Abstract
Mesenchymal stem cell (MSC)-based therapy for promoting vascular regeneration is a promising strategy for treating ischemic diseases. However, low engraftment and retention rate of MSCs at the target site highlights the importance of paracrine signaling of MSCs in the reparative process. Thus, harnessing MSC-secretome is essential for rational design of MSC-based therapies. The role of microenvironment in regulating the paracrine signaling of MSCs is not well known. In this study, human bone marrow-derived MSCs were seeded on matrices with varying stiffness or cell adhesive sites, and conditioned media was collected. The concentrations of angiogenic molecules in the media was measured via ELISA. In addition, the bioactivity of the released molecules was investigated via assessing the proliferation and capillary morphogenesis of human umbilical vein endothelial cells (HUVECs) incubated with conditioned media. Our study revealed that secretion of vascular endothelial growth factor (VEGF) is dependent on substrate stiffness. Maximal secretion was observed when MSCs were seeded on hydrogel matrices of 5.0 kPa stiffness. Proliferation and tubulogenesis of HUVECs supported ELISA data. On the other hand, variation of cell adhesive sites while maintaining a uniform optimal stiffness, did not influence the pro-angiogenic activity of MSCs.
Keywords: Mesenchymal stem cells, Matrix rigidity, Growth factors, Angiogenesis
Graphical abstract
Introduction
Angiogenesis, i.e. the growth of new blood vessels from a pre-existing vessel, is necessary for physiological conditions including embryonic development, wound healing, and reproduction [1]. The complex multi-step process of angiogenesis is regulated by a precise balance of stimulatory (e.g. vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet derived growth factor (PDGF), angiopoentin-1, and matrix metalloproteinases (MMPs)) and inhibitory (e.g. angiostatin, thrombospondin, tissue inhibitor of metallopeptidase (TIMP)) factors [1]. Dysregulation of this balance is a hallmark in many diseases including cancer, psoriasis, arthritis, osteoporosis, age-related blindness, diabetic ulcers, and cardiovascular diseases [2, 3]. Taking into account that the “common denominator” in many of these deadly and debilitating conditions is insufficient vessel maintenance or growth, therapeutic angiogenesis aiming to revascularize ischemic tissues has drawn worldwide attention. Strategies to enhance vascular remodeling include delivery of potent angiogenic factors, promotion of angiogenic genes expression, and delivery of stem or progenitor cells [4]. While administration of proangiogenic factors to induce blood vessel formation is considered safe with the added advantage of “offthe-shelf” availability, uncontrolled and abnormal vasculature [4] limits its application. Gene delivery via viral vectors upregulates the expression of angiogenic genes resulting in enhanced production of angiogenic molecules. However, viral vectors can potentially trigger immune responses [4, 5]. On the other hand, nonviral vectors are less efficient [6, 7].
Alternatively, designing cell-based therapies for promoting vascular regeneration have attracted widespread efforts to treat ischemic diseases [8]. Compared to the other therapies, cell-based therapy is considered to be more comprehensive due to the possibility of delivering multiple proangiogenic factors. Improved understanding of stem cell biology has resulted in extensive investigation of the therapeutic potential of mesenchymal stem cells (MSCs) for developing cell-based therapies [9, 10]. MSCs are multipotent adult stem cells originating from various tissues including bone marrow, adipose tissue, umbilical cord tissue (Wharton’s jelly), and amniotic fluid and capable of differentiating to multiple cell types [11]. Transdifferentiation of MSCs to endothelial cells [12, 13] has been attributed to the efficacy of MSC-based therapy in stimulating vascularization [14] and consequently, accelerating wound healing, protecting cardiomyocytes, decreasing infarct size, and improving cardiac function as well as fracture healing [15–20]. However, the low engraftment and retention rate of MSCs at the target site due to a harsh microenvironment with death promoting stimuli e.g., hypoxia, reactive oxygen species, cytotoxic cytokines, and absence of extracellular matrix (ECM) for MSC attachment [21] refutes the hypothesis that the donor cells can functionally integrate with the damaged/ischemic tissue.
Mounting evidence corroborates that the paracrine mechanisms mediated by factors released by the MSCs play a critical role in the reparative process [15, 22–25]. This diverse array of biomolecules, including cytokines, chemokines, angiogenic factors, growth factors, extracellular matrix proteases, and hormones, known as “secretome,” is believed to be potentially involved in MSC-mediated tissue vascularization [15, 22–25]. This underscores the importance of harnessing MSC secretome to improve MSC-based therapies. Current methods for modulation of MSC secretome include physiological (hypoxic or anoxic), pharmacological, or growth factor/cytokine pre-conditioning and/or genetic manipulations prior to transplantation [26–30]. While these strategies have shown promise in inducing expression/secretion of pertinent factors, they are limited by transient impact post-transplantation (physiological or pharmacological preconditioning) and by challenges associated with clinical translation due to viral modification of the target gene (genetic manipulation). In addition, none of these pre-conditioning methods can improve the limited engraftment and viability of MSCs in situ. While the role of cellular microenvironment in regulating the commitment decisions of stem cells has been extensively explored [31], strategy involving modulation of the microenvironment to harness MSC secretome has been largely overlooked. Recent studies have reported enhanced expression of interleukin-8 (IL-8) as well as VEGF with increase in matrix stiffness [32]. In our earlier studies, we have observed that matrix stiffening enhanced pro-angiogenic signaling of breast cancer cells as reflected from increased VEGF secretion [33]. On the other hand, human dermal fibroblasts preferred compliant matrices for optimal angiogenic activity [34]. Based on these observations we hypothesized that secretion of pro-angiogenic factors by MSCs can be modulated by manipulating the microenvironmental cues (via matrix stiffness and cell adhesive sites).
In this study, mechanically tunable gelatin methacrylate (GelMA)-poly (ethylene glycol) diacrylate (PEGDA) hybrid matrices were developed to leverage the biocompatibility of gelatin with the stability and reproducibility of PEG. GelMA, obtained via conjugation of methacrylate groups to gelatin (denatured collagen), provides the cell-adhesive as well as MMP sensitive sites [35]. Human bone marrow-derived MSCs (well characterized in terms of their paracrine signaling [15, 19, 20]) were seeded on the matrices with varying stiffness yet uniform cell adhesive sites. Enhanced angiogenic activity was observed when MSCs were seeded on matrices of optimal rigidity. Compliant or stiffer matrices resulted in reduced pro-angiogenic signaling as manifested from lower VEGF secretion and consequently reduced sprouting in Matrigel culture of human umbilical vein endothelial cells (HUVECs). HUVECs were selected because of their well-known sprouting activity [36]. In addition, the density of cell adhesive sites was altered by varying GelMA concentration while maintaining constant matrix stiffness. No significant effect of cell-binding sites on pro-angiogenic signaling was observed. Our study suggests that matrix stiffness but not the available cell-adhesive sites plays a critical role in pro-angiogenic signaling of MSCs.
Materials and Methods
2.1. Materials
Poly (ethylene) glycol diacrylate 10,000 kDa (PEGDA) was procured from Laysan Bio Inc. (Alabama). Gelatin from porcine skin, photo-initiator (2-Hydroxy-4’-(2-hydroxyethoxy)-2-methylpropiophenone), methacrylic anhydride, ethylene glycol, and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (St. Louis, MO). Geltrex™ LDEV-free reduced growth factor basement membrane, Dulbecco’s Phosphate Buffer Saline (DPBS), Dulbecco’s Modified Eagle Medium (DMEM), Penicillin-Streptomycin (Pen Strep), 0.25% Trypsin-EDTA, Fetal Bovine Serum (FBS), and collagenase Type I were acquired from Gibco by Life Technologies (Grand Island, NY). Hoechst 33342, Alexa Fluor™ 488 phalloidin, methanol, acetone, and Slide-A-Lyzer Dialysis Cassette G2 (10k MWCO) were obtained from Thermofisher Scientific (Waltham, MA). XTT Proliferation Assay kit was purchased from ATCC (Manassas, VA). Endothelial cell basal medium-2 (EBM-2) and EGM-2 SingleQuots growth factors were obtained from Lonza (Walkersville, MD).
2.2. Synthesis of Gelatin Methacrylate (GelMA)
Gelatin Methacrylate (GelMA) was synthesized as described previously [34]. Gelatin type A (from porcine skin) was added to Dulbecco’s Phosphate Buffer Saline (DPBS) and stirred continuously at 50 °C until fully dissolved to prepare a 10% (w/v) solution. To achieve methacrylation of gelatin, methacrylic anhydride was added at a rate of 0.5mL/min to get a 10% (v/v) solution and allowed to react for 3 h at 60 °C. To remove salts and unreacted acid, the reaction mixture was then diluted 5X with warm DPBS at 50 °C and dialyzed against deionized distilled H2O (dH2O) at 50 °C for 10 days using Slide-A-Lyzer (10K MWCO) Dialysis Cassettes G2. After 10 days, the GelMA solution was frozen at −80 °C, lyophilized, and collected as a porous foam until further use [34].
2.3. Fabrication of Hydrogel Scaffolds
Hydrogels were fabricated by adding 50 μL of the pre-polymer solution containing PEGDA, GelMA, 1% (w/v) photo initiator, and dH2O to the wells of a 96-well plate and exposing the solution to UV (CL-1000 Ultraviolet Crosslinker, 365 nm) for 5 min.
To vary hydrogel stiffness without affecting the cell adhesive sites, the concentration of PEGDA in the pre-polymer solution was varied from 2.5% to 10% (w/v) while maintaining the concentration of GelMA constant at 5% (w/v).
To vary concentration of cell adhesive sites, the ratio of PEGDA to GelMA in the pre-polymer solution was varied to achieve a total polymer concentration of 10% with PEGDA and GelMA combined.
2.4. Characterization of Hydrogel Scaffolds
2.4.1. Swelling Ratio
To measure swelling ratio (Qm), the hydrogels were incubated for 72 h in DPBS on a rotator shaker at 50 rpm at room temperature. Following incubation, the weights of the hydrated samples were measured (Wwet). The hydrogels were then dried at 60 °C, and their weights (Wdry) were measured after 24 h. The swelling ratio of the hydrogels was then calculated using the following equation:
2.4.2. Compression
To measure the stiffness of the hydrogels, the pre-polymer solutions were placed in cylindrical molds measuring 10 mm in diameter and approximately 3 mm in thickness and UV cured as described earlier. To remove unreacted monomers, the scaffolds were then incubated in DPBS for 72 h. The hydrated samples were compressed using uniaxial testing machine (TestResources, USA) at a loading rate of 1.2 mm/min and a precision load up to 9 N. Maximum strain and stress at the moment of fracture was recorded, and the compression modulus was calculated from the initial 10% compression [36].
2.4.3. Degradation
To measure the rate of hydrogel degradation, the samples were incubated in 2.5 units/mL collagenase Type I solution on a rotator shaker at room temperature. The weights of the hydrogels were recorded each day for 10 consecutive days. The degradation ratio was determined by comparing the weights of the samples at each day to the weights of the samples on the day of hydrogel fabrication.
2.5. Cell Culture and angiogenic activity
Mesenchymal stem cells (MSCs) derived from bone marrow were purchased from American Type Culture Collection (ATCC) and expanded in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin. Human umbilical vein endothelial cells (HUVECs) were procured from (ATCC) and expanded in endothelial cell basal medium-2 (EBM-2) supplemented with 1% (v/v) penicillin-streptomycin and EGM-2 SingleQuots (containing FBS, hydrocortisone, hFGF, VEGF, R3-IGF-1, ascorbic acid, hEGF, GA-1000, and heparin). Cells were cultured and incubated until confluence at 37 °C and 5% CO2. In this study, cells up to passage 6 were used.
2.5.1. Cell Proliferation
Resuspended MSCs, following detachment from the flasks using 0.25% Trypsin-EDTA, were seeded on the scaffolds in 96-well plates at a density of 2.0 ×104 cells/gel and incubated in 200 μL of complete DMEM media at 37 °C and 5% CO2. 48 h post-seeding, activated XTT reagent was added to the cells along with fresh media and incubated at 37 °C and 5% CO2 for 4 h. The absorbance was then measured using BioTek Eon Microplate reader at a wavelength of 450 nm. Cell proliferation was calculated as a percentage using the following equation:
The cells seeded on tissue culture well plates served as control. The data was collected from at least three independent experiments, each of which was carried out in three replicates.
2.5.2. Cell Morphology
MSCs were seeded on scaffolds (fabricated within wells of 48 well plates) at a density of 2 × 103 cells/gel and incubated at 37 °C and 5% CO2 for 96 h. Cell images from each sample were captured using Zeiss Axio Observer A1 microscope with integrated CCD camera. A minimum of five images were captured at random per scaffold. Area, length, and width of the cells were measured using Axiovision Software (Release 4.8.2). Three independent experiments were performed with three replicates per experiment.
2.5.3. Immunostaining
MSCs seeded on scaffolds with varying stiffness or cell adhesive properties were stained with 1% (v/v) Hoechst 33342 dye for 5 min. Following which, the cells were fixed and permeabilized with 1:1 acetone:methanol solution at −20 °C for 20 min. The cells were then blocked with 5% (w/v) BSA solution for 1 h, washed three times with 1X DPBS, and incubated with 2.5% (v/v) Alexa Fluor 488-phalloidin antibody at 37 °C for 1 h. After washing the cells three times with 1X DPBS, images were captured using Olympus laser scanning confocal microscope (FV1200) to study the effect of hydrogel properties on organization of actin stress fibers.
2.5.4. Quantification of MSC secreted growth factors
To assess the release of growth factors by MSCs as a function of microenvironment, cells were seeded on matrices with varying stiffness or cell adhesive properties at a density of 2 × 104 cells/gels in 48 well plates. 48 h post-seeding, the media was collected and centrifuged to remove cellular debris. The concentration of the angiogenic factors in the collected media were measured using Human Quantikine VEGF, FGF, and PDGF ELISA kit from R&D Systems (Minneapolis, MN) as per manufacturer’s instruction. Eon Microplate Reader from BioTek was utilized to analyze the samples at 450 nm. Three replicates were performed for each condition. Conditioned media was also collected from cells seeded on wells. The final densities of cells were also measured, and the data was reported as mean protein released/1000 cells ± S.E.M.
2.5.5. Assessment of bioactivity of released angiogenic molecules
To investigate the bioactivity of the angiogenic molecules released by MSCs, HUVECs were incubated with conditioned media collected 48 h post-seeding MSCs on gels of varying stiffness and cell adhesion sites.
2.5.5.1. Proliferation of HUVECs
HUVECs were seeded in 96 well plate at a density of 2.0 ×104 cells/well and incubated in 200 μL of conditioned media for 48 h at 37 °C and 5% CO2. Cells incubated in endothelial cell media served as positive control. Cell proliferation was measured as described earlier using XTT cell proliferation assay kit.
2.5.5.2. Capillary morphogenesis of HUVECs
50 μL of Geltrex™ LDEV-free reduced growth factor basement membrane was added to 96 well plates and kept at room temperature for 30 min to facilitate gel formation. HUVECs were seeded on gels at a density of 5 × 104 cells/gel and incubated in the presence of conditioned media for 15 h at 37 °C and 5% CO2. Cells incubated in endothelial cell media served as the control. After 15 h, images of capillary sprouting formation were captured using Zeiss Axio Observer A1 microscope with integrated CCD camera. Total mesh area was calculated using Angiogenesis Analyzer in Image J (Fig S1), and the mesh area for different samples were normalized with respect to the control using the following equation:
2.6. Statistical Analysis
All experiments were conducted in triplicates and repeated three times at least. The data represents the mean ± S.E.M of three independent experiments. Statistical analyses were carried out using one-way ANOVA with post-hoc Tukey HSD test. Differences between two sets of data were considered significant at p-value < 0.05.
Results
3.1. Mechanically tunable hydrogel fabrication and characterization
The mechanical properties of the hydrogel matrices were altered by varying the concentration of PEGDA from 2.5% to 10% (w/v) while the concentration of GelMA was held constant at 5%. As demonstrated in Table 1, an increase in PEGDA concentration which enhanced the crosslinking groups per volume resulted in matrices with higher compression moduli (1.3 ± 0.5 kPa for 2.5% PEGDA to 23 ± 1.0 kPa for 10% PEGDA). The impact of increasing the PEGDA concentration and consequently, the compression modulus on the swelling ratio of the hydrogels was investigated. As shown in Table 1, the swelling ratio (Qm) of the hydrogels decreased as the concentration of PEGDA (and consequently stiffness of the gels) increased due to higher crosslinking of PEGDA.
Table 1:
Comparison of compression modulus and swelling ratio of hydrogels as a function of composition
| PEGDA(%) | Compression modulus (kPa) | Swelling Ratio (Qm) |
|---|---|---|
| 2. 5 | 1.3 ± 0.5 | 44.0 ± 10.40 |
| 5.0 | 5.0 ± 1.0 | 29.9 ± 4.30 |
| 7.5 | 13.0 ± 1.2 | 23.1 ± 0.02 |
| 10.0 | 23.0 ± 1.3 | 19.7 ± 0.02 |
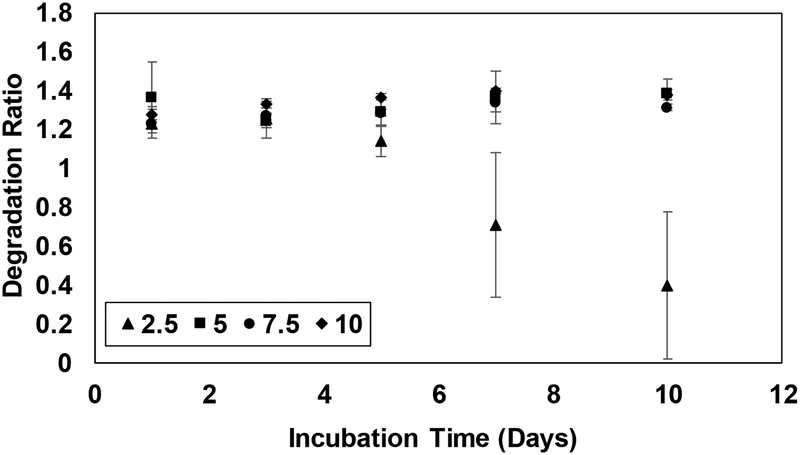
The stability of the hydrogels was assessed by measuring the rate of degradation of the matrices. Degradation ratio (weight of hydrogels at different time points normalized with respect to the weight following fabrication) of the hydrogels remained constant for 6 days (Fig 1). After 6 days of incubation in collagenase solution, the weights of the hydrogels composed of 2.5% PEGDA decreased indicating the breakdown of polymeric network. However, all matrices retained their physical integrity during the period of the experiments. Taken together, the studies confirm the fabrication of hydrogel matrices with mechanical properties mimicking that of physiologically relevant tissues [36, 37]. Maintenance of uniform presentation of cell adhesive sites (constant GelMA concentration at 5% (w/v)) ascertained that the observed changes in the functions of MSCs can be correlated to the compliances of hydrogels only.
Figure 1.
Degradation of hydrogel matrices (2.5%, 5%, 7.5%, and 10% (w/v) PEGDA with GelMA maintained at 5% (w/v)) monitored over 10 days. Error bar S.E.M (N=3).
3.2. Effect of hydrogel rigidity on MSC-material interactions
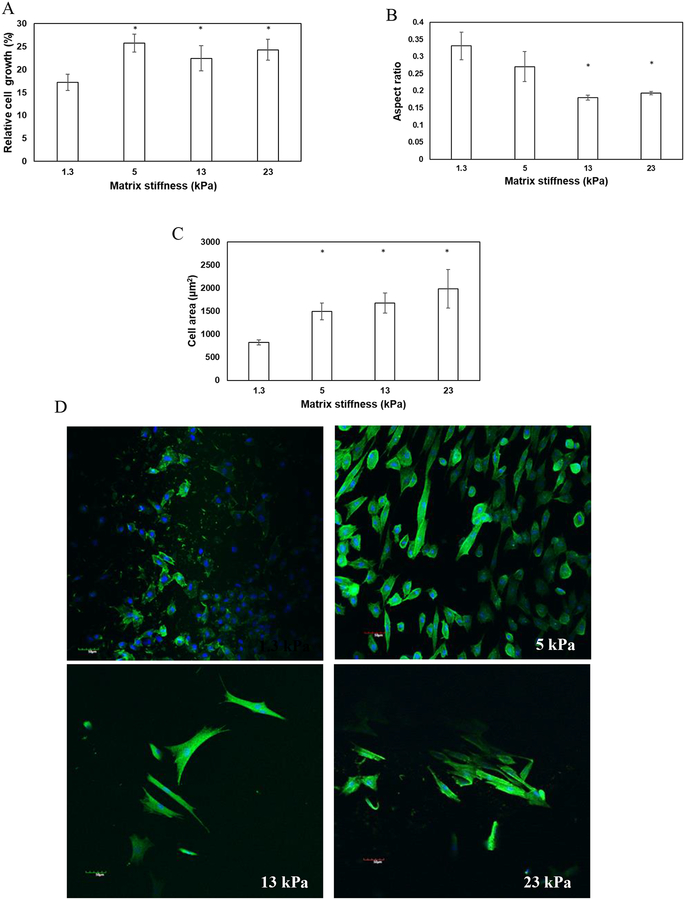
To assess the impact of hydrogel compliances on cell-material interactions, human bone-marrow derived MSCs were seeded on the top of hydrogels with different rigidities. XTT assay was used to measure the proliferation of MSCs after 48 h. As demonstrated in Figure 2A, the proliferation of MSCs increased with increase in matrix stiffness. These observations suggest that while MSCs can proliferate irrespective of matrix compliances, stiffer gels stimulate their growth (p-value < 0.05 compared to 1.3 kPa gels). However, no significant difference in proliferation of MSCs was observed when the stiffness of the gels was varied from 5 kPa to 23 kPa. To elucidate the effect of matrix mechanics on the morphology of MSCs, cells were sparsely plated on the top of the gels. It was observed that rigid matrices promoted elongated morphology of MSCs as manifested from significant attenuation in aspect ratio (cell width/length) of the cells seeded on stiffer gels (0.3 ± 0.04 for 1.3 kPa gels vs. 0.2 ± 0.004 for 23 kPa gels) (p-value < 0.05) (Fig 2B). Quantitative analysis also revealed that cell area increased significantly from 822 ± 53 μm2 when seeded on matrices of 1.3 kPa to 1985 ± 415 μm2 for matrices of 23 kPa (p-value < 0.05) (Fig 2C). Increase in cell-projected area and adaptation of elongated morphology are consistent with literature that has correlated increased cellular spreading with enhanced stiffness of matrices [38]. Furthermore, to explore the effect of matrix stiffness with cytoskeletal reorganization, formation of actin stress fibers was evaluated. Confocal images of MSCs seeded on top of the gels showed that cells seeded on compliant gels were devoid of stress fibers; instead, the actin fibers had a cortical arrangement (Fig 2D). When the rigidity of the gels was enhanced, MSCs responded to the altered microenvironment via formation of actin stress fibers (Fig 2D).
Figure 2.
MSCs were seeded on hydrogel matrices of varying stiffness and the impact of matrix mechanics on proliferation and morphology was investigated. Increase in matrix rigidity (A) enhanced the proliferation, (B) reduced the aspect ratio, and (C) increased the area of the cells. Error bar S.E.M (N=3). * p-value< 0.05. (D) The confocal images of the cells displaying the arrangement of actin fibers (green). Hoechst 33342 (blue) stained the nuclei of the cells. The scale bar corresponds to 50 μm.
3.3. Effect of matrix mechanics on the angiogenic activity of MSCs
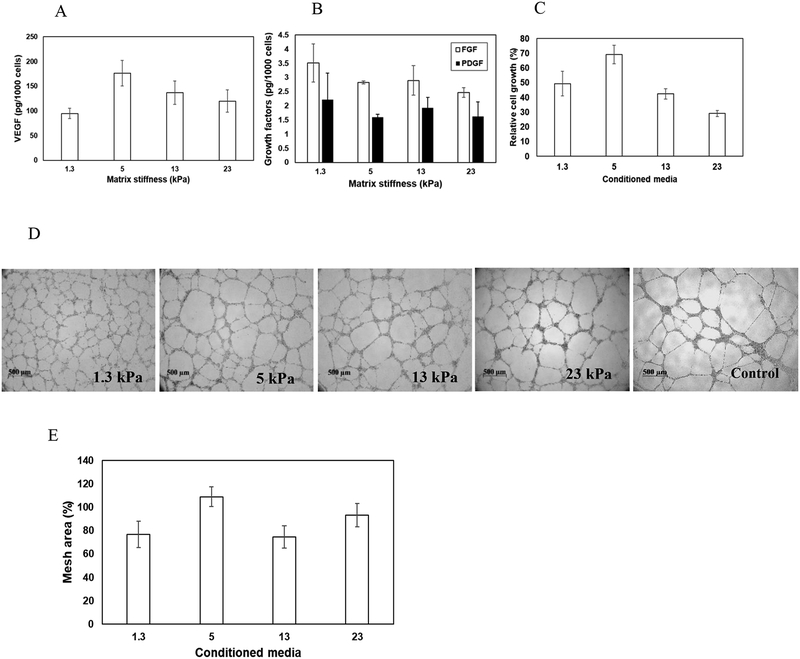
To investigate how matrix compliances influence the pro-angiogenic signaling of MSCs, cells were seeded on gels of varying stiffness. 48 h post-seeding, conditioned media was collected. The concentration of angiogenic growth factors including VEGF, bFGF, and PDGF in the collected media was measured via ELISA. To account for the variable growth rate of cells on different hydrogels, the final density of cells was assessed, and the data was represented as amount of growth factors released per 1000 cells (pg/1000 cells). As observed in Fig 3A, increase in substrate rigidity enhanced the secretion of VEGF by MSCs. However, maximal release of VEGF was observed when MSCs were seeded on gels with stiffness of 5 kPa. On the other hand, substrate compliances did not alter the secretion profile of FGF and PDGF (Fig 3B). These observations suggest the need for optimal level of matrix compliances for enhanced expression/secretion of VEGF by MSCs.
Figure 3.
Effect of matrix mechanics on pro-angiogenic activity of MSCs. The concentration of (A) VEGF and (B) FGF and PDGF released by MSCs seeded on gels of different rigidity assayed via ELISA. (C) Effect of released angiogenic factors on proliferation of HUVECs. The proliferation of HUVECs in the presence of conditioned media was normalized with respect to that in presence of HUVECs media (control). (D) Typical images of sprout formation during Matrigel culture of HUVECs in presence of conditioned media as well as normal HUVEC growth media. (E) Quantitative analysis of sprouts formed. Mesh area formed by HUVECs in presence of conditioned media was normalized with respect to control (normal HUVEC growth media). Error bar S.E.M (N=3).
To investigate the bioactivity of the released VEGF, conditioned media was collected 48 h post-seeding of MSCs on gels of varying stiffness. HUVECs were seeded at a density of 2.0 ×104 cells/well in 96-well plates, cultured in the presence of conditioned media, and proliferation was measured. HUVECs growth media acted as the positive control. As demonstrated in Fig 3C, maximal proliferation of HUVECs was observed when incubated in presence of conditioned media collected from MSCs seeded on 5 kPa gels. This observation corroborates the ELISA data. Further, in presence of conditioned media from 5 kPa gels the growth of HUVECs were observed to be around 70% of that observed in presence of HUVECs growth media (supplemented with cocktail of growth factors). Effect of matrix-mediated pro-angiogenic activity of MSCs were further confirmed via capillary morphogenesis of HUVECs. Towards this, HUVECs were seeded on growth factor reduced ECM (rECM) gels in the presence of conditioned media, and formation of capillary sprouts was measured. HUVECs in the presence of fresh endothelial cell medium acted as positive control. Typical images of rECM gel assay captured 15 h post-seeding are illustrated in Fig 3D. To quantify capillary morphogenesis of endothelial cells, the area covered by sprouts in the presence of conditioned media was measured as a percentage of mesh area of cells incubated in normal endothelial cells media. Figure 3E shows a subtle but not significant increase in percentage mesh area when HUVECs were incubated in conditioned media collected from MSCs seeded on matrices with a stiffness of 5 kPa.
3.4. Effect of concentration of cell adhesive sites on pro-angiogenic signaling of MSCs.
To investigate whether the density of cell adhesive sites influences MSC functions, hydrogel matrices were fabricated by varying the ratio of PEGDA to GelMA concentration to achieve a total polymer concentration of 10% (w/v). Table 2 shows compliances and swelling ratios of these gels as a function of PEGDA:GelMA ratio. Hydrogels with PEGDA:GelMA ratio of 0%:10%, 3%:7%, 7%:3%, and 9%:1% displayed compression moduli comparable to matrices with 5% PEGDA and 5% GelMA (Table 2) which elicited optimal angiogenic activity of MSCs. However, the swelling ratio (Qm) of 0%:10% gels was significantly different. Hence, the hydrogels with composition 3%:7%, 7%:3%, and 9%:1% (hence forth, represented as 3:7, 5:5, 7:3, and 9:1, respectively) were selected for further studies. The swelling ratio of the selected hydrogels displayed no difference in comparison to the hydrogels with 5:5 matrices (Table 2).
Table 2:
Comparison of compression modulus and swelling ratio of gels as a function of matrix composition
| PEGDA (%) | GelMA (%) | Compression modulus (kPa) |
Swelling Ratio (Qm) |
|---|---|---|---|
| 0 | 10 | 4.7 ± 2.3 | 18.9 ± 1.2 |
| 1 | 9 | 1.1 ± 0.1 | 27.1 ± 2.5 |
| 2 | 8 | 2.2 ± 0.4 | 23.0 ± 3.1 |
| 3 | 7 | 3.4 ± 0.2 | 26.8 ± 3.8 |
| 4 | 6 | 5.2 ± 0.1 | 26.4 ± 3.2 |
| 5 | 5 | 5.0 ± 1.0 | 32.0 ± 1.9 |
| 6 | 4 | 7.0 ± 0.5 | 26.3 ± 0.8 |
| 7 | 3 | 6.2 ± 0.3 | 28.8 ± 1.8 |
| 8 | 2 | 8.3 ± 0.1 | 32.7 ± 3.3 |
| 9 | 1 | 7.5 ± 0.1 | 28.8 ± 1.6 |
The degradation ratio of the hydrogels remained constant over a period of 6 days (Fig 4). Beyond 6 days, the 3:7 matrices were susceptible to faster degradation. In summary, variation of PEGDA to GelMA ratio permitted decoupling of protein concentration (cell adhesive sites) from intertwined matrix properties namely, compliances, swelling ratio, and degradation of hydrogel matrices. This underscores that any changes observed in cellular behaviors when MSCs were seeded on 3:7, 5:5, 7:3, and 9:1 gels can be correlated to density of cell binding motifs.
Figure 4.
Degradation of hydrogel matrices (3:7, 5:5, 7:3, and 9:1 ratio of PEGDA:GelMA, represented as 37, 55, 73, and 91 in the legend) monitored over 10 days. Error bar S.E.M (N=3).
The impact of variation of cell adhesive sites (GelMA concentration) on MSC proliferation was investigated. No significant difference in cell growth was observed when MSCs were seeded on 3:7, 5:5, and 7:3 gels (Fig 5A) (p-value > 0.05). However, reduced cell adhesive sites in 9:1 gels contributed to attenuation in cell proliferation (p-value < 0.05 compared to 37 gels). In addition, MSCs displayed an increasing tendency to adopt a rounded morphology with decreasing cell adhesive sites as manifested from increased aspect ratio and reduced cell area (Fig 5B and C) (p-value < 0.05). This observation suggested that when seeded on hydrogels with less cell adhesive sites, MSCs exhibited preference for intercellular adhesion. Phalloidin-staining confirmed that rounded morphology and increased intercellular adhesion in 7:3 and 9:1 gels are associated with cortical arrangement of actin fibers (Fig 5D).
Figure 5.
Comparison of the effect of variation of cell adhesive sites (3:7, 5:5, 7:3, and 9:1 ratio of PEGDA:GelMA, represented as 37, 55, 73, and 91 in the plot) on (A) proliferation, (B) aspect ratio, (C) area of MSCs. Error bar S.E.M (N=3). # p-value < 0.05 with respect to 3:7 * p-value< 0.05 with respect to 5:5. (D) The confocal images of the cells displaying the arrangement of actin fibers (green) and nuclei (blue) of the cells. The scale bar corresponds to 50 μm.
The impact of cell adhesive sites on angiogenic potential of MSCs was explored via quantification of proliferation and capillary morphogenesis of HUVECs. As demonstrated in Fig 6A, upon incubation of HUVECs with conditioned media, no significant difference in cell proliferation was observed as a function of varying adhesive sites (p-value > 0.05). Similarly, altering the density of cell binding motifs yielded no significant difference in sprouting propensity of HUVECs as manifested from similar mesh area (Fig 6B and C) (p-value > 0.05).
Figure 6.
Bioactivity of MSC-secreted growth factors. HUVECs were incubated in the presence of conditioned media collected from MSCs seeded on gels with varying cell adhesive sites (3:7, 5:5, 7:3, and 9:1 ratio of PEGDA:GelMA, represented as 37, 55, 73, and 91 in the plot). (A) Effect of angiogenic molecules on proliferation of HUVECs in the presence of conditioned media from MSCs cultured on gels of varying composition. The proliferation was normalized with respect to HUVECs growth media and represented as relative cell growth. (B) Typical images of sprout formation during Matrigel culture of HUVECs in presence of conditioned media as well as HUVEC growth media (control). (C) Quantitative analysis of sprouts formed in the presence of conditioned media from different gels. Mesh area observed in presence of conditioned media was normalized with respect to control. Error bar S.E.M (N=3).
Discussion
While the precise mechanism that governs MSC-mediated tissue revascularization remains controversial, only recently the role of myriad of chemokines, cytokines, and growth factors [4, 39] in stimulating angiogenesis has started to be elucidated. Harnessing the spatiotemporal secretion of immunomodulatory and trophic factors for stimulating tissue vascularization is critical for success of cell-based therapies. Along with biochemical cues, matrix elasticity has been shown to modulate lineage specification of stem cells [40]. Even though much efforts have been directed in investigating how mechanical signaling from local cellular environment regulates cell fate [41], the influence of matrix compliances in modulating the angiogenic activity of cells is less understood. Experimental evidence established in recent years indicates a significant role of substrate stiffness in guiding the paracrine signaling of various cell types [33, 34, 42, 43]. However, differential responses of the cells to matrix rigidity highlights the importance of deeper understanding of the impact of matrix mechanics on the expression of trophic factors by MSCs, which in turn can augment their therapeutic efficacy. The primary focus of this study is testing whether variation in substrate compliances would influence the angiogenic activity of MSCs. Variation of matrix compliances using naturally derived materials such as collagen, fibrin, and Matrigel involves alteration in the cell adhesion sites, the protease susceptibility, and porous architecture of the matrices [44]. This in turn can influence the angiogenic activity of MSCs. To deconstruct the multivariate instructive role of ECM in guiding pro-angiogenic signaling of MSCs, a mechanically tunable biomaterial platform was developed for exploring the correlation between ECM compliance and angiogenic signaling of MSCs. The rigidity of the matrices varying from 1.3 ± 0.5 kPa to 23 ± 1.0 kPa covers a broad range of tissues [36, 37, 40], thereby highlighting the physiological relevance of these matrices.
Cells sense the changes in the mechanical resistivity of the matrices and accordingly respond by altering the cell-material interactions and cytoskeletal organization. This, in turn, has a decisive impact on various cellular functions including cell spreading, proliferation, and migration. Our study, consistent with other studies [38, 43], demonstrated that in response to different matrix compliances, MSCs adopted different morphologies- from rounded on compliant gels to more elongated (or isotropic spreading) morphology with robust cytoskeleton on stiffer gels. Investigation of the influence of matrix compliances on angiogenic signaling of MSCs revealed stiffness dependent expression of VEGF while no effect on FGF and PDGF expression was observed. Further, the study revealed maximal secretion of VEGF when MSCs were seeded on gels of intermittent (5.0 kPa) stiffness. In contrast to other studies that reported upregulation of trophic factors in the presence of stiffer gels [43, 45], the observation in the current study indicated the requirement of an optimal level of ECM compliance for maximal expression of VEGF. This inconsistency in the observations can potentially be due to differences in the ranges of matrix compliances studied. Seib and coworkers demonstrated that irrespective of length of culture, secretion of VEGF is higher on stiffer gels (15–20 kPa) than on compliant matrices (1–2 kPa) [32]. Similarly, Abdeen and coworkers reported enhanced expression of trophic factors including VEGF on stiffer gels (40 kPa) compared to soft matrices (0.5 kPa) [43]. In addition, unlike the earlier studies, we normalized the expression of angiogenic molecules with respect to cell numbers to account for the difference in the rate of cell growth on gels of varying stiffness. Towards this goal, we measured the amount of growth factors released per 1000 cells. We observed enhanced concentrations of VEGF secreted by MSCs when seeded on gels with higher rigidity compared to 1.3 kPa gels. However, the concentration of VEGF released in medium was maximal when cells were seeded on 5.0 kPa gels. To explore whether the enhanced MSC secretome would translate to better capillary morphogenesis of endothelial cells, the bioactivity of the secreted factors was confirmed via rECM gel-based angiogenesis assay. Since all the hydrogel systems utilized in this study incorporated identical quantities of gelatin, the differential angiogenic activity observed in this study could be primarily attributed to substrate compliances. Investigation on the role of adhesive sites in regulating MSC angiogenic activity revealed no significant impact on pro-angiogenic signaling of MSCs.
While other factors such as oxygen tension in vivo will influence trophic factor secretion by MSCs, we demonstrated in this study that MSC secretome can be harnessed by regulating the substrate stiffness. Future work involving temporal profiling of angiogenesis related factors (both pro-and anti-angiogenic molecules) will permit elucidating the complex interplay between different microenvironmental factors in guiding angiogenesis.
Conclusions
Harnessing MSC secretome can potentially improve the efficacy and clinical translation of cell-based therapies. In this study, the influence of matrix stiffness and density of cell adhesive sites in guiding the pro-angiogenic activity of MSCs were investigated. Our study demonstrated that an appropriate level of matrix compliance is required for maximal paracrine activity by MSCs. Variation of cell adhesive sites, however, has limited influence in stimulating pro-angiogenic potential of MSCs.
Supplementary Material
Highlights.
Cell area and stress fiber formation of MSCs increase with increasing matrix mechanics
Appropriate level matrix compliance for maximal paracrine activity of MSCs
Cell adhesive sites have limited influence on angiogenic signaling of MSCs
Acknowledgement
The authors would like to thank NIH (1R03EB026526–01), NSF (1531217), Alternatives Research and Development Foundation (AWD005747), and University of Michigan Office of Research (U049922) for the financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ucuzian AA, Gassman AA, East AT, Greisler HP, Molecular mediators of angiogenesis. J. Burn Care Res 31 (2010) 158–175. 10.1097/BCR.0b01381c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carmeliet P, Angiogenesis in health and disease. Nat. Med 9 (2003) 653–660. 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- [3].Carmeliet P, Jain RK, Angiogenesis in cancer and other diseases. Nature. 407 (2000) 249–257. 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- [4].Chu H, Wang Y, Therapeutic angiogenesis: controlled delivery of angiogenic factors. Ther Deliv. 3 (2012) 693–714. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564557/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, De Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M, LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 302 (2003) 415–419. 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- [6].Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R, Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 105 (2002) 1233–1239. [DOI] [PubMed] [Google Scholar]
- [7].Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG, Polymeric materials for gene delivery and DNA vaccination. Adv. Mater 21 (2009) 847–867. 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang L, Xu Q, Stem/Progenitor Cells in Vascular Regeneration. Arter. Thromb. Vasc. Biol 34 (2014) 1114–1119. 10.1161/ATVBAHA.114.303809. [DOI] [PubMed] [Google Scholar]
- [9].Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY, The meaning, the sense and the significance: translation the science of mesenchymal stem cells into medicine. Nature medicine. 19 (2013) 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E, Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg. Sports Traumatol. Arthrosc 21 (2013) 1717–1729. 10.1007/s00167-012-2329-3. [DOI] [PubMed] [Google Scholar]
- [11].Phelps J, Sanati-Nezhad A, Ungrin M, Duncan NA, Sen A, 2018. Bioprocessing of mesenchymal stem cells and their derivatives: Toward cell-free therapeutics. Stem Cells Int. 2018, 9415367 10.1155/2018/9415367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wingate K, Bonani W, Tan Y, Bryant SJ, Tan W, Compressive elasticity of three-dimensional nanofiber matrix directs mesenchymal stem cell differentiation to vascular cells with endothelilal or smooth muscle cell markers. Acta. Biomater 8 (2012) 1440–1449. 10.1016/j.actbio.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lozito TP, Kuo CK, Taboas JM, Tuan RS, Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J. Cell. Biochem 107 (2009) 714–722. 10.1002/jcb.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Losordo DW, Dimmeler S, Therapeutic angiogenesis and vasculogenesis for ischemic disease. Circulation. 109 (2004) 2692–2697. [DOI] [PubMed] [Google Scholar]
- [15].Williams AR, Hatzistergos KE, Addicott B, et al. , McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM, Enhanced effect of combining human cardia stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 127 (2012) 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Walter MNM, Wright KT, Fuller HR, MacNeil S, Johnson WEB, Mesenchymal stem cell-conditioned medium accelerates wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp. Cell Res. 316 (2010) 1271–1281. 10.1016/j.yexcr.2010.02.026. [DOI] [PubMed] [Google Scholar]
- [17].Kanki S, Segers VFM, Wu W, Kakkar R, Gannon J, Sys SU, Sandrasagra A, Lee RT, Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circulation. 4 (2011) 509–518. [DOI] [PubMed] [Google Scholar]
- [18].Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AAM, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DPV, Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell. Res 6 (2011) 206–214. 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [19].Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, Liu X, Qi S, 2014. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells echances wound healing in mice. PloS one. 9, e96161 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hwang B, Liles WC, Waworuntu R, Mulligan MS, Pretreatment with bone marrow-derived mesenchymal stromal cell-conditioned media confers pulmonary ischemic tolerance. J. Thor. Cardio. Surg 151 (2015) 841–849. 10.1016/j.jtcvs.2015.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Song H, Cha MJ, Song BW, Kim IK, Chang W, Lim S, Choi EJ, Ham O, Lee SY, Chung N, Jang Y, Hwang KC. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 28 (2010) 555–563. 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- [22].Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK, Transplanted stem cell-secreted vascular endothelial growth factor effects post-stroke recovery, inflammation, and vascular repair. Stem Cells. 29 (2011) 274–285. 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mureli S, Gans CP, Bare DJ, Geenen DL, Kumar NM, Banach K, Mesenchymal stem cells improve cardiac conduction by upregulation of connexin 43 through paracrine signaling. American J. Phys 304 (2012) H600–609. 10.1152/ajpheart.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liang X, Ding Y, Zhang Y, Tse HF, Lian Q, Paracrine mechanisms of mesenchymal stem cell-based therapy; current status and perspectives. Cell Transplant. 23 (2014) 1045–1059. 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- [25].Kamprom W, Kheolamai P, U-Pratya Y, Supokawej A, Wattanapanitch M, Laowtammathron C, Issaragrisil S, Effects of mesenchymal stem cell-derived cytokines on the functional properties of endothelial progenitor cells, Euro. J. Cell Biol 95 (2016) 153–163. 10.1016/j.ejcb.2016.02.001. [DOI] [PubMed] [Google Scholar]
- [26].Afzal MR, Haider HK, Idris NM, Jiang S, Ahmed RP, Ashraf M, Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid. Redox. Signal 12 (2010) 693–702. 10.1089/ars.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kamota T, Li TS, Morikage N, Murakami M, Ohshima M, Kubo M, Kobayashi T, Mikamo A, Ikeda Y, Matsuzaki M, Hamano K, Ischemic pre-conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J. Am. Coll. Cardio 53 (2009) 1814–1822. 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [28].Shi RZ, Wang JC, Huang SH, Wang XJ, Li QP, Angiotensin II induces vascular endothelial factor synthesis in mesenchymal stem cells. Exp. Cell Res 315 (2009) 10–15. 10.1016/j.yexcr.2008.09.024. [DOI] [PubMed] [Google Scholar]
- [29].Tang JM, Wang JN, Guo LY, Kong X, Yang JY, Zheng F, Zhang L, Huang YZ, Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol. Cells 29 (1010) 9–19. 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- [30].Ranganath SH, Levy O, Inamdar MS, Jeffrey KM, Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 10 (2012) 244–258. 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barthes J, Özçelik H, Hindié M, Ndreu-Halili A, Hasan A, Vrana NE, 2014. Cell microenvironment engineering and monitoring for tissue and regenerative medicine: The recent advances. BioMed Res. Int 2014, 921905 https://doi.org/10/1155/2014/921905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Seib FP, Prewitz M, Werner C, Bornhäuser M, Matrix elasticity regulates the secretary profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs). Biochem. Biophys. Res. Commun 389 (2009) 663–667. 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- [33].Li J, Wu Y, Schimmel N, Al-Ameen MA, Ghosh G, Breast cancer cells mechanosensing in engineered matrices: Correlation with aggressive phenotype. J. Mech. Beh. Biomed. Mat 61 (2016) 208–220. 10.1016/j.jmbbm.2016.01.021. [DOI] [PubMed] [Google Scholar]
- [34].El-Mohri H, Wu Y, Mohanty S, Ghosh G, Impact of matrix stiffness on fibroblast function. Mat. Sci. Engi 74 (2017) 146–151. 10.1016/j.msec.2017.02.001. [DOI] [PubMed] [Google Scholar]
- [35].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A, Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 31 (2010) 5536–5544. 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu Y, Al-Ameen MA, Ghosh G, Integrated effects of matrix mechanics and vascular endothelial growth factor (VEGF) on capillary sprouting, Ann. Biomed. Eng 42 (2014) 1024–1036. 10.1007/s10439-014-0987-7. [DOI] [PubMed] [Google Scholar]
- [37].Achterberg V, Buscemi L, Diekmann H, Smith-Clerc J, Schwengler H, Miester J, Wenck H, Gallinat S, Hinz B, The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol 134 (2014) 1139–1143. [DOI] [PubMed] [Google Scholar]
- [38].Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S, The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TFG-β. Biomaterials. 32 (2011) 3921–3930. 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Keeley EC, Mehrad B, Strieter RM, Chemokines as mediators of neovascularization. Arteriosclerosis, Thrombosis, and Vascular Biology. 28 (2008) 1928–1936. 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Engler AJ, Sens S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification. Cell. 126 (2006) 677–689. https://doi.org/10/1016/j/cell/2006.06.044. [DOI] [PubMed] [Google Scholar]
- [41].Gilbert PM, Weaver VM, Cellular adaptation to biomechanical stress across length scales in tissue homeostasis and disease. Seminars in Cell and Developmental Biol. 67 (2017) 141–152. 10.1016/j.semcdb.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kusuma GD, Carthew J, Lim R, Frith JE, Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells and Development. 26 (2017) 617–631. 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- [43].Abdeen AA, Weiss JB, Lee J, Kilian KA, Matrix composition and mechanics direct proangiogenic signaling from mesenchymal stem cell. Tissue Eng. Part A 20 (2014) 2737–2745. 10.1089/ten/tea.2013.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mason BN, Reinhart-King CA, Controlling the mechanical properties of three-dimensional matrices via non-enzymatic collagen glycation. Organogenesis. 9 (2013) 70–75. 10.4161/org.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Murphy KC, Stilhano RS, Mitra D, Zhou D, Batarni S, Silva EA, Leach JK, Hydrogel biophysical properties instruct coculture-mediated osteogenic potential. The FASEB Journal. 30 (2016) 477–486. 10.1096/fj.15-279984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.