Abstract
Background:
In infants with ductal-dependent pulmonary blood flow (PBF), initial palliation with patent ductus arteriosus (PDA) stent or modified Blalock-Taussig (BT) shunt have comparable mortality but discrepant length of stay (LOS), procedural complication rates and reintervention burdens, which may influence cost. The relative economic impact of these palliation strategies is unknown.
Methods and Results:
Retrospective study of infants with ductal-dependent PBF palliated with PDA stent (n=104) or BT shunt (n=251) from 2008–15 at 4 centers of the Congenital Catheterization Research Collaborative. Inflation-adjusted inpatient hospital costs were calculated for first-year-of-life (FYOL) using Pediatric Health Information System (PHIS) data. Costs derived from outpatient catheterizations not in PHIS were imputed. Costs were compared using propensity score adjusted multivariable models, to account for baseline differences between groups. After propensity score adjustment, FYOL costs were significantly lower in PDA stent ($215,825 [190,644–244,333]) than BT shunt ($249,855 [230,693–270,609]) patients (p=0.05). Following addition of imputed costs, FYOL costs were not significantly different between PDA stent ($226,403 [200,274–255,941]) and BT shunt ($252,072 [232,955–272,759]) groups (p=0.15). Patient characteristics associated with higher costs included: younger gestational age, genetic syndrome, non-cardiac diagnoses, procedural complications, ECMO, duration of ventilation, ICU and hospital LOS and re-intervention (p≤0.02 for all).
Conclusions:
In this first multicenter comparative study of PDA stent or BT shunt as palliation for infants with ductal-dependent PBF, adjusted for baseline differences, PDA stent was associated with lower to equivalent costs over the FYOL. Combined with previous evidence suggesting clinical non-inferiority, these findings suggest that PDA stent provides competitive health care value.
Keywords: Congenital heart disease, palliation, intervention, surgery, cost, value
Introduction
Infants with cyanotic congenital heart disease (CHD) and ductal-dependent pulmonary blood flow (PBF) may require initial palliation to provide adequate PBF until such time that definitive repair or second stage palliative surgery can be performed. For decades, this initial palliation was accomplished with surgical placement of a Blalock-Taussig (BT) shunt, or equivalent.1–3 More recently, innovation in clinical practice has facilitated use of a less invasive technique, with transcatheter placement of a patent ductus arteriosus (PDA) stent to secure PBF.4–7 Two large multicenter comparisons of BT shunt and PDA stent therapies, adjusted to account for differences in baseline factors, were recently published.8, 9
In both studies, mortality following PDA stent was found to be non-inferior (or superior) to BT shunt with significantly shorter hospital length of stay (LOS) and fewer procedural complications and other adverse events.8, 9 At the same time, during follow-up, the risk of reintervention was significantly higher after PDA stent. In the context of these competing considerations, analysis of differences in cost of care is potentially relevant. An empirical comparison of costs not only determines which procedure delivers greater healthcare value (lower price for similar clinical outcomes) but also provides a means of combining multiple heterogenous clinical outcomes into a single evaluable and quantitative metric.
To our knowledge, no previous studies have evaluated the relative costs of PDA stent and BT shunt in infants with ductal dependent pulmonary blood flow. We utilized a large multicenter cohort to empirically compare the cost of BT shunt and PDA stent as palliative strategies for cyanotic infants with ductal-dependent PBF. We hypothesized that the shorter LOS and decreased morbidity after PDA stent would be reflected in a cost-advantage for PDA stent during the incident hospitalization. We also hypothesized that these cost benefits would overcome the increased costs associated with the higher risk of re-intervention and, thus, that this cost-advantage would persist through the first year of life.
Methods
Data Sources
The Congenital Catheterization Research Collaborative (CCRC) is a multicenter collaborative, comprised of investigators from the Children’s Hospital of Philadelphia, Cincinnati Children’s Hospital Medical Center, Children’s Healthcare of Atlanta, and Texas Children’s Hospital, as described previously.10, 11 Data collection was performed by individual centers under the direction of site principal investigators using common data collections tools and definitions. Data were collected, cleaned, and analyzed at one center (Children’s Healthcare of Atlanta), which serves as the data coordinating center (DCC) for the CCRC. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The Pediatric Health Information Systems (PHIS) database is an administrative database that contains data from inpatient, emergency department, ambulatory surgery, and observation encounters from 47 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children’s Hospital Association (Overland Park, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. The data warehouse function for the PHIS database is managed by Truven Health Analytics (Ann Arbor, MI). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. These data include department-specific charges that are adjusted using local wage-price indices. Details about the PHIS database have been reported previously.12
Study Population
A retrospective multicenter cohort study was performed using the population from a previous study that evaluated differences in clinical outcomes between the two palliative strategies.9 The study population included all infants with ductal-dependent PBF and confluent pulmonary arteries palliated at <1 year of age with either a BT shunt (from 1/1/12) or PDA stent (from 1/1/08) through 11/1/15 at the 4 member hospitals of the CCRC.9 PDA stenting was adopted at each of the centers in the CCRC over different dates. The different ranges of study period for the two strategies were chosen to 1) capture all PDA stent subjects at all four centers and 2) generate a contemporaneous cohort of BT shunt subjects with a goal ratio of approximately 2 BT shunt cases for each PDA stent case. All subjects included in the prior analysis were eligible for inclusion in this study. This study was approved by the Institutional Review Board at each participating center with a waiver of the need for informed consent.
Data Acquisition
Subject eligibility was established by direct review of patient charts. In eligible subjects, demographic, clinical, and procedural characteristics were extracted by further chart review as previously described.9 The wage and price index (published annually in the Federal Register) adjusted charges for each unit of service, and annual hospital- and department-specific ratio of cost to charge were obtained from PHIS. Adjusted inpatient and observation treatment costs were calculated by multiplying the adjusted charge by the relevant ratio of cost to charge13–15, which was then further inflated to 2016 United States dollars (US $) using the medical care services component of the Consumer Price Index.16 Costs for each day of hospitalization were summarized into the following categories: clinical, imaging, laboratory, pharmacy, supply and room. Adjusted overall cost and total costs for each category were calculated for each patient as the sum of the daily costs during the entire hospitalization. Analyses include direct medical costs accrued during inpatient and observation admissions. Cost data were extracted and cleaned by the Healthcare Analytics Unit at the Children’s Hospital of Philadelphia, prior to analysis at the DCC for the CCRC.
Statistical Analysis
Descriptive statistics were calculated for all variables of interest and include means and standard deviations, medians and ranges or counts and percentages, as appropriate. The primary exposure in the study was choice of palliative strategy: either BT shunt or PDA stent. The primary outcomes were the total inpatient (including observation) costs during 1) index hospitalization (the entire hospitalization during which the palliation was performed) and 2) over the first year of life (extending from the date of birth through day of life 365). Secondary outcomes were the department-specific costs over these same time periods. Given these definitions, the duration of the index hospitalization could possibly exceed that of the first year of life, in the case of a patient who underwent initial palliation and remained admitted to the hospital beyond day of life 365.
As detailed previously, there were significant differences in the clinical characteristics of the PDA stent and BT shunt groups that might affect both assignment to palliation strategy and cost of care.9 As a result, inverse probability of treatment weighting (IPTW) was used to address this potential source of confounding by indication, as described previously.9 IPTW was used to calculate the adjusted cost, which is presented in US dollars as well as a cost ratio (CR), which is reported as PDA stent relative to BT shunt, which served as the reference group. Variables used in the propensity score included center, expected ultimate physiology (one- vs. two-ventricle), presence of antegrade pulmonary blood flow, underlying anatomic diagnosis, pre-intervention inotrope use, and pre-intervention mechanical ventilation.
As expected, cost data followed a skewed right distribution. As a result, prior to statistical modeling, data were log-transformed and analysis was conducted on the transformed data. Model-based least square mean estimates of log-cost with associated 95% confidence intervals were estimated for each treatment group. Estimates were back transformed, via exponentiation, to obtain estimates in the original units (2016 US $). In addition, estimated differences in cost, on the log scale, were exponentiated resulting in a ratio of estimated cost between the two treatment groups. Since BT shunt was the reference group, a CR < 1 implies that the average cost in the PDA stent group was lower than the BT shunt group. Conversely, a CR > 1 implies that the average cost in PDA stent group was higher than the BT shunt group. Because there is no “gold-standard” frequency distribution for modeling cost data, each strategy ameliorates skew to varying degrees. As a sensitivity analysis, untransformed costs were also analyzed using a gamma distribution, which in other settings has been used to analyze cost data.13, 17, 18 The gamma distribution was heavily influenced by outliers and failed to provide a better fit to the data when compared to the log-transformation. However, resulting estimates of ratios of costs did not differ when compared to the log-transformed results (data not shown). Similar analyses were used to compare department-specific costs.
Imputed Costs
Due to concern that some catheterization procedures were performed as “outpatient” encounters and would not be included in PHIS, additional analyses were performed to avoid bias. The counts of catheterization procedures in PHIS were compared with catheterization procedures in the medical record. Catheterization procedures were identified in PHIS using International Classification of Disease version 9 (ICD-9) codes (372.1–372.3, 372.5–372.7) or ICD-10 (4A0.23N6, 4A0.23N6, 4A0.23N7, 4A0.23N8, 02B.K3ZX, 02B.L3ZX, 02B.M3ZX) codes. All encounter-related charges were captured and converted to costs, as described above. To minimize bias due to missing procedures, the costs for each procedure identified in the medical record and missing in PHIS were estimated and imputed. Because only “outpatient” catheterization procedures were expected to be missing from PHIS, the costs of procedures were estimated by averaging, on a site-specific basis, the costs related to catheterization procedures with LOS <2 days that were represented in PHIS. These site-specific averaged estimated costs were then used as the imputed values for all procedures at each site that were found to be unrepresented in PHIS. A sensitivity analysis was then performed, using the same technique as the primary analyses, comparing total adjusted FYOL costs between treatment groups.
Cost Drivers
To evaluate for factors associated with adjusted cost for the 1) index hospitalization and 2) first year of life, the population was analyzed across the entire cohort. Data were analyzed in a similar manner to what is described above with analyses performed on the log-scale and resulting estimates back-transformed via exponentiation. Factors included patient-level factors and procedural outcomes. Associations are presented as a CR with associated 95% confidence intervals. For categorical factors, ratios are presented relative to a reference group (e.g. diagnosis category), whereas continuous variables are presented as an increase (or decrease) associated with a 1 unit change (e.g. 1 day increase in duration of mechanical ventilation) in the variable of interest. For example, a ratio of 1.01 would be interpreted as a 1% increase in cost associated with a 1 unit increase in the predictor.
Statistical analyses were performed by using SAS v9.4 (SAS Institute, Inc, Cary, NC) and statistical significance was assessed at the 0.05 level, unless otherwise noted. P-values were not adjusted for multiple comparisons.
Results
Patients and Outcomes
A total of 355 infants with cyanotic CHD and ductal-dependent PBF underwent initial palliation with BT shunt (n=251) or PDA stent (n=104) and had matched PHIS data available for analysis. Two patients (0.5%) from the original study cohort were not able to be matched within the PHIS database. Baseline characteristics of the cohorts, and related clinical outcomes, were published previously with key elements displayed in Table 1.9 There were a number of important differences in baseline characteristics and procedural outcomes present between groups.
Table 1:
Baseline Demographics and Observed Clinical Outcomes
| Covariate or Outcome | PDA Stent n=104 | BT Shunt n=251 | p Value |
|---|---|---|---|
| Anatomic diagnosis, n (%) Isolated PS PA/IVS TA with PA or PS VSD/PA VSD/PS |
10 (9.6) 46 (44.2) 5 (4.8) 17 (16.3) 26 (25.0) |
1 (0.4) 50 (20) 39 (15.5) 99 (39.4) 62 (24.7) |
<0.001 |
| Expected two ventricle ultimate physiology, n (%) | 64 (61.5) | 112 (44.6) | 0.004 |
| Gestational age, weeks | 38.0 (36.4–39.0) | 38.0 (37.0–39.0) | 0.450 |
| Genetic syndrome, n (%) | 13 (12.5) | 38 (15.3) | 0.501 |
| Other co-morbid medical conditions, n (%) | 12 (11.7) | 49 (19.6) | 0.073 |
| Invasive ventilation pre-intervention, n (%) | 29 (28.8) | 78 (31.3) | 0.551 |
| Procedural complications, n (%) | 13 (12.5) | 54 (21.5) | 0.048 |
| Need for ECMO during recovery, n (%) | 2 (1.9%) | 14 (5.6) | 0.166 |
| Total duration of ventilation, days | 1 (0–2) | 3 (1–5) | <0.001 |
| Total ICU LOS, days | 3 (2–10) | 7 (4–16) | <0.001 |
| Total hospital LOS, days | 10 (6–20) | 13 (9–27) | <0.001 |
| Unplanned reintervention to treat cyanosis, n (%) | 12 (11.5) | 52 (20.7) | 0.041 |
| Planned/other reintervention, n (%) | 37 (35.6) | 4 (1.6) | <0.001 |
| Death, n (%) | 7 (6.7) | 26 (10.4) | 0.284 |
| Time to definitive surgical repair, days | 184 (122–310) | 150 (118–205) | <0.001 |
| Presence of any definitive surgical repair (Stage II palliation or definitive repair), n (%) | 68 (66.7) | 207 (82.5) | <0.001 |
| Presence of Stage II palliation, n (%) | 34 (33.3) | 130 (52.8) | <0.001 |
| Presence of definitive repair, n (%) | 34 (33.3) | 77 (31.3) | 0.694 |
| Pulmonary arterioplasty performed at definitive repair, n (%) | 33 (37.5) | 89 (42.2) | 0.511 |
Data are reported as median (25th-75th percentile) or n (%). ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; IVS = intact ventricular septum; LOS = length of stay; PA = pulmonary atresia; PS = pulmonary stenosis; TA = tricuspid atresia; VSD = ventricular septal defect.
Index Hospitalization
Costs incurred during the index hospitalization are presented in Table 2. The median total cost for index hospitalization in the PDA stent group was US $106,152 [$89,576 – $125,796] whereas in the BT shunt group it was US $128,822 [$115,485 – $143,700] (p=0.06). Following adjustment for baseline factors, the point-estimate for the difference in cost between PDA stent and BT shunt groups again suggested cost savings with PDA stent (CR 0.82) but the difference was not statistically significant (95% CI: 0.67 – 1.01, p=0.06).
Table 2:
Summary of Costs for the Index Hospitalization
| Cost Metric |
Group | Unadjusted Cost, Mean (95% CI) |
Cost Ratio (95% CI) |
p value | Propensity Score Adjusted Cost, Mean (95% CI) |
Cost Ratio (95% CI) |
p value |
|---|---|---|---|---|---|---|---|
| Total Cost | PDA Stent N=104 |
$106,152 ($89,576 – $125,796) |
0.82 (0.67 – 1.01) |
0.06 | $106,976 ($90,126 – $126,975) |
0.82 (0.67 – 1.01) |
0.06 |
| BT Shunt (ref) N=251 |
$128,822 ($115,485 – $143,700) |
$129,898 ($116,339 – $145,036) |
|||||
| Clinical | PDA Stent N=104 |
$13,721 ($10,792 – $17,444) |
0.99 (0.74 – 1.31) |
0.93 | $12,829 ($10,063 – $16,356) |
0.91 (0.68 – 1.21) |
0.51 |
| BT Shunt (ref) N=251 |
$13,893 ($11,904 – $16,215) |
$14,142 ($12,097 – $16,533) |
|||||
| Imaging | PDA Stent N=104 |
$13,855 ($12,105 – $15,858) |
1.56 (1.33 – 1.83) |
<0.001 | $13,648 ($11,861 – $15,705) |
1.47 (1.25 – 1.74) |
<0.001 |
| BT Shunt (ref) N=251 |
$8,887 ($8,147 – $9,694) |
$9,259 ($8,460 – $10,133) |
|||||
| Lab | PDA Stent N=104 |
$13,867 ($11,493 – $16,732) |
0.68 (0.54 – 0.85) |
<0.001 | $13,777 ($11,366 – $16,699) |
0.66 (0.53 – 0.83) |
<0.001 |
| BT Shunt (ref) N=251 |
$20,488 ($18,155 – $23,120) |
$20,841 ($18,415 – $23,586) |
|||||
| Pharmacy | PDA Stent N=104 |
$8,727 ($6,876 – $11,077) |
0.88 (0.66 – 1.17) |
0.38 | $8,296 ($6,501 – $10,587) |
0.81 (0.61 – 1.09) |
0.16 |
| BT Shunt (ref) N=251 |
$9,911 ($8,501 – $11,555) |
$10,205 ($8,724 – $11,938) |
|||||
| Supply | PDA Stent N=104 |
$6,942 ($4,791 – $10,058) |
1.25 (0.81 – 1.94) |
0.32 | $6,885 ($4,748 – $9,984) |
1.37 (0.88 – 2.14) |
0.16 |
| BT Shunt (ref) N=251 |
$5,548 ($4,370 – $7,044) |
$5,015 ($3,949 – $6,369) |
|||||
| Room | PDA Stent N=104 |
$38,207 ($32,064 – $45,527) |
0.66 (0.54 – 0.82) |
<0.001 | $40,068 ($33,569 – $47,825) |
0.69 (0.56 – 0.85) |
<0.001 |
| BT Shunt (ref) N=251 |
$57,614 ($51,467 – $64,496) |
$57,974 ($51,737 – $64,963) |
Cost is reported in 2016 US $. BT = Blalock-Taussig. PDA = patent ductus arteriosus. BT Shunt was the reference group for Cost Ratio.
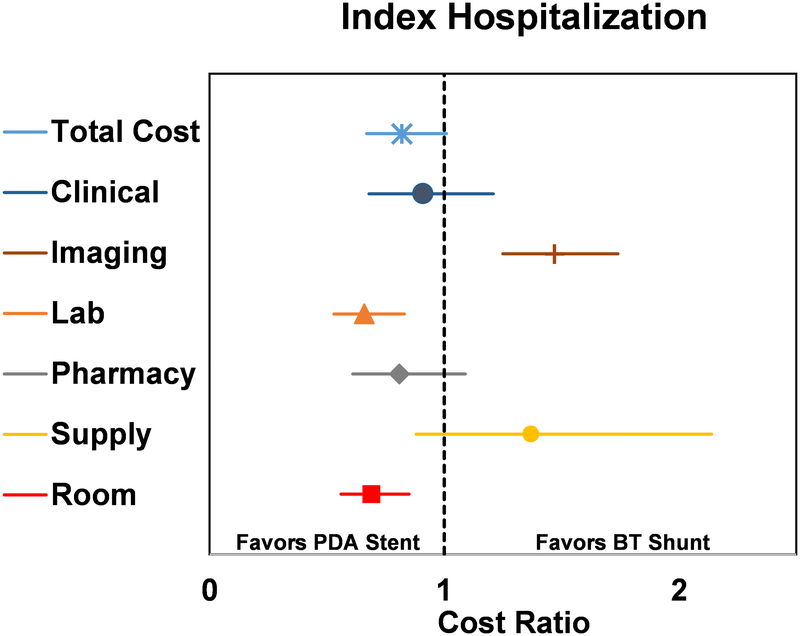
Adjusted departmental-level costs were studied to evaluate if the drivers of cost differed between groups (Figure 1). In the PDA stent group, laboratory (CR 0.66 [0.53 – 0.83], p<0.001) and room charges (CR 0.69 [0.56 – 0.85], p<0.001) were lower, while imaging costs were higher (CR 1.47 [1.25 – 1.74], p<0.001), when compared to the BT shunt group.
Figure 1.
Forest plot of propensity score adjusted total and departmental costs accrued during the index hospitalization, displayed as cost ratio. Cost ratio used BT shunt as the reference group. The vertical dashed line represents a cost ratio of 1, which is associated with equal costs for both groups. For each cost ratio displayed, the symbol depicts the mean and the line represents the 95% CI. Lines which do not cross the dashed vertical line are statistically significant and favor either PDA stent (to the left of the dashed line) or BT shunt (to the right of the dashed line).
First Year of Life
Costs incurred over the entirety of the first year of life are presented in Table 3. The observed median cost over this period for the PDA stent group was US $187,534 [$164,896 – $213,280], while in the BT shunt group it was US $254,469 [$234,246 – $276,439] (p<0.001). After adjustment, there continued to be a significant cost savings associated with PDA stent therapy (CR 0.86 [0.75 – 1.00], p=0.05). Importantly, a comparison of FYOL cardiac catheterization “counts” identified in the study database and in the PHIS dataset revealed discrepant values, with a total of 122 catheterization encounters unrepresented in PHIS, including 73 in the PDA stent group and 49 in the BT shunt group. Following imputation of estimated costs associated with these unrepresented catheterization encounters, the observed median cost over the FYOL for the PDA stent group increased, but remained significantly less than in the BT shunt group (US $199,955 vs. $257,022, p=0.001). After adjustment, the point estimate still favored the PDA stent group, but the difference was no longer significant (CR 0.90 [0.78 – 1.04], p=0.149).
Table 3:
Summary of Costs for the First Year of Life
| Cost Metric |
Group | Unadjusted Cost, Mean (95% CI) |
Cost Ratio (95% CI) |
p value | Propensity Score Adjusted Cost, Mean (95% CI) |
Cost Ratio (95% CI) |
p value |
|---|---|---|---|---|---|---|---|
| Total Cost | PDA Stent N=104 |
$187,534 ($164,896 – $213,280) |
0.74 (0.63 – 0.86) |
<0.001 | $215,825 ($190,644 – $244,333) |
0.86 (0.75 – 1.00) |
0.05 |
| BT Shunt (ref) N=251 |
$254,469 ($234,246 – $276,439) |
$249,855 ($230,693 – $270,609) |
|||||
| Total Cost with Missing Cath Cost Imputed | PDA Stent N=104 |
$199,955 ($176,130 – $227,002) |
0.78 (0.67 – 0.90) |
0.001 | $226,403 ($200,274 – $255,941) |
0.90 (0.78 – 1.04) |
0.149 |
| BT Shunt (ref) N=251 |
$257,022 ($236,867 – $278,892) |
$252,072 ($232,955 – $272,759) |
|||||
| Clinical | PDA Stent N=104 |
$25,712 ($21,483 – $30,772) |
0.84 (0.68 – 1.04) |
0.11 | $27,855 ($23,305 – $33,293) |
0.94 (0.76 – 1.16) |
0.57 |
| BT Shunt (ref) N=251 |
$30,652 ($27,304 – $34,410) |
$29,635 ($26,424 – $33,237) |
|||||
| Imaging | PDA Stent N=104 |
$21,380 ($19,072 – $23,968) |
1.22 (1.06 – 1.39) |
0.005 | $23,014 ($20,529 – $25,798) |
1.27 (1.11 – 1.46) |
<0.001 |
| BT Shunt (ref) N=251 |
$17,590 ($16,343 – $18,932) |
$18,109 ($16,827 – $19,490) |
|||||
| Lab | PDA Stent N=104 |
$24,520 ($21,345 – $28,168) |
0.66 (0.56 – 0.78) |
<0.001 | $28,752 ($25,140 – $32,882) |
0.78 (0.67 – 0.92) |
0.002 |
| BT Shunt (ref) N=251 |
$37,102 ($33,934 – $40,567) |
$36,854 ($33,805 – $40,177) |
|||||
| Pharmacy | PDA Stent N=104 |
$15,180 ($12,902 – $17,861) |
0.74 (0.61 – 0.89) |
0.002 | $17,561 ($14,995 – $20,566) |
0.85 (0.71 – 1.03) |
0.09 |
| BT Shunt (ref) N=251 |
$20,585 ($18,539 – $22,856) |
$20,642 ($18,648 – $22,849) |
|||||
| Supply | PDA Stent N=104 |
$15,795 ($13,112 – $19,027) |
1.0 (0.80 – 1.25) |
1.0 | $18,132 ($15,076 – $21,807) |
1.25 (1.01 – 1.56) |
0.04 |
| BT Shunt (ref) N=251 |
$15,789 ($14,006 – $17,798) |
$14,469 ($12,849 – $16,293) |
|||||
| Room | PDA Stent N=104 |
$72,370 ($63,157 – $82,927) |
0.63 (0.53 – 0.74) |
<0.001 | $86,810 ($76,239 – $98,848) |
0.77 (0.66 – 0.90) |
<0.001 |
| BT Shunt (ref) N=251 |
$115,207 ($105,539 – $125,760) |
$112,989 ($103,935 – $122,831) |
Cost is reported in 2016 US $. BT = Blalock-Taussig. PDA = patent ductus arteriosus. Cost Ratio is presented with BT shunt as the reference group.
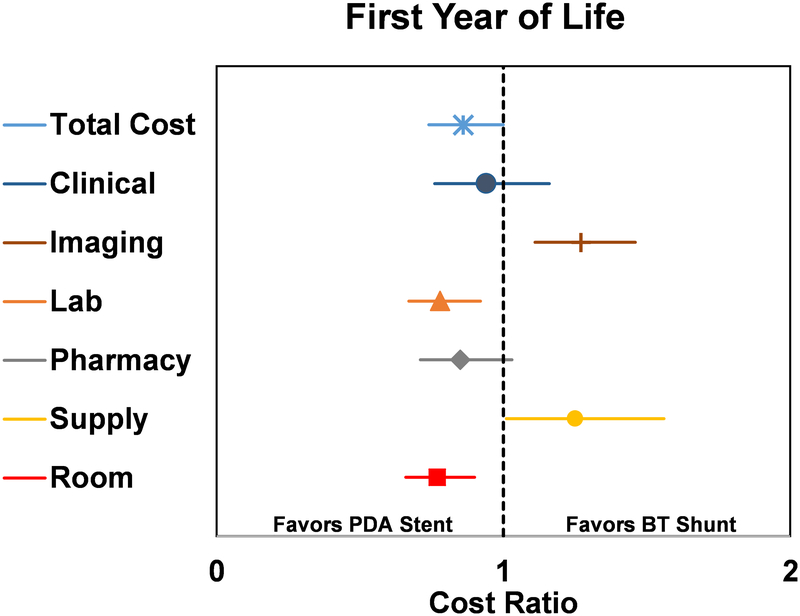
In the analysis of adjusted departmental-level costs (Figure 2), laboratory (CR 0.78 [0.67 – 0.92], p=0.002) and room charges (CR 0.77 [0.66 – 0.90], p<0.001) were lower in the PDA stent group, while imaging (CR 1.27 [1.11 – 1.46], p<0.001) and supply (CR 1.25 [1.01 – 1.56], p=0.044) costs were higher.
Figure 2.
Forest plot of propensity score adjusted total and departmental costs accrued during the first year of life, displayed as cost ratio. Cost ratio used BT shunt as the reference group. The vertical dashed line represents a cost ratio of 1, which is associated with equal costs for both groups. For each cost ratio displayed, the symbol depicts the mean and the line represents the 95% CI. Lines which do not cross the dashed vertical line are statistically significant and favor either PDA stent (to the left of the dashed line) or BT shunt (to the right of the dashed line).
Factors Associated with Increased Cost
A number of patient-level factors were associated with increased cost during the index hospitalization (Table 4), including anatomic diagnosis, genetic syndrome, prematurity, non-cardiac co-morbid conditions, invasive ventilation prior to initial palliation, procedural complications and need for ECMO post-palliation. The use of ECMO was associated with a 159% increase in cost. Total hospital and ICU LOS and total duration of mechanical ventilation were also significantly associated with cost, each representing a 1% increase in cost for every 1 day increase in the covariate.
Table 4:
Patient Factors Associated with Index Hospitalization Costs
| Risk Factor | Cost Ratio | 95% CI | p value |
|---|---|---|---|
| Anatomic diagnosis Isolated PS PA/IVS TA with PA or PS VSD/PA VSD/PS |
1.96 1.76 1.16 1.67 Ref. |
1.17 – 3.27 1.39 – 2.24 0.84 – 1.6 1.32 – 2.1 |
0.01 <0.001 0.36 <0.001 Ref. |
| Expected two ventricle ultimate physiology | 0.89 | 0.74 – 1.08 | 0.24 |
| Gestational age, weeks | 0.93 | 0.90 – 0.97 | <0.001 |
| Genetic syndrome | 1.56 | 1.20 – 2.04 | 0.001 |
| Other co-morbid medical conditions | 2.04 | 1.60 – 2.61 | <0.001 |
| Invasive ventilation pre-intervention | 2.04 | 1.69 – 2.46 | <0.001 |
| Procedural complications | 1.47 | 1.17 – 1.84 | 0.001 |
| Need for ECMO | 2.59 | 1.71 – 3.9 | <0.001 |
| Total duration of ventilation, days | 1.01 | 1.01 – 1.02 | <0.001 |
| Total ICU LOS, days | 1.01 | 1.01 – 1.02 | <0.001 |
| Total hospital LOS, days | 1.01 | 1.01 – 1.01 | <0.001 |
CI = confidence interval. ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; IVS = intact ventricular septum; LOS = length of stay; PA = pulmonary atresia; PS = pulmonary stenosis; TA = tricuspid atresia; VSD = ventricular septal defect.
Similarly, a number of patient characteristics were associated with increased cost when analyzed over the first year of life (Table 5). Genetic syndrome, prematurity, non-cardiac co-morbid conditions, invasive ventilation prior to initial palliation, procedural complications and need for ECMO post-palliation all remained significant drivers of cost in this analysis. Likewise, total hospital and ICU LOS and total duration of mechanical ventilation remained significantly associated with increased cost. Unplanned reintervention was found to be associated with increased cost. Importantly, death, interstage duration, presence of stage II palliation, presence of definitive cardiac repair and surgical pulmonary artery arterioplasty at definitive repair or palliation were not found to be factors significantly associated with cost difference over the first year of life.
Table 5:
Patient Factors Associated with First Year of Life Costs
| Risk Factor | Cost Ratio | 95% CI | p value |
|---|---|---|---|
| Anatomic diagnosis Isolated PS PA/IVS TA with PA or PS VSD/PA VSD/PS |
0.93 1.10 1.06 1.23 Ref. |
0.63 – 1.36 0.92 – 1.32 0.83 – 1.35 1.03 – 1.46 |
0.69 0.29 0.65 0.02 Ref. |
| Expected two ventricle ultimate physiology | 0.95 | 0.83 – 1.08 | 0.44 |
| Gestational age, weeks | 0.94 | 0.92 – 0.97 | <0.001 |
| Genetic syndrome | 1.45 | 1.20 – 1.75 | <0.001 |
| Other co-morbid medical conditions | 1.59 | 1.33 – 1.91 | <0.001 |
| Invasive ventilation pre-intervention | 1.69 | 1.48 – 1.93 | <0.001 |
| Procedural complications | 1.18 | 1.00 – 1.39 | 0.05 |
| Need for ECMO | 1.98 | 1.47 – 2.66 | <0.001 |
| Total duration of ventilation, days | 1.01 | 1.01 – 1.01 | <0.001 |
| Total ICU LOS, days | 1.01 | 1.01 – 1.01 | <0.001 |
| Total hospital LOS, days | 1.01 | 1.01 – 1.01 | <0.001 |
| Unplanned reintervention to treat cyanosis (categorical) | 1.44 | 1.22 – 1.69 | <0.001 |
| Unplanned reintervention to treat cyanosis (continuous) | 1.4 | 1.23 – 1.6 | <0.001 |
| Death | 1.11 | 0.88 – 1.39 | 0.39 |
| Time to definitive surgical repair | 1.0 | 0.99 – 1.01 | 0.94 |
| Presence of any definitive surgical repair (Stage II palliation or definitive repair) | 0.98 | 0.81 – 1.2 | 0.86 |
| Presence of Stage II palliation | 0.96 | 0.84 – 1.11 | 0.6 |
| Presence of definitive repair | 1.0 | 0.86 – 1.16 | 0.95 |
| Pulmonary arterioplasty performed at definitive repair | 1.07 | 0.92 – 1.26 | 0.37 |
CI = confidence interval. ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; IVS = intact ventricular septum; LOS = length of stay; PA = pulmonary atresia; PS = pulmonary stenosis; TA = tricuspid atresia; VSD = ventricular septal defect.
Discussion
In this retrospective multicenter cohort study, PDA stent palliation was associated with lower to equivalent costs incurred over the index hospitalization and first year of life. Differences in costs resulted from greater LOS (room charges) and laboratory testing in the BT shunt group, which offset higher imaging and supply costs in the PDA stent group, when evaluated across the first year of life. Major and minor factors associated with excess costs were identified and included non-modifiable patient characteristics, potentially modifiable characteristics and modifiable factors surrounding initial palliation (including post-operative ECMO and procedural complications).
A number of cost comparison studies have been performed within congenital heart disease, several specifically evaluating for differences between interventional and surgical approaches to a specific procedure (e.g. atrial septal defect closure or pulmonary valve replacement), where clinical outcomes are reasonably similar.13, 15, 17, 19, 20 No prior cost comparison studies have been performed in a population of infants with cyanotic congenital heart disease and ductal-dependent pulmonary blood flow. This first investigation into the cost of PDA stent vs. BT shunt therapy offers an opportunity to assess the overall value of care delivery for this population, now that the clinical outcomes of these therapies have been largely defined.8, 9 To the extent that PDA stent and BT shunt therapies have differing hospital LOS, complication rates and reintervention burdens, rigorous investigation of the relative economic impact of these strategies is necessary, as the findings may not be as predictable as with prior surgical and interventional cost comparisons.13, 17, 19, 20 In so doing, adjusted cost of care may act as a single and continuous overall barometer of the impact of potentially disparate and competing clinical outcomes. This is not to suggest that cost, or even healthcare value, ought to be the sole driver of clinical decision making but, rather, that adjusted cost can serve as a unique and unifying output measure taking into account multiple heterogenous clinical outcomes.
Importantly, this study is unique in its use of a high-fidelity direct linkage (99.5% matching) between a highly granular clinical dataset sourced directly from the medical record and audited cost data obtained from PHIS, which provides for a rigorous comparison of costs with adjustment for clinical differences between the groups. This adjustment for confounding by indication relied upon access to rich clinical details which would not be available in the absence of such a strong linkage. Further, the availability of detailed clinical data provided the opportunity to identify, and then correct for, unrepresented catheterization procedures in the PHIS dataset utilizing a novel technique for imputation of estimated costs. Development of this hybrid approach to a multi-center cost study utilizing both a PHIS linkage and the addition of site-specific encounter data serves to augment the validity of this cost analysis. It should be noted that derivation of the estimated average site-specific “outpatient” catheterization cost relied upon “inpatient” catheterization encounters (LOS <2 days) captured in PHIS, which would tend to overestimate the true cost of an “outpatient” catheterization, which is almost by its definition an uncomplicated procedure. As such, this additional analysis likely represents an overestimate of these costs.
Regardless of therapeutic strategy employed, the economic cost burden of initial palliation and associated care is high, averaging nearly US $107,000 for PDA stent and $130,000 for BT shunt patients. Including the entirety of the first year of life, these numbers rise substantially, averaging over US $226,000 for PDA stent and $252,000 for BT shunt palliation, reflective of a 10% cost savings in the PDA stent group. Not surprisingly, some factors associated with increased cost relate to innate patient characteristics and are not readily modifiable, including genetic syndrome, non-cardiac co-morbid conditions and cardiac anatomic diagnosis. However, there are also both potentially modifiable patient factors (such as gestational age, pre-operative ventilatory status) and procedural outcomes (use of ECMO, complications, ICU and hospital LOS, unplanned reinterventions) that have substantial impact on cost. Most of these modifiable outcomes favored the PDA stent group and were thus largely responsible for the cost-advantage favoring the PDA stent group. Therefore, the same group of adverse patient outcomes which tends to favor PDA stent palliation on clinical grounds also does so on a strictly financial basis. A culture of quality improvement that targets reductions in adverse events, hospital LOS and the incidence of unplanned reintervention would be expected to improve patient outcomes and eliminate excess cost burden, regardless of palliation strategy selected.21, 22 The high cost of care over the first year of life in both groups should provide ample opportunity for cost reduction in the treatment of this population.
Departmental costs differed by initial palliation strategy and may provide insight into opportunities for reduction of modifiable costs. The PDA stent group incurred excess imaging costs during the index hospitalization and excess imaging and supply costs during the first year of life. Differences in imaging costs probably reflect the cardiac catheterization (angiography) for the PDA stent implant procedure itself, but also include subsequent catheterization procedures associated with planned reintervention, which was more common in the PDA stent cohort (35.8 vs. 1.6% in the BT shunt group).9 It is also possible that PDA stent candidates underwent more advanced imaging (e.g. CT angiography) in an effort to better evaluate the ductal, aortic and pulmonary arterial anatomy, prior to PDA stent palliation. Excess supply costs in the PDA stent group, incurred over the first year of life, probably reflect the expense of catheterization equipment (catheters, angioplasty balloons and stents) used during PDA stent reintervention procedures. It is noteworthy to mention that costs associated with innovative technologies and procedures tend to be at their peak during the period of implementation and initial uptake into clinical practice. Early in the clinical experience, intense post-procedural monitoring is justified in the absence of existing outcome data, whereas this surveillance tends to decrease over time in the face of increasing provider familiarity and comfort. As such, we are likely to be capturing the cost of PDA stent patients at their highest during the current era; it is reasonable to anticipate that this cost will fall significantly in coming years.
Laboratory costs were greater in the BT shunt group and reflect excess cost burden accumulated during the index hospitalization (the difference in mean adjusted laboratory costs was similar between index hospitalization and first year of life [US $7,064 vs $8,102]). Although multifactorial in nature, this excess laboratory cost probably reflects a greater burden of procedural complications, greater duration of mechanical ventilation and inotrope use, and longer hospital and ICU LOS in the BT shunt cohort. Room costs also favored the PDA stent group, reflecting the longer hospital and ICU LOS in the BT shunt group. Moreover, despite a greater burden of post-palliation reinterventions in the PDA stent group, the difference in adjusted mean room costs between groups increased following initial hospital discharge to favor the PDA stent group to a greater degree at the end of the first year of life (the difference in mean adjusted rooms costs for the 1) index hospitalization: $17,906 and 2) first year: $26,179). This finding may possibly reflect a greater burden of interstage readmissions in the BT shunt group (e.g. for failure to thrive or feeding intolerance), as opposed to catheter-based reinterventions performed on an outpatient or observation basis in the PDA stent group, which would serve to further accentuate the total hospital LOS disparity between groups. Data related to non-reintervention hospital readmissions are not yet available for this cohort but are the subject of intended future study.
There are several limitations to the current study. The study is retrospective, and, despite our best efforts, there may be unmeasured or residual confounding. Also, despite matching patients to PHIS data at the center level, 2/357 patients were not successfully matched, and thus could not be included in the study. When compared with existing literature linking across multiple databases, however, we believe that this match rate (99.5%) far exceeds the anticipated norm and we do not imagine that the inability to include the costs for these two patients would significantly alter our conclusions.23–25 There are limitations to our measurement of cost. As mentioned previously, outpatient costs were not measured. Some reintervention catheterization procedures may be coded as an outpatient encounter and thus would not be captured in the PHIS dataset. However, we attempted to account for this underrepresentation with the inclusion of imputed site-specific estimated costs associated with catheterization procedures identified in the study database but not found in the PHIS dataset. Additionally, if inpatient costs were incurred at non-study center hospitals, they would not be counted. Further, this study was performed on an as treated basis. As such, a patient that underwent catheterization for PDA stent placement but ultimately did not receive a stent and instead underwent BT shunt placement, was counted as a BT shunt patient. This patient would be expected to drive up the cost of the BT shunt group. A prospective approach to this study could utilize an intent to treat basis which would mitigate this concern. Patient selection, practice, and resource utilization between hospitals also could differ but these differences would be expected to be a source of statistical noise and the durability of the observed findings reaffirms their validity. We were unable to control for inter- and intra-center practice variation that may affect cost outcomes. Lastly, although this is the largest cohort reported to date, what appear to be substantial point estimates for several cost ratios nevertheless had confidence intervals that either just approached or crossed 1, indicating that our statistical power remains somewhat limited. With an even larger sample, our power to make firmer statistical conclusions would likely improve.
In summary, in this first multicenter cost comparison of PDA stent or BT shunt as initial palliation for cyanotic congenital heart disease and ductal-dependent pulmonary blood flow, adjusted for differences in baseline factors, we found that PDA stent palliation was associated with lower to equivalent costs over the first year of life. Combined with previous evidence suggesting clinical non-inferiority, these findings suggest that PDA stent provides competitive health care value and further support the use of PDA stenting, as a reasonable alternative to BT shunt placement, in select cyanotic infants with ductal dependent pulmonary blood flow.
Central Figure.
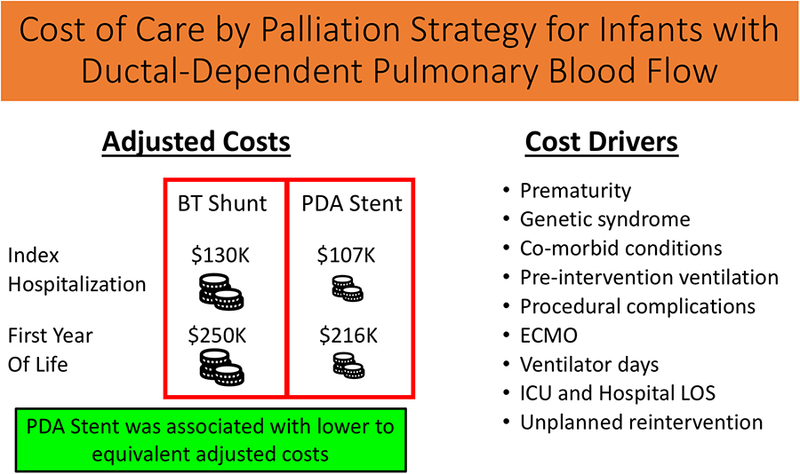
This central illustration depicts the main findings of this study evaluating the cost of care by palliation strategy, for infants with ductal-dependent pulmonary blood flow. Adjusted costs are displayed in the left hand pane, in separate rows for index hospitalization and first year of life. In each case, adjusted costs are equivalent to greater in the BT Shunt group. Factors found to be significant drivers of cost accrued over the first year of life are displayed in the right hand pane. Our overall study conclusion is displayed in the green box, at the bottom of the figure.
What is Known
Blalock-Taussig (BT) shunt placement and patent ductus arteriosus (PDA) stent implantation are alternative strategies for initial palliation of infants with cyanotic congenital heart disease and ductal-dependent pulmonary blood flow. Two large multicenter studies, adjusted to account for baseline differences, have recently demonstrated equivalent to superior mortality and reduced morbidity following PDA stent compared with BT shunt, although the economic impact of each therapy is unknown.
What the Study Adds
In this first multicenter cost comparison of PDA stent or BT shunt, adjusted for differences in baseline factors, PDA stent palliation was found to be associated with lower to equivalent costs over the first year of life. Combined with previous evidence suggesting clinical non-inferiority, these findings suggest that PDA stent provides competitive health care value and further support the use of PDA stenting, as a reasonable alternative to BT shunt placement, in select cyanotic infants with ductal-dependent pulmonary blood flow.
Sources of Funding
Financial support for this research was derived, in part, from the Kennedy Hammill Pediatric Cardiac Research Fund, the Liam Sexton Foundation and A Heart Like Ava.
Footnotes
Disclosures The authors have no relevant disclosures to report.
References
- 1.Blalock A, Taussig HB. Landmark article May 19, 1945: The surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. JAMA. 1984;251:2123–38. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor MJ, Ravishankar C, Ballweg JA, Gillespie MJ, Gaynor JW, Tabbutt S, Dominguez TE. Early systemic-to-pulmonary artery shunt intervention in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2011;142:106–12. [DOI] [PubMed] [Google Scholar]
- 3.Petrucci O, O’Brien SM, Jacobs ML, Jacobs JP, Manning PB, Eghtesady P. Risk factors for mortality and morbidity after the neonatal Blalock-Taussig shunt procedure. Ann Thorac Surg. 2011;92:642–51; discussion 651–2. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs JL, Rothman MT, Rees MR, Parsons JM, Blackburn ME, Ruiz CE. Stenting of the arterial duct: a new approach to palliation for pulmonary atresia. Br Heart J. 1992;67:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alwi M, Choo KK, Latiff HA, Kandavello G, Samion H, Mulyadi MD. Initial results and medium-term follow-up of stent implantation of patent ductus arteriosus in duct-dependent pulmonary circulation. J Am Coll Cardiol. 2004;44:438–45. [DOI] [PubMed] [Google Scholar]
- 6.Santoro G, Gaio G, Giugno L, Capogrosso C, Palladino MT, Iacono C, Caianiello G, Russo MG. Ten-years, single-center experience with arterial duct stenting in duct-dependent pulmonary circulation: early results, learning-curve changes, and mid-term outcome. Catheter Cardiovasc Interv. 2015;86:249–57. [DOI] [PubMed] [Google Scholar]
- 7.Santoro G, Gaio G, Palladino MT, Iacono C, Carrozza M, Esposito R, Russo MG, Caianiello G, Calabro R. Stenting of the arterial duct in newborns with duct-dependent pulmonary circulation. Heart. 2008;94:925–9. [DOI] [PubMed] [Google Scholar]
- 8.Bentham JR, Zava NK, Harrison WJ, Shauq A, Kalantre A, Derrick G, Chen RH, Dhillon R, Taliotis D, Kang SL, Crossland D, Adesokan A, Hermuzi A, Kudumula V, Yong S, Noonan P, Hayes N, Stumper O, Thomson JDR. Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates With Duct-Dependent Pulmonary Blood Flow: Associations With Clinical Outcomes in a Multicenter National Study. Circulation. 2018;137:581–588. [DOI] [PubMed] [Google Scholar]
- 9.Glatz AC, Petit CJ, Goldstein BH, Kelleman MS, McCracken CE, McDonnell A, Buckey T, Mascio CE, Shashidharan S, Ligon RA, Ao J, Whiteside W, Wallen WJ, Metcalf CM, Aggarwal V, Agrawal H, Qureshi AM. Comparison Between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants With Ductal-Dependent Pulmonary Blood Flow: Insights From the Congenital Catheterization Research Collaborative. Circulation. 2018;137:589–601. [DOI] [PubMed] [Google Scholar]
- 10.Petit CJ, Glatz AC, Qureshi AM, Sachdeva R, Maskatia SA, Justino H, Goldberg DJ, Mozumdar N, Whiteside W, Rogers LS, Nicholson GT, McCracken C, Kelleman M, Goldstein BH. Outcomes After Decompression of the Right Ventricle in Infants With Pulmonary Atresia With Intact Ventricular Septum Are Associated With Degree of Tricuspid Regurgitation: Results From the Congenital Catheterization Research Collaborative. Circ Cardiovasc Interv. 2017;10:e004428. [DOI] [PubMed] [Google Scholar]
- 11.Petit CJ, Qureshi AM, Glatz AC, McCracken CE, Kelleman M, Nicholson GT, Meadows JJ, Shahanavaz S, Zampi JD, Law MA, Pettus JA, Goldstein BH. Comprehensive Comparative Outcomes in Children with Congenital Heart Disease: The Rationale for the Congenital Catheterization Research Collaborative. Congenit Heart Dis. 2018. [DOI] [PubMed] [Google Scholar]
- 12.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299:2048–55. [DOI] [PubMed] [Google Scholar]
- 13.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). Am J Cardiol. 2016;117:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Byrne ML, Shinohara RT, Grant EK, Kanter JP, Gillespie MJ, Dori Y, Rome JJ, Glatz AC. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: An observational study using data from the Pediatric Health Information Systems database. Am Heart J. 2017;192:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, Kim D, Kogon B, Oster ME. Transcatheter Versus Surgical Closure of Atrial Septal Defects in Children: A Value Comparison. JACC Cardiovasc Interv. 2016;9:79–86. [DOI] [PubMed] [Google Scholar]
- 16.US Bureau of Labor Statistics. Consumer Price Index (http://www.bls.gov/cpi/). 2018.
- 17.O’Byrne ML, Glatz AC. Importance of Cost-Comparison Analysis in Comparing Operative and Tanscatheter Closure of Atrial Septal Defects. JACC Cardiovasc Interv. 2016;9:1085. [DOI] [PubMed] [Google Scholar]
- 18.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24:465–88. [DOI] [PubMed] [Google Scholar]
- 19.Gatlin SW, Kim DW, Mahle WT. Cost analysis of percutaneous pulmonary valve replacement. Am J Cardiol. 2011;108:572–4. [DOI] [PubMed] [Google Scholar]
- 20.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. Am Heart J. 2015;169:727–735 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahle WT, Nicolson SC, Hollenbeck-Pringle D, Gaies MG, Witte MK, Lee EK, Goldsworthy M, Stark PC, Burns KM, Scheurer MA, Cooper DS, Thiagarajan R, Sivarajan VB, Colan SD, Schamberger MS, Shekerdemian LS,Pediatric Heart Network I. Utilizing a Collaborative Learning Model to Promote Early Extubation Following Infant Heart Surgery. Pediatr Crit Care Med. 2016;17:939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf MJ, Lee EK, Nicolson SC, Pearson GD, Witte MK, Huckaby J, Gaies M, Shekerdemian LS, Mahle WT, Pediatric Heart Network I Rationale and methodology of a collaborative learning project in congenital cardiac care. Am Heart J. 2016;174:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs JP, Pasquali SK, Austin E, Gaynor JW, Backer C, Hirsch-Romano JC, Williams WG, Caldarone CA, McCrindle BW, Graham KE, Dokholyan RS, Shook GJ, Poteat J, Baxi MV, Karamlou T, Blackstone EH, Mavroudis C, Mayer JE Jr., Jonas RA, Jacobs ML. Linking the congenital heart surgery databases of the Society of Thoracic Surgeons and the Congenital Heart Surgeons’Society: part 2--lessons learned and implications. World J Pediatr Congenit Heart Surg. 2014;5:272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquali SK, Hall M, Li JS, Peterson ED, Jaggers J, Lodge AJ, Marino BS, Goodman DM, Shah SS. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation. 2010;122:2123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquali SK, Jacobs JP, Shook GJ, O’Brien SM, Hall M, Jacobs ML, Welke KF, Gaynor JW, Peterson ED, Shah SS Li JS. Linking clinical registry data with administrative data using indirect identifiers: implementation and validation in the congenital heart surgery population. Am Heart J. 2010;160:1099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]