Abstract
Hypoparathyroidism is a rare disorder that is associated with abnormal bone properties. Recombinant human parathyroid hormone (1–84) [rhPTH(1–84)] in short-term studies has beneficial skeletal effects. Although rhPTH(1–84) will likely be used indefinitely, long-term effects on skeletal microstructure are unknown. We therefore studied histomorphometric changes with transiliac crest bone biopsies before and after 8.3±1 years of rhPTH(1–84) in 13 hypoparathyroid subjects compared with 45 controls. Before institution of rhPTH(1–84), skeletal remodeling indices were markedly suppressed. With long-term treatment, indices of bone remodeling increased. Mineralizing surface increased by 26-fold (0.3±1 to 7.9±7%, p=0.003), bone formation rate increased by 15-fold (0.003±0.01 to 0.047±0.05 µm2/µm/day, p=0.007), osteoid width doubled (1.9±1 to 4.3±1 lamellae, p=0.017), and osteoid surface tripled (3.3±3 to 10.8±6%, p=0.011). Bone resorption as measured by eroded surface increased (4.6±2 to 7.5±3%, p=0.021). Structural changes demonstrated intratrabecular tunneling, with increases in cancellous bone volume (19.6±5 to 29.1±11%, p=0.017) and trabecular number (1.8±1 to 2.5±1 #/mm, p=0.025). Cortical porosity tended to increase (6.3±5 to 9.5±3%, p=0.07). Mineralizing surface, osteoid surface, and eroded surface surpassed control levels, as did cancellous bone volume, trabecular number, and cortical porosity. These data, the first to reflect such long exposure of any PTH for any disease, illustrate that PTH establishes and maintains a new skeletal state for at least 8 years in hypoparathyroidism.
Keywords: HYPOPARATHYROIDISM, HISTOMORPHOMETRY, RHPTH(1-84), TRABECULAR TUNNELING, CORTICAL POROSITY
Introduction
Hypoparathyroidism (HypoPT) is a rare disorder characterized by hypocalcemia and insufficient or absent PTH levels.(1) In addition to characteristic biochemical abnormalities, bone remodeling is markedly reduced(1–5) with BMD that is above average.(1,2,6–8) We previously reported that percutaneous iliac crest bone biopsies from subjects with hypoparathyroidism demonstrated skeletal abnormalities including greater cancellous bone volume and cortical thickness.(2) Histomorphometric analyses also showed that remodeling indices, including mineralizing surface and bone formation rate, were profoundly suppressed in hypoparathyroid subjects.(2)
In 2015, the US Food and Drug Administration approved the use of human recombinant PTH(1–84) [rhPTH(1–84)] for the management of hypoparathyroidism.(9) Marked improvements in hypoparathyroid bone properties have been observed with the short-term use of rhPTH(1–84). We reported, for example, that after 2 years of rhPTH(1–84) therapy, trabecular number and cortical porosity increased.(6) Histomorphometrically measured remodeling parameters, including mineralizing surface, increased as early as 3 months, peaked at 1 year and remained elevated at 2 years.(6) These data suggested that rhPTH(1–84) treatment for 2 years in hypoparathyroidism has the potential to restore abnormal skeletal properties towards normal. Similar findings were reported for the short-term treatment of hypoparathyroidism with PTH(1–34).(10) However, given the chronic nature of hypoparathyroidism and the likelihood that rhPTH(1–84) will be used long-term in this disease, more extended data on the skeletal effects of PTH are needed. We hypothesized that the long-term use of rhPTH(1–84) in hypoparathyroidism would have persistent effects to restore abnormal skeletal structure and function to within the normal range.
Subjects and Methods
The diagnosis of chronic hypoparathyroidism in women and men was established by the requirement for supplemental calcium and/or active vitamin D to maintain serum calcium in the low-normal range along with an undetectable or insufficient serum PTH concentration. Hypoparathyroidism was present for at least 1 year to establish the chronic hypoparathyroid state. Subjects were excluded if they had ever been treated with PTH (1–34) or rhPTH(1–84). Patients with a history consistent with a CaSR mutation (eg, a family history of hypocalcemia or a lack of hypocalcemic symptoms with concomitant low serum calcium levels) were also excluded. Patients were recruited from the Metabolic Bone Diseases Unit of Columbia University Medical Center (New York, NY, USA) and from the Hypoparathyroidism Association (Idaho Falls, ID, USA). Of the 13 patients in this report, 12 were described in the previous 2-year report.(6) The prior report included paired biopsies at baseline and at 1 or 2 years of rhPTH(1–84) treatment, or a quadruple-label protocol, in which two sets of tetracycline labels were sequentially administered, before rhPTH(1–84) initiation and after 3 months of PTH(1–84) administration.(6) The subject added to the current report underwent baseline and long-term biopsies, but did not have a short-term biopsy at 1 or 2 years.
Forty-five control subjects were randomly selected from four previous studies, which included 22 postmenopausal women,(11) 12 premenopausal women,(12) and 11 men.(13) There was no history of low trauma fractures in any of the controls, as well as no history of medical illness or drug therapy known to affect bone metabolism. The study was approved by the Institutional Review Boards of Columbia University Medical Center (New York, NY, USA) and Helen Hayes Hospital (West Haverstraw, NY, USA). All subjects gave written informed consent.
Protocol
Hypoparathyroid subjects self-administered rhPTH(1–84), provided by NPS Pharmaceuticals (Bedminster, NJ, USA)/Shire Pharmaceuticals (Lexington, MA, USA) continuously for 6 to 10 years. At the initiation of the study, rhPTH(1–84) was only available at the 100-mg dose; we initially used 100 µg s.c. every other day for all subjects because we found that this regimen restored reduced bone turnover markers in hypoparathyroidism to levels that are within the normal range.(14) The availability of lower doses of rhPTH(1–84) during the course of this study made it possible to follow a titration schedule in which the dose could be increased or decreased.(15) The mean length of treatment with PTH 100 µg s.c. every other day before the switch to daily dosing was 2.9±1 years. The dose was adjusted during the study for almost all patients. rhPTH(1–84) dosages at study conclusion were 25 mg/day (n=1), 50 µg/day (n=7), 75 µg/day (n=3), and 100 µg/day (n=2). Calciotropic parameters and BMD were monitored as previously described.(15)
Histomorphometry
At baseline, two tetracycline labels were administered (Sumycin 250 mg 4 times daily) using a standard 3 days-on, 12 days-off, 3 days-on regimen in 8 subjects immediately prior to initiation of rhPTH(1–84).(6) Baseline percutaneous iliac crest biopsies were performed 1 week after labeling. The other 5 subjects underwent the quadruple-label protocol, in which two sets of tetracycline labels were sequentially administered before rhPTH (1–84) initiation and 2 weeks prior to the biopsy, which was obtained after 3 months of PTH(1–84) administration.(6) Baseline structural parameters could not be obtained from the five quadruple-label biopsies, but baseline remodeling data were obtained from the first set of double labels. For the long-term biopsy, the labeling schedule was repeated for all subjects (n=13) between 6 to 10 years of rhPTH(1–84) treatment, with percutaneous iliac crest biopsies performed 1 week after the long-term double-label protocol.
Biopsy specimens were processed and subjected to histomorphometric analysis as previously described in detail from our laboratory.(16) Histomorphometry was performed using a digitizing image-analysis system OsteoMeasure (OsteoMetrics, Inc., Atlanta, GA, USA). Cancellous and cortical bone structures were assessed by measuring cancellous bone volume (BV/TV), trabecular width (Tb.Wi), trabecular number (Tb.No), trabecular separation (Tb. Sp), cortical width (Ct.Wi), and cortical porosity (Ct. Po). Bone remodeling activity was evaluated on cancellous, endocortical, and intracortical bone surfaces and expressed by the variables of osteoid surface (OS/BS), osteoid width (O.Wi), mineralizing surface (MS/BS), mineral apposition rate (MAR), bone formation rate (BFR), and eroded surface (ES/BS). We expressed all indices according to the recommendations of the ASBMR nomenclature committee.(17)
Statistical analysis
Statistical analyses were performed using PASW Statistics 18 for Windows (SPSS, Chicago, IL, USA). All continuous data are presented as mean value±SD. The significance of changes within hypoparathyroid paired samples from baseline to long- term was assessed with the Wilcoxon signed rank test to account for any violations of normality. Hypoparathyroid subjects were compared with controls (n=45) at baseline and at conclusion by regression with adjustment for age and sex as covariates. An additional comparison with controls was performed by selecting 13 age- (±5 years) and sex-matched control subjects from all controls (n=45) and comparing them with the 13 hypoparathyroid patients at baseline (8 matched controls were selected for the 8 hypoparathyroid patients with baseline structural data). Thirteen older age- (±5 years) and sex-matched controls were selected from all controls (n=45) and compared with the 13 hypoparathyroid subjects at conclusion. The Mann-Whitney U test was used to determine significance of differences between hypoparathyroid subjects and controls at baseline and at conclusion. A value of p < 0.05 was considered significant.
Results
Subjects
The hypoparathyroid subjects were predominantly women, consistent with the demographics of the disease (Table 1). The control subjects included 11 men and 34 women (17 postmenopausal). Approximately half of the hypoparathyroid etiologies were the result of postsurgical consequences and the other half were caused by an idiopathic process. The patients with idiopathic hypoparathyroidism did not show involvement of other endocrine glands. Eight subjects had the first biopsy at baseline; 5 subjects had an initial quadruple label biopsy after 3 months of rhPTH(1–84). All subjects (n=13) had a repeat biopsy after 6 to 10 years of continuous rhPTH(1–84) treatment; the average duration of therapy was 8.3±1 years. TSH levels were generally within normal limits, but in 4 subjects the TSH level was below (range 0.02 to 0.06 µU/mL) the normal range. No individuals had evidence for clinical hypo- or hyperthyroidism. Abnormal TSH values had no effect on the outcomes (see below).
Table 1.
Characteristics of Hypoparathyroid Cohort at Baseline ± SD
| Hypoparathyroid cohort n = 13 | Cohort range | Normal range | |
|---|---|---|---|
| Age, years | 44.7±16.0 | 25.0–71.0 | |
| Sex | Male: 4 Female: 9 (premenopausal: 6, postmenopausal: 3) |
||
| Etiology of hypoparathyroidism | Postsurgical = 7 Idiopathic = 6 |
||
| Duration, years | 14.2±14.0 | 3.0–41.0 | |
| Calcium supplements, mg/day (median) | 3276±2857 | 1000–11,000 (2400) | |
| Calcitriol supplements, μg/day (median) | 0.8±1.0 | 0.0–3.0 (0.5) | |
| Parent vitamin D supplements, IU/day n = 9 (median) | 20,263±34,683 | 400–100,000 (1000) | |
| Thiazide dose, mg, n = 6 (median) | 37.5±34.0 | 12.5–100.0 (25) | |
| Serum calcium, mmol/La | 2.1±1.0 | 1.8–2.5 | 2.1–2.6 |
| PTH, ng/L | 2.9±3.0 | <3.0–14.0 | 10.0–65.0 |
| Phosphate, mmol/L | 1.4±1.0 | 1.0–1.7 | 0.8–1.5 |
| Total alkaline phosphatase, U/L | 58.4±8.0 | 47.0–71.0 | 33.0–96.0 |
| 24-hour urinary calcium excretion, mmol/24 hours | 6.5±2.0 | 2.9–10.3 | |
| 25-hydroxyvitamin D, nmol/L | 127.6±110 | 49.4–429.3 | 50.0–125.0 |
| TSH, µU/mL | 0.9±1.0 | 0.02–2.9 | 0.4–4.7 |
| Bone mineral density DXA Z-scores | |||
| Lumbar spine | +1.9±1.0 | −0.41− + 4.21 | |
| Femoral neck | +1.4±1.0 | −0.39− +3.50 | |
| Total hip | +1.5±1.0 | +0.09−+4.36 | |
| Distal 1/3 radius | +0.9±1.0 | −1.70−+2.09 | |
Measured 3 times.
Untreated hypoparathyroid subjects versus controls
Baseline biopsies for structural analysis were obtained on 8 hypoparathyroid subjects. These were compared with data from controls, both by comparison with all 45 controls with adjustment for age and sex and by matching with 8 age- and sex-matched controls. Hypoparathyroid subjects had increased cortical width as compared with controls, whereas cancellous bone volume, trabecular width, trabecular number, trabecular separation, and cortical porosity did not differ (Table 2). In comparison with all 45 controls with adjustment for age and race, baseline histomorphometric indices of bone turnover (Table 2) in the 13 hypoparathyroid subjects (8 with the standard tetracycline protocol and on the pretreatment set of labels in the 5 subjects with the quadruple label 1protocol) showed significantly reduced mineralizing surface on the cancellous, endocortical, and intracortical surfaces. Osteoid width and bone formation rate were also lower in the cancellous and endocortical surfaces as compared with controls, with decreased osteoid surface and mineral apposition rate in cancellous and intracortical surfaces and reduced adjusted apposition rate in the cancellous surface. The comparison of bone turnover indices using the 13 matched controls showed similar results.
Table 2.
Hypoparathyroid Samples at Baseline Versus Control Samples ± SD
| Hypo-BL | Matched control-BL | P-value Hypopara-BL vs matched control-BL | Control | P-value Hypopara-BL vs control adjusted for age and gender | |
|---|---|---|---|---|---|
| Bone structure | n = 8 | n = 8 | n = 45 | ||
| Age, years (range) |
50.9±16 (25–71) |
50.1±16 (22–71) |
0.93 | 50.4±13 (20–71) |
|
| Gender (n) | |||||
| Male | 3 | 3 | 11 | ||
| Premenopausal | 3 | 3 | 17 | ||
| Postmenopausal | 2 | 2 | 17 | ||
| BV/TV % | 19.6±5.0 | 16.1±4.0 | 0.16 | 19.8±5.0 | 0.90 |
| Tb.Wi µm | 111.7±22 | 101.9±13 | 0.20 | 114.8±20.0 | 0.70 |
| Tb.N #/mm | 1.8±01 | 1.6±1.0 | 0.33 | 1.74±1.0 | 0.55 |
| Tb.Sp mm | 474.0±117 | 573.5±201.0 | 0.44 | 484.7±131 | 0.59 |
| Ct.Wi µm | 1162.3±640 | 652.4±186.0 | 0.021 | 748.4±247 | 0.003 |
| Cortical porosity % | 6.3±5 | 5.3±1 | 0.88 | 7.4±4.0 | 0.36 |
| Bone remodeling | n = 13 | n = 13 | n = 45 | ||
| Age, years (range) |
44.7±16.0 (25–71) |
44.3±16.0 (20–71) |
0.95 | 50.4±13 (20–71) |
|
| Gender | |||||
| Male | 4 | 4 | 11 | ||
| Premenopausal | 6 | 6 | 17 | ||
| Postmenopausal | 3 | 3 | 17 | ||
| Cancellous envelope | |||||
| O.Wi Lamellar# | 1.9±1.0 | 3.9±2.0 | 0.009 | 4.1±1.0 | <0.001 |
| OS/BS % | 3.3±3.0 | 6.7±3.0 | 0.011 | 7.2±4.0 | 0.010 |
| MS/BS % | 0.3±1.0 | 4.0±3.0 | <0.001 | 4.3±3.0 | <0.001 |
| MAR µm/day | 0.4±1.0 | 0.8±1.0 | 0.003 | 0.7±1.0 | 0.001 |
| BFR/BS | 0.003±0.003 | 0.034±0.02 | <0.001 | 0.033±0.03 | 0.001 |
| Mm/µm2/day | |||||
| Aj.AR µm/day | 0.2±1.0 | 0.6±1.0 | 0.003 | 0.5±1.0 | 0.010 |
| ES/BS % | 4.6±2.0 | 4.6±2.0 | 0.90 | 4.2±2.0 | 0.571 |
| Endocortical envelope | |||||
| O.Wi Lamellar# | 1.9±1.0 | 3.1±2.0 | 0.091 | 3.2±1.0 | <0.001 |
| OS/BS, % | 10.5±11.0 | 11.3±7.0 | 0.55 | 12.6±7.0 | 0.405 |
| MS/BS % | 1.4±2.0 | 7.9±7 | <0.001 | 9.9±10 | 0.009 |
| MAR mm/d | 0.5±1.0 | 0.8±0.3 | 0.046 | 0.7±1.0 | 0.061 |
| BFR/BS % | 0.012±0.02 | 0.070±0.07 | 0.001 | 0.077±0.08 | 0.018 |
| Mm/µm2/day | |||||
| Aj.AR µm/d | 0.3±1.0 | 1.2±3.0 | 0.045 | 0.80±2.0 | 0.128 |
| ES/BS % | 5.1±3.0 | 6.7±4.0 | 0.42 | 6.4±4.0 | 0.367 |
| Intracortical envelope | |||||
| O.Wi Lamellar# | 2.7±1.0 | 3.5±1.0 | 0.17 | 3.4±1.0 | 0.052 |
| OS/BS % | 5.8±5.0 | 9.0±7.0 | 0.11 | 10.2±7.0 | 0.047 |
| MS/BS % | 3.1±4.0 | 11.6±9.0 | 0.002 | 9.4±8.0 | 0.004 |
| MAR µm/day | 0.80±1.0 | 1.01±1.0 | 0.12 | 0.81±1.0 | 0.839 |
| BFR/BS | 0.035±0.05 | 0.120±0.10 | 0.003 | 0.088±0.08 | 0.030 |
| Mm/µm2/day | |||||
| Aj.AR µm/day | 1.0±1.0 | 1.7±2.0 | 0.24 | 1.1±1.0 | 0.783 |
| ES/BS % | 2.6±2.0 | 6.1±5.0 | 0.01 | 4.6±4.0 | 0.135 |
Hypo-BL = hypoparathyroid baseline; Matched Control-BL = control baseline; BV/TV = trabecular bone volume; Tb.Wi = trabecular width; Tb. N = trabecular number; Tb.Sp = trabecular separation; Ct.Wi = cortical width; O.Wi = osteoid width; OS/BS = osteoid surface; MS/BS = mineralizing surface; MAR = mineral apposition rate; BFR/BS = bone formation rate; AjAR = adjusted apposition rate; ES/BS = eroded surface.
Bold indicates significance at p < 0.05.
Long-term treatment of hypoparathyroid subjects with rhPTH(1–84)
With long-term rhPTH(1–84) treatment, cancellous bone volume and trabecular number increased significantly with a reciprocal, significant decrease in trabecular separation (Table 3, Fig. 1). These changes were consistent with trabecular tunneling (Fig. 2). Remodeling parameters showed an increase in osteoid width, mineralizing surface, bone formation rate, and eroded surface in all three envelopes, with an increase in osteoid surface at the cancellous and intracortical envelopes. Mineral apposition rate and adjusted apposition rate did not change in any envelope. At the cancellous envelope, osteoid width doubled, osteoid surface tripled, mineralizing surface increased 26-fold, and bone formation rate increased 15-fold (Table 3). In the postmenopausal women at baseline, the changes in structural (n=2) and remodeling (n=3) parameters did not reach significance, but they demonstrated similar trends as compared with the rest of the cohort (data not shown).
Table 3.
Paired Hypoparathyroid Samples Before and After Long-Term rhPTH(1–84) ± SD
| Hypo-BL | Hypo-LT | P-value | |
|---|---|---|---|
| Bone structure | n = 8 | n = 8 | |
| BV/TV % | 19.6±5.0 | 29.1±11.0 | 0.017 |
| Tb.Wi µm | 111.7±22.0 | 115.9±21.0 | 0.48 |
| Tb.N #/mm | 1.8±1.0 | 2.5±1.0 | 0.025 |
| Tb.Sp µm | 474.0±117.0 | 321.4±145 | 0.025 |
| Ct.Wi µm | 1162.3±640.0 | 967.2±347 | 0.67 |
| Cortical porosity % | 6.3±5.0 | 9.5±3.0 | 0.07 |
| Bone remodeling | n = 13 | n = 13 | |
| Cancellous envelope | |||
| O.Wi Lamellar# | 1.9±1.0 | 4.3±1.0 | 0.017 |
| OS/BS % | 3.3±3.0 | 10.8±6.0 | 0.011 |
| MS/BS % | 0.3±1.0 | 7.9±7.0 | 0.003 |
| MAR µm/day | 0.4±1.0 | 0.6±1.0 | 0.284 |
| BFR/BS Mm/µm2/day | 0.003±0.01 | 0.047±0.05 | 0.007 |
| Aj.AR µm/day | 0.2±1.0 | 0.4±1.0 | 0.161 |
| ES/BS % | 4.6±2.0 | 7.5±3.0 | 0.021 |
| Endocortical envelope | |||
| O.Wi Lamellar# | 1.9±1.0 | 3.5±2.0 | 0.016 |
| OS/BS % | 10.5±11 | 13.7±9.0 | 0.20 |
| MS/BS % | 1.4±2.0 | 11.0±12.0 | 0.005 |
| MAR µm/day | 0.5±1.0 | 0.5±1.0 | 0.225 |
| BFR/BS Mm/µm2/day | 0.012±0.02 | 0.070±0.07 | 0.011 |
| Aj.AR mm/day | 0.3±1.0 | 0.4±1.0 | 0.345 |
| ES/BS % | 5.1±3.0 | 9.7±5.0 | 0.014 |
| Intracortical envelope | |||
| O.Wi Lamellar# | 2.7±1.0 | 4.0±1.0 | 0.004 |
| OS/BS % | 5.8±5.0 | 11.1±7.0 | 0.028 |
| MS/BS % | 3.1±4.0 | 11.3±8.0 | 0.004 |
| MAR µm/day | 0.8±1.0 | 0. 7±1.0 | 0.26 |
| BFR/BS Mm/µm2/day | 0.035±0.05 | 0.078±0.06 | 0.033 |
| Aj.AR µm/day | 1.0±1.0 | 0.7±1.0 | 0.59 |
| ES/BS % | 2.6±2.0 | 7.1±4.0 | 0.006 |
Hypo-BL = hypoparathyroid baseline; Hypo-LT = hypoparathyroid long-term; BV/TV = trabecular bone volume; Tb.Wi = trabecular width; Tb.N = trabecular number; Tb.Sp = trabecular separation; Ct.Wi = cortical width; O.Wi = osteoid width; OS/BS = osteoid surface; MS/BS = mineralizing surface; MAR = mineral apposition rate; BFR/BS = bone formation rate; AjAR = adjusted apposition rate; ES/BS = eroded surface.
Bold indicates significance at p < 0.05.
Fig. 1.
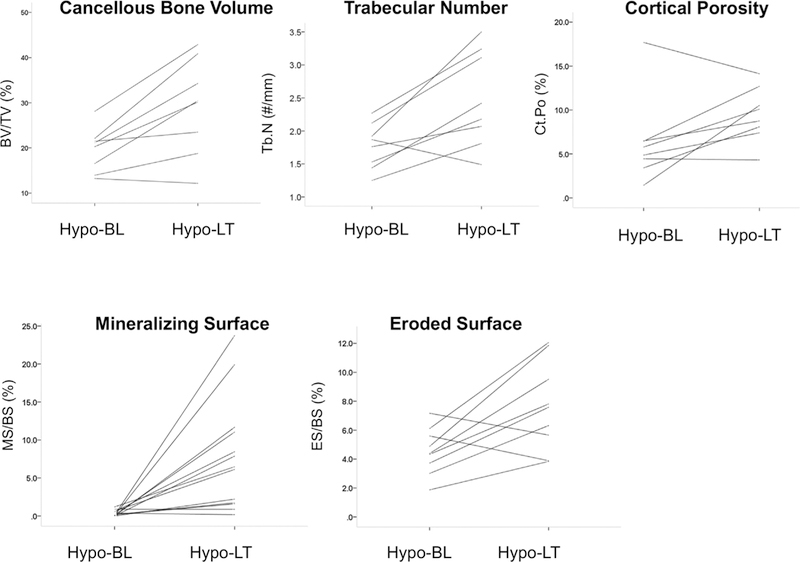
Histomorphometric variables in controls and in hypoparathyroid subjects before and after long-term rhPTH(1–84) treatment. Cancellous bone volume, trabecular number, cortical porosity, mineralizing surface, and eroded surface in the cancellous envelope increased. Hypo-BL = hypoparathyroid baseline; Hypo-LT = hypoparathyroid long-term.
Fig. 2.
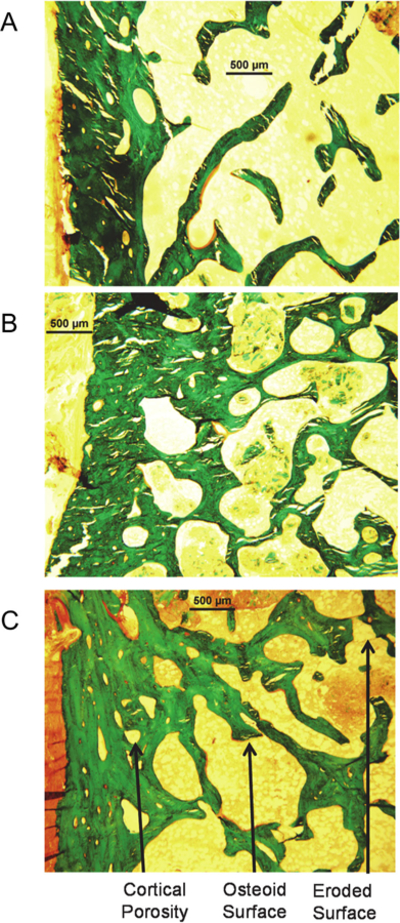
Representative histomorphometric images in a control subject and in a hypoparathyroid subject before and after 8 years of rhPTH(1–84) treatment. In comparison with the control image (A), the baseline untreated hypoparathyroid image (B) demonstrates increased cortical width. The long-term image (C) shows an increase in cortical porosity and trabecular tunneling, as well as in unmineralized osteoid (osteoid surface, in red) and eroded surface (arrow) as compared with the baseline hypoparathyroid image.
Earlier biopsies posttreatment initiation were available in 7 of the subjects, after either 1 (n=5) or 2 (n=2) years of rhPTH (1–84). In the 5 subjects who had biopsies at baseline, 1 year, and long-term, dynamic indices and cortical porosity did not increase further after the first year. MS/BS increased from baseline to year 1 (0.61±0.5 to 7.41±7.0%, p=0.04), but did not increase further between year 1 to long-term (5.23±4.1%, p=0.69 versus year 1). MAR decreased from year 1 to long- term (0.65±0.1 to 0.55±0.1 µm/day, p=0.04), with a trend towards a decrease in cortical porosity (11.77±5.4 to 9.08±3.5%, p=0.08). Conversely, trabecular indices continued to change long-term. Over the first year, Tb.N tended to increase (1.90±0.3 to 2.48±0.5 #/mm, p=0.08), with a further increase at long-term (2.87±0.6 #/µm, p=0.04 versus year 1). Trabecular separation reciprocally decreased from year 1 to long-term (310.5±116 to 242.2±82 mm, p=0.04 versus year 1). Data from the 2 patients with year 2 biopsies were not analyzed because of the small n.
In comparison with controls, cancellous bone volume and trabecular number were greater after long-term rhPTH(1–84), both by matching with 13 age- and sex-matched controls and by comparison with all 45 controls with adjustment for age and sex, whereas trabecular separation was lower (Table 4). Cortical porosity also exceeded control values. Eroded surface with long-term rhPTH(1–84) was greater at all envelopes and osteoid and mineralizing surfaces were greater in the cancellous envelope in comparison with all 45 controls with adjustment for age and sex. The other remodeling parameters did not differ from control values.
Table 4.
Hypoparathyroid Samples: Long-Term Versus Control Samples ± SD
| Hypo-LT (n = 13) | Matched control-LT (n = 13) | P-value Hypopara-LT vs matched control-LT | Control (n = 45) | P-value Hypopara-LT vs control adjusted for age and gender | |
|---|---|---|---|---|---|
| Age, years (range) |
52.9±16.0 (32–79) |
52.3±12.0 (34–71) |
0.18 | 50.4±13.0 (20–71) |
|
| Gender (n) | 1.0 | ||||
| Male | 4 | 4 | 11 | ||
| Premenopausal | 5 | 5 | 17 | ||
| Postmenopausal | 4 | 4 | 17 | ||
| Bone structure | |||||
| BV/TV % | 29.2±9.0 | 19.0±6.0 | 0.007 | 19.8±5.0 | <0.001 |
| Tb.Wi µm | 114. 9±20.0 | 115.0±26.0 | 0.76 | 114.8±20.0 | 0.94 |
| Tb.N #/mm | 2.5±1.0 | 1.6±1.0 | <0.001 | 1.7±1.0 | <0.001 |
| Tb.Sp µm | 308.3±120.0 | 530.9±182.0 | <0.001 | 484.7±131.0 | <0.001 |
| Ct.Wi µm | 851.05±327.0 | 676.1±207.0 | 0.14 | 748.4±247.0 | 0.23 |
| Cortical porosity % | 10.0±3.0 | 8.1±4.0 | 0.044 | 7.4±4.0 | 0.036 |
| Bone remodeling | |||||
| Cancellous envelope | |||||
| O.Wi Lamellar# | 4.3±1.0 | 4.1± 2.0 | 0.92 | 4.1±1.0 | 0.54 |
| OS/BS % | 10.8±6.0 | 8.9±6.0 | 0.45 | 7.2±4.0 | 0.023 |
| MS/BS % | 7.9±7.0 | 4.5±4.0 | 0.39 | 4.3±3.0 | 0.012 |
| MAR mm/day | 0.6±1.0 | 0.7±1.0 | 0.09 | 0.7±1.0 | 0.044 |
| BFR/BS | 0.047±0.05 | 0.034±0.03 | 0.61 | 0.033±0.03 | 0.112 |
| Mm/µm2/day | |||||
| Aj.AR µm/day | 0.4±1.0 | 0.3±1.0 | 0.76 | 0.5±1.0 | 0.362 |
| ES/BS % | 7.5±3.0 | 4.3±2.0 | 0.002 | 4.2±2.0 | <0.001 |
| Endocortical envelope | |||||
| O.Wi Lamellar# | 3.5±2.0 | 3.3±1.0 | 0.76 | 3.2±1.0 | 0.44 |
| OS/BS, % | 13.7±9.0 | 15.1±7.0 | 0.61 | 12.6±7.0 | 0.64 |
| MS/BS % | 11.0±12.0 | 9.9±7.0 | 0.92 | 9.9±10.0 | 0.72 |
| MAR µm/day | 0.5±1.0 | 0.7±1.0 | 0.12 | 0.7±1.0 | 0.066 |
| BFR/BS | 0.070±0.07 | 0.077±0.06 | 0.35 | 0.077±0.08 | 0.903 |
| Mm/µm2/day | |||||
| Aj.AR µm/day | 0.4±1.0 | 0.5±1.0 | 0.98 | 0.8±2.0 | 0.48 |
| ES/BS % | 9.7±5.0 | 5.9±3.0 | 0.029 | 6.4±4.0 | 0.015 |
| Intracortical envelope | |||||
| O.Wi Lamellar# | 4.0±1.0 | 3.6±1.0 | 0.42 | 3.4±1.0 | 0.195 |
| OS/BS % | 11.1±7.0 | 9.4±6.0 | 0.48 | 10.2±7.0 | 0.720 |
| MS/BS % | 11.3±8.0 | 8.9±6.0 | 0.58 | 9.4±8 | 0.431 |
| MAR µm/day | 0.7±1.0 | 0.9±1.0 | 0.06 | 0.8±1.0 | 0.238 |
| BFR/BS | 0.078±0.06 | 0.090±0.07 | 0.92 | 0.088±0.08 | 0.723 |
| Mm/µm2/day | |||||
| Aj.AR µm/day | 0.7±1.0 | 1.0±1.0 | 0.29 | 1.1±1.0 | 0.482 |
| ES/BS % | 7.1±4.0 | 4.5±4.0 | 0.057 | 4.6±4.0 | 0.015 |
Hypo-BL = hypoparathyroid baseline; Hypo-LT = hypoparathyroid long-term; Matched Control-LT = matched control long-term; BV/TV = trabecular bone volume; Tb.Wi = trabecular width; Tb.N = trabecular number; Tb.Sp = trabecular separation; Ct.Wi = cortical width; O.Wi = osteoid width; OS/ BS = osteoid surface; MS/BS = mineralizing surface; MAR = mineral apposition rate; BFR/BS = bone formation rate; AjAR = adjusted apposition rate; ES/ BS = eroded surface.
Bold indicates significance at p < 0.05.
Exclusion of the subjects with abnormal TSH values from the analysis did not alter any of these findings.
Discussion
Long-term treatment with rhPTH(1–84) in hypoparathyroid subjects induced significant and sustained changes in remodeling and structural indices of bone. These findings extend our earlier report that demonstrated reversal of baseline abnormalities in hypoparathyroid bone properties after 2 years of rhPTH(1–84) treatment.(6) We now show that marked increases in previously suppressed remodeling indices persist after an average of 8 years of treatment. With long-term treatment, indices of bone remodeling become comparable to or exceed euparathyroid values. Structural changes with long-term rhPTH(1–84) treatment include significant increases in cancellous bone volume, intratrabecular tunneling, and cortical porosity to levels that exceed those in controls. Given the chronic nature of hypoparathyroidism and the likelihood that rhPTH(1–84) will be used long term, these data provide key insights into the new skeletal state that is established and maintained with long-term PTH treatment.
In the absence of PTH, hypoparathyroidism is associated with low bone turnover, increased BMD, and altered skeletal micro-architecture.(2,3,6) Similar to our previous reports,(2,6) we found a reduction in bone formation prior to PTH treatment, as demonstrated by a profound reduction of tetracycline labeling compared with controls. Remodeling variables in cancellous, endocortical, and intracortical envelopes showed that bone formation indices, including bone formation rate and mineralizing surface, were consistently and substantially reduced across all envelopes. Reduced bone remodeling is likely to contribute to greater cortical width, as well as the greater cancellous bone volume as shown previously.(6) These findings are consistent with data obtained in hypoparathyroid subjects by mCT(18,19) and high-resolution peripheral CT.(20) The impact of these hypoparathyroid skeletal abnormalities on fracture risk is unclear.(21–23) Notably, in Danish case-control studies of hypoparathyroid patients in comparison with the general population, no difference in overall fracture rates was detected.(21,22)
We previously showed that bone formation increased as early as 3 months with rhPTH(1–84) administration, with indices peaking at 1 year and remaining higher than baseline at 2 years.(6) With an average of 8 years of treatment, we now observe that on all bone surfaces, osteoid width and bone formation rate increased significantly into the euparathyroid range. Certain remodeling parameters, interestingly, increased to values above controls: The osteoid surface and mineralizing surface exceeded control levels at the cancellous envelope and the eroded surface significantly exceeded normal values in all envelopes. Thus, with long-term rhPTH(1–84), many of the untreated baseline parameters are restored to and maintained at normal values with a suggestion that for some indices values above controls are seen.
With regard to trabecular changes, we previously showed that 2 years of rhPTH(1–84) led to a decrease in trabecular width at 1 year and an increase in trabecular number at 1 and 2 years.(6) After 8 years of rhPTH(1–84), the increase in trabecular number persists, along with a reciprocal decrease in trabecular separation and a 1.5-fold increase in cancellous bone volume. Intratrabecular tunneling, or splitting of thickened trabeculae by osteoclast penetration, was observed. Intratrabecular tunneling was also previously observed in our 1- and 2-year biopsies, as well as in a 6-month trial of rhPTH (1–84) utilizing quantitative CT.(19) Given that trabecular measures such as number and volume correlate with areal BMD (aBMD),(24) these long-term biopsy data are consistent with the increases in lumbar spine aBMD that we reported after 6 years of treatment.(15) Taken together, these data indicate that after nearly a decade of rhPTH(1–84), when given as a daily injection, a prolonged and continuous anabolic effect at cancellous sites is evident.
Enhanced intracortical resorption was also observed, as well as increased eroded surface on the endocortex. Our prior data showed that cortical porosity increased after 1 and 2 years of rhPTH(1–84),(6) as it similarly did in the 6-month rhPTH(1–84) study.(19) The overall increase in cortical porosity, long-term, is consistent with the persistent increase in intracortical mineralizing surface, as well as the trend toward a decrease in cortical width, presumably based on enhanced endocortical resorption and trabecularization of the cortex. These changes indicate a long-term and ongoing effect of rhPTH(1–84) to stimulate intracortical and endocortical resorption. It is consistent with the progressive decrease in cortical aBMD that we observed after 6 years of rhPTH(1–84).(15) Although the persistent nature of the cortical porosity might raise a concern vis-àvis cortical bone strength, the pores represent only a small percentage (<10%) of the total cortical area and the cortices are still thicker than normal. Notably, in hypoparathyroidism a different paradigm exists than in that of postmenopausal osteoporosis, where an increase in cortical remodeling accompanied by a decrease in cortical thickness could be problematic. In the setting of hypoparathyroidism though, it is theoretically possible that increased remodeling might result in the replacement of older, overly mature bone with younger and more resilient bone. A contrary view is that given that fracture risk is not increased in hypoparathyroidism, the increases in cortical porosity and eroded surface might indicate that bone turnover is overstimulated with our regimen. Fracture data are ultimately needed to determine whether the histomorphometric changes we observed after 8 years of rhPTH(1–84) injections have clinical consequences. Other rhPTH(1–84) dosing regimens, such as twice daily administration or a continuous infusion via a pump, might mitigate overstimulation of turnover, if it is present.
The structural and remodeling patterns that we observed are reminiscent of the effects of PTH treatment when used in osteoporosis, although the osteoporosis data are generally limited to 2 to 3 years of PTH exposure. In osteoporosis, rhPTH(1–34)—admittedly a foreshortened fragment of the full-length peptide—stimulates bone remodeling and increases cancellous bone volume, cortical thickness, and trabecular connectivity.(25–27) rhPTH(1–34) also induces intratrabecular tunneling in patients with osteoporosis(25) as does rhPTH(1–84),(28) supporting a similar mechanism of action as rhPTH(1–84) in hypoparathyroidism. Notably, the anabolic effect of teriparatide wanes over time in postmenopausal osteoporosis.(29) It is possible that in hypoparathyroidism, homeostatic adaptation to PTH treatment by regulators such as sclerostin(30) might be absent,(31) leading to persistent skeletal stimulation, although this has not been studied. Whether other effects on bone quality also transpire with rhPTH(1–84) in hypoparathyroidism, as they do in the treatment of postmenopausal osteoporosis, is unclear. With rhPTH(1–34) in osteoporosis, the replacement of old bone with newer bone results in reduced microcrack accumulation and improved quality and elasticity of the organic matrix, with altered matrix mineralization, mineral crystallinity, and collagen crosslink ratios.(32–35) In this regard, we reported transient changes in bone mineralization density distribution in hypoparathyroidism after 1 year of rhPTH(1–84).(36) It is possible that other mineral and matrix changes that alter bone quality become evident with long-term treatment.
A main strength of our study is the long duration of treatment with rhPTH(1–84), with some of our patients being treated for up to 10 years. With the exception of a single case,(37) this is the longest report, to our knowledge, of any PTH treatment for any condition. An additional strength is quantification of both endocortical and intracortical bone surfaces, in addition to cancellous surfaces. We recognize that the relatively small sample size is a limitation. Our sample size also precluded a comparison between surgical and nonsurgical hypoparathyroid groups. Given the rarity of this disease, however, and the fact that there are no large experimental cohorts, one could also consider the acquisition of 13 biopsies as a strength of the study. The lack of a control group untreated for the same period is a limitation, but the changes that we observed are inescapably attributable to PTH given our knowledge of the natural history of the disease. Our cohort was also limited by heterogeneity in our labeling protocol, although this did not affect the number of remodeling or long-term data points. Finally, as noted above, the impact of skeletal changes with long-term rhPTH(1–84) on fracture risk is unknown. Fracture incidence might be difficult to determine because of the rarity of this disease and the need for a larger number of subjects that are typically needed to ascertain fracture incidence in a tested population.
This study has shown, for the first time in a cohort of patients with hypoparathyroidism, that long-term treatment with rhPTH (1–84) induces significant and sustained changes in remodeling and structural indices of bone. Indices of bone remodeling and structure became comparable to or exceeded euparathyroid values. These findings, from the first systematic, histomorphometric study to reflect such a long exposure of any PTH for any disease, provide essential insights given the potential long-term use of rhPTH(1–84) in hypoparathyroidism. They suggest that a new skeletal state is established and sustained with long-term PTH treatment.
Acknowledgments
This work was supported by grants NIH R01 DK069350, DK32333, K23DK067619, R01 FD002525, and by NPS/Shire Pharmaceuticals.
Footnotes
Authors’ roles: Study design: MRR, DWD, NEC, and JPB. Study conduct: MRR, NEC, RM, BO, and MG. Data collection: MRR, HZ, NEC, TLN, RM, BO, and MG. Data analysis: HZ and MRR. Data interpretation: MRR, DWD, and JPB. Drafting manuscript: MRR, DWD, and JPB. MRR takes responsibility for the integrity of the data analysis.
Disclosures
JPB is a consultant for Amgen, Radius, Shire, Ultragenyx and Regeneron. MRR receives research support from Shire and Amgen. TLN receives research support from Amgen. DWD is a consultant for Eli Lilly, Amgen, Radius, and Tarsa and has received research support from Eli Lilly, Amgen and Radius. NEC is on the Speakers Bureau for Shire.
Public clinical trial registration: NCT00473265 Bone Properties in Hypoparathyroidism: Effects of PTH
References
- 1.Shoback D Clinical practice. Hypoparathyroidism. N Engl J Med 2008. July;359(4):391–403. Epub 2008 Jul 25. [DOI] [PubMed] [Google Scholar]
- 2.Rubin MR, Dempster DW, Zhou H, et al. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res 2008. December;23(12):2018–24. Epub 2008 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langdahl BL, Mortensen L, Vesterby A, Eriksen EF, Charles P. Bone histomorphometry in hypoparathyroid patients treated with vitamin D. Bone 1996. February;18(2):103–8. Epub 1996 Feb 1. [DOI] [PubMed] [Google Scholar]
- 4.Kruse K, Kracht U, Wohlfart K, Kruse U. Biochemical markers of bone turnover, intact serum parathyroid horn and renal calcium excretion in patients with pseudohypoparathyroidism and hypoparathyroidism before and during vitamin D treatment. Eur J Pediatr 1989. April;148(6):535–9. [DOI] [PubMed] [Google Scholar]
- 5.Mizunashi K, Furukawa Y, Miura R, Yumita S, Sohn HE, Yoshinaga K. Effects of active vitamin D3 and parathyroid hormone on the serum osteocalcin in idiopathic hypoparathyroidism and pseudohypoparathyroidism. J Clin Invest 1988. September;82(3):861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin MR, Dempster DW, Sliney J Jr., et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res 2011. November;26(11):2727–36. Epub 2011 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilezikian JP, Khan A, Potts JT, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 2011. October;26(10):2317–37. Epub 2011 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012. December;97(12):4507–14. Epub 2012 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Food and Drug Administration. NATPARA package insert 2015. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125511s000lbl.pdf Accessed July 30, 2018.
- 10.Gafni RI, Brahim JS, Andreopoulou P, et al. Daily parathyroid hormone 1–34 replacement therapy for hypoparathyroidism induces marked changes in bone turnover and structure. J Bone Miner Res 2012. August;27(8):1811–20. Epub 2012 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Recker RR, Kimmel DB, Parfitt AM, Davies KM, Keshawarz N, Hinders S. Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. J Bone Miner Res 1988. April;3(2):133–44. Epub 1988 Apr 1. [DOI] [PubMed] [Google Scholar]
- 12.Parisien M, Cosman F, Morgan D, et al. Histomorphometric assessment of bone mass, structure, and remodeling: a comparison between healthy black and white premenopausal women. J Bone Miner Res 1997. June;12(6):948–57. Epub 1997 Jun 1. [DOI] [PubMed] [Google Scholar]
- 13.Clarke BL, Ebeling PR, Jones JD, et al. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab 1996. June;81(6):2264–70. Epub 1996 Jun 1. [DOI] [PubMed] [Google Scholar]
- 14.Rubin MR, Sliney J Jr., McMahon DJ, Silverberg SJ, Bilezikian JP. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int 2010. November;21(11):1927–34. Epub 2010 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin MR, Cusano NE, Fan WW, et al. Therapy of hypoparathyroidism with PTH(1–84): a prospective six year investigation of efficacy and safety. J Clin Endocrinol Metab 2016. July;101(7):2742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempster DW, Parisien M, Silverberg SJ, et al. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 1999. May; 84(5):1562–6. [DOI] [PubMed] [Google Scholar]
- 17.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2013. January;28(1): 2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin MR, Dempster DW, Kohler T, et al. Three dimensional cancellous bone structure in hypoparathyroidism. Bone 2010. January;46(1):190–5. Epub 2009 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sikjaer T, Rejnmark L, Thomsen JS, et al. Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH (1–84): a randomized controlled study. J Bone Miner Res 2012. April;27(4):781–8. Epub 2011 Dec 14. [DOI] [PubMed] [Google Scholar]
- 20.Cusano NE, Nishiyama KK, Zhang C, et al. Noninvasive assessment of skeletal microstructure and estimated bone strength in hypoparathyroidism. J Bone Miner Res 2016;31(2):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Postsurgical hypoparathyroidism—risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res 2014. November;29(11): 2504–10. [DOI] [PubMed] [Google Scholar]
- 22.Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. The epidemiology of nonsurgical hypoparathyroidism in Denmark: a nationwide case finding study. J Bone Miner Res 2015. September;30(9):1738–44. [DOI] [PubMed] [Google Scholar]
- 23.Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J Bone Miner Res 2013. November;28(11):2277–85. [DOI] [PubMed] [Google Scholar]
- 24.Cosman F, Schnitzer MB, McCann PD, Parisien MV, Dempster DW, Lindsay R. Relationships between quantitative histological measurements and noninvasive assessments of bone mass. Bone 1992;13 (3):237–42. Epub 1992 Jan 1. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 2003. November;18(11):1932–41. Epub 2003 Nov 11. [DOI] [PubMed] [Google Scholar]
- 26.Stepan JJ, Burr DB, Li J, et al. Histomorphometric changes by teriparatide in alendronate-pretreated women with osteoporosis. Osteoporos Int 2010. December;21(12):2027–36. [DOI] [PubMed] [Google Scholar]
- 27.Ma YL, Zeng QQ, Chiang AY, et al. Effects of teriparatide on cortical histomorphometric variables in postmenopausal women with or without prior alendronate treatment. Bone 2014. February;59:139–47. [DOI] [PubMed] [Google Scholar]
- 28.Recker RR, Bare SP, Smith SY, et al. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone 2009. January;44(1):113–9. Epub 2008 Nov 6. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997;350(9077):550–5. [DOI] [PubMed] [Google Scholar]
- 30.Silvestrini G, Ballanti P, Leopizzi M, et al. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol 2007. August;38(4):261–9. [DOI] [PubMed] [Google Scholar]
- 31.Costa AG, Cremers S, Rubin MR, et al. Circulating sclerostin in disorders of parathyroid gland function. J Clin Endocrinol Metab 2011. December;96(12):3804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksen EF, Keaveny TM, Gallagher ER, Krege JH. Literature review: the effects of teriparatide therapy at the hip in patients with osteoporosis. Bone 2014. October;67:246–56. [DOI] [PubMed] [Google Scholar]
- 33.Dobnig H, Stepan JJ, Burr DB, et al. Teriparatide reduces bone microdamage accumulation in postmenopausal women previously treated with alendronate. J Bone Miner Res 2009. December;24(12):1998–2006. [DOI] [PubMed] [Google Scholar]
- 34.Paschalis EP, Glass EV, Donley DW, Eriksen EF. Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the fracture prevention trial. J Clin Endocrin Metabol 2005. August;90(8):4644–9. Epub 2005 May 26. [DOI] [PubMed] [Google Scholar]
- 35.Hofstetter B, Gamsjaeger S, Varga F, et al. Bone quality of the newest bone formed after two years of teriparatide therapy in patients who were previously treatment-naive or on long-term alendronate therapy. Osteoporos Int 2014. December;25(12):2709–19. [DOI] [PubMed] [Google Scholar]
- 36.Misof BM, Roschger P, Dempster DW, et al. PTH(1–84) Administration in hypoparathyroidism transiently reduces bone matrix mineralization. J Bone Miner Res 2016. January;31(1):180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theman TA, Collins MT, Dempster DW, et al. PTH(1–34) replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J Bone Miner Res 2009. May;24 (5):964–73. Epub 2008 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]


