ABSTRACT
Heavy metals are highly toxic elements that contaminate the global food supply and affect human and wildlife health. Purification technologies are often too expensive or not practically applicable for large-scale implementation, especially in impoverished nations where heavy metal contamination is widespread. Lactobacillus rhamnosus GR-1 (LGR-1) was shown in previous work to reduce heavy metal bioaccumulation in a Tanzanian cohort of women and children through indeterminant mechanisms. Here, it was hypothesized that LGR-1 could sequester the heavy metals lead (Pb) and cadmium (Cd), thereby reducing their absorption across intestinal epithelium. LGR-1 and other lactobacilli significantly reduced the amount of Pb and Cd in solution at all concentrations tested (0.5 mg/L – 50 mg/L) and exhibited sustained binding profiles over a 48-hour period. Relative binding efficiency of LGR-1 decreased as Pb concentration increased, with an absolute minimum binding threshold apparent at concentrations of 2 mg/L and above. Electron microscopy revealed that Pb formed irregular cell-surface clusters on LGR-1, while Cd appeared to form intracellular polymeric clusters. Additionally, LGR-1 was able to significantly reduce apical-to-basolateral translocation of Pb and Cd in a Caco-2 model of the intestinal epithelium. These findings demonstrate the absorbent properties of LGR-1 can immobilize Pb and Cd, effectively reducing their translocation across the intestinal epithelium in vitro. Oral administration of heavy metal-binding Lactobacillus spp. (many of which are known human symbionts and strains of established probiotics) may offer a simple and effective means to reduce the amount of heavy metals absorbed from foods in contaminated regions of the world.
KEYWORDS: heavy metals, xenobiotics, Probiotics, detoxification, cadmium, lead, environmental contaminants, dietary toxins, sequestration, lactobacilli
Introduction
Increased anthropologically-generated heavy metal pollution is a concern for human and wildlife health.1–3 Characterized by their high relative atomic densities (> 5 g/cm3), heavy metals are non-degradable inorganic pollutants that are often toxic to a wide variety of living biological organisms. Cadmium (Cd), lead (Pb), copper (Cu), manganese (Mn), and zinc (Zn) are the most prevalent heavy metals and major contributors to soil contamination.1 Whereas metals such as Cu, Mn, and Zn act as necessary cofactors in enzymatic reactions, no biological role is known for metals such as Cd and Pb. Lead batteries, coal combustion, petrol production, mining processes, and usage of pesticides and fertilizers are believed to be the primary sources of Cd and Pb contamination.4,5 Since these metals are not degraded in the environment, they are prone to bioaccumulation and biomagnification in the food chain.
The health effects of long-term low-dose exposure to Cd and Pb are well documented.6–8 The major risks associated with Cd exposure are renal damage9, osteoporosis10, and cancer.11,12 Alternatively, Pb exposure is associated with decreased birth weight, pre-term delivery, cognitive development delays in children, and neuropsychological deficits in adults that affect memory, mood, visuospatial and motor skills, attention, and executive functioning.8 These examples demonstrate that heavy metal exposure can pose significant health risks even at environmentally-relevant concentrations.
Regulatory guidelines that exist in many developed countries include screening of food, soil, and water sources for metal contamination. However, similar programs and technologies are not cost-effective options in developing nations where metal contamination is widespread with a lack of regulatory enforcement. For instance, lead is frequently found in drinking water at concentrations exceeding the WHO guidelines of 10 μg/L13-15, commonly due to corrosion of Pb-containing plumbing. Lead exposure may also come from ingestion of dust, soil and Pb-containing paints. Blood lead levels in Africa have been reported between 0.05 – 0.45 ppm, depending on occupational exposure.16–18 Beyond the widely known exposure risks of Cd through recreational tobacco use, non-smokers can be exposed to Cd through contaminated foods19 and proximity to industrial facilities. Blood cadmium levels have been reported from 0.02 – 0.11 ppm in Africa.17,18 In short, Cd and Pb can enter the gastrointestinal tract following consumption of contaminated food and water and/or ingestion of particles cleared through the respiratory mucociliary escalator.19 Intriguingly, the absorption of numerous xenobiotics (including toxic heavy metals) is known to be influenced by the collection of microorganisms residing in the gastrointestinal tract, known as the microbiota. For example, germ-free mice have been utilized to demonstrate that the intestinal microbiota can mitigate mercury20, Cd, and Pb21 absorption following oral exposure. Because of food regulations and environmental condition in Africa, the gut microbiota can be exposed to up to 2 ppm of Cd and 2 ppm of Pb, thus we will consider these levels as the reference for typical human gut exposure.22,23
Remediation of heavy metals using inorganic chelators and filters have shown success in decontaminating various water sources, however, widespread implementation is prohibited by cost, practicality, and sustainability.24,25 Several applications utilizing biomass to absorb metals (biosorption) from soil, water, and other media have also been described.26,27 In addition, adsorptive and absorptive properties of fungi, bacteria, and plants display great promise for environmental bioremediation of heavy metals.28–30 Specifically, cell wall peptidoglycan and lipoteichoic acid of Gram-positive bacteria are known to undergo electrostatic-mediated ion-exchange reactions with heavy metals, resulting in extracellular immobilization through precipitation and complexation with nitrogen and oxygen moieties.31,32
Despite studies supporting the use of microbes in bioremediation of environmental pollutants, much less is known about the effects of food-grade bacteria on preventing heavy metal uptake. However, it has been suggested that orally supplemented probiotic bacteria could also be useful for bioremediation of toxic heavy metals33, via sequestration from concurrently consumed food items and subsequent elimination through defecation. Lactobacillus is a promising genus of Gram-positive bacteria due to their safety, affordability, and widespread use in products such as supplemental probiotics, yogurt, cheese, sauerkraut, kimchi, kefir, pickles, and other fermented foods. Additionally, many strains of lactobacilli have been shown to bind and sequester a variety of heavy metals.34–36
Previous work demonstrated that mercury and arsenic bioaccumulation were reduced in a Tanzanian cohort of pregnant women and children supplemented with Lactobacillus rhamnosus GR-1 (LGR-1) yogurt compared to controls.17 However, the mechanisms of this LGR-1 mediated effect remains poorly characterized. We hypothesized that heavy metal binding properties of LGR-1 would immobilize Pb and Cd, thereby affecting their translocation across a Caco-2 model of the intestinal epithelium.
Results
In vitro sequestration of Pb and Cd by lactobacilli
L. plantarum 14917T has previously been shown to sequester Cd in vitro, thus it was used as a positive control.37 LGR-1, L. plantarum 14917T, and L. casei 393T were able to significantly reduce the amount of Pb and Cd in solution (Figure 1A-F). There was a trend of decreasing relative binding capacity at higher concentrations of Pb and Cd (Figure 1H). Relative binding capacity of Pb by LGR-1 was significantly greater at lower concentrations (0.5 mg/L; 75.56 ± 2.03% bound) compared to higher concentrations (50 mg/L; 39.83 + 2.08% bound; one-way ANOVA, P < 0.0001; Figure 1H-I). A similar trend was observed with LGR-1 being able to bind more Cd at low concentrations (0.5 mg/L; 56.78 ± 4.21% bound) than at high concentrations (50 mg/L; 44.29 ± 0.63% bound; one-way ANOVA, P = 0.0691). For cross-species comparison between different bacteria, particularly Gram-negative organisms, binding capacities of two Escherichia coli strains were also assessed. E. coli Co1 and E. coli 25922 were able to significantly reduce Cd in solution (1 mg/L; 72.41 ± 5.91% and 71.43 ± 3.94% bound, respectively; one-way ANOVA, P < 0.01), though neither strain could significantly reduce Pb (Figure 1J-K).
Figure 1.
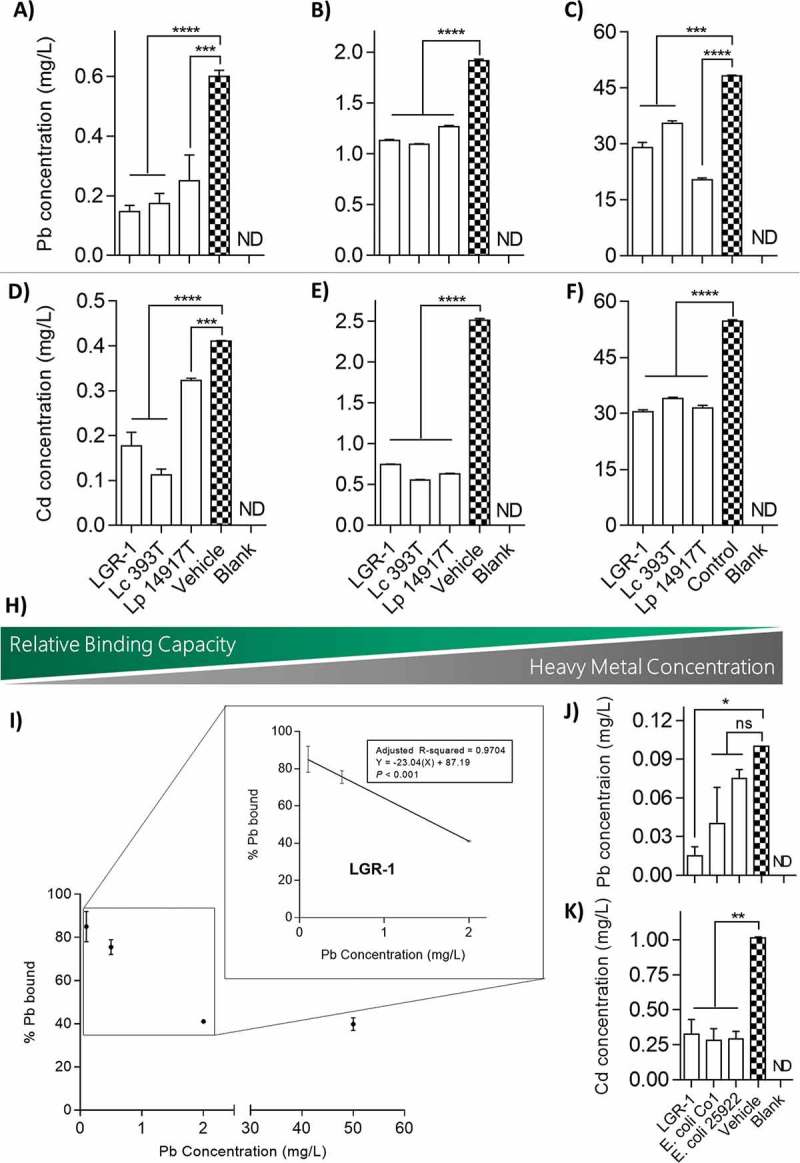
In vitro sequestration of Pb and Cd by lactobacilli. Overnight cultures of lactobacilli were re-suspended in 50 mM HEPES buffer with Pb at concentrations of (A) 0.5 mg/L, (B) 2.0 mg/L, and (C) 50 mg/L, or alternatively with Cd at concentrations of (D) 0.5 mg/L, (E) 2.5 mg/L, and (F) 50 mg/L. (H) Visual representation of the inverse relationship between relative binding capacity of lactobacilli and concentration of Pb and Cd in solution. (I) Linear regression analysis of relative binding potential of LGR-1 compared to amount of Pb in solution. Binding potential of (J) Pb at a concentration of 0.1 mg/L and (K) Cd at a concentration of 1.0 mg/L was compared between LGR-1 and two strains of lab-strain E. coli. All experiments were performed from 3 independent experiment and analyzed in triplicate. Pb and Cd were quantified using an inductively coupled plasma atomic emission spectrophotometer. Error bars represent ± standard error. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. ND = not detected. ns = not significant.
Binding of Pb and Cd by lactobacilli is consistent over time and independent of cell viability
To determine binding kinetics of lactobacilli, bacterial strains were incubated with the metals of interest and free Pb or Cd was measured at various time points over a 48-hour period. Minimal change in bound Pb or Cd was observed from 15 min to 48 h post-incubation for both LGR-1 and L. plantarum 14917T (Figure 2A-B). To characterize the heavy metal sequestration mechanism as a passive or impactive process, bacteria were heat-inactivated and then incubated with the metals of interest. Heat-killed LGR-1 showed no significant difference in binding of Pb or Cd (unpaired, two-tailed t tests, P = 0.5654 and P = 0.9948, respectively) compared to live LGR-1, which suggested a passive binding mechanism (Figure 2C-D).
Figure 2.
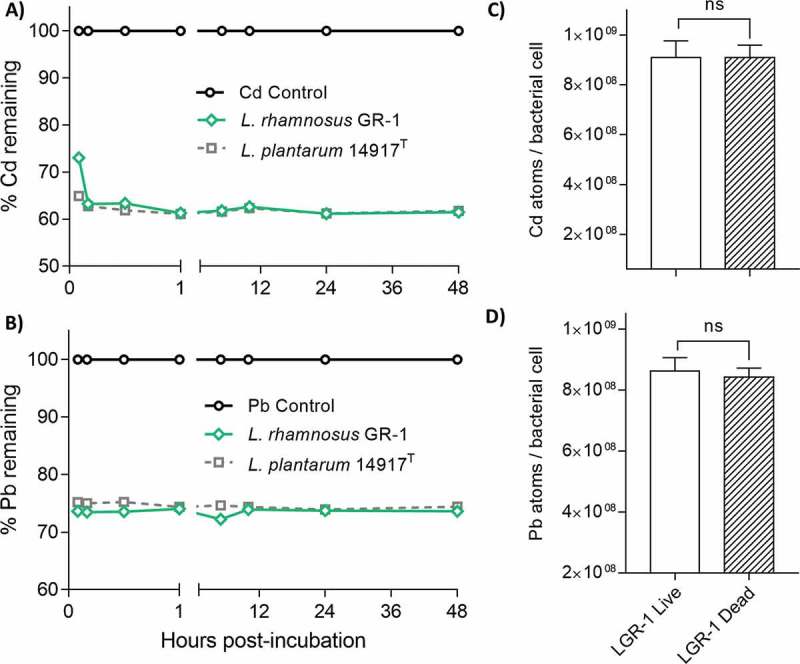
Binding of Pb and Cd by lactobacilli is consistent over time and independent of cell viability. Overnight cultures of lactobacilli were re-suspended in 50 mM HEPES buffer 0.5 mM (A) Pb and (B) Cd and subsequently monitored over a 48-hour period. Pb and Cd vehicle control groups represent non-inoculated 50 mM HEPES buffer containing 0.5 mM Pb or Cd. Each graph line represents a different biological replicate measured consecutively in triplicate over 48 hours. The binding efficiency between live and dead LGR-1 was compared by heat-inactivating LGR-1 cultures at 80 C for 1 hour. Live and dead LGR-1 were suspended in 50 mM HEPES with 0.5 mg/L (C) Cd and (D) Pb and incubated for 5 mins prior to quantification. Experiments were performed 3 times and analyzed in triplicate. Heat-inactivation experiments were performed in biological triplicate. Pb and Cd were quantified using an inductively coupled plasma atomic emission spectrophotometer. Error bars represent ± standard error. ns = not significant.
Scanning electron microscopy (SEM) and x-ray analysis revealed metal precipitates present in LGR-1
To gain a better visual representation of sequestration, LGR-1 was incubated with 0.1 mM Pb or Cd and then visualized using SEM. Micrographs showed bright white particle clusters on the surface of LGR-1 cells (Figure 3A-B), suggesting potential surface binding of Pb (which distinctively illuminates bright white upon exposure to X-rays). Excitation by X-rays combined with a backscatter detector was used to excite the elements and visualize them (Figure 3C-D). Energy dispersive X-ray (EDX) microanalysis confirmed the identity of visualized precipitates to be Pb or Cd, and not any other non-specific signals (Figure 3E-F). Different peaks of the metals appear in the spectrum due to X-ray-mediated excitation of electrons in different orbitals (K, L or M), each displaying a unique signal. To assay for heavy metal internalization, transmission electron microscopy (TEM) was used on ultra thin sections of lactobacilli incubated with either Pb or Cd at 0.1 mM. Analysis of thin sections of LGR-1 indicated the occurrence of intracellular colloidal Cd bioaccumulation (Figure 3E-G). Cd generally appeared as two dense and dark (resultant of characteristic electron reflection) precipitates inside the cell compared to the surrounding area, giving them distinctive contrast inside LGR-1 cells (Figure 3G). In corroboration with these findings, no similar precipitates were found in control cells (Figure 3A) or cells treated with Pb alone (Figure 3A-B).
Figure 3.
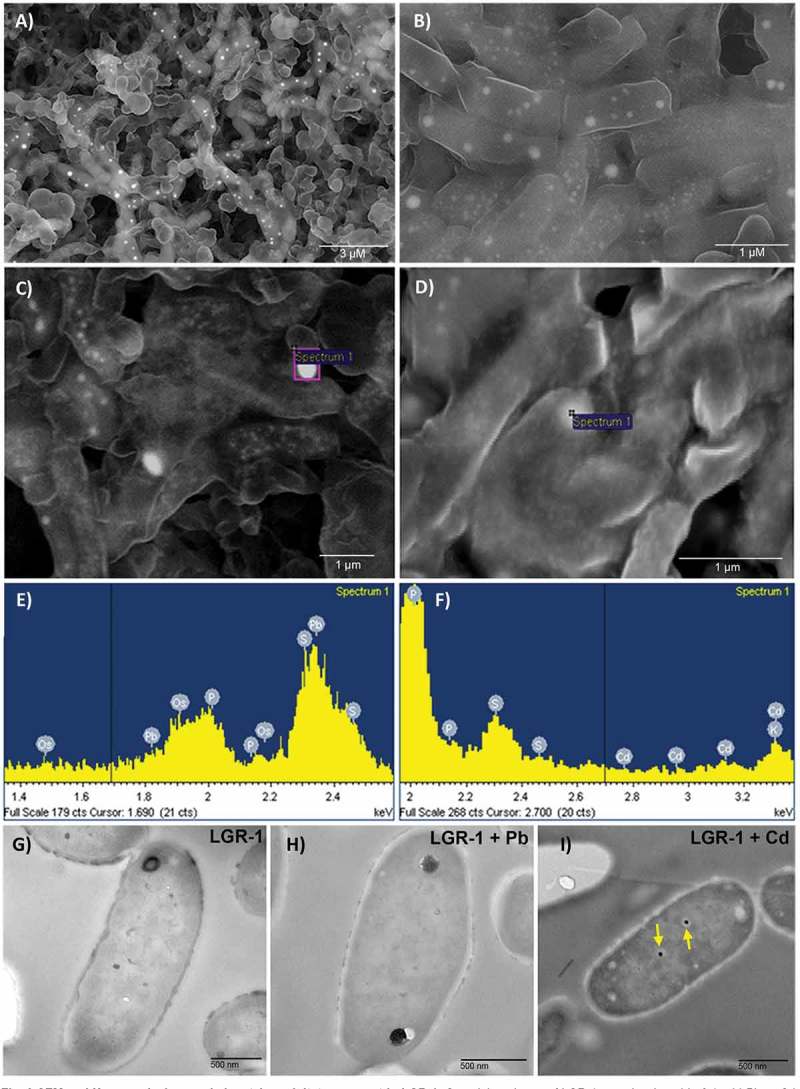
SEM and X-ray analysis revealed metal precipitates present in LGR-1. Overnight cultures of LGR-1 were incubated in 0.1 mM Pb or Cd prior to visualization at the microscopic level. (A-B) SEM micrographs of LGR-1 incubated with 0.1 mM Pb. Bright white clusters represent cell surface precipitates of Pb excited by X-rays with an excitation energy source of 15 kV. (C-F) Energy dispersive X-ray analysis of metal treated bacterial samples. Spectra show all elements detected in the area of analysis, the heavy metals Pb (C) and Cd (D) are highlighted in each sample confirming their presence. Beam energy was set in the range of 5 kV-25 kV to excite bound metals as needed and INCA® software used for analysis (E-F) TEM micrographs from ultra thin sections of (G) control LGR-1 cells, (H) LGR-1 incubated with 0.1 mM Pb, and (I) LGR-1 incubated with 0.1 mM Cd. Yellow arrows highlight the two colloidal precipitates of cadmium. SEM images were taken using a LEO 1540XB FIB lithography filter. Ultra thin slices were viewed at 60 kV with a Philips EM410 TEM.
Lactobacilli reduce toxicity and apical-to-basolateral translocation of Pb and Cd in a caco-2 cell model of the intestine epithelium
Caco-2 cell (intestinal cell line) survival following exposure to Cd for 5-hours was significantly improved when pre-supplemented with LGR-1 (Figure4 A-D). In contrast, the concentrations of Pb used in this study did not display any toxicity towards the Caco-2 cell line (data not included).
Figure 4.
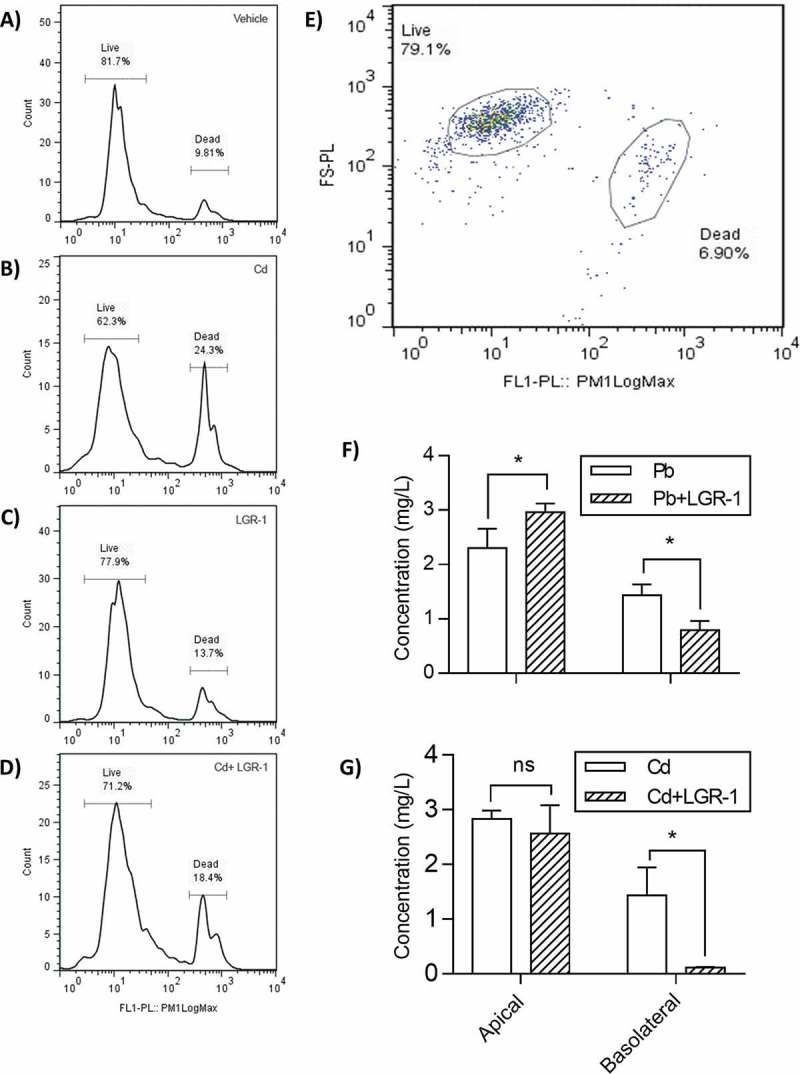
Lactobacilli reduce toxicity and apical-to-basolateral translocation of Pb and Cd in a Caco-2 cell model of the intestine epithelium. Flow cytometry was used to quantify viability of Cd-exposed Caco-2 cells (A-D) Histogram populations of the Caco-2 cells after incubation with the metals Pb or Cd, with or without pre-treatment with LGR-1. (E) Visible representation of gating used to distinguish and quantify live and dead populations of Caco-2 cells. A significant separation occurs between live and dead cells allowing for accurate labelling (F-G). To determine effect of LGR-1 supplementation on intestinal absorption of (F) Pb and (G) Cd, a Caco-2 Transwell model of the intestinal epithelium was used. Caco-2 apical and basolateral measurements of Pb and Cd were performed in biological triplicate (n = 3 for each treatment group). Error bars represent ± standard error. * p > 0.05. ns = not significant.
A Caco-2 Transwell model of the intestinal epithelium (with separate apical and basolateral compartments) was used to evaluate the effect of LGR-1 mediated heavy metal sequestration on Pb and Cd absorption in vitro. The amount of Pb remaining in the apical compartment of Caco-2 exposed cells was significantly greater in LGR-1 supplemented cells compared to vehicle treated controls (two-way ANOVA, P = 0.0354; Figure 4). In addition, the amount of Pb absorbed (measured by concentration of Pb appearing in the basolateral chamber after 5 hours) was significantly reduced in LGR-1 supplemented cells compared to vehicle-treated controls (two-way ANOVA, P = 0.0400; Figure 4F). Similar findings were observed for Cd, with significantly reduced concentrations of Cd found in the basolateral chamber of LGR-1 treated cells compared to vehicle-treated controls (two-way ANOVA, P = 0.0104; Figure 4).
Discussion
This study demonstrated the utilization of various lactobacilli, with particular focus on LGR-1 to bind and remove Pb and Cd from solution. Notably, LGR-1 effectively reduced apical-to-basolateral translocation of both toxic metals in a Caco-2 cell model of the intestinal epithelium (Figure 4F-G). These findings expand on our previous work demonstrating that LGR-1 could reduce mercury and arsenic absorption in vivo.17 This study suggests that the mechanism of lactobacilli-mediated reduction in human heavy metal exposure is via sequestration and subsequent excretion upon defecation.
Mechanisms related to heavy metal tolerance in environmentally-isolated bacteria often include alteration of cell wall components, modified efflux pumps, and binding/sequestration of metals into particles both on the cell surface and intracellularly.38,39 Our findings suggest that lactobacilli strains tested here primarily bind (Pb) and sequester (Cd) toxic metals into particles that are less dangerous to their hosts. Cell surface binding properties of lactobacilli (especially those enriched in negatively charged EPS layers, like LGR-1) might explain why the removal of metals like Pb and Cd from solution was so effective.40 Variations between sequestration abilities of lactobacilli may be due to differences in cell wall properties between strains. Studies looking at chemical properties of cell walls show that hydrophobic cell walls (associated with the presence of proteins) and cell walls with electron donating groups (Lewis base) are shown to bind high amounts of metals.41,42 Also, cells with high amounts of soft Lewis bases would bind more Cd and Pb because they are soft Lewis acids.43 Interestingly, electron microscopy revealed that the Pb and Cd did not appear to coat the bacterial surfaces evenly, and instead formed small microparticles that ranged in size and generally clustered together. It has been suggested by others that formation of these interspersed particles could be the result of ion-exchange reactions between heavy metals and cell wall peptidoglycan and lipoteichoic acid of Gram-positive bacteria.32
By looking at cross-species comparison of lactobacilli with E. coli, a trend of reduced heavy metal concentration despite an alternate mechanism of sequestration was observed. Similar to other Gram negative species, E.coli use biofilms to sequester heavy metals.44,45 The positively charged metal ions (Lewis acids) are bound to the negatively charged carboxyl, phosphate, and sulfate functional groups (Lewis bases) found in the extracellular polymeric substance. Diversity in biofilm composition results in different Lewis bases that have different binding affinities, thus causing variations in heavy metal sequestration.46
Using electron profiles unique to each element, it was possible to confirm the identity of Pb and Cd in metal treated samples. EDX and associated spectra analyses demonstrated the occurrence of densely localized Pb and Cd clusters on the cell surface of LGR-1. These findings supported the premise that cell surface components of LGR-1 could strongly attract heavy metals and suggests nucleation sites may be present and acting to facilitate the precipitation and immobilization of heavy metals outside of the cell.32 Since the excitation energy used (15 kV) can also permit the excitation of intracellular metals, TEM analysis was performed to eliminate the possibility that SEM-visualized extracellularly-located heavy metal precipitates were instead intracellular metals excited by the strong energy source. Images acquired using TEM demonstrated the formation of colloidal Cd particles, but not Pb, inside the cells of LGR-1. In Figure 3G, TEM images show Cd-exposed LGR-1 displaying two small and dense polymeric Cd clusters inside the intracellular compartment. Similar precipitation of interior microparticles has been shown in B. subtilus using gold47 and in other Lactobacillus species using Cd.48 Formation of intracellular metal particles is a widespread phenomenon that could play a role in metal resistance, as these largely insoluble clusters act to immobilize heavy metals and thereby lessen their reactivity and overall toxicity to the cell. Polymeric clustering of heavy metals with low molecular weight sulphur-rich proteins (such as metallothionein) and segregation into vesicles have been proposed as mechanisms for this type of bioaccumulation in bacteria.49 It has been shown that L. plantarum has a nutrient dependent manganese transporter, which may account for the presences of intracellular Cd. However, due to the low amount of intracellular Cd (but not Pb) compared with the extracellular bound heavy metals, we consider cell binding of heavy metals to be the primary mechanisms of heavy metal sequestration.
Unlike some studies that have used excessively high metal concentrations not found in vivo, we used concentrations known to reach the human gut (~ 1 mg/L;1 ppm50). Through evaluating binding efficiencies across a wide range of metal concentrations, we identified an inverse correlation between Pb concentration and relative binding of efficiencies of tested lactobacilli (Figure 1G). This suggests lactobacilli may be more prodigious in their binding ability to sequester heavy metals at environmentally-relevant concentrations, and that studies evaluating binding of heavy metals at higher concentrations may underestimate efficiency relevant to human exposure.
Flow cytometry of Caco-2 cells exposed Cd showed that pre-treatment with LGR-1 was able to ameliorate Cd-induced toxicity, as illustrated by a significant reduction in dead Caco-2 cells following exposure to Cd. Though Cd is known to exhibit negative effects on epithelial viability and structure51, Pb is considerably less cytotoxic towards intestinal epithelial cells52 and consequently did not affect viability of Caco-2 cells at any concentration in this study. Importantly, our results demonstrated LGR-1 could reduce apical-to-basolateral translocation of Pb and Cd across a Caco-2 Transwell model of the intestinal epithelium (gold-standard model for in vitro evaluation of intestinal absorption.53) Together, these results suggest lactobacilli may also be useful for detoxifying the gastrointestinal tract by mitigating intestinal absorption of heavy metals.
From a global perspective, human risk of exposure is steadily increasing with reports of high concentrations of toxic metals present in the global food supply and unlikely to be solved in the immediate future.54 The findings from this study suggest the use of food-grade and probiotic lactobacilli to reduce absorption of heavy metals, like Pb and Cd, may offer a safe, simple, and affordable solution for reducing exposure and consequent health risks of these inorganic and non-degradable toxic contaminants.
Material and methods
Bacterial strains and culture conditions
Bacterial strains were obtained from the American Type Collection Centre (ATCC). Lactobacillus casei 393T (ATCC 393), Lactobacillus plantarum 14917T (ATCC 14917) and LGR-1 (ATCC 55826) were routinely cultured anaerobically at 37°C using de Man, Rogosa, and Sharpe (MRS) broth or agar (catalog number: 288130; BD Difco). Lactobacilli used for experimental purposes were grown anaerobically at 37°C for 24 h and then washed twice with ultra pure ddH2O (Milli-Q Plus, Millipore S.A.), followed by immediate use at a final concentration of 109 colony forming units (CFU)/mL. E. coli 25922T (ATCC 25922) and E. coli Co1 (strong biofilm producer) strains were grown aerobically in Luria-Bertani (LB) media at 37°C for 24 h, washed twice with ddH2O, and used for experiments at a final concentration of 109 CFU/mL.
Bacterial sequestration of heavy metals in solution
Bacterial pellets were re-suspended in 5 mL of 50 mM HEPES buffer containing either Pb (CAS: 7439–92-1; Sigma) or Cd (CAS: 7440–43-9; Sigma), with pH maintained at 7.0 (chosen to mimic the small intestinal conditions where much metal uptake is most likely to occur in the gastrointestinal tract). After incubation for 5 min to 48 h at 37°C with gentle shaking, the samples were centrifuged (10,000 × g, 10 min). The supernatant was then removed and preserved for downstream metal analysis using ultra pure HNO3 (Fluka Chemie). Blank groups (negative controls) contained 50 mM HEPES buffer only, while vehicle groups (positive controls) contained various concentrations of Pb or Cd. Groups containing bacteria were suspended in vehicle-matched concentrations of Pb or Cd. Each experiment was performed in triplicate.
Heavy metal quantification
Pb and Cd were quantified using an inductively coupled plasma atomic emission spectrometer (ICP-AES, Perkin-Elmer Optima-3300 Dual View) following established methodologies for metal quantification (EPA method #6010; USA). Dilutions of 100 ppm ultra-pure stock solutions were used as calibration standards (0.05 to 5.0 mg/L). Analytical functionality was assessed as sufficient (< 10% relative reproducibility standard deviations for most samples) and method detection limits for Pb and Cd were 0.01 mg/L.
SEM and EDX analysis of bacteria incubated with pb and cd
Twice-washed bacterial pellets were re-suspended in 50 mM HEPES buffer at 109 CFU/mL. Bacterial suspensions were then diluted 10-fold into 50 mM HEPES buffer containing either Pb or Cd (final mixture concentration of 0.5mM). Samples were incubated for 30 min at 37°C, vortexed briefly, and passed through a 0.45µm nitrocellulose filter. After air drying until complete moisture evaporation, filters were coated with a 5nm film of osmium tetraoxide using an osmium plasma coater (Model# OPC 80T, SPI, PA) to establish conductivity for downstream visual analysis with SEM. Preparation of the samples for analysis was conducted in a Class 10,000 Clean Room (US FED STD 209E) thereby reducing the risks of sample environmental contamination (Nanofabrication Facility, University of Western Ontario, ON, Canada). Imaging and analysis was carried out using a LEO 1540XB FIB lithography (Zeiss, Canada) fitted with an x-ray system (Oxford Instruments). Sample variation and intrinsic differences in heavy metal properties necessitated optimization of system settings (including magnification, intensity of electron beam, X-ray parameters and type of analysis) for each individual sample. Briefly, beam energy was set in the range of 5 kV-25 kV to excite bound metals as needed for EDX analysis. Magnification was adjusted depending on frame of view and picture quality, but generally ranged from 10 x overview to 1000 x for closer detailed analysis. For EDX metal analysis, INCA® software (Stuttgart, Germany) was used with no additional changes to the default workflow. Analysis and spectrum verification of bound metals was also carried out using INCA® software with the manufacturer’s default settings. All imaging and analysis was carried out at the Nanofabrication Facility, University of Western Ontario.
TEM and ultra thin sectioning
Lactobacilli were grown in MRS anaerobically at 37°C for 24 h. Following centrifugation (10,000 x g, 10 min), bacterial cells were washed three times in 50 mM HEPES buffer, diluted 10-fold into 50 mM HEPES buffer containing Pb or Cd, for a final concentration of 0.1 mM for each metal. Following incubation for 5 hrs at 37°C, cells were centrifuged (10,000 x g, 10 min), washed three times in sterile 50 mM HEPES buffer, and finally re-suspended in 0.1 M cacodylate buffer with 2% glutaraldehyde and a pH of 7.3. Cells were then fixed overnight (16 h) at room temperature. The bacteria were then washed in 0.1 M cacodylate buffer, enrobed in 5% noble agar, dehydrated in graded ethanol series for 10 min per concentration: 50, 70, 85, 95, and 100%, and infiltrated with a 50:50 LR White resin (London Resin Company Limited, England) and absolute ethanol mixture at room temperature for 30 min Subsequently, samples were infiltrated with 100% L R White for three hours and then with a second change of 100% L R White overnight. Samples were embedded and polymerized at 60°C for 24 hours, then thin sectioned on an ultra-microtome (Reichert OmU3, Vienna, Austria) and viewed at 60 kV with a Philips EM410 TEM (Philips/FEI Corporation, Eindhoven, Holland).
Caco-2 tissue culture growth and maintenance
The human epithelial colorectal adenocarcinoma cells (Caco-2) were used to represent the intestinal barrier, with cells only being used between 30–40 passages. The cell lines were maintained in Eagles Minimum Essential Medium (EMEM, ATCC) supplemented with 1.5 g/L sodium bicarbonate, 10 % final concentration Fetal Bovine Serum (FBS), 1% final concentration penicillin-streptomycin and 2mM L-glutamine. All ingredients were mixed together in warm EMEM (37°C) and filtered through a 0.2 µm filter (VWR® Mississauga, Canada). Media was aliquoted and stored at 4°C until use. Cells were maintained in 75 cm2 or 25 cm2 flasks, 12-well and 24-well plates (BD Falcon™) depending on stage of growth and experimental setup, enough media to cover the bottom of the flask or wells was added to each container. Cells were left in culture until 90% confluent before passing with media changed every other day, with growth at 37°C under a 5 % CO2 atmosphere in a Hera Cell tissue culture incubator (Mandel® Guelph, Canada). The Caco-2 cells were passed by removing the media with a glass Pasteur pipette connected to a vacuum line. Five mL of PBS was then added to the flask to wash cells and remove residual FBS which was the removed by aspiration. Four mL of 0.25% (w/v) trypsin was added to the flask and incubated for 5–10 mins until cell detachment at 37°C. Once cells were detached, 6 mL of media was added to quench trypsin, and the media was vigorously pipetted 4–5 times to break up any cell clumps. All the media was then transferred to a 15 mL conical tube (BD Falcon™) and centrifuged at 3000 rpm for 5 mins. The supernatant was discarded, and cells re-suspended in 6 mL of fresh media, followed by seeding into a new flask or tissue plate at set dilutions depending on experimental setup.
Metal translocation studies
The ability of metals to transport across a Caco-2 cell monolayer and have this transportation blocked or reduced by lactobacilli was examined using Transwell® Permeable Supports (Costar®, Corning, MA). Transwell inserts (12 mm, 0.4 µm polyester membrane) were pre-treated by the addition of 100 µl of 50 µg/ml rat tail collagen to the insert, inserts were air dried in the clean hood for 3 hrs or until all solution was dry. Following drying, 1.5 mL of media was added to the basolateral chamber and to the apical chamber of the Transwell plate, then incubated overnight at 37°C. The following day, media in the apical chamber was aspirated and Caco-2 cells were seeded onto a Transwell insert at a concentration of at least 1 × 105 cells/ml. Viable cell concentration was determined by counting cells on a haemocytometer (Assisten, Sondheim/Rhön, Germany) using a trypan blue exclusion stain. Cells were grown on the insert for two weeks until fully confluent. Monolayer integrity was determined by measuring trans-epithelial electrical resistance (TEER) using a Millicell ERS® Volt-Ohm meter (Millipore) only values of 500 Ώ per well were accepted for use. On day 14, once monolayer integrity was confirmed, media in the apical chamber was removed and replaced either with new media containing lactobacilli strains of interest (preparation of lactobacilli strains for addition to cell culture described previously) or media without lactobacilli (control wells), with incubation for 2 hrs at 37°C. Media in the apical chamber was removed and replaced with Pb or Cd containing media at various concentrations. The control did not contain metal. Cells were incubated for 5 hrs at 37°C, then media removed from the apical and basolateral chambers and supernatant placed into separate 1.5 mL conical tubes (Diamed). 20 % nitric acid was added to sample tubes, final concentration of 2%, and samples were frozen at −20°C until analysis.
Flow cytometry
Viability of Caco-2 cells was measured using the Guava ViaCount Assay for flow cytometry to determine the toxic effects of metals and protective role of lactobacilli. Following incubation in select conditions, cell medium was removed by aspiration and Caco-2 cells were washed once gently with warm HEPES buffer (pH 6.8), and then detected from the flask using 500 μL of 0.25% (w/v) trypsin. Next, 500 μL of cell media were added to stop the trypsin reaction and total volume of each well was transferred to a separate sterile 1.5 mL centrifuge tube (Diamed). The cell suspension was mixed and diluted by a factor of 10 by combining 50 μL of cell suspension with 450 μL of Guava ViaCount® Reagent (Millipore, catalogue number: 4000–0041) in a clean sample tube. Cells were stained for 5 minutes and then analyzed using the Guava ViaCount Assay on the Guava EasyCyte Mini bench top flow cytometer based on viability, forming two distinct populations: live and dead. Cell populations were analyzed and statistically compared using FlowJo (TreeStar™ Ashland, USA) analysis software for flow cytometry data.
Funding Statement
This work was supported by the Natural Sciences and Engineering Research Council of Canada [RGPIN-2014-05188].
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council of Canada. We thank Dr. Martin Stillman for his invaluable input.
Disclosure of Potential Conflicts of Interest
GR held patents related to lactobacilli probiotics for urogenital health in women until 2008 but has had no financial interests in these since. He is an advisor to Seed, a company selling probiotics, none of which are discussed nor their intended use in this manuscript.
References
- 1.Buchauer MJ. Contamination of soil and vegetation near a zinc smelter by zinc, cadmium, copper, and lead. Environ Sci Technol. 1973;7(2):131–135. doi: 10.1021/es60074a004. [DOI] [Google Scholar]
- 2.Camobreco VJ, Richards BK, Steenhuis TS, Peverly JH, McBride MB. Movement of heavy metals through undisturbed and homogenized soil columns. Soil Sci. 1996;161(11):740–745. doi: 10.1097/00010694-199611000-00003. [DOI] [Google Scholar]
- 3.Fierens S, Mairesse H, Heilier J-F, Focant JF, Eppe G, De Pauw E, Bernard A. Impact of iron and steel industry and waste incinerators on human exposure to dioxins, PCBs, and heavy metals: results of a cross-sectional study in Belgium. J Toxicol Environ Health Part A. 2007;70(3–4):222–226. doi: 10.1080/15287390600884628. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Lin C, Cheng H, Duan X, Lei K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol and Environ Saf. 2015;113:391–399. doi: 10.1016/j.ecoenv.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Notices. 2011;2011(402647):1–20. [Google Scholar]
- 6.Jiang G, Xu L, Song S, Zhu C, Wu Q, Zhang L, Wu L. Effects of long-term low-dose cadmium exposure on genomic DNA methylation in human embryo lung fibroblast cells. Toxicol. 2008;244(1):49–55. doi: 10.1016/j.tox.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Järup L, Åkesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int. 2014;2014(840547):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi E, Suwazono Y, Dochi M, Honda R, Kido T. Influence of consumption of cadmium-polluted rice or Jinzu River water on occurrence of renal tubular dysfunction and/or Itai-itai disease. Biol Trace Elem Res. 2009;127(3):257–268. doi: 10.1007/s12011-008-8239-z. [DOI] [PubMed] [Google Scholar]
- 11.Waalkes MP, Rehm S, Riggs CW, Bare RM, Devor DE, Poirier LA, Wenk ML, Henneman JR Cadmium carcinogenesis in male Wistar rats: dose-response analysis of effects of zinc on tumor induction in the prostate, in the testes, and at the injection site. Cancer Res. 1989;49(15):4282–4288. [PubMed] [Google Scholar]
- 12.McCredie M, Ford JM, Stewart JH. Risk factors for cancer of the renal parenchyma. Int J Cancer. 1988;42(1):13–16. [DOI] [PubMed] [Google Scholar]
- 13.WHO Guidelines for drinking-water quality (3rd ed.). Available at: http://www.who.int/water_sanitation_health/dwq/GDWQ2004web.pdf. Accessed February16, 2018.
- 14.Wyatt CJ, Fimbres C, Romo L, Méndez RO, Grijalva M. Incidence of heavy metal contamination in water supplies in northern Mexico. Environ Res. 1998;76(2):114–119. doi: 10.1006/enrs.1997.3795. [DOI] [PubMed] [Google Scholar]
- 15.Mathee A, von Schirnding Y, Montgomery M, Röllin H. Lead poisoning in South African children: the hazard is at home. Rev Environ Health. 2004;19(3–4):347–361. [PubMed] [Google Scholar]
- 16.Ngueta G, Ndjaboue R. Blood lead concentrations in sub-Saharan African children below 6 years: systematic review. Trop Med Int Health. 2013;18(10):1283–1291. doi: 10.1111/tmi.12179. [DOI] [PubMed] [Google Scholar]
- 17.Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, Reid G. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. MBio. 2014;5(5):e01580–1514. doi: 10.1128/mBio.01580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alli LA. Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip Toxicol. 2015;8(3):146–150. doi: 10.1515/intox-2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett. 2003;137(1–2):65–83. doi: 10.1016/S0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura I, Hosokawa K, Tamura H, Miura T. Reduced mercury excretion with feces in germfree mice after oral administration of methyl mercury chloride. Bull Environ Contam Toxicol. 1977;17(5):528–533. doi: 10.1007/BF01685974. [DOI] [PubMed] [Google Scholar]
- 21.Breton J, Massart S, Vandamme P, De Brandt E, Pot B, Foligné B. Ecotoxicology inside the gut: impact of heavy metals on the mouse microbiome. BMC Pharmacol and Toxicol. 2013;14(62):1–12. doi: 10.1186/2050-6511-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabulo G, Oryem-Origa H, Diamond M. Assessment of lead, cadmium, and zinc contamination of roadside soils, surface films, and vegetables in Kampala City, Uganda. Environ Res. 2006;101(1):42–52. doi: 10.1016/j.envres.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 23.South African Department of Health (DOH). Foodstuffs, cosmetics and disinfectants act (act no. 54 of 1972) 2004; Republic of South Africa: South African Department of Health; 1972. [Google Scholar]
- 24.Gracia RC, Snodgrass WR. Lead toxicity and chelation therapy. Am J Health Syst Pharm. 2007;64(1):45–53. doi: 10.2146/ajhp060175. [DOI] [PubMed] [Google Scholar]
- 25.George GN, Prince RC, Gailer J, Buttigieg GA, Denton MB, Harris HH, Pickering IJ. Mercury binding to the chelation therapy agents DMSA and DMPS and the rational design of custom chelators for mercury. Chem Res Toxicol. 2004;17(8):999–1006. doi: 10.1021/tx049904e. [DOI] [PubMed] [Google Scholar]
- 26.Bhakta JN, Ohnishi K, Munekage Y, Iwasaki K, Wei MQ. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J Appl Microbiol. 2012;112(6):1193–1206. doi: 10.1111/j.1365-2672.2012.05284.x. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilescu M. Removal of heavy metals from the environment by biosorption. Eng Life Sci. 2004;4(3):219–233. doi: 10.1002/elsc.200420026. [DOI] [Google Scholar]
- 28.Mullen MD, Wolf DC, Ferris FG, Beveridge TJ, Flemming CA, Bailey GW. Bacterial sorption of heavy metals. Appl Environ Microbiol. 1989;55(12):3143–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raskin I, Smith RD, Salt DE. Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol. 1997;8(2):221–226. [DOI] [PubMed] [Google Scholar]
- 30.Matsunaga T, Takeyama H, Nakao T, Yamazawa A. Screening of marine microalgae for bioremediation of cadmium-polluted seawater. J Biotechnol. 1999;70(1–3):33–38. doi: 10.1016/S0168-1656(99)00055-3. [DOI] [PubMed] [Google Scholar]
- 31.Fein JB, Martin AM, Wightman PG. Metal adsorption onto bacterial surfaces: development of a predictive approach. Geochimica Et Cosmochimica Acta. 2001;65(23):4267–4273. doi: 10.1016/S0016-7037(01)00721-9. [DOI] [Google Scholar]
- 32.Beveridge TJ, Murray RG. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141(2):876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monachese M, Burton JP, Reid G. Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol. 2012;78(18):6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halttunen T, Collado MC, El-Nezami H, Meriluoto J, Salminen S. Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett Appl Microbiol. 2008;46(2):160–165. doi: 10.1111/j.1472-765X.2007.02276.x. [DOI] [PubMed] [Google Scholar]
- 35.Halttunen T, Finell M, Salminen S. Arsenic removal by native and chemically modified lactic acid bacteria. Int J Food Microbiol. 2007;120(1–2):173–178. doi: 10.1016/j.ijfoodmicro.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim F, Halttunen T, Tahvonen R, Salminen S. Probiotic bacteria as potential detoxification tools: assessing their heavy metal binding isotherms. Can J Microbiol. 2006;52(9):877–885. doi: 10.1139/w06-043. [DOI] [PubMed] [Google Scholar]
- 37.Hao Z, Reiske HR, Wilson DB. Characterization of cadmium uptake in Lactobacillus plantarum and isolation of cadmium and manganese uptake mutants. Appl Environ Microbiol. 1999;65(11):4741–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choudhary S, Islam E, Kazy SK, Sar P. Uranium and other heavy metal resistance and accumulation in bacteria isolated from uranium mine wastes. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47(4):622–637. doi: 10.1080/10934529.2012.650584. [DOI] [PubMed] [Google Scholar]
- 39.Naik MM, Pandey A, Dubey SK. Biological characterization of lead-enhanced exopolysaccharide produced by a lead resistant Enterobacter cloacae strain P2B. Biodegradation. 2012;23(5):775–783. doi: 10.1007/s10532-012-9552-y. [DOI] [PubMed] [Google Scholar]
- 40.Polak-Berecka M, Waśko A, Paduch R, Skrzypek T, Sroka-Bartnicka A. The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Anton Van Leeuwen. 2014;106:751–762. doi: 10.1007/s10482-014-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirillova AV, Danilushkina AA, Irisov DS, Bruslik NL, Fakhrullin RF, Zakharov YA, Bukhmin VS, Yarullina DR. Assessment of resistance and bioremediation ability of Lactobacillus strains to lead and cadmium. Int J Microbiol, 2017;2017. doi: 10.1155/2017/9869145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalita D, Joshi SR. Study on bioremediation of Lead by exopolysaccharide producing metallophilic bacterium isolated from extreme habitat. Biotechnol Rep (Amst). 2017;16:48–57. doi: 10.1016/j.btre.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobman JL, Crossman LC. Bacterial antimicrobial metal ion resistance. J Med Microbiol. 2015;64(Pt 5):471–497. doi: 10.1099/jmm.0.023036-0. [DOI] [PubMed] [Google Scholar]
- 44.Templeton AS, Trainor TP, Traina SJ, Spormann AM, Brown GE Jr. Pb(II) distributions at biofilm-metal oxide interfaces. Proc Natl Acad Sci USA. 2001;98(21):11897–11902. doi: 10.1073/pnas.201150998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teitzel GM, Parsek MR. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol. 2003;69(4):2313–2320. doi: 10.1128/AEM.69.4.2313-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nocelli N, Bogino PC, Banchio E, Giordano W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of Rhizobia. Materials (Basel). 2016;9(6). doi: 10.3390/ma9060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Southam G, Beveridge TJ. The in vitro formation of placer gold by bacteria. Geochim Et Cosmo Acta. 1994;58(20):4527–4530. doi: 10.1016/0016-7037(94)90355-7. [DOI] [Google Scholar]
- 48.Prasad K, Jha AK. Biosynthesis of CdS nanoparticles: an improved green and rapid procedure. J Colloid Int Sci. 2010;342(1):68–72. doi: 10.1016/j.jcis.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Chen P, Munoz A, Nettesheim D, Shaw CF 3rd, Petering DH. Stoichiometry and cluster specificity of copper binding to metallothionein: homogeneous metal clusters. Biochem J. 1996;317:395–402. doi: 10.1042/bj3170395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihedioha JN, Okoye COB. Dietary intake and health risk assessment of lead and cadmium via consumption of cow meat for an urban population in Enugu State, Nigeria. Ecotoxicol Environ Saf. 2013;93:101–106. doi: 10.1016/j.ecoenv.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Boveri M, Pazos P, Gennari A, Casado J, Hartung T, Prieto P. Comparison of the sensitivity of different toxicological endpoints in Caco-2 cells after cadmium chloride treatment. Arch Toxicol. 2004;78(4):201–206. doi: 10.1007/s00204-003-0532-1. [DOI] [PubMed] [Google Scholar]
- 52.Keogh JP, Steffen B, Siegers CP. Cytotoxicity of heavy metals in the human small intestinal epithelial cell line I-407: the role of glutathione. J Toxicol Environ Health. 1994;43(3):351–359. doi: 10.1080/15287399409531926. [DOI] [PubMed] [Google Scholar]
- 53.Araújo F, Sarmento B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int J Pharm. 2013;458(1):128–134. doi: 10.1016/j.ijpharm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Boonprasert K, Kongjam P, Limpatanachote P, Ruengweerayut R, Na-Bangchang K. Urinary and blood cadmium levels in relation to types of food and water intake and smoking status in a Thai population residing in cadmium-contaminated areas in Mae Sot. J Trop Med Public Health. 2011;42(6):1521–1530. [PubMed] [Google Scholar]


