevalent AF patients included in this meta-analysis could have had an intrinsic resistance to AF-induced cognitive damage, thus blunting possible differences between prevalent and incident AF subgroups. Another possible reason is that, considering the normal baseline cognitive function, prevalent AF patients may have been affected by the atrial arrhythmia for a relatively short time before the start of the follow-up. Finally, there is a possibility that cognitive decline influences AF awareness and ultimate presentation for diagnosis and treatment. If so, then patients with healthier cognition are those that are more likely to present for clinical evaluation and management of AF.
Limitations
First, even if the overall risk of bias was very low, the included studies were observational in design, consequently limiting the inferential power of our results. Another limitation is that systematic data regarding thromboembolic risk profiles of the patients in the included studies and their eventual use of anticoagulation medications is lacking, however, it is reasonable to assume that proper guideline-based management of these patients in terms of OAT will prevail. Moreover, based on methods of each studies, definitions of AF and dementia may result heterogeneous. Finally, the included studies do not report data on subclinical cerebral lesions, which would provide more insights to interpret the present findings.
Conclusion
AF confers a nearly 30% increased risk of dementia, independently from clinically relevant cerebrovascular events (CVAs/TIAs). These data possibly suggest that an aggressive approach towards AF, including early identification and optimised management that spans anticoagulation to careful management of rhythm and rate could help in preventing progression of cognitive decline and dementia, with favourable consequences in patients’ quality of life and in terms of social/public health costs.
Footnotes
Contributors: AS, MA, MM contributed to study design, articles search and analysis. AS, MA, MM, FG, TJB, VJ contributed to the writing of the manuscript.
Funding: This study was performed thanks to the support of the “Compagnia di San Paolo” within the project “Progetti di Ricerca di Ateneo–2016: Cerebral hemodynamics during atrial fibrillation (CSTO 60444)” of the University of Turin, Italy.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
References
- 1. Hui DS, Morley JE, Mikolajczak PC, et al. Atrial fibrillation: a major risk factor for cognitive decline. Am Heart J 2015;169:448–56. 10.1016/j.ahj.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 2. Anselmino M, Scarsoglio S, Saglietto A, et al. Transient cerebral hypoperfusion and hypertensive events during atrial fibrillation: a plausible mechanism for cognitive impairment. Sci Rep 2016;6 10.1038/srep28635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dagres N, Chao T-F, Fenelon G, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: What is the best practice? Journal Arrhythmia 2018;34:99–123. 10.1002/joa3.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dublin S, Anderson ML, Haneuse SJ, et al. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc 2011;59:1369–75. 10.1111/j.1532-5415.2011.03508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marzona I, O'Donnell M, Teo K, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. Can Med Assoc J 2012;184:E329–E336. 10.1503/cmaj.111173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Bruijn RFAG, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015;72:1288–7. 10.1001/jamaneurol.2015.2161 [DOI] [PubMed] [Google Scholar]
- 7. Singh-Manoux A, Fayosse A, Sabia S, et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. European Heart Journal 2017;38:2612–8. 10.1093/eurheartj/ehx208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen LY, Norby FL, Gottesman RF, et al. Association of Atrial Fibrillation With Cognitive Decline and Dementia Over 20 Years: The ARIC‐NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc 2018;7 10.1161/JAHA.117.007301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graves KG, May HT, Jacobs V, et al. Atrial fibrillation incrementally increases dementia risk across all CHADS 2 and Cha 2 Ds 2 VASc strata in patients receiving long-term warfarin. Am Heart J 2017;188:93–8. 10.1016/j.ahj.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 10. Gaita F, Corsinovi L, Anselmino M, et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol 2013;62:1990–7. 10.1016/j.jacc.2013.05.074 [DOI] [PubMed] [Google Scholar]
- 11. Selim M, Diener H-C. Atrial fibrillation and microbleeds. Stroke 2017;48:2660–4. 10.1161/STROKEAHA.117.017085 [DOI] [PubMed] [Google Scholar]
- 12. Lei C, Lin S, Tao W, et al. Association between cerebral microbleeds and cognitive function: a systematic review. J Neurol Neurosurg Psychiatry 2013;84:693–7. 10.1136/jnnp-2012-303948 [DOI] [PubMed] [Google Scholar]
- 13. Bunch TJ, May HT, Bair TL, et al. Atrial fibrillation patients treated with Long‐Term warfarin anticoagulation have higher rates of all dementia types compared with patients receiving Long‐Term warfarin for other indications. J Am Heart Assoc 2016;5:e003932 10.1161/JAHA.116.003932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs V, Woller SC, Stevens S, et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm 2014;11:2206–13. 10.1016/j.hrthm.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 15. Jacobs V, Woller SC, Stevens SM, et al. Percent time with a supratherapeutic Inr in atrial fibrillation patients also using an antiplatelet agent is associated with long-term risk of dementia. J Cardiovasc Electrophysiol 2015;26:1180–6. 10.1111/jce.12776 [DOI] [PubMed] [Google Scholar]
- 16. Stefansdottir H, Arnar DO, Aspelund T, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke 2013;44:1020–5. 10.1161/STROKEAHA.12.679381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gardarsdottir M, Sigurdsson S, Aspelund T, et al. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. Europace 2018;20:1252–8. 10.1093/europace/eux220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santangeli P, Di Biase L, Bai R, et al. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm 2012;9:1761–8. 10.1016/j.hrthm.2012.07.026 [DOI] [PubMed] [Google Scholar]
- 19. Kalantarian S, Stern TA, Mansour M, et al. Cognitive impairment associated with atrial fibrillation. Ann Intern Med 2013;158:338–46. 10.7326/0003-4819-158-5-201303050-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwok CS, Loke YK, Hale R, et al. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology 2011;76:914–22. 10.1212/WNL.0b013e31820f2e38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2018-000984supp001.docx (82.8KB, docx)