ABSTRACT
Water is a fundamental part of any in vivo microbiome experiment however, it is also one of the most overlooked and underreported variables within the literature. Currently there is no established standard for drinking water quality set by the Canadian Council on Animal Care. Most water treatment methods focus on inhibiting bacterial growth within the water while prolonging the shelf-life of bottles once poured. When reviewing the literature, it is clear that some water treatment methods, such as water acidification, alter the gut microbiome of experimental animals resulting in dramatic differences in disease phenotype progression. Furthermore, The Jackson Lab, one of the world’s leading animal vendors, provides acidified water to their in-house animals and is often cited in the literature as having a dramatically different gut microbiome than animals acquired from either Charles River or Taconic. While we recognize that it is impossible to standardize water across all animal facilities currently conducting microbiome research, we hope that by drawing attention to the issue in this commentary, researchers will consider water source as an experimental variable and report their own water sources to facilitate experimental reproducibility. Moreover, researchers should be cognisant of potential phenotypic differences observed between commercial animal vendors due to changes in the gut microbiome as a result of various sources of water used.
KEYWORDS: Gut microbiome, bacteriome, microbial ecology, drinking water source, mice, in vivo, acidified water, Jax vs Taconic Non-obese diabetic mice, experimental variable; Muc2-/- mice; spontaneous colitis model
Overview
The field of microbiome research has grown exponentially over the past two decades due to rapid advances in technology and bioinformatic tools resulting in a better understanding of how gut microbes impact human health and disease susceptibility. Additionally, evidence has revealed that various factors including: diet, the environment and genetics effect the gut microbiome. As the microbiome is influenced by so many factors, in vivo animal models serve as an invaluable tool, allowing us to demonstrate cause and effect relationships by conducting experiments within a controlled environment. Microbiome researchers go to great lengths to alleviate as many extraneous variables as possible when designing their in vivo experiments and many researchers have been rightly assiduous in reporting factors like specific pathogen free (SPF) facility, irradiated or autoclaved food, autoclaved bedding, temperature controlled, ventilated racks, 12:12 light/dark cycle, etc. Recently, microbiome researchers have had to mitigate another confounding factor, cage effects; a term used to describe the fact that mice co-housed have a more similar microbiome than mice housed in other cages, within the same facility, due in large part to coprophagia.1 Attempts have been made to overcome cage effects through various statistical methods and adequate sample sizes.1 Still, one often overlooked and underreported variable is perhaps the most basic and fundamental of them all – water. Of the 76 primary research articles examined for this commentary, 47 studies failed to report their water source, while 18 of the studies reported some aspects of their water source (Figure 1). Only 14% of the 76 studies reported sufficient detail regarding water source for the study to be replicated by another researcher. A list defining adequate detail concerning water source can be found in Table 1. When reported, it becomes clear that a wide array of water sources, including acidified, hyper-chlorinated, autoclaved, UV sterilized, reverse osmosis, and municipal tap water are being used (Table 2). Treatments such as autoclaving, hyper-chlorination and acidification are performed with the intent of inhibiting bacterial growth. However, this may affect the bacteria within the gut of these animals and in fact may alter the phenotype of the animal being studied.
Figure 1.
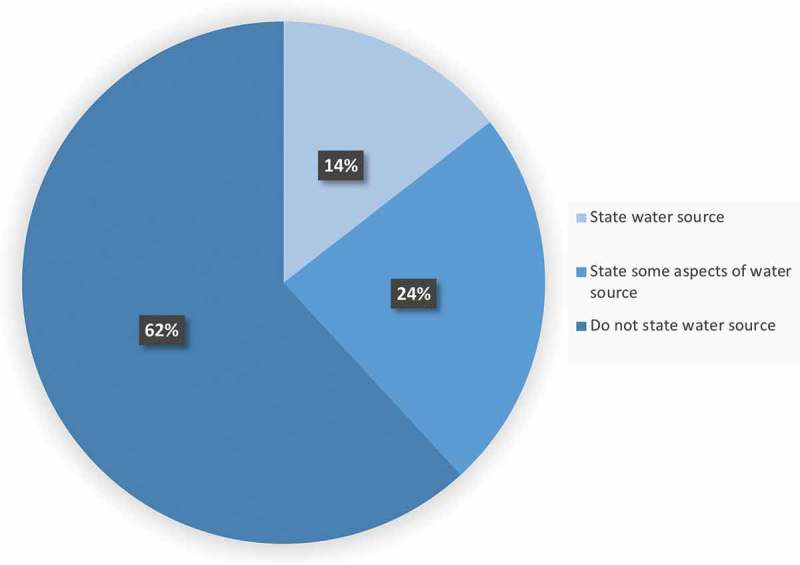
The majority of microbiome research literature does not report the complete details of water source. Of the 76 primary research articles surveyed for the current commentary, 62% of the articles did not state any information regarding the water source provided to their in vivo animals (47 articles.) While 24% stated some aspect of their water source, but not enough to be repeated by another researcher (18 articles.) Only 14% of the articles listed what the current authors would consider adequate detail with regards to the water source being provided to experimental animals (11 articles.).
Table 1.
Summary of what key words currently found in literature regards to water source compared to complete details required when reporting water source for in vivo animal experiments.
| No water source | Incomplete water source | Complete water source |
|---|---|---|
| No details provided on water | “Acidified water” (pH, water source and type of acid not specified) | “Autoclaved municipal tap water” |
| “Animals received water” | “Autoclaved water” (water source not specified) | “Reverse osmosis water acidified to a pH of 2.3 via the addition of HCl” |
| “Regular drinking water” | “Sterile water” (water source and method of sterilization not specified) | “Municipal tap water” |
| “Drinking water” | “Non-acidified water” (water source not specified) | “Reverse osmosis water” |
| “Filtered water” (water source and filtration method not specified) | “UV sterilized municipal tap water” |
Table 2.
Summary of various microbiome studies to highlight discrepancies in water sources and reporting.
| Author | Facility Type | Strain | Vendor | Water Source | Sex | Diet | LD Cycle | DOI/PMID |
|---|---|---|---|---|---|---|---|---|
| Van der Sluis et al.2 | SPF | 129Sv-Muc2−/− 129Sv-Muc2+/+ 129Sv-Muc2+/− | 129Sv acquired from Charles River Muc2−/− raised in house | Acidified tap water (pH not specified) [administered prior to experiment] Autoclaved tap water [administered during DSS exposure] | Male | Standard rodent pellets (Special Diet Services, Witham, Essex, England) given ad libitum | 12:12 LD | 10.1053/j.gastro.2006.04.020 |
| Burger-van Paassen et al.3 | SPF | 129Sv-Muc2−/− 129Sv-Muc2+/+ | 129Sv acquired from Charles River Muc2−/− raised in house | Acidified tap water (pH not specified) | Male | Standard rodent pellets (Special Diet Services, Witham, Essex, England) given ad libitum | 12:12 LD | 10.1371/journal.pone.0038798 |
| Lu et al.4 | SPF | 129Sv-Muc2−/− 129Sv-Muc2+/+ | 129Sv acquired from Charles River Muc2−/− raised in house | Acidified tap water (pH not specified) | Not specified | Standard rodent pellets (Special Diet Services, Witham, Essex, England) given ad libitum | 12:12 LD | 10.1002/ibd.21592 |
| Morampudi et al.5 | SPF | C57BL/6-Muc2−/− C57BL/6-Retnlb−/− C57BL/6 C57BL/6-Muc2−/−/Retnlb−/− | All mice raised in house | Autoclaved water (source not specified) | Not specified | Autoclave food (type not specified) | Not Specified | 10.1038/mi.2015.140 |
| Wolf et al.6 | SPF | NOD/ShiLtJ | NOD/ShiLtJ were acquired from JAX | Original breeder pairs received Birmingham city water which had been chlorinated and autoclaved Animals were then split between neutral pH (pH ~7.0) and acidified water (pH ~3.2) via the addition of HCL | Not specified | Autoclaved N1H-31 rodent diet (Harlan Teklan, Madison, WI) given ad libitum | Not Specified | 10.1369/0022155413519650 |
| Sofi et al.7 | SPF | NOD/ShiLtJ C57BL/6 | NOD/ShiLtJ were acquired from JAX or acquired in-house from a SPF colony of NOD/ShiLtJ Original C57BL/6 mice were purchased from Taconic Farms | Mice were maintained on either autoclaved neutral (pH7.0–7.2) or acidic (pH 3.0–3.2) water Water was acidified using HCL as per JAX instructions (http://jaxmice.jax.org/genetichealth/health_program.html; http://craniofacial.jax.org/new_standard_protocols.html |
Female | Not specified | Not Specified | 10.2337/db13-0981 |
| Graham et al.8 | SPF | Fox1nu/Fox1nu (Nude mice) | Animals were acquired from three vendors: Jax, Charles River and Taconic Farms | Not specified | Male | Irradiated Rodent Diet (Diet 2919 Teklad Global 19% protein rodent diet, Harland Laboratories) provided ad libitum | 12:12 LD | PMC3155402 |
| Brown et al.9 | SPF | NOD/ShiLtJ NOR/LtJ | Animals were acquired from JAX | Filtered, UV sterilized water | Female | Sterile chow (Laboratory Rodent Diet 5001, Purina Mills) ad libitum | 12:12 LD | http://doi.org/10.1038/ismej.2015.114 |
Search criteria
A systemic search of the literature was conducted using PubMed, EMBASE and Medline for the terms “in vivo”, “gut microbiome”, “rodent”, “animal”, “hyper-chlorinated water”, “acidified water”, “reverse osmosis water”, “autoclaved water”, “deionized water”, “drinking water”, “municipal tap water”, and “tap water”. Searches containing relevant synonyms and combinations of the above terms were also utilized. Studies were limited to primary articles conducted within the past 15 years. Studies utilizing rodents other than rats or mice (i.e.: guinea pigs) and review article were excluded from the current commentary.
Water treatment methods
According to the Canadian Council on Animal Care (CCAC) the standards set for the quality of drinking water for laboratory animals has not been subject to the same requirements as those for defining laboratory animal diets.10 Traditionally, researchers have predominantly focused on inhibiting bacterial growth when choosing a water source for their laboratory animals. Common water sanitization methods include autoclaving, UV sterilization, or the addition of acids, such as hydrochloric acid (HCl), in an effort to inhibit bacterial growth and increase the shelf life of water bottles once filled. Water-acidification is a common method of water sanitization and has been shown to be effective at killing Gram-negative bacteria11 including Pseudomonas spp.12 Most SPF rodent facility exclusion lists contain several Gram-negative pathogens including: Pseudomonas aeruginosa, Klebsiella pneumoniae and Pasteurella pneumotropica and it’s for this reason water acidification remains a popular water treatment method. However, not all Gram-negative bacteria are pathogenic. Bacteroidetes is comprised of three classes of Gram-negative bacteria and is the dominant phyla found within the murine gut microbiome.13 The potential presence of microbes is not the only variable that should be considered when choosing a water sanitation method for laboratory animals. The organic and inorganic contents of potable water vary significantly depending on geographical location or water treatment method employed. For example, water purified using reverse osmosis has a negligible level of ions and molecules such as sodium, manganese, iron, fluoride, lead and calcium including salt,14 whereas autoclaved municipal tap water or UV sterilized water retain much of their ion content. The presence or absence of ions may have implications for the animal’s gut microbiota as ions including manganese,15 iron16,17 and calcium magnesium20 have been shown to alter the gut microbiome, gut inflammation and disease susceptibility both in vitro and in vivo. Additionally, ions impact the taste of drinking water, and may influence the amount of water consumed by an animal.18 Furthermore, acidified drinking water has been shown to leach heavy metals from the water dispensing system itself.19
Effects of acidified water on the non-obese diabetic mouse microbiome
There have been studies conducted examining the effects of drinking water pH on the gut microbiome and subsequent disease susceptibility. One such study administered either neutral (N) or acidified water to non-obese diabetic NOD/ShiLtJt mice and monitored the impact on microbial composition and incidence of type-1 diabetes (T1D).6 NOD/ShiLtJt mice were obtained from Jackson Lab (Bar Harbor, Maine) and were housed under SPF conditions.6 Animals received autoclaved NIH-31 rodent diet (Harlan Teklan) and autoclaved and chlorinated Birmingham city water ad libitum.6 Breeding pairs were split between neutral (pH ~ 7) and acidified water (pH ~ 3.2) groups.6 Water was acidified with the addition of HCl (1 mL of 1 N HCl/500 mL water).6 Pups born from each of the breeding pairs were maintained on their specific water source.6 Blood glucose measurements were taken weekly, and a diagnosis of diabetes was given if the mouse exhibited two adjacent weekly readings of over 200 mg/dl or a single reading over 400 mg/dl.6 Mice maintained on the neutral pH water (NOD-N) exhibited an increase in the development of TID, with only 11% of NOD-N mice remaining diabetes-free by 30 weeks of age.6 NOD-N mice also displayed significantly decreased levels of Firmicutes in their stool compared to their acidified water counterparts, including a reduction in the amount of Lactobacillus spp. and Clostridium coccoides. Conversely, NOD-N animals also exhibited an increase in Bacteroides spp., Actinobacteria and Proteobacteria prior to disease initiation.6 These changes in the intestinal microbiome composition were observed as early as 2 weeks of age.6 Immunological differences were also observed between the NOD-N and acidified water groups, with NOD-N mice exhibiting decreased expression of intracellular IL-17 in CD4+ T-cells and a decreased level of Foxp3 expression in Treg cells.6 These findings lead the authors to suggest that early dietary manipulation of the intestinal microbiota may allow for the delay of T1D onset in genetically susceptible individuals.6
Four months after the Wolf et al., publication a second study emerged examining the effects of drinking water pH, the gut microbiome and the incidence of T1D. However, unlike the previous study, these authors concluded female NOD mice, maintained on acidified-water, developed insulitis and hyperglycemia rapidly compared with animals receiving neutral pH water.7 Like the previous study, the pH of the drinking water altered the gut microbiota, with mice receiving acidified water exhibiting a substantial decrease in the diversity of their microbes, characterized by a reduction in Bacteroides spp. and an increase in the proportion of Lactobacillus spp. – the exact opposite of what Wolf et al. found in their 2014 study.7 Both studies housed their animals in a SPF facility, acidified their water to a pH of 3.0–3.2 with the addition of HCl and obtained NOD/ShiLtJ mice from Jax®.6,7 However only, the study conducted by Sofi and associates, used mice from their in-house SPF colony of NOD/ShiLtJ mice.7 These in-house NOD mice were 2–3 generations old and had been maintained on autoclaved neutral pH water (pH ~7.0–7.4).7 Thus, we cannot rule out the possibility that the differences between these two studies was influenced by differences in the water sources used to maintain the respective breeding colonies.
Effects of acidified water on the Muc2−/− microbiome
The effects of water source may also affect other models of inflammatory diseases besides T1D, including colitis. Mice deficient in the gene encoding for mucin 2 (Muc2−/− mice) develop colonic inflammation which increases with age and serves as a model of spontaneous colitis. Mucin 2 is a gel-like oligomeric protein secreted by goblet cells in the colonic epithelium and this gel creates a physical barrier preventing enteric microbes and noxious substances from crossing over into the intestinal lumen. In the Muc2−/− mice, spontaneous colitis has been shown to depend on the commensal microbes.5 To understand if water source was important in this model, we analyzed stool samples collected from two different Muc2−/− colonies located in British Columbia, Canada. Several sets of breeders from the Child and Research Family Institute in Vancouver were sent to the Biosciences Animal facility at the Okanagan campus in Kelowna. Both colonies were bred in-house, maintained under SPF conditions with similar pathogen exclusion lists, received similar breeding and maintenance chows ad libitum, however the colony located at the Vancouver campus supplied their Muc2−/− animals with autoclaved municipal tap water, whereas the Okanagan campus provided water which had been acidified to a pH of ~2.3 via the addition of HCl. In total, 28 stool samples were obtained from the Okanagan in vivo facility and 27 stool samples collected from age-matched males and females located at the UBC Vancouver animal facility (courtesy of Dr. Bruce Vallance). Microbial DNA was isolated from the stool using the QIAamp DNA Stool Mini Kit (Qiagen CAT No: 51504). Amplicon sequencing was performed using primers targeting the V3-V4 region of the 16S rRNA and sequenced using Illumina MiSeq paired-end sequencing as previously performed by our lab.24 Sequencing data was analyzed using QIIME2 pipelines22 and the microbial communities present within the fecal samples collected from the two facilities were ordinated using the Bray-Curtis distance metrics. Principal coordinate analysis (PCoA) based on the Bray-Curtis dissimilarity metric was conducted in QIIME2 and the results of this analysis can be observed in Figure 3. It can be seen that PCoA plots constructed using Bray-Curtis (Figure 3) show two distinct clusters corresponding to the two facilities supplying different water sources. While food, age, and sex were consistent between the two Muc2−/− facilities analyzed here, we also recognize that the differences observed in beta-diversity could also be influenced by the two different facility locations. A future controlled experiment is needed before drawing any definitive conclusions on the effect of acidified water and the Muc2−/− microbiome. However, the results at least show that water source could be an important factor in spontaneous colitis due to changes in the gut microbiome.
Figure 3.
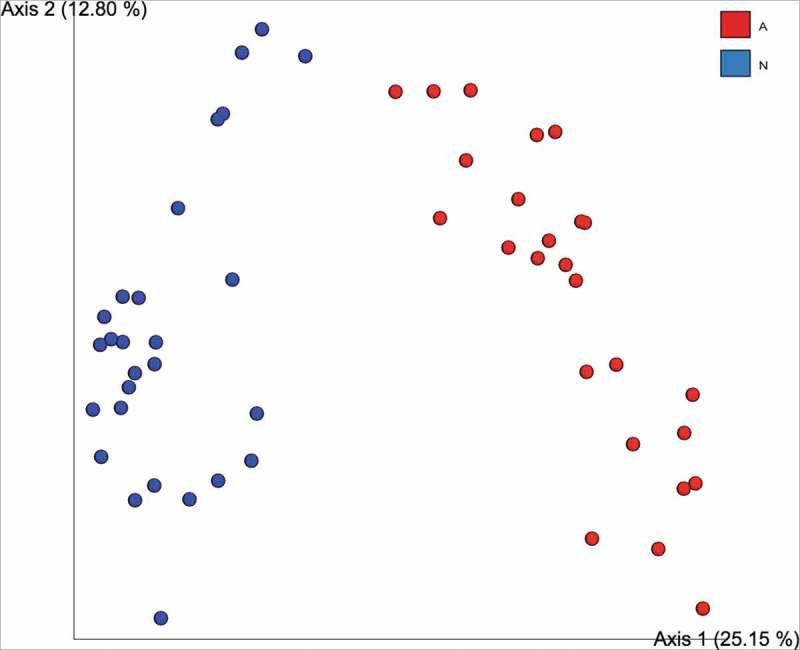
The fecal microbiome clusters to water source in a mouse model of spontaneous colitis. Beta-diversity between two in vivo facilities supplying different water sources to Muc2−/− mice show clear clustering based on Bray-Curtis dissimilarity measures. Red dots represent the UBC Okanagan cohort (receiving acidified water at pH of ~2.3 via the addition of HCL) and blue dots represent the UBC Vancouver cohort (who received autoclaved municipal tap water.).
Vendor differences
The potential effects of water quality on parental animals becomes of even greater importance when one considers that the top three commercial animal vendors: Jackson Laboratories (Jax®), Taconic and Charles River, differ in the drinking water treatments for laboratory animals. Both Charles River and Taconic filter their water at multiple levels and hyper-chlorinate it. Charles River takes things one step further and also UV sterilizes the water before delivering it to their in-house animals. On the other hand, Jax® acidifies their water to a pH of 2.5–3.0 via the addition of HCl (Table 3). Perhaps on a related note, Jax® animals are often cited as having a radically different microbiome compared to mice obtained from Charles River or Taconic.7,21,23,25 Notably, Sofi and associates set out to conduct their 2014 study as they observed their in-house NOD mice developed diabetes more slowly than that reported by Jax®.7 These researchers observed a lower incidence of T1D when their in-house NOD mice were given neutral water or when Jax® mice were switched to neutral water at an early age (3–4 weeks old).7 However pre-diabetic mice purchased from Jax® (8 weeks of age) developed hyperglycemia rapidly compared with those from their in-house SPF breeding colony.7 This suggests that the age at which acidified water is discontinued plays an important role in the developing murine gut microbiome and subsequent disease progression.
Table 3.
Table summarizing the water sources of the top three commercial mouse vendors.
| Vendor | Water source |
|---|---|
| Jax®^ | Water acidified to pH 2.5–3.0 by the addition of HCL. |
| Charles River* | Water filtered at multiple levels, hyper-chlorinated and UV sterilized. |
| Taconic* | Hyper-chlorinated water (2 to 8 ppm) passed through a series of 0.2-micron filters. |
*Contacted vendor for information
^ Taken from Jax® website: https://www.jax.org/news-and-insights/jax-blog/2014/april/top-five-tips-to-get-ready-for-your-new-research-mice
Vendor differences may be responsible for the conflicting results found by two similar studies conducted by independent research groups. In their 2012 study, Hansen et al., assessed the effect of early life vancomycin exposure and subsequent diabetes onset.26 NOD/BomTac mice (Taconic, Lille Skensved, Denmark) were purchased and bred in house and maintained under SPF conditions.26 Animals had free access to food and water where the water source was not specified.26 Hansen and associates found that exposure to the antibiotic vancomycin (0.5 g/l) from birth until weaning, resulted in a significant decrease in diabetes onset, while exposure later in life resulted in a non-significant decrease in cumulative diabetes onset.26 Simultaneously, a study from our group9 examined the effects of prolonged antibiotic treatment on NOD mice and the incidence of diabetes. NOD and NOR mice were acquired from Jax® and were housed and bred under SPF conditions.9 Animals received sterile chow and filtered UV sterilized water ad libitum throughout the experiment.9 Pregnant dams were exposed to vancomycin (0.5 g/l) just prior to birth and throughout lactation and until diabetes onset.9 Contrary to what Hansen et al. found, we found that exposure of the pregnant dams to vancomycin resulted in accelerated diabetes onset in NOD pups.9 These conflicting results may be due to water sources provided at either the animal vendor and/or the research facility considering it had been shown that acidified water in the NOD mice alters the gut microbiome and diabetic phenotype.6,7 The differences observed in Jax® mice compared to other commercial vendors extends beyond diabetes research, a summary of differences observed in the literature between the top three commercial animal vendors is summarized in Figure 2.
Figure 2.
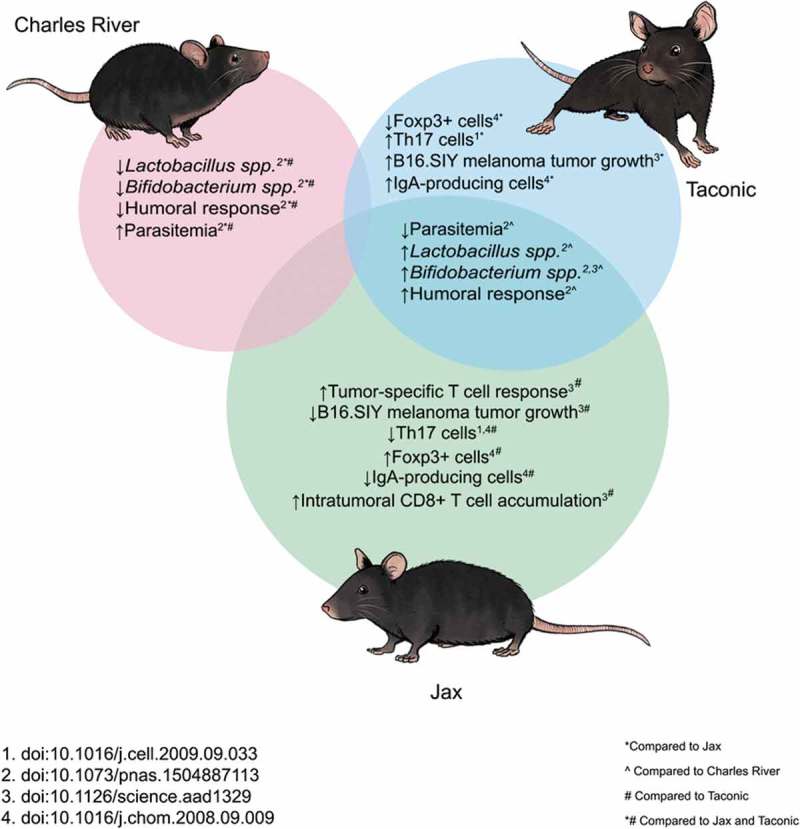
Summary of differences observed in C57Bl/6 mice obtained from the top three commercial animal vendors: Charles River, Taconic and Jax®.
Conclusion
Water is a fundamental necessity of life – however its importance is often underappreciated in the field of in vivo microbiome research. Water can be a significant source of variability depending on the geographical location of the animal facility, the time of year, the presence or absence of agriculture within the surrounding area, and the sterilization and filtration methods employed by the animal facility itself. While it is impossible to standardize water across all animal facilities currently conducting microbiome work throughout the world, more care should be taken on the part of the researcher to report as much detail as possible regarding their own facilities water source and treatment to facilitate reproducibility. Finally, it is important to consider individual commercial vendor differences in water treatment when purchasing animals for microbiome studies considering the gut microbiome plays an important role in the development of several disease phenotypes like diabetes and colitis.
Funding Statement
This work was supported by grants from Natural Sciences and Engineering Research Council (NSERC) and Crohn's and Colitis Canada (CCC).
Acknowledgments
Thanks to Dr. Bruce Vallance for the donation of the Muc2−/− breeders and fecal samples collected at the Child and Family Research Institute animal care unit. Thanks to Kevin Fraser for his mouse illustrations featured in Figure 2. Thanks to Gibson lab members for the data used in Figure 3 and for the critical reading of this manuscript. Grant funding to DLG through Crohn’s and Colitis Canada and Natural Sciences and Engineering Research Council support this research.
Author Contributions
Both authors actively contributed to the conceptual development of this commentary.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lundberg R, Bahl MI, Licht TR, Toft MF, Hansen AK.. Microbiota composition of simultaneously colonized mice housed under either a gnotobiotic isolator or individually ventilated cage regime. Sci Rep. 2017;7. doi: 10.1038/srep42245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Einerhand AWC, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Burger-van Paassen N, Loonen LMP, Witte-Bouma J, Korteland-van Male AM, de Bruijn ACJM, van der Sluis M, Renes IB, Van Goudoever JB, Wells JM, Dekker J, et al. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3β, Reg3γ and Angiogenin-4. PLoS ONE. 2012;7(6):1–11. doi: 10.1371/journal.pone.0038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu P, Burger-Van Paassen N, Van der Sluis M, Witte-Bouma J, Kerckaert JP, Van Goudoever JB, Renes IB, Renes IB. Colonic gene expression patterns of mucin muc2 knockout mice reveal various phases in colitis development. Inflamm Bowel Dis. 2011;17(10):2047–2057. doi: 10.1002/ibd.21592. [DOI] [PubMed] [Google Scholar]
- 5.Morampudi V, Dalwadi U, Bhinder G, Sham HP, Gill SK, Chan J, Bergstrom KSB, Huang T, Ma C, Jacobson K, et al. The goblet cell-derived mediator RELM-β drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016;9:1218–1233. doi: 10.1038/mi.2015.140. [DOI] [PubMed] [Google Scholar]
- 6.Wolf KJ, Daft JG, Tanner SM, Hartmann R, Khafipour E, Lorenz RG. Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J Histochem Cytochem. 2014;62(4):237–250. doi: 10.1369/0022155413519650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofi MH, Gudi R, Karumuthil-Melethil S, Perez N, Johnson BM, Vasu C. PH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes. 2014;63(2):632–644. doi: 10.2337/db13-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham ML, Janecek JL, Schuurman H. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources materials and methods. Comp Med. 2011;61(August 2014):356–360. [PMC free article] [PubMed] [Google Scholar]
- 9.Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, Gibson DL, Chan Y, Chan JM, Lochner A, et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016;10(2):321–332. doi: 10.1038/ismej.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schofield L, Bailey M, Bélanger R, Fry S, Kirk M, McKay D, … Griffin G. Canadian Council on Animal Care guidelines on: laboratory animal facilities — characteristics. Des Dev. 2003;115. [Google Scholar]
- 11.Ave B. Drinking water acidification. Waterford, WI: Edstrom Industries, Inc.; 2003. [Google Scholar]
- 12.Tanner RS, James SA. Rapid bactericidal effect of low pH against Pseudomonas aeruginosa. J Ind Microbiol. 1992;10(3–4):229–232. doi: 10.1007/BF01569771. [DOI] [Google Scholar]
- 13.Mullin GE. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Nutr Clin Pract. 2010; doi: 10.1177/0884533610368714. [DOI] [PubMed] [Google Scholar]
- 14.Binnie C, Kimber M. Basic water treatment. 3rd ed. Royal Society of Chemistry; 2014. doi: 10.1680/bwt.58163. [DOI] [Google Scholar]
- 15.Chi L, Gao B, Bian X, Tu P, Ru H, Lu K. Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol Appl Pharmacol. 2017;331:142–153. doi: 10.1016/j.taap.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natoli M, Felsani A, Ferruzza S, Sambuy Y, Canali R, Scarino ML. Mechanisms of defence from Fe(II) toxicity in human intestinal Caco-2 cells. Toxicol Vitro. 2009;23(8):1510–1515. doi: 10.1016/j.tiv.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Ferruzza S, Scarino ML, Gambling L, Natella F, Sambuy Y. Biphasic effect of iron on human intestinal Caco-2 cells: early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell Mol Biol (Noisy-Le-Grand). 2003;49(1):89–99. [PubMed] [Google Scholar]
- 18.Zocchi D, Wennemuth G, Oka Y. The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci. 2017;20(7):927–933. doi: 10.1038/nn.4575. [DOI] [PubMed] [Google Scholar]
- 19.Nunamaker EA, Otto KJ, Artwohl JE, Fortman JD. Leaching of heavy metals from water bottle components into the drinking water of rodents. J Am Assoc Lab Anim Sci. 2013;52(1):22–27. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3548197&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley EK, Long-Smith CM, Murphy A, Patterson E, Murphy K, O’Gorman DM, Nolan YM, Nolan Y. Dietary supplementation with a magnesium-rich marine mineral blend enhances the diversity of gastrointestinal microbiota. Mar Drugs. 2018;16:6. doi: 10.3390/md16060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov II, Frutos RDL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Littman DR, Littman DR. Specific microbiota direct the differentiation of IL-17-Producing T-Helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.QIIME 2 : Reproducible, interactive, scalable, and extensible microbiome data science. Peer J Preprints 6:e27295v1. 10.7287/peerj.preprints.27295v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Littman DR, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quin C, Estaki M, Vollman DM, Barnett JA, Gill S, Gibson DL. Probiotic supplementation and associated infant gut microbiome and health: A cautionary retrospective clinical comparison. Sci Rep. 2018; doi: 10.1038/s41598-018-26423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villarino NF, LeCleir GR, Denny JE, Dearth SP, Harding CL, Sloan SS, Schmidt NW, Campagna SR, Wilhelm SW, Schmidt NW. Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci U S A. 2016;113(8):2235–2240. doi: 10.1073/pnas.1504887113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen CHF, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, Hansen AK. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012; doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]


