Abstract
Neurotropic RNA virus infections cause a major neurological disease burden. Due to the morbidity and mortality rates of viral encephalitides worldwide, there is a need to develop clinical treatments. Features of the central nervous system (CNS), including interconnected cell types and limited regeneration, provide unique challenges. Viral encephalitis and antiviral immunity can disrupt the CNS environment, leaving patients with poor neurological out-comes despite virologic control. The cellular mechanism(s) underlying neurological recovery are not fully understood, but involve neuroimmune interactions that, until recently, primarily focused on microglia. With increasing evidence that astrocytes also have significant roles in inflammatory responses to viruses, here we summarize recent astrocyte contributions to acute virologic control and neurological impairments during recovery from viral infection.
Neurotrophic Viruses Cause Acute and Chronic Neuropathology
The continued emergence of neurotropic RNA viruses throughout the globe has led not only to increased cases of acute viral infections of the CNS, but also to recognized sequelae that persist as chronic neurologic diseases, which, in some cases, continue to worsen with age (reviewed in [1]). These include both motor and cognitive disabilities that may be due to injury or loss of neurons, or to persistent activation of inflammatory cascades that negatively impact neural cell homeostasis or function, respectively. Acute effects of CNS viral infections lead to classical, adaptive sickness behaviors, including anorexia, fatigue, hypersomnia, depressed activity, decreased social interactions, and inability to concentrate (Box 1). Many of these responses can be traced to the derailment of normal physiological functions of resident macrophages and microglia, endothelial cells, ependymal cells, neurons, and glia (astrocytes and oligodendrocytes), in favor of innate immune responses that recruit peripheral immune cells for the purpose of CNS virologic control (reviewed in [2]). Although recruited antiviral lymphocytes and myeloid cells are generally efficient and successful in clearing viral pathogens, their actions may impact genetic and phenotypic characteristics of resident neural cells, including alterations in their expression of neurotoxic versus neuroprotective proteins and/or morphological characteristics that impact function. These effects may depend on the brain region targeted or heterogeneous responses of infected and/or bystander neural cell types. In this review, we discuss the effects of neuroinvasive RNA viruses on neural cell functions, especially with regard to their capacity to promote viral clearance via activation of innate and adaptive immune responses (reviewed in [3]). Given newly identified types and functions of astrocytes within the inflamed CNS, we especially focus on new data delineating novel roles for this cell type in viral clearance and recovery.
Box 1. Symptomatic and Neuropathological Differences among Flaviviruses
While most individuals infected with flaviviruses are asymptomatic, ~25% of patients may present with mild or neuroinvasive symptoms. JEV causes symptomatic infection in the very young and older patients, whose immune systems have not fully developed or are deteriorating, respectfully. Infection causes a mild illness in most symptomatic patients with a mortality rate of ~30%. Of the survivors, ~20–30% exhibit motor and/or cognitive deficits and recurrent seizures [5]. JEV causes neuronal damage in the thalamus, hippocampus, substantia nigra, and medulla oblongata, as well as an increase of inflammatory infiltrates into the CNS [63]. By contrast, WNV illness is mostly seen in adults. Approximately half of symptomatic patients have febrile illness, while the remainder present with neurological diseases, including meningitis and encephalitis, leading to seizures, movement disorders, and neurocognitive deficits [5]. Neuropathology is present in the hippocampus, temporal lobe, and brainstem [8,9]. Historically, ~70–80% of patients infected with ZIKV were asymptomatic. Symptomatic patients reported mild presentation that included fever, conjunctivitis, and fatigue, lasting approximately 1 week. Since the outbreak in 2007, there has been an increase in reported cases of microcephaly, retinal lesions, and Guillain–Barré syndrome associated with ZIKV infection [5]. ZIKV has been shown to infect neural progenitor cells, neurons, and glial cells, leading to inhibition of neural differentiation [13]. Pregnant women with ZIKV have been correlated with fetal abnormalities, such as microcephaly and spontaneous abortion. Newborns with microcephaly tend to have major neurological dysfunctions, affecting development milestones and/or cognitive abilities later in life [5]. While mosquitoes serve as a reservoir of the previously mentioned viruses, TBEV is a tick-borne encephalitic virus. Due to potentially more compromised immune systems, clinical symptoms and sequelae are more severe in adult and older patients compared with younger individuals. Most TBEV infections are asymptomatic; however, symptomatic cases typically have neurological disease, including meningitis, encephalitis, or a combination of both. Similar to other flaviviruses, TBEV causes neuronal damage, leading to movement deficits and neurological sequelae [95].
Box 2. Flaviviruses Differentially Target IFNAR Signaling
Type I IFNs signal to the Jak/STAT pathway to amplify the innate immune response and initiate the adaptive immune response. Activation of IFNAR by type I IFNs initiates Jak1 and Tyk2, which facilitate the phosphorylation of STAT1 and STAT2. This results in the recruitment of IRF9 to form the ISGF3 transcription factor, driving the transcription of hundreds of ISGs (reviewed in [98]). Flaviviruses, including WNV, JEV, and TBEV, are highly sensitive to the antiviral effects of type I IFNs. Some flaviviruses, including WNV and JEV, antagonize type IIFN responses at the IFN-1 receptor, resulting in downstream inhibition of ISG expression [99,100]. By contrast, other flaviviruses, such as DENV and yellow fever, inhibit the type I IFN cascade by degrading STAT2 or by preventing ISGF3 from binding to DNA [101,102]. Most of these viruses appear to inhibit IFN signaling through the NS5 protein. In DENV, NS5 expression degrades the STAT2 signaling molecule [101]. In WNV-infected cells, NS5 directly inhibited the IFNAR subunit to prevent ISG expression [99]. However, a more recent study showed that WNV infection caused a decrease in the IFNAR 1 protein level without the presence of NS5, implying that WNV and other flaviviruses have evolved several distinct mechanisms to modulate the innate immune pathway [103].
Box 3. Clinician’s Corner
Upon entry into the CNS, neurotropic viruses variably infect neurons and glial cells, leading to both virus- and immune-mediated CNS dysfunction.
Viral infection presentations vary, from completely asymptomatic to inflammation of the meninges (meningitis), parenchyma (encephalitis), spinal cord (flaccid paralysis), or even obtundation (coma) leading to death.
Almost half of symptomatic patients tend to develop a flu-like illness, experiencing mild fevers or headaches. The progression to nausea, vomiting, or severe neural cell damage, as in meningitis or encephalitis, occurs in the remainder of cases.
Viral strain and inocula and viral cell targets are major factors affecting neuroinvasiveness, tropism, and viral persistency.
Encephalitis can present with focal or nonfocal neurologic signs, with altered mental status ranging from delirium to coma.
Viral Infections of the CNS
Members of the Picornaviridae family, such as polioviruses (PV) and coxsackieviruses, and the Togaviridae family, including Eastern (EEEV), Western (WEEV), and Venezuelan equine encephalitis viruses (VEEV), can infect neurons and astrocytes, which can ultimately lead to viral-induced apoptosis [4,5] (Table 1). Enterovirus (EV)71 is a common cause of hand, food, and mouth disease (HFMD), in which ulcerative lesions occur at these anatomic sites. Unfortunately, a few cases of acute infection have been associated with fatal neurological symptoms, including brain stem encephalitis and aseptic meningitis. Evidence from postmortem EV71-infected brains confirmed viral infection of neurons along with a dramatic immune response [6]. Similarly, alphaviruses can indirectly cause neuronal death via the release of proinflammatory cytokines and enhanced neurotoxicity [7].
Table 1.
In Vivo Viral Infection Pathogenesisa
| Virus | Genus | Family | Reservoir | Route of infection | Cellular tropism | Effects on neural cells | Refs |
|---|---|---|---|---|---|---|---|
| Poliovirus | Enterovirus | Picomaviridae | Humans | NMJs, BBB | Neurons | Inhibits proliferation | [4] |
| Coxsackievirus | Humans | BBB | NSPC, neurons | Inhibits proliferation | [93] | ||
| TMEV | Cardiovirus | Rodents | BBB | Neurons (acute), oligodendrocytes, microglia, astrocytes (chronic) | Oligodendrocyte and neuronal apoptosis, axonal degeneration, demyelination | [85] | |
| EEEV | Alphavirus | Togaviridae | Mosquito | BBB | Neurons, astrocytes, microglia | Neuronal apoptosis | [5] |
| WEEV | Mosquito | BBB | Neurons | Neuronal apoptosis | [5,94] | ||
| VEEV | Mosquito | BBB | Neurons, astrocytes, microglia | Neuronal apoptosis | [5,7] | ||
| TBEV | Flavivirus | Flaviviridae | Ticks | BMVECs, circulating immune cells | Neurons | Neuronal apoptosis | [95] |
| JEV | Pigs, wild birds | Peripheral nerve, BBB, BMVECs | Neurons, NSPCs, astrocytes, microglia | Inhibits proliferation | [63] | ||
| WNV | Mosquito | Peripheral nerve, BBB, BMVECs | Neurons | Neuronal apoptosis, synaptic elimination | [8,9] | ||
| ZIKV | Mosquito | BMVECs | Neurons, astrocytes, NSPCs | Inhibits neural differentiation, NPSCs and neuronal apoptosis | [12,13] | ||
| LCMV | Arenavirus | Arenaviridae | Rodents | BBB | Immature glial cells, NSPCs | Inhibits neuronal differentiation | [44] |
| Measles virus | Morbillivirus | Paramyxoviridae | Unknown | BBB | Neurons (acute), oligodendrocytes, astrocytes, endothelial cells (chronic) | Neuronal apoptosis, demyelination | [96] |
| Rabies virus | Lyssavirus | Rhabdoviridae | Bats, raccoons, skunks, foxes | NMJs | Neurons | Neuronal apoptosis | [40] |
| HIV | Lentivirus | Retroviridae | Humans | BBB, circulating immune cells | Microglia, astrocytes, NSPCs | Neuronal apoptosis, inhibits neuronal differentiation, synaptic elimination | [80,81] |
Abbreviations: JEV, Japanese encephalitis virus; LCMV, lymphocytic choriomeningitis virus; TBEV, tick-borne encephalitis virus; TMEV, Theiler’s encephalomyelitis virus; WNV, West Nile virus; ZIKV, Zika virus.
A second group of viruses that target neural cell types are the Flaviviridae (Box 2). Neuronal cell death, glial activation, breakdown of the blood–brain barrier (BBB), and immune cell infiltrates have been observed in humans infected with West Nile (WNV), tick-borne encephalitis (TBEV), Zika (ZIKV), and Japanese encephalitis virus (JEV), and have been similarly found in animal models (reviewed in [5]). Infected neurons in WNV specifically induce apoptosis via the capase-3-dependent pathway, while synapse loss is mediated by the classical complement proteins via microglia and infected neurons [8,9]. In studies using well-established murine models (reviewed in [10]), WNV-infected neurons have been shown to release proinflammatory cytokines and chemokines that activate both microglia and astrocytes, and recruit lymphocytes, respectively. The responses of microglia and astrocytes each contribute to neural pathogenesis and long-term neurocognitive deficits via differential mechanisms that include regulating cell death and/or neurotoxicity [11]. ZIKV infection additionally targets neural lineage cells [12], suggesting that chronic effects are due to the loss of neural precursor cells and their progeny. However, a recent study demonstrated that ZIKV can activate astrocytes, which, along with microglia, may become viral reservoirs in the absence of innate immune-mediated viral clearance [13] (Box 3).
Some RNA viruses, including retroviruses, can cause latent infection of the CNS via integration into the host genome. The best example, HIV-1, primarily enters the CNS via infected, circulating CD4+ T cells or as free virions (reviewed in [14]). Microglia and astrocytes were recently implicated as viral reservoirs within the CNS, although this remains controversial [15,16]. As the immune system progressively weakens during infection, glial cells acting as viral reservoirs pose a challenge to HIV eradication. Given that HIV-1 does not directly infect neurons, the development of a neurotoxic environment after peripheral infection may be the underlying cause of neuronal loss with long-term neurological dysfunction. The addition of exogenous HIV-1 proteins, Including Tat and gp120, injured cultured neurons via calcium-mediated excitotoxicity and stimulated the release of inflammatory cytokines and chemokines, including IL-1β, TNF, IL-6, RANTES/CCL5, and reactive oxygen species (ROS) [17,18].
Response of Astrocytes to Viral Infection
Astrocytes and BBB Function during Viral Encephalitis
Astrocytes and their end-feet processes surround the brain microvascular endothelial cells (BMECS), which comprise the BBB. At homeostasis, astrocytes support and maintain a functional BBB, preventing toxins and other substances from entering the brain (reviewed in [19]). Aberrant BBB permeability and the role of astrocytes in the dysregulation, during and after viral infection, has a critical impact on viral neuropathogenesis. WNV infection of the cultured astrocyte increases matrix metalloproteinases (MMPs), which are key mediators of tight junction proteins of the BBB [20]. Specifically, MMP9 upregulation occurs in a variety of viral infections, including HIV-1, JEV, and WNV, and disrupts the BBB, leading to viral entry into the brain [21–23].
Expressed on neurons and glia cells, Toll-like receptors (TLRs), including TLR3,7, and 9, and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) are examples of pattern recognition receptors (PPRs) that are activated by viral pathogen-associated molecular patterns (PAMPs). TLR and RLR activation contributes to neuronal damage, maintenance of glial activation, and the production of cytokines, such as type I interferons (IFNs) [24,25]. Infection of cultured astrocytes with viruses, including rabies virus, Theiler’s murine encephalomyelitis virus (TMEV), TBEV, WNV, ZIKV, and JEV, also induces the production of type I IFNs [25–27]. Type I IFNs, along with various other innate cytokines, including TNF, IL-6, IL-1β, and IFN-γ, regulate BBB integrity through several mechanisms, including activation of MMP9, regulation of Rho GTPases, and suppression of proinflammatory cytokines [28]. Mouse models of WNV demonstrated the importance of type I IFNs in BBB integrity, because astrocyte-specific deletion of IFNAR, a type IIFN downstream receptor, led to enhanced BBB permeability. Interestingly, the increase in BBB permeability, as well as viral neuroinvasion and immunopathologic neuronal cell death, occurred primarily in the cerebellum of mice, indicating heterogeneous roles for type I IFN in hindbrain and forebrain astrocytes. Cerebellar astrocytes also have higher basal levels of PRRs, which recognize PAMPs, and IFN-stimulated gene (ISG) expression [29]. Regional and individual heterogeneity of astrocyte function has long been appreciated [30], supporting the need to evaluate differential astrocyte responses in viral infections with regard to effects on both BBB integrity and immunological responses.
Astrocytic end-feet are rich in glutamate transporters and potassium channels. Both these types of protein have vital roles in ion and glutamate homeostasis (reviewed in [31]). HIV-1 alters glutamate uptake and the activity of potassium channels, suggesting that these alterations affect the integrity of the BBB [32]. It has also been observed that HIV-1 Tat proteins alter the expression of tight junction proteins, resulting in a breakdown of BBB permeability [33]. These studies support the idea that astrocyte dysfunction contributes to the increasing permeability of the BBB, resulting in increased penetration of virus and/or infiltrating immune cytokines.
Acute Infection: Amplification of Inflammatory Responses
Similar to microglia, astrocyte participation in innate immune responses via PRR detection of viral PAMPS, along with Nod-like receptors (NLRs), AIM2-like receptors, mitochondrial antiviral signaling (MAVS), C-type lectin receptors, and cytokine receptors, promote the expression of hundreds of ISGs, including those that limit viral replication and contribute to inflammatory cell infiltration, which are critical for virologic control [34]. The increased expression of ISGs has been associated with the increased production of inflammatory cytokines and chemokines in astrocytes, including TNF and CXCL10 [35]. Hence, in murine models of lymphocytic choriomeningitis virus (LCMV) and WNV, global deletion of IFNAR led to an inability to clear virus and increased mortality, respectively [26,27]. Astrocyte-derived CXCL10 has been implicated as a major driver of antibody-secreting cells (ASCs) in the JHM strain of mouse hepatitis virus (JHMV)-infected mice [36]. ASCs produce local antibodies (Abs) and are vital for the ultimate clearance of virus and the prevention of viral persistence or reappearance [37]. Ab-mediated control of viral infection has been observed in numerous RNA viruses, including mouse hepatitis virus (MHV), Sindbis virus and rabies virus [38–40]. In the CNS, CXCR3 mediates ASC trafficking [39,104]. More recently, CXCL10, a CXCR3 ligand, was shown to regulate ASC recruitment into the CNS following JHMV infection. In this infection model, CXCL10 colocalized primarily with astrocytes [36,41]. CXCL10 production by astrocytes is consistent with models of MHV infection [42], supporting a role for astrocytes in viral clearance.
Chronic Infection: Astrocyte Role in T Cell Recruitment
Chemokine ISGs regulate T cell immunity by either promoting or inhibiting T cell activation, proliferation, and survival (reviewed in [43]). T cells have a vital role in eliminating acute infection and controlling viral loads during chronic infection. In addition to microglia, activated astrocytes continue to regulate the adaptive immune response via its role in recruiting T cells to the CNS. Astrocytes were shown to be a significant producer of CXCL10 in the neural parenchyma in a murine model of LCMV infection [44]. At the site of CNS viral infection, CXCL10 has been implicated as an important ligand for CXCR3 on CD8+T cells [45]. In a murine model of WNV infection, the recruitment of CXCR3+ T cells to the CNS led to the control of viral infection and promoted survival [44]. Flaviviruses also induce the expression of MHC class I molecules, which are involved in the activation of CD8+ T cells [46]. While astrocytes normally express low levels of MHC class I molecules, infection with JHMV in vivo caused an increase in these molecules [47]. Another more recent example demonstrated that Infection of culture astrocytes with St Louis encephalitis virus (SLEV) increased the surface expression of MHC class I [48]. Further studies examining the functionality of the MHC class I molecules in recognizing T cells are warranted.
Astrocyte–Microglia Interactions in Viral Infections
As the resident macrophages of the CNS, microglia are important immunological players after CNS insults, including viral infections. Similar to astrocytes, activated microglia have the capacity to dramatically alter their morphology and upregulate the expression of various receptors and produce a multitude of secretory chemokines that can contribute to both the defense and damage of the infected brain (reviewed in [49]). Astrocytes also express many of these receptors and chemokines, suggesting that chemokines serve as a communication signal between astrocytes and microglia.
Astrocytes exposed to HIV-1 upregulate biologically active complement C3. HIV-1 viral proteins, including Net and gp41, are also able to upregulate C3 [50]. C3 is a central component of the cascade that mediates the clearance of immune complexes, modulates T cell function, and participates in synapse maturation (reviewed in [51]). The microglial mediated-classical complement cascade, which includes C3, regulates synaptic elimination during development and after CNS injuries, including viral Infections, leading to CNS-related pathology [9,52,53]. This astrocyte–microglia interaction adds to our understanding of the role of the complement pathway in promoting antiviral immunological functions after viral infections and supports the notion that innate immune molecules exert CNS-specific functions that Impact recovery from infection.
More recently, the interaction between reactive astrocytes, activated microglia, and invading monocytes has been studied, albeit in nonviral models. In a stab injury mouse model, proliferating astrocytes were shown to regulate CCR2+ monocytes by either reducing their invasion, proliferation, differentiation, or survival in the brain. Conversely, the loss of CCR2+ monocytes led to an increase in astrocyte proliferation and microglia activation at the lesion site, revealing a feedback regulation loop between invading monocytes and astrocytes [54]. How this crosstalk between astrocytes, microglia, and invading monocytes regulates viral infection and recovery from viral infection needs to be further studied.
Activated Astrocytes in Viral Infections
Astrocytes perform a variety of neuroprotective functions, including regulation of synaptic function, facilitating neuronal repair, and maintaining BBB integrity. In addition, these glial cells produce neurotrophic factors and anti-inflammatory cytokines, such asTGF-β, IFN-γ, and brain-derived neurotrophic factor (BDNF) (reviewed in [55]). However, in response to most CNS injuries, including viral infections, astrocytes undergo a dramatic morphological and functional transformation referred to as astrogliosis (reviewed in [56]). Astrogliosis can be detected during the acute infection period and can persist over much longer periods. This transformation is characterized by increased genetic expression of glial fibrillary acid protein (GFAP), vimentin, and nestin, hypertrophy of processes, and, in some cases, proliferation of new astrocytes and scar formation.
Reactive astrocytes have generally been accepted to be beneficial because they have to ability to remove toxic molecules and restore the BBB. They also promote extracellular matrix components to promote axonal growth and repair (reviewed in [57]). However, reactive scar-forming astrocytes can also inhibit neuronal repair and regeneration by upregulating numerous inhibitory molecules, including chondroitin sulfate proteoglycan, NG2-proteoglycan, and MMPs [57]. The duality of reactive astrocytes is prominent in many models of infection. For example, in HIV-1 infection, activated astrocytes increase their production of cytokines and chemokines, including IFN-γ and RANTES/CCL5, which may inhibit viral replication [58,59]. HIV-1 infection of astrocytes themselves is associated with the upregulation of HIV proteins, such as Tat, Nef, and Rev [60]. Tat and gp120 specifically alter astrocyte growth and promote the elevation of neurotoxic proteins, including TNF, IL-6, IL-1β, RANTES, CXCL10, and glutamate [41,61,62].
While virus-mediated neuronal cell death and cytokines derived from immune infiltrates may activate astrocytes [56,63], emerging studies have also implicated microglia as a key player in this process. Microglial cells release proinflammatory cytokines, such as IL-1β, TNF, and IFN-γ, which may favor astrogliosis during viral infections and other CNS insults [64,65], because some GFAP+ astrocytes incorporated 5-bromo-2′-deoxyuridine (BrdU) or stained positively for Ki67 (a marker of proliferation). Whether this is indicative of astrogenesis (the dividing and generation of new astrocytes) or astrogliogenesis (the proliferation of astrocytes) is still unclear. The source of newly generated adult astrocytes that may contribute to the reactive astrocytic pool is not well established, but include radial glia, neuronal progenitors in the subventricular zone (SVZ) and subgranularzone (SGZ), locally proliferating glia, and NG2+ cells [66]. While still debated, proliferation of astrocytes appears to be context dependent. Astrogenesis has been observed in murine stab wound and neurodegenerative disease models, including Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS), yet does not occur after injection of bacterial cell wall endotoxin lipopolysaccharide (LPS), a bacterial PAMP that triggers PRR activation [67–70]. In a recent study using an adult murine model of WNV, increased astrogenesis, as indicated by BrdU incorporation, was observed after viral infection [71].
Reactive astrocytes are also known to enhance the expression or dysfunction of ion channels, glutamate and glucose transporters, and gap junctional proteins, including Connexin 43 (Cx43) [72]. For example, in Borna disease virus infection, a global reduction in Cx43 mRNA was observed [73]. Astrocytic infection of HIV-1 promotes the opening of Cx43 channels, resulting in the aberrant secretion of dickkopf-1 (DKK1) protein. DKK1 is an inhibitor of Wnt signaling, a pathway that promotes neurogenesis in vivo [74]. Given that gap junctions allow the movement of small molecules between neuronal cells, providing metabolic support and maintaining homeostasis, they may also have a role in intercellular communication. MHV infection of primary astrocytes induced the downregulation of Cx43, reducing gap junctional communication as demonstrated by a LY dye transfer assay. Murine infection of MHV reduced Cx43 levels during acute infection, reflecting culture studies [75].
Astrocytes in Postinfectious Syndromes
Many viral infections, including WNV, JEV, ZIKV, and HIV-1, have been associated with chronic sequelae [1]. These chronic complications, including long-term cognitive impairment, motor deficits, and acute immune neuropathies, such as Guillain-Barré syndrome, can persist long after viral clearance. While immune responses triggered by viral infection or the persistent presence of viral RNA have been suggested to contribute to neurological sequalae, there is little known about the direct molecular mechanisms involved.
WNV is one of the most common causes of mosquito-borne neuroinvasive disease (WNND) [5]. Approximately 50% of survivors will experience debilitating, long-term cognitive sequelae, such as deficits in visuospatial learning and memory [5]. Recently, a murine model of WNND was developed, mimicking rates of human survival and neurocognitive deficits [9]. WNND-recovered mice favored hippocampal astrogenesis at the expense of neurogenesis, which persisted up to 30 days post infection. These mice also exhibited a lack of hippocampal synapse recovery. Further findings demonstrated the preferential generation of proinflammatory reactive astrocytes over neurons that ultimately become a source of antineurogenic cytokines, such as IL-1β. IL-1R1–/– WNND-recovered mice exhibited normal neurogenesis, recovery of hippocampal synapses, and resistance to spatial learning defects, suggesting a role for reactive astrocyte cytokine release in the persistent neurological sequelae [71]. TNF can also regulate neural correlates of memory by influencing synaptic plasticity through the regulation of AMPA receptors. TNF also controls glutamatergic gliotransmission in healthy and disease states [76,77]. Recently, astrocytes were shown to release proinflammatory TNF, resulting in altered synaptic transmission and impaired cognition in a mouse model of multiple sclerosis (MS) [77].
Astrocytes have also been implicated in other viral-related neurocognitive syndromes, including HIV-associated neurocognitive disorder (HAND). Nearly half of HIV-1 infections lead to neurological complications, such as loss of attention and concentration, motor deficits, and behavioral changes [78]. Vpr is a HIV accessory protein in the cytoplasm of astrocytes near the inflammation site. In cultured rat hippocampal neurons, Vpr contributes to cell death as well as inducing several inflammatory products, including CCL5, IL-6, IL-8, and MCP-1 [79,80]. A recent study demonstrated the functionality of this protein, because Vpr-infused rats showed impaired novel location and novel object recognition correlated with the loss of presynaptic terminals in the CA3 region of the hippocampus [81], suggesting that astrocytes participate in creating a neurotoxic environment after infection. Furthermore, membrane HIV-1 Tat protein released from astrocytes results in mitochondrial dysfunction and neuronal cell death in culture [82]. Astrocyte activation by HIV-1 Tat also secretes cytokines, including TNF and IL-β, chemokines, such as MCP-1 and IP-10, and nitric oxide (NO) [83]. Similar to other viruses, these cytokines and chemokines regulate the BBB and neuronal injury and apoptosis pathway in the context of HIV-1 infection (Table 2).
Table 2.
Neurotoxic Effects of Common Cytokines and Chemokines
| Cytokine/chemokine | CNS origin | Neurotoxic effect | Refs |
|---|---|---|---|
| TNF | Microglia, astrocytes, neurons | Neuronal apoptosis, decreases neural proliferation and differentiation, and synaptic plasticity | [77,97] |
| IL-1β | Microglia, astrocytes, neurons | Neuronal apoptosis, decreases neural proliferation and differentiation, and neural migration, increases astrocyte differentiation | [69,94] |
| IL-6 | Microglia, astrocytes, endothelial cells | Decreases neuronal proliferation and differentiation | [94] |
| INF-γ | Microglia, astrocytes | Decreases neuronal proliferation and differentiation | [97] |
| F1ANTES/CCL5 | Microglia, astrocytes | Increases neuronal excitotoxicity | [59,79] |
| CXCL10 | Microglia, astrocytes, neurons | Neuronal apoptosis | [61] |
| NO, ROS | Microglia, astrocytes | Neuronal apoptosis, increases neuronal excitotoxicity, decreases synaptic plasticity | [33] |
Glutamate excitotoxicity is a proposed mechanism for neuronal injury in viral infections as well as other neurological diseases, such as ALS and AD (reviewed in [84]). Exposure of cultured astrocytes to HIV-1 or gp120 resulted in the reduction of glutamate transporters, such as EAAT2, and the impairment of extracellular transport of glutamate by astrocytes [32]. HIV-1 and other viral proteins, such as Tat, modulate the expression of transporter EAAT1 [60]. The altered expression and function of EAAT1 and 2, in conjunction with reactive astrocyte over production of glutamate, can cause excess extracellular glutamate, inducing the increase of intracellular calcium levels in astrocytes. Intracellular calcium levels can, in turn, release even more glutamate from the cell. Excess extracellular glutamate can lead to the overactivation of NMDARs, resulting in enhanced intracellular calcium and free radicals [83]. Together, these alterations may contribute to neuronal injury in HIV-1 and lead to HAND pathogenesis.
Infection with various viruses, including the mouse strains of TMEV, MHV, and Semliki Forest virus, induce a demyelinating disease, mimicking certain pathologies similar to those seen with human MS [85]. These viral models have been informative in our understanding of the role of astrocytes in demyelination. TMEV infection of primary astrocyte cultures induced the expression of proinflammatory cytokines, including IL-12, IL-1, IL 6, TNF, and IFN-β, through the NF-κB pathway [86]. In vitro, TNF has been shown to limit the phagocytosis of damaged myelin as well as to negatively affect oligodendrocyte maturation [87]. However, it is possible that TNF increases the production of plate-derived growth factor (PDGF), a factor that enhances oligodendrocyte precursor cells in demyelinating lesions, in the demyelinated spinal cord of MHV-infected mice [88].
Stimulation of proinflammatory cytokines, such as IL-1β and TNF, reduced glycogen storage and lactate transport necessary for providing an energy source to axons remyelinated after viral infection [89]. The astrocyte-neuron lactate transport is an important mechanism providing energy to induce long-term potentiation in the hippocampus. A similar transport system has been implicated during demyelination. Astrocytes can store glycogen that can be metabolized to initiate glycogenolysis and provide lactate as an energy source to neurons and nearby axons [90]. The transport of lactate is mediated by monocarboxylate transporters (MCTs). MCT1 and MCT4 are expressed on astrocytes to release lactate, while MCT2 is expressed primarily on neurons to receive lactate [91]. Recent evidence showed that oligodendrocytes can also transfer lactate via MCT1 and MCT2, suggesting that at least some astrocytic lactate production is targeted to myelinating oligodendrocytes [92].
Concluding Remarks
Although significant findings have provided the field with a better understanding of astrocytic contributions during viral infection, many questions remain (see Outstanding Questions). The activation of astrocytes and their subsequent role in the immune response have been implicated in the pathogenesis of viral infection as well as in the persistence of neurological complications following acute infection. In many instances, the role of reactive astrocytes in disease is not completely clear. Given that astrocytic activation may be beneficial in some instances and detrimental in others (Figure 1), it is likely that regional and individual heterogeneity in astrocyte responses contributes to outcomes after infection and injury. Furthermore, crosstalk between neurons, astrocytes, and microglia adds another layer of complexity to the web of signaling pathways modulating the immune responses during and after viral infection. These factors will be important considerations when developing therapies targeting astrocytic mechanisms in neurological deficits. Moreover, elucidating the role of reactive astrocytes and their interactions with other neural and glial cell types may provide insights into the progression of other neurological diseases.
Outstanding Questions.
How does viral infection promote the generation of proinflammatory reactive astrocytes? Through what signaling pathway is this process mediated?
What are the cellular targets (i.e., neurons, astrocytes, microglia, etc.) of astrocytic proinflammatory cytokines that limit the recovery of neurological deficits after viral infection?
What is the source of newly generated adult astrocytes that may contribute to the reactive astrocytic pool (radial glia, neuronal progenitors, locally proliferating glia, and/or NG2+ cells)?
How does astrocyte-neuron or astrocyte-microglia communication shape the immune environment during and after viral infection?
Do similar astrocytic mechanisms underlie the immune response in other neurological CNS diseases?
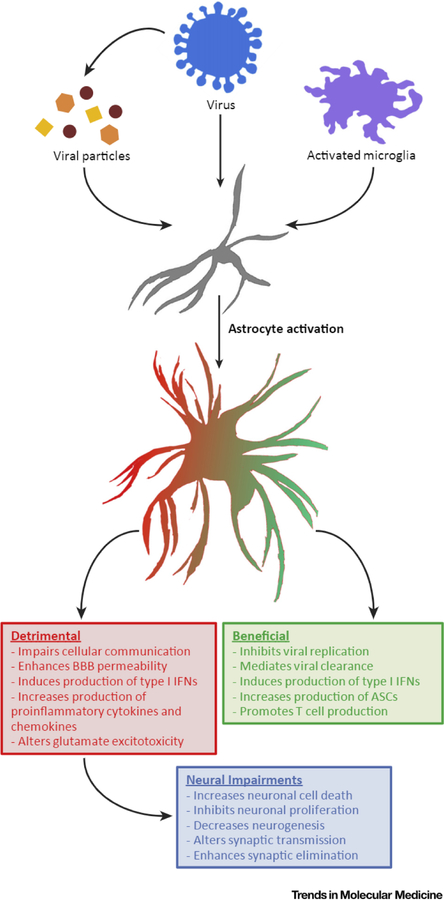
Figure 1. The Duality of Reactive Astrocytes during and after Viral Infection.
Astrocytes transition into an activated state through multiple mechanisms, such as direct viral infection or indirectly via viral particles and/or activated microglia. Reactive astrocytes can exhibit both beneficial (green box) and detrimental (red box) roles during viral infection and recovery from viral infection. Damaging roles of activated astrocytes have been linked to immunopathology that may lead to more long-term neurological disease symptoms (blue box) after recovery from viral infection. Abbreviations: ASC, Antibody-secreting cells; BBB, blood-brain barrier; CNS, central nervous system; INF, interferon.
Highlights.
Neurotropic RNA viruses invade the CNS, often eliciting serious chronic neurological impairments.
Astrocytes are activated during acute viral infection and can persist over a long period of time.
Astrocyte activation during acute infection is vital for viral control and clearance.
However, persistent activation of astrocytes has been implicated as a cause of long-term neurological impairments after viral infection.
References
- 1.van den Pol AN (2009) Viral infection leading to brain dysfunction: more prevalent than appreciated? Neuron 64, 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein RS et al. (2017) Infectious immunity in the central nervous system and brain function. Nat. Immunol 18, 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender C et al. (2012) Role of astrocytes in viral infections. In Astrocytes: Structure, Functions and Role in Disease (González-Pérez O, ed.), pp. 109–124, Nova Science Publishers [Google Scholar]
- 4.Nagata N et al. , (2004) Differential localization of neurons susceptible to Enterovirus 71 and Poliovirus type 1 in the central nervous system of cynomolgus monkeys after intravenous inoculation. J. Gen. Virol 85, 2981–2989 [DOI] [PubMed] [Google Scholar]
- 5.Salimi H et al. (2016) Encephalitic arboviruses: emergence, clinical presentation, and neuropathogenesis. Neurotherapeutics 13, 514–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong KT et al. (2008) The distribution of inflammation and virus in human Enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J. Neuropathol. Exp. Neurol 67, 162–169 [DOI] [PubMed] [Google Scholar]
- 7.Schoneboom BA et al. (2000) Inflammation is a component of neurodegeneration in response to Venezuelan equine encephalitis virus infection in mice. J. Neuroimmunol 109, 132–146 [DOI] [PubMed] [Google Scholar]
- 8.Samuel MA et al. (2007) Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol 81,2614–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasek MJ et al. (2016) A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agner SC and Klein RS (2018) Viruses have multiple paths to central nervous system pathology. Curr. Opin. Neurol 31, 313–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar M et al. (2010) Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J. Neuroinflammation 7, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang H et al. (2016) Zika virus infects human cortical neural precursors and attenuates their growth. Cell Stem Cell 18,587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muffat J et al. (2018) Human induced pluripotent stem cell-derived glial cells and neural progenitors display divergent responses to Zika and dengue infections. Proc. Natl. Acad. Sci. U. S. A 115, 7117–7122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruelas DS and Greene WC (2013) An integrated overview of HIV-1 latency. Cell 155, 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellano P et al. (2017) HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep 7, 12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G-H et al. (2016) Astrocytes as an HIV reservoir: mechanism of HIV infection. Curr. HIV Res 14, 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein RS et al. (1999) Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J. Immunol 163, 1636–1646 [PubMed] [Google Scholar]
- 18.Holden CP et al. (1999) Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience 91,1369–1378 [DOI] [PubMed] [Google Scholar]
- 19.Cardoso FL et al. (2010) Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res. Rev 64, 328–363 [DOI] [PubMed] [Google Scholar]
- 20.Verma S et al. (2010) Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 397, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C-M et al. (2012) Japanese encephalitis virus induces matrix metalloproteinase-9 expression via a ROS/c-Src/PDGFR/PI3K/Akt/MAPKs-dependent AP-1 pathway in rat brain astrocytes. J. Neuroinflammation 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju SM et al. (2009) Extracellular HIV-1 Tat up-regulates expression of matrix metalloproteinase-9 via a MAPK-NF-κB dependent pathway in human astrocytes. Exp. Mol. Med 41, 86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P et al. (2008) Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J. Virol 82, 8978–8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crill EK et al. (2015) RIG-I is required for VSV-induced cytokine production by murine glia and acts in combination with DAI to initiate responses to HSV-1. Glia 63, 2168–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfefferkom C et al. (2016) Abortively infected astrocytes appear to represent the main source of interferon beta in the virus-infected brain. J. Virol 90, 2031–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuel MA and Diamond MS (2005) Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol 79, 13350–13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou S et al. (2010) Induction and inhibition of type I interferon responses by distinct components of lymphocytic choriomeningitis virus. J. Virol 84, 9452–9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels BP et al. (2014) Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. mBio 5, e01476–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels BP et al. (2017) Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J. Clin. Invest 127, 843–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein RS and Flicker LD (1992) Heterogeneous expression of carboxypeptidase E and proenkephalin mRNAs by cultured astrocytes. Brain Res 569, 300–310 [DOI] [PubMed] [Google Scholar]
- 31.Olsen ML et al. (2015) New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J. Neurosci 35, 13827–13835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z et al. (2003) Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology 312,60–73 [DOI] [PubMed] [Google Scholar]
- 33.Cisneros IE and Ghorpade A (2012) HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr. HIV Res 10, 392–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui J et al. (2015) Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin. Immunother 10, 3270–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X et al. (2008) Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev 226, 41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phares TW et al. (2013) Astrocyte-derived GXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. J. Virol 87, 3382–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin MT et al. (1999) Antibody prevents virus reactivation within the central nervous system. J. Immunol 162, 7358–7368 [PubMed] [Google Scholar]
- 38.Hooper DC et al. (2009) The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl. Trop. Dis 3, e535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyor WR and Griffin DE (1993) Virus specificity and isotype expression of intraparenchymal antibody-secreting cells during Sindbis virus encephalitis in mice. J. Neuroimmunol 48,37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hooper DC et al. (1998) Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol 72, 3711–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Hage N et al. (2005) Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50, 91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane TE et al. (1998) Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol 160, 970–978 [PubMed] [Google Scholar]
- 43.Crouse J et al. (2015) Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol 15, 231–242 [DOI] [PubMed] [Google Scholar]
- 44.Christensen JE et al. (2009) Fulminant lymphocytic choriomeningitis virus-induced inflammation of the CNS involves a cytokine-chemokine-cytokine-chemokine cascade. J. Immunol 182, 1079–1087 [DOI] [PubMed] [Google Scholar]
- 45.Zhang B et al. (2008) CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J. Immunol 180, 2641–2649 [DOI] [PubMed] [Google Scholar]
- 46.Cheng Y et al. (2004) Major histocompatibility complex class I (MHC-I) induction by West Nile virus: involvement of 2 signaling pathways in MHC-I up-regulation. J. Infect. Dis 189, 658–668 [DOI] [PubMed] [Google Scholar]
- 47.Hamo L et al. (2007) Distinct regulation of MHC molecule expression on astrocytes and microglia during viral encephalomyelitis. Glia 55, 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuza AL et al. (2016) Astrocyte response to St. Louis encephalitis virus. Virus Res 217, 92–100 [DOI] [PubMed] [Google Scholar]
- 49.Cherry JD et al. (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation 11,98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Speth C et al. (2001) Human immunodeficiency virus type 1 induces expression of complement factors in human astrocytes. J. Virol 75, 2604–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ricklin D et al. (2010) Complement: a key system for immune surveillance and homeostasis. Nat. Immunol 11, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafer DP et al. (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong S et al. (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frik J et al. (2018) Cross-talk between monocyte invasion and astrocyte proliferation regulates scarring in brain injury. EMBO Rep 19, e45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colombo E and Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37, 608–620 [DOI] [PubMed] [Google Scholar]
- 56.Liddelow SA and Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 [DOI] [PubMed] [Google Scholar]
- 57.Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32, 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W et al. (2011) IFN-γ mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the β-catenin pathway (DKK1) in a STAT 3-dependent manner. J. Immunol 186, 6771–6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Appay V and Rowland-Jones SL (2001) RANTES: a versatile and controversial chemokine. Trends Immunol 22, 83–87 [DOI] [PubMed] [Google Scholar]
- 60.Zhou BY et al. (2004) Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol. Cell. Neurosci 27, 296–305 [DOI] [PubMed] [Google Scholar]
- 61.Williams R et al. (2009) HIV-1 Tat co-operates with IFN-γ and TNF-α to increase CXCL10 in human astrocytes. PLoS One 4, e5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah A et al. (2011) HIV-1 gp120 induces expression of IL-6 through a nuclear factor-kappa B-dependent mechanism: suppression by gp120 specific small interfering RNA. PLoS One 6, e21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C-J et al. (2010) Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J. Gen. Virol 91, 1028–1037 [DOI] [PubMed] [Google Scholar]
- 64.Liddelow SA et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541,481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Röhl C et al. (2007) The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res 1129,43–52 [DOI] [PubMed] [Google Scholar]
- 66.Ge W-P and Jia J-M (2016) Local production of astrocytes in the cerebral cortex. Neuroscience 323, 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simon C et al. (2011) Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59, 869–881 [DOI] [PubMed] [Google Scholar]
- 68.Kamphuis W et al. (2012) Differential cell proliferation in the cortex of the APPswePS1dE9 Alzheimer’s disease mouse model. Glia 60, 615–629 [DOI] [PubMed] [Google Scholar]
- 69.Sirko S et al. (2013) Reactive glia in the injured brain acquire stem cell properties in response to Sonic Hedgehog. Cell Stem Cell 12, 426–439 [DOI] [PubMed] [Google Scholar]
- 70.Lepore AC et al. (2008) Selective ablation of proliferating astrocytes does not affect disease outcome in either acute or chronic models of motor neuron degeneration. Exp. Neurol 211, 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garber C et al. (2018) Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat. Immunol 19, 151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theodoric N et al. (2012) Role of gap junction protein Connexin43 in astrogliosis induced by brain injury. PLoS One 7, e47311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Köster-Patzlaff C et al. (2007) Persistent Boma disease virus infection changes expression and function of astroglial gap junctions in vivo and in vitro. Brain Res 1184, 316–332 [DOI] [PubMed] [Google Scholar]
- 74.Orellana JA et al. (2014) HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. J. Neurochem 128, 752–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basu R et al. (2016) Mouse hepatitis virus infection remodels Connexin43-mediated gap junction intercellular communication in vitro and in vivo. J. Virol 90, 2586–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santello M et al. (2011) TNFα controls glutamatergic gliotrans-mission in the hippocampal dentate gyrus. Neuron 69, 988–1001 [DOI] [PubMed] [Google Scholar]
- 77.Habbas S et al. (2015) Neuroinflammatory TNFα Impairs memory via astrocyte signaling. Cell 163, 1730–1741 [DOI] [PubMed] [Google Scholar]
- 78.Heaton RK et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75, 2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gangwani MR et al. (2013) Human immunodeficiency virus type 1 viral protein R (Vpr) induces CCL5 expression in astrocytes via PI3K and MAPK signaling pathways. J. Neuroinflammation 10, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferrucci A et al. (2013) Extracellular HIV-1 viral protein R affects astrocytic glyceraldehyde 3-phosphate dehydrogenase activity and neuronal survival. J. Neurovirol 19, 239–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torres L and Noel RJ (2014) Astrocytic expression of HIV-1 viral protein R in the hippocampus causes chromatolysis, synaptic loss and memory impairment. J. Neuroinflammation 11,53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chauhan A et al. (2003) Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J. Biol. Chem 278, 13512–13519 [DOI] [PubMed] [Google Scholar]
- 83.Kaul M et al. (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 [DOI] [PubMed] [Google Scholar]
- 84.Maragakis NJ. and Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol 2, 679–689 [DOI] [PubMed] [Google Scholar]
- 85.Das Sarma J (2010) A mechanism of virus-induced demyelination. Interdiscip. Perspect. Infect. Dis 2010, 109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palma JP et al. (2003) Infection with Theiler’s murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-κB activation: potential role for the initiation of demyelinating disease. J Virol 77, 6322–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fischer R et al. (2014) Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia 62, 272–283 [DOI] [PubMed] [Google Scholar]
- 88.Vana AC et al. (2007) Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J. Neuropathol. Exp. Neurol 66, 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gavillet M et al. (2008) Modulation of astrocytic metabolic phenotype by proinflammatory cytokines. Glia 56, 975–989 [DOI] [PubMed] [Google Scholar]
- 90.Magistretti PJ and Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron 86, 883–901 [DOI] [PubMed] [Google Scholar]
- 91.Suzuki A et al. (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fünfschilling U et al. (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485,517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feuer R et al. (2005) Coxsackievirus targets proliferating neuronal progenitor cells in the neonatal CNS. J. Neurosci 25, 2434–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phelps AL et al. (2017) Susceptibility and lethality of Western equine encephalitis virus in Balb/c mice when infected by the aerosol route. Viruses 9, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansfield KL et al. (2009) Tick-borne encephalitis virus – a review of an emerging zoonosis. J. Gen. Virol 90, 1781–1794 [DOI] [PubMed] [Google Scholar]
- 96.Patterson CE et al. (2003) Measles virus infection induces chemokine synthesis by neurons. J. Immunol 171,3102–3109 [DOI] [PubMed] [Google Scholar]
- 97.del Rey A et al. (2013) A cytokine network involving brain-borne IL-1β, IL-1rα, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behav. Immun 33, 15–23 [DOI] [PubMed] [Google Scholar]
- 98.MacMicking JD (2012) Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol 12, 367–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo J-T et al. (2005) West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol 79, 1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin R-J et al. (2006) Blocking of interferon-induced Jak-Stat signaling by Japanese encephalitis virus NS5 through a protein tyrosine phosphatase-mediated mechanism. J. Virol 80,5908–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ashour J et al. (2009) NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol 83, 5408–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laurent-Rolle M et al. (2014) The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe 16, 314–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans JD et al. (2011) West Nile Virus infection induces depletion of IFNAR1 protein levels. Viral Immunol 24, 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marques CP et al. (2011) CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J. Virol 85, 6136–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]