ABSTRACT
Enterobactin (Ent), a prototypical bacterial siderophore known for its unparalleled affinity for iron, is widely conserved among members of the Enterobacteriaceae family of Gram-negative bacteria. In this study, we demonstrated that, aside from mediating iron acquisition, Ent also dampened the macrophages (MΦs) antimicrobial responses against intracellular infection by Salmonella enterica serovar Typhimurium. Accordingly, the loss of Ent expression (ΔentB) in Salmonella demoted their survivability against MΦs. Addition of exogenous Ent not only rescued the survival of ΔentB Salmonella, but also augmented WT Salmonella to better withstand the microbicidal activity of MΦs. The protection conferred to WT Salmonella was observed only when Ent was administered as iron-free, thus indicating the requirement of iron chelation in this context. In contrast, the exogenous iron-bound Ent retained its ability to promote the survival of ΔentB Salmonella, albeit modestly. Assessment on MΦs labile iron pool (LIP) revealed that iron-free Ent is able to permeate into MΦs, chelate the intracellular LIP, and regulate the expression of several key iron-regulatory proteins, i.e., divalent metal transporter 1, ferroportin, and hepcidin. Chelation of iron by Ent was also observed to promote the MΦs towards M2 polarization. Collectively, our findings demonstrated that Ent not only facilitates bacterial iron uptake but also disrupts MΦs iron homeostasis and M1/M2 polarization to safeguard intracellular bacteria against the anti-bacterial effects of their host.
KEYWORDS: Enterochelin, iron, Salmonella, iron chelation
Introduction
Macrophages (MΦs) are professional phagocytes, which reside virtually in every organ, such as the liver, spleen and intestines, the latter being the largest reservoir.1 MΦs patrol these organs for microbial encroachment and eliminate potential pathogens via phagocytosis. These features are central to the role of MΦs in maintaining homeostasis between the host and bacteria at mucosal surfaces, specifically in the gastrointestinal tract. Aside from their roles in innate immunity, MΦs play an equally important role in maintaining iron homeostasis by virtue of its ability to phagocytose senescent red blood cells and recycle heme iron for de novo hemoglobin biosynthesis.2 Moreover, the MΦs are equipped with several iron-regulatory mechanisms3–8 that are dynamically regulated to facilitate the onset of hypoferremia/anemia of inflammation.9 MΦs could also rapidly deplete their labile iron pool (LIP) to curtail intracellular pathogens,10 although the extent to which the depletion of LIP could impair its effector functions is not well understood.
Salmonella is a food-borne enteropathogen from the Enterobacteriaceae family of Gram-negative bacteria. The majority of Salmonella infections result in self-limiting gastroenteritis and diarrhea; however, the dissemination of this pathogen into systemic circulation often leads to life-threatening septicemia. As an intracellular pathogen, Salmonella has the machinery to invade the gut epithelia and MΦs, with the latter serving as the carrier to spread the infection systemically. After being engulfed by the MΦs, the Salmonella resides in a modified phagosome known as the Salmonella-containing vacuoles. Several mechanisms, which permit Salmonella to survive within the MΦs, have been documented, including blocking the fusion of lysosome with the Salmonella-containing vacuoles.11 The intracellular survival of Salmonella has also been purported to be dependent on its ability to exploit and utilize the diverse nutrients in the MΦs12, especially iron, which is essential for Salmonella growth and replication.13
The acquisition of iron is one of the important aspects that underlie the host-pathogen interaction during Salmonella infection. To counter bacterial infection, MΦs are known to upregulate diverse iron-regulatory proteins (e.g. ferroportin), which limit the accessibility of LIP to pathogens.10 The scarcity of iron, in turn, cues the Salmonella to express enterobactin (Ent; a catecholate siderophore), which has an unmatched affinity for iron to sequester iron from the host iron-binding proteins.14 In response, the host expresses the innate immune protein lipocalin 2 (Lcn2), which binds Ent in its binding pocket and thus prevents bacterial uptake of ferric Ent.15 Previously, we have demonstrated that Ent could also impede neutrophil bactericidal activity by dampening formation of neutrophil extracellular traps and reactive oxygen species (ROS).16
In the present study, we investigated to which extent Ent could provide a survival advantage to Salmonella. We demonstrated the better intracellular survival of wild type (WT) Salmonella when compared to its isogenic Ent-deficient mutant strain; the survival of both strains was further augmented in MΦs pre-treated with exogenous Ent. Our molecular study revealed that Ent can chelate the intracellular LIP and disrupt the iron-dependent effector mechanisms in the MΦs to favor bacterial growth. Taken together, our current study identified an important role of Ent during the host-pathogen interaction, which can be targeted as a therapeutic model to treat the infection.
Results
Ent provides survival advantage to Salmonella in macrophages.
To investigate whether Ent allow Salmonella to survive better in MΦs, we took advantage of WT Salmonella enterica serovars Typhimurium (which express Ent and salmochelin, a glucosylated form of Ent) and its isogenic mutant (ΔentB) deficient in both Ent and salmochelin. We first confirmed that WT Salmonella secreted more siderophores than ΔentB based on the degree of halo formation on the Chrome Azurol S (CAS) agar plate (Figure 1A) and CAS liquid assay (Figure 1A and B). However, ΔentB mutant can still produce some of the intermediates of the Ent biosynthetic pathway, namely chorismate and isochorismate17; this may partially explain why ΔentB mutant could still form halo, but to a much lesser extent, in the CAS assay.
Figure 1.
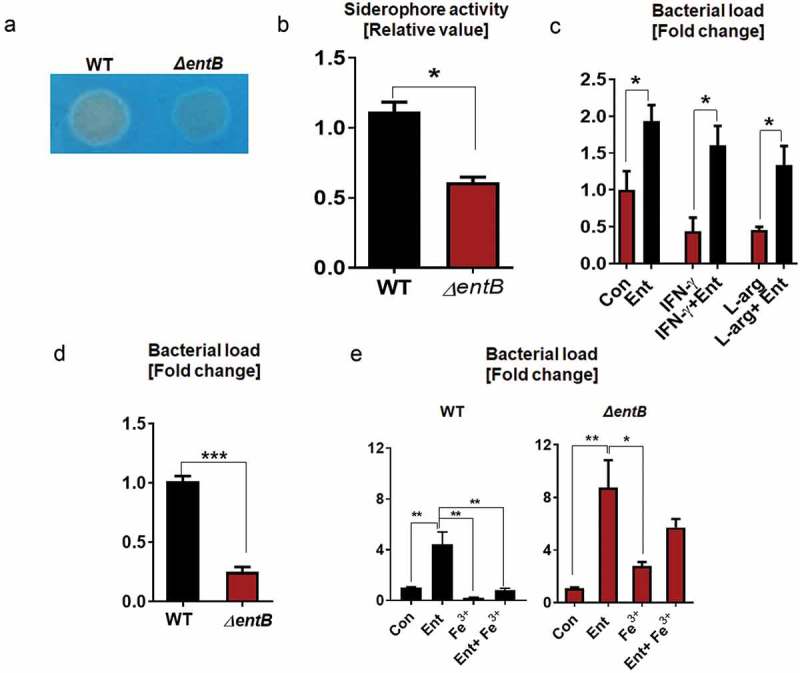
Ent provides an intracellular survival advantage to Salmonella.
Production of Ent by Salmonella and its isogenic mutants (ΔentB) were assessed using CAS assay. (a) The formation of orange halo indicates siderophore production by WT Salmonella and ΔentB mutant on CAS agar plate. (b) Bar graphs indicate the relative siderophore activity in the bacterial culture supernatant detected via CAS liquid assay. (c) BMDMs were pre-incubated with Ent, then infected with WT Salmonella or ΔentB mutant at MOI of 100, and treated with Ent for 24h in presence of gentamicin (20 µg/ml). Intracellular bacterial survival was determined by serial dilution and plating on LB plate. The impact of Ent on the intracellular survival of WT Salmonella activated with IFN-γ (20 ng/ml) or L-arginine (500 µM). (d) BMDMs were infected with WT Salmonella or ΔentB mutant for 24h in presence of gentamicin. (e) MΦs were pre-incubated with Ent, FeCl3, or Ent+ FeCl3 (1:1 ratio, 1h) then infected with WT Salmonella or ΔentB mutant and treated with Ent, FeCl3 or Ent+ FeCl3 for 24h in presence of gentamicin. In vitro assays were performed in triplicate and data represented as mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001.
To further investigate the importance of Ent-mediated effects on MΦs in the context of infection, we performed the gentamicin protection assay. The MΦs were pre-treated with or without Ent and then infected with Salmonella. After one hour of infection, the extracellular bacteria were eliminated by adding gentamicin, thus allowing us to quantify the bacteria that were internalized. As anticipated, Ent treatment substantially protected WT-Salmonella from MΦ-mediated intracellular killing, i.e., a 2-fold increase in bacterial survival when compared to control (Figure 1C).
We also primed the MΦs with IFN-γ18 or pretreated the MΦs with L-arginine, a substrate for iNOS19, to augment bacterial killing via increased oxidative burst and NO production, respectively, prior to infection. The bacterial survival decreased by approximately 2-fold in both the IFN-γ-treated and L-arginine supplemented MΦ when compared to the control; however, such decrease in bacterial survival was substantially reversed in Ent + IFN-γ and Ent + L-arginine-treated cells (Figure 1C).
To assess whether endogenous Ent from Salmonella could impact the MΦ antibacterial activity, we infected the MΦs with WT Salmonella or ΔentB mutant. Ent deficiency impaired the survival of ΔentB mutant by ~ 4-fold, compared with WT Salmonella (Figure 1D). Addition of exogenous Ent substantially improved the survival of WT Salmonella by ~ 4-fold when compared to vehicle-treated WT Salmonella-infected control (Figure 1E). The increase in survival was more pronounced when ΔentB mutant-infected MΦs were treated with Ent, i.e., ~ 8.5-fold more survival relative to ΔentB-infected control (Figure 1E). The addition of Fe3+ alone seemed to further impede bacterial survival, which we presumed to be due to the ability of iron to participate in Fenton reaction and thus augment the MΦ oxidative responses (Figure 1E). We observed that the iron-free Ent was effective in improving the survival of both WT and ΔentB Salmonella (Figure 1E). Intriguingly, Ent-Fe+3 was more capable of improving the survival of ΔentB than WT (Figure 1E) Salmonella, which we postulate to be due to the increased uptake Ent-Fe+3 by ΔentB.
Iron inaccessibility is an environmental stimulus for bacteria to upregulate the expression of a set of genes for iron uptake modulated by the ferric uptake regulator (Fur) regulon, which comprises genes such as TonB (encodes for high-affinity iron transport)20, FepA (encodes for Ent receptor) and FeS (encodes for an esterase degrading Ent-Fe+3).21 Our results indicate that ΔentB mutant displayed an increasing trend in TonB expression, but comparable FepA and FeS expression, relative to WT Salmonella (Figure 2A-C). Provision of exogenous iron-free Ent to ΔentB mutant did not affect their TonB expression, but upregulated expression of FepA and FeS (Figure 2A-C). Such upregulation in FepA and FeS was not apparent in WT Salmonella given iron-free Ent. Moreover, iron-bound Ent did not upregulate FepA and FeS, but instead downregulated TonB, in both strains. Next, we asked whether other bacterial siderophore could also alter Salmonella gene expression. However, we observed that addition of deferoxamine (DFO) did not affect the expression of TonB, FepA and FeS in Salmonella (data not shown).
Figure 2.
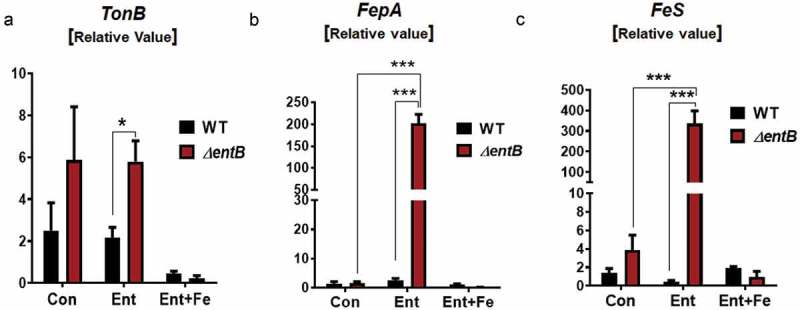
Exogenous ent upregulates iron uptake regulatory genes in ent-deficient Salmonella.
The quantitative RT-PCR analysis was used to quantify bacterial (WT Salmonella and mutant ΔentB) mRNA expression of (a) TonB, (b) FepA and (c) FeS upon treatment with Ent or Ent+ FeCl3 (1h). mRNA values are represented as fold-change normalized to the Gyrase B housekeeping gene. In vitro assays were performed in triplicate and data represented as mean ± SEM. * p < 0.05, and *** p < 0.001.
Aside from MΦs, Salmonella have to withstand other immune cells, such as dendritic cells, which also mediate antimicrobial activity. Hence, we next examined the survival of WT and ΔentB Salmonella against bone marrow-derived dendritic cells (BMDCs). We observed that WT and ΔentB Salmonella survived similarly. Nevertheless, addition of exogenous Ent promoted their survival by 1.5 to 1.8 -fold in WT Salmonella and ΔentB mutant, respectively (supplementary Figure 1). The extent of Salmonella killing by BMDCs was less pronounced when compared to MΦs. Such disparity could be due to the fact that MΦs are specialised phagocytic cells whereas dendritic cells are more specialised towards antigen presentation. Accordingly, MΦs have a more prominent repertoire of ROS and iron-dependent responses that not only mediate better killing activity, but also are more susceptibility to be inhibited by Ent. The lack of such responses in BMDCs may explain why we do not see differences between the intracellular survival of WT and ΔentB Salmonella.
Ent promotes the Salmonella-infected MΦ towards the M2 phenotype.
To study whether Ent can influence M1/M2 polarization, we pre-treated the MΦs with or without Ent, and then infected the MΦs with Salmonella (MOI of 100). The MΦs were analyzed via flow cytometry and gated for F4/80+CD38+CD206− cells (M1-MΦs)22 and F4/80+CD38−CD206+ cells (M2-MΦs)23, (Figure 3A). The Salmonella-infected MΦs were polarized towards the M1 phenotype as evidenced by the 2.8-fold increase in the percentage of M1-MΦs. In contrast, Ent treatment on Salmonella-infected MΦs substantially reduced the percentage of M1-MΦs to 1.5-fold more than control (Figure 3B). Interestingly, the percentage of M2-MΦs populations was significantly increased in Salmonella-infected MΦs upon treatment with Ent (2 folds) (Figure 3C). Such capacity of Ent to promote MΦ polarization towards the M2 phenotype may be another potential mechanism by which Ent exert its protective effects on intracellular Salmonella.
Figure 3.
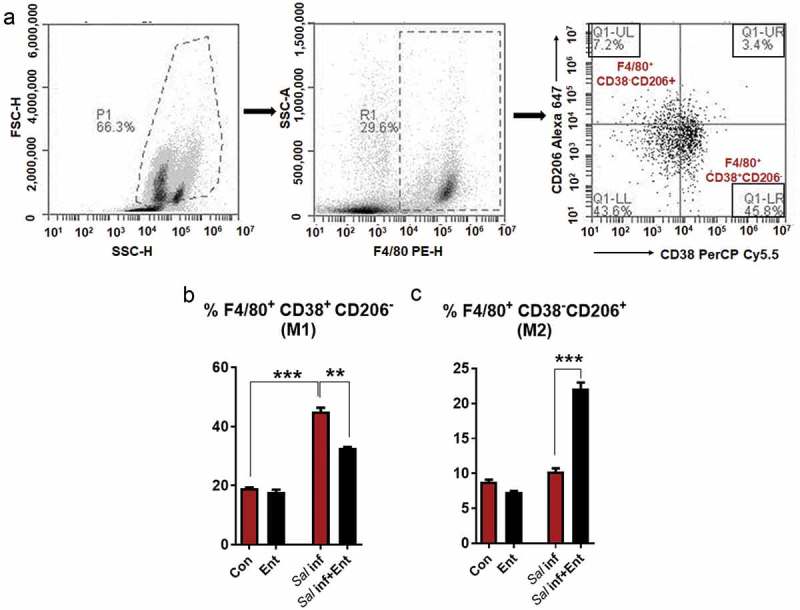
Ent polarizes the Salmonella-infected M1-MΦs towards the M2 phenotype.
Mice BMDMs were pre-treated with or without Ent, and then infected with Salmonella (MOI of 100). After washing, cells were incubated for 18h in presence of Ent. Cells were stained with anti-mouse F4/80, CD38, and CD206 antibodies. (a) F4/80+ MΦs were gated and analyzed for % positivity of F4/80+CD38+ CD206− (M1-MΦs) and F4/80+ CD38−CD206+ (M2-MΦs). (b and c) Bar graphs represented the % positivity of F4/80+CD38+CD206− and F4/80+ CD38−CD206+ cells in basal and Salmonella infected MΦs treated with Ent. In vitro assays were performed in triplicate and data represented as mean ± SEM. ** p < 0.01, and *** p < 0.001.
Ent chelates intracellular LIP and disrupts iron homeostasis in Mϕs
Intracellular pathogens have been shown to counter the antimicrobial activity of MΦs by gaining access to their intracellular LIP.24,25 To measure the cytosolic LIP that are accessible to Ent, we employed the cytochemical calcein-AM, a cell-permeable dye whose fluorescence is quenched when bound to LIP, but is elevated in the presence of a competitive iron chelator.26,27 Addition of Ent to uninfected calcein-stained MΦs increased their mean fluorescence intensity, thus implicating that Ent can indeed permeate MΦs and sequester their LIP (Figure 4A). Ent was also capable of chelating MΦs LIP during Salmonella infection, albeit to a lesser extent (Figure 4B-C). When using DFO in place of Ent, we observed that DFO could likewise chelate LIP in both uninfected and infected MΦs, but to a lesser extent when compared to Ent (supplementary Figure 2). This outcome is consistent with the assertion that Ent can chelate iron rapidly due to having the highest known affinity (Kd of 10−49M) for iron,28 whereas DFO is known to chelate iron slowly.29
Figure 4.
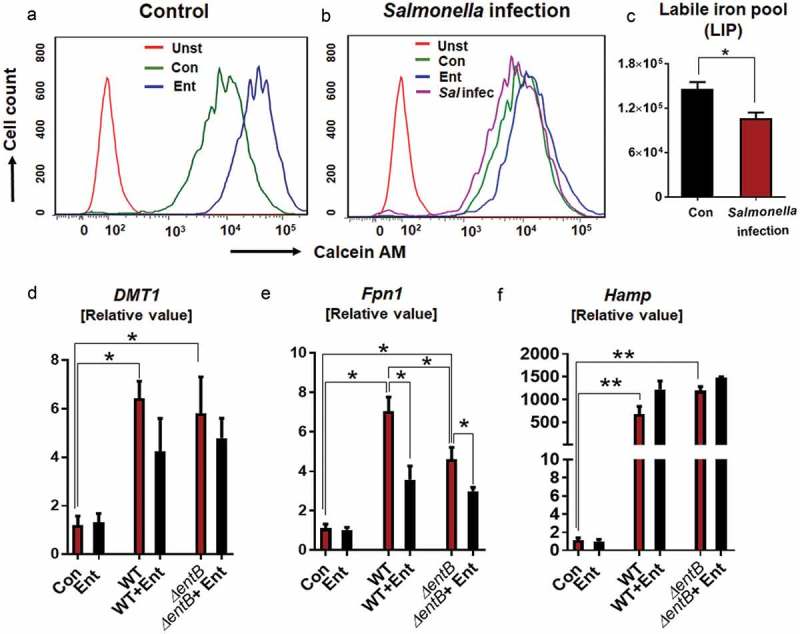
Ent disrupts the intracellular iron pool (LIP) in MΦs.
(a) Control and (b) Salmonella-infected BMDMs were incubated with Ent (25 µM) and cytosolic LIP was quantitated using the Calcein-AM method. Histograms represent the flow cytometric measurement of LIP in control and Salmonella-infected BMDMs, quantified as mean fluorescence intensity (MFI). (c) Bar graphs represent the LIP chelated by Ent in control and Salmonella-infected MΦs. In infection assay, BMDMs were pre-incubated with Ent then infected with Salmonella (WT strain or its isogenic Ent mutant ΔentB) at MOI of 100 and treated with Ent for 8h in presence of gentamicin (20 µg/ml). Cells were harvested and quantitative RT-PCR analysis was used to quantify mRNA expression of (d) DMT1 (e) Fpn1, and (f) Hamp. Values are represented as fold-change normalized to 36B4 housekeeping gene. In vitro assays were performed in triplicate and data represented as mean ± SEM. * p < 0.05 and ** p < 0.01.
To determine whether Ent could influence the MΦ iron-regulatory mechanism, we assessed the expression levels of Dmt1 and Fpn1, which facilitate cellular iron influx and efflux, respectively. Salmonella infected MΦs were observed to upregulate their expression of Dmt1 and Fpn1, whereas Ent pre-treatment modestly reduced the Dmt1 expression (albeit not significantly) but substantially down regulated Fpn1 expression. The decrease in the levels of Fpn1 in the Ent-treated MΦs was inversely correlated with the increase in the expression of hepcidin (Hamp) (Figure 4D-F), a master regulator of iron homeostasis known to promote the internalization and degradation of ferroportin.7,8
Discussion
Enterobactin is a prototype of Gram-negative bacterial siderophores with the unparalleled affinity (Kd of 10−49M) towards ferric iron.28 When colonizing or invading the host, both commensal and pathogenic Enterobacteriaceae deploy Ent to acquire iron, an essential nutrient for their replication and growth.17 Considering the number of specific enzymes involved in its biosynthesis, the production of Ent can be regarded as an expensive metabolic process.17 Accordingly, it is reasonable to presume that Ent may play more unique and adaptable roles, beyond iron procurement, for bacteria to preserve its production as cost-effective. In this respect, several studies have sought to elucidate the pleiotropic properties of Ent,30 including its role in facilitating bacterial colonization and persistence,31 promoting development of mature biofilms,32 and protecting bacteria from oxidative stress and microbicidal effects of paraquat, H2O2, ROS, myeloperoxidase (MPO) and neutrophil extracellular traps.16,33–36
Amongst Enterobacteriaceae, the enteropathogen Salmonella has the ability to subvert the phagocytotic feature of MΦs to establish an intracellular niche that supports bacterial growth and replication.37 To survive within the MΦs, Salmonella employs its type III secretion system (T3SS) to deliver virulence factors, which prevent the delivery of NADPH oxidase and iNOS-generated products into the Salmonella-containing vacuoles.38–40 These virulence factors and the mechanisms underlying their capacity to modulate the MΦ antimicrobial activities, however, are not fully characterized. In this study, we sought to investigate whether Ent could be part of the mechanism employed by the pathogen to survive inside the MΦs, based on the assertions that Ent is a virulence factor for Salmonella41 and that Ent-deficient Salmonella survives poorly in MΦs.33
As part of their immune strategy to combat infection, the MΦs are known to initiate a state of ‘iron starvation/nutritional immunity’ to ensure that the amount of intracellular LIP is far less than the amount required for the bacteria to replicate and grow, thus facilitating bacterial clearance.9 An adequate supply of LIP, however, still need to be retained in the MΦs to facilitate their effector functions, many of which are dependent on the redox property of iron [e.g., oxidative burst responses by NADPH oxidase and inducible nitric oxide synthase (iNOS)]. Moreover, the major enzymes involved in the antimicrobial responses, i.e., MPO (albeit restricted to neutrophils), NADPH oxidase and iNOS, are heme proteins which require iron for their bioactivity. We accordingly tested the hypothesis, on whether Ent could have mediated its bacterial protective effects via depletion of MΦ LIP, by comparing the effects of apo-Ent (iron-free Ent) and ferric-Ent. Our results demonstrated that apo-Ent is overall more effective than iron-bound Ent in promoting the protective functions to intracellular Salmonella, thus suggesting that the effector functions of Ent are likely to be dependent on its iron-chelating property.42 Intriguingly, we found that Ent is also capable of modulating the expression of several key iron-regulatory proteins, i.e., Dmt1, Fpn1, and Hamp (hepcidin), which orchestrate MΦ intracellular iron homeostasis. Ent treatment modestly reduced the expression of Dmt1, but substantially decreased the expression of Fpn1 in the infected MΦ, thus resulting in a net effect which promotes the retention of cytosolic LIP that favors bacterial growth.43
The iron chelation effects of Ent may not only benefit Salmonella specifically, but also could provide a survival advantage to a wider spectrum of Ent-producing bacteria, encompassing both pathogens and commensals. Furthermore, the production of Ent could benefit other non-Ent-producing bacteria as well, particularly those which express the receptor for Ent44 and thus could ‘pirate’ Ent to support the growth of other bacteria45 via polymicrobial cross-feeding46 and antagonism.47 The property of Ent to scavenge free radicals,34 for instance, could be pivotal in allowing microbial communities to withstand the high levels of ROS/NO present in the mammalian gut.48 It seems reasonable to suggest that the ability of Enterobacteriaceae to bloom and outgrow other gut bacteria during inflammatory bowel disease49,50 could be facilitated, in whole or in part, by the iron chelation property of Ent. A deeper understanding on the host immune responses and iron regulation property of Ent, as well as other siderophores would be beneficial for developing Ent42,51 as a biological therapeutic to treat inflammatory diseases.
Materials and methods
Reagents
Iron-free Ent (Escherichia coli), deferoxamine mesylate (DFO), ferric chloride, Histopaque®-1077 and 1119, RPMI, Triton X-100, gentamicin, calcein acetoxymethyl ester (Calcein AM), L-arginine, and murine recombinant IFN-γ were purchased from Sigma (St Louis, MO). SYBR® Green mix and qScript cDNA synthesis kit were procured from Quanta Biosciences, USA. Chrome Azurol S (CAS) was purchased from Acros Organics (Geel, Belgium).
Mice
C57BL/6J wild type (WT) mice were procured from the Jackson Laboratory (Bar Harbor, ME). These mice were bred and maintained in the animal facility at The Pennsylvania State University and University of Toledo. The Institutional Animal Care and Use Committee (IACUC) at The Pennsylvania State University and University of Toledo approved all the animal experiments.
Bone marrow-derived macrophages (BMDMs) culture
Bone marrow cells from WT mice were used to generate BMDMs as described by Zhang et al..52 Briefly, the BMDM cells were cultured in the presence of macrophage colony-stimulating factor (M-CSF; 100 U/ml, R&D Systems) at 37°C, 5% CO.253 On every other day, 60% of the media was replaced with fresh complete media containing M-CSF. After 7 days, non-adherent cells were removed and adherent cells were re-plated on 6 or 24 well plates at a density of 1.0 × 106 cells/ml and pre-treated with Ent (25 μM), FeCl3 (25 μM) or Ent+ FeCl3 (1:1 ratio) for 1h, before the addition of IFN-γ (20 IU).
Isolation of bone marrow-derived dendritic cells (BMDCs):
BMDCs were generated from bone marrow cells from WT mice femurs and tibias as described earlier.54 In brief, bone marrow cells were cultured in complete RPMI 1640 media and in presence of GM-CSF (20 ng/ml). On every 3rd day, 60% of media was replaced with fresh complete medium containing GM-CSF. Cells were collected on day 7.
Bacterial culture and growth conditions
WT Salmonella typhimurium (ATCC 14028) and its isogenic ΔentB mutant (entB::MudJ, kanamycin-resistant)21 were a gift from Dr. A. J. Baumler, University of California, Davis. Bacterial strains were grown for 15 h in Luria-Bertani (LB) media at 37°C with shaking (200 rpm). Equal numbers of bacterial CFUs were adjusted based on their optical density at 600 nm, pelleted and suspended in incomplete RPMI media, and added to MΦ at a multiplicity of infection (MOI) of 100.
Quantification of bacterial survival in gentamicin protection assay
BMDMs or BMDCs (2.0x106 cells/well) were prepared in 1.0 ml incomplete phenol red free RPMI media without antibiotics and plated in 12-well plates. The cells were treated with either Ent (25 μM), FeCl3 (25 μM), Ent + FeCl3 (1:1 ratio), recombinant murine IFN-γ (20 ng/ml) or L-arginine (500 µM) and incubated for 1h at 37ºC, 5% CO2. Next, the cells were infected with either WT Salmonella or its isogenic ΔentB mutant at MOI of 100 and incubated for 1h. The cells were washed twice with sterile PBS and incubated with fresh Ent (25 μM), FeCl3 (25 μM), or Ent + FeCl3 (1:1 ratio), IFN-γ (20 ng/ml) or L-arginine (500 µM) for 24h in the presence of gentamicin (20 µg/ml). The cells were pelleted, washed and resuspended in 1 ml of hypotonic solution (containing 50% PBS and 0.1% Triton X-100) to release intracellular bacteria, serially diluted and plated on LB agar plates and incubated overnight at 37°C. Bacterial CFU was represented as relative fold change to the WT Salmonella-treated group, whereby the infection was considered as 100%.
Chrome azurol s (CAS) assay
CAS agar plates and liquid reagent were prepared as previously described by Schwyn and Neilands.29,55 The principle of the assay is that CAS remains blue when complexed with iron, but changes to orange when other iron chelators chelate iron. Equal CFUs of WT Salmonella and ΔentB mutant were incubated on CAS agar plate overnight at 37°C and monitored for formation of orange halo. To assess Ent released by Salmonella, the overnight culture supernatants (diluted 4x in 100 µl) were added to the CAS liquid reagent (100 μl), incubated for 20 min at room temperature, and the change in absorbance was measured at 630 nm. Results were estimated using a standard curve generated from iron-free Ent (0–25 µM).
Quantification of intracellular iron
Unstimulated, WT Salmonella-infected BMDMs were incubated with 0.5 μM Calcein-AM for 15 min. The cells were washed 2x with PBS and treated with either Ent or DFO (25 µM) for 2h. After washing, cells were analyzed by using LSR Fortessa and the mean fluorescence intensity (MFI) was determined using the FlowJo Software. The levels of intracellular labile iron (ΔF) were calculated by subtracting the difference in the MFI, before and after treatment with the iron chelators as previously described.56
Flow cytometric analysis of macrophage surface markers
To verify the M1 (F4/80+CD38+) and M2 (F4/80+CD206+) markers in mouse BMDMs, 5.0 × 105 cells were seeded in 24-well culture plates in complete RPMI media. After 8h, the cells were incubated with or without Ent, FeCl3 or Ent+ FeCl3 (1:1 ratio) for another 1h, and then infected with Salmonella (MOI of 100, 1h incubated then washed) for an additional 18h. Cells were washed and stained with fluorescent conjugated anti-mouse monoclonal antibodies directed against the following cell surface proteins: F4/80-PE (BD Biosciences), CD38-PerCP-Cy 5.5 (BD Biosciences) and CD206-AlexaFluor-647 (BD Biosciences). Cells were analyzed on Accuri c6 flow cytometer (BD Biosciences); F4/80−positive MΦs were gated and assessed for the % cells positive for CD38 and CD206.
Quantitative and semi-quantitative RT-PCR for bacterial genes
Equal CFU of WT Salmonella and ΔentB mutant (from overnight culture) were incubated with either Ent or DFO (25 µM) and Ent + FeCl3 or DFO + FeCl3 (1:1 ratio) for 1h. Bacteria was pelleted and washed with PBS. Total bacterial RNA was isolated from bacteria using Pure Link® RNA Mini Kit (Life Technologies). RNA concentration and purity were determined using Nano drop. RNA was treated with DNase to avoid genomic DNA (gDNA) contamination. Furthermore, PCR was run with RNA alone to confirm the lack of gDNA contamination, DNase-treated RNA was reverse-transcribed to cDNA using iScript cDNA synthesis kit (Bio-Rad). For the semi- quantitative RT-PCR, the sequences of the primers were as follows (sense and antisense respectively): Gyrase B (housekeeping gene/internal loading control) 5ʹ- TGCGTGAACTGTCATTCCTG −3ʹ; 5ʹ-GTGGATCGGCGTTTTATTCTTG −3ʹ; TonB 5ʹ- CAAACCTAAGCCAAAACCCAAG; 5ʹ-GCTATTTTCAAACGGTGAGGC; FepA 5ʹ- GTGGTTCGTTGGGATTTTGC-3ʹ; 5ʹ-GTTGGTTCGTATTGGTGTTCTG-3ʹ; FeS 5ʹ- GACCCGCTTAATTCGCAAAG-3ʹ; 5ʹ-ACGTTCGCTATGCCACTG-3ʹ. Real-time PCR amplifications were performed using SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad). Amplification cycle was as follows: Polymerase activation and initial DNA denaturati-on of 30 sec at 95°C, second denaturation of 15 sec at 95°C, and annealing and extension for 30 sec at 62°C, second denaturation and annealing and extension cycles were repeated for 40 cycles, followed by melt curve analysis from 55°-95°C in 0.5°C increments with 2 sec/step.ΔΔCq values were calculated for each sample (triplicate) and used for analysis of transcript abundance or loss with respect to the internal control.
Real-time PCR for macrophages genes
Total RNA was isolated from unstimulated and stimulated BMDMs by using TRIzol reagent (Sigma) as described in the manufacturer’s protocol. mRNA was used to synthesize cDNA for qRT-PCR using SYBR green (Quanta) according to manufacturer’s protocol. qRT-PCR was performed in StepOnePlusTM real time PCR instrument (Life technologies). Sequence of primers used for qRT-PCR were (sense and antisense respectively): 36B4 5ʹ-TCCAGGCTTTGGGCATCA-3ʹ and 5ʹ-CTTTATTCAGCTGCACATCACTCAGA-3ʹ57; Fpn1 (ferroportin 1) 5ʹ-TTGTTGTTGTGGCAGGAGAA-3ʹ and 5ʹ-AGCTGGTCAATCCTTCTAATGG-3ʹ58; Hamp (hepcidin) 5ʹ-AGAAAGCAGGGCAGACATTG-3ʹ and 5ʹ-CACTGGGAATTGTTACAGCATT-3ʹ58; Dmt-1 (divalent metal transporter-1) 5ʹ GGCTTTCTTATGAGCATTGCCTA-3ʹ and 5ʹ-GGAGCACCCAGAGCAGCTTA-3ʹ.59 36B4 was used to normalize the relative mRNA expression using the comparative Ct (2−ΔΔCt) method. Fold change was determined by comparison to the untreated cells.
Statistical analysis
All in vitro experiments were performed in triplicate and data were presented as representative of three independent experiments. Results were expressed as mean ± SEM. Statistical significance between two groups was analyzed using unpaired, two-tailed t-test. Data from more than two groups was compared using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test (to compare the mean of each column with the mean control column) or Tukey’s multiple comparison tests (when to compare the mean of each column with the mean of every other column). All statistical analyses were performed with the GraphPad Prism 7.0 software (GraphPad Inc, La Jolla, CA). p < 0.05 was considered as statistically significant and denoted as * p < 0.05 and ** p < 0.01 and *** p < 0.001.
Funding Statement
This work was supported by a grant from the National Institutes of Health R01 (DK097865) to MV-K and AI077917 and AI123521 to GSK. P.S. is supported by CCFA׳s Research Fellowship Award. We thank Dr. A. J. Baumler (University of California, Davis) for providing the WT Salmonella typhimurium and its isogenic ΔentB mutant. We acknowledge the Huck Institutes of the Life Sciences for seed funding through the Flow Cytometry Facility.
Abbreviations
- MΦ
macrophage
- Ent
enterobactin
- Dmt1
divalent metal transporter 1
- Fpn1
ferroportin 1
- Hamp
hepcidin antimicrobial peptide
- LIP
labile iron pool
- BMDM
bone marrow-derived macrophage
- M-CSF
macrophage colony-stimulating factor
- MFI
mean fluorescence intensity
- WT
wild-Type
- DFO
deferoxamine mesylate
Authorship
Contribution: P.S. performed the experiments, analyzed the data and co-wrote the paper; X.X. performed the experiments, analyzed the data and co-wrote the paper; B.S.Y. analyzed the results and co-wrote the paper; Q.C. contributed to the experimental design and flow cytometry analysis, B.K. performed bacterial gene analysis. G.S.K. contributed to the experimental design and analysis and co-wrote the paper. M.V.-K. conceptually developed the project, designed the experiments, analyzed the data, and co-wrote the manuscript.
Supplemental material
Supplementary material for this article can be accessed here
Disclosure of potential conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Smith PD, Ochsenbauer-Jambor C, Smythies LE.. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206: 149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 2.Nairz M, Theurl I, Swirski FK, Weiss G. “Pumping iron”-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers archiv: European. J Physiol. 2017;469: 397–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nairz M, Weiss G. Molecular and clinical aspects of iron homeostasis: from anemia to hemochromatosis. Wien Klin Wochenschr. 2006;118: 442–462. doi: 10.1007/s00508-006-0653-7. [DOI] [PubMed] [Google Scholar]
- 4.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102: 1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss G. Modification of iron regulation by the inflammatory response. Best Pract Res Clini Haematol. 2005;18: 183–201. doi: 10.1016/j.beha.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5: 299–309. [DOI] [PubMed] [Google Scholar]
- 7.Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur J Immunol. 2008;38: 1923–1936. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306: 2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EE, Wessling-Resnick M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012;14: 207–216. doi: 10.1016/j.micinf.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9: 2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 11.Coburn B, Sekirov I, Finlay BB. Type III secretion systems and disease. Clin Microbiol Rev. 2007;20: 535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steeb B, Claudi B, Burton NA, Tienz P, Schmidt A, Farhan H, Mazé A, Bumann D, Nassif X. Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog. 2013;9: e1003301. doi: 10.1371/journal.ppat.1003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dostal A, Gagnon M, Chassard C, Zimmermann MB, O’Mahony L, Lacroix C. Salmonella adhesion, invasion and cellular immune responses are differentially affected by iron concentrations in a combined in vitro gut fermentation-cell model. PLoS One. 2014;9: e93549. doi: 10.1371/journal.pone.0093549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischbach MA, Lin H, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol. 2006;2: 132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 15.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 16.Saha P, Yeoh BS, Olvera RA, Xiao X, Singh V, Awasthi D, Subramanian BC, Chen Q, Dikshit M, Wang Y, et al. Bacterial siderophores hijack neutrophil functions. J Immunol. 2017;198: 4293–4303. doi: 10.4049/jimmunol.1700261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A. 2003;100: 3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009;11: 1365–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahiri A, Das P, Chakravortty D. Arginase modulates Salmonella induced nitric oxide production in RAW264.7 macrophages and is required for Salmonella pathogenesis in mice model of infection. Microbes Infect. 2008;10: 1166–1174. doi: 10.1016/j.micinf.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Noinaj N, Guillier M, Barnard TJ, Buchanan SK. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol. 2010;64: 43–60. doi: 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsolis RM, Baumler AJ, Stojiljkovic I, Heffron F. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 1995;177: 4628–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M, Olszewski MA. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10: e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015: 816460. doi: 10.1155/2015/125380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13: 509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg ED. Iron availability and infection. Biochim Biophys Acta. 2009;1790: 600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Cabantchik ZI, Glickstein H, Milgram P, Breuer W. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal Biochem. 1996;233: 221–227. doi: 10.1006/abio.1996.0032. [DOI] [PubMed] [Google Scholar]
- 27.Glickstein H, El RB, Shvartsman M, Cabantchik ZI. Intracellular labile iron pools as direct targets of iron chelators: a fluorescence study of chelator action in living cells. Blood. 2005;106: 3242–3250. doi: 10.1182/blood-2005-02-0460. [DOI] [PubMed] [Google Scholar]
- 28.Loomis LD, Raymond KN. Solution equilibria of enterobactin and metal enterobactin complexes. Inorg Chem. 1991;30: 906–911. doi: 10.1021/ic00005a008. [DOI] [Google Scholar]
- 29.Xiao X, Yeoh BS, Saha P, Tian Y, Singh V, Patterson AD, Vijay-Kumar M. Modulation of urinary siderophores by the diet, gut microbiota and inflammation in mice. J Nutr Biochem. 2016;41: 25–33. doi: 10.1016/j.jnutbio.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holden VI, Bachman MA. Diverging roles of bacterial siderophores during infection. Metallomics. 2015;7: 986–995. doi: 10.1039/c4mt00333k. [DOI] [PubMed] [Google Scholar]
- 31.Pi H, Jones SA, Mercer LE, Meador JP, Caughron JE, Jordan L, Newton SM, Conway T, Klebba PE, Heimesaat MM. Role of catecholate siderophores in gram-negative bacterial colonization of the mouse gut. PLoS One. 2012;7: e50020. doi: 10.1371/journal.pone.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May T, Okabe S. Enterobactin is required for biofilm development in reduced-genome Escherichia coli. Environ Microbiol. 2011;13: 3149–3162. doi: 10.1111/j.1462-2920.2011.02607.x. [DOI] [PubMed] [Google Scholar]
- 33.Achard ME, Chen KW, Sweet MJ, Watts RE, Schroder K, Schembri MA, McEwan AG. An antioxidant role for catecholate siderophores in Salmonella. Biochem J. 2013;454: 543–549. doi: 10.1042/BJ20121771. [DOI] [PubMed] [Google Scholar]
- 34.Peralta DR, Adler C, Corbalan NS, Paz Garcia EC, Pomares MF, Vincent PA. Enterobactin as part of the oxidative stress response repertoire. PLoS One. 2016;11: e0157799. doi: 10.1371/journal.pone.0157799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler C, Corbalan NS, Peralta DR, Pomares MF, de Cristobal RE, Vincent PA. The alternative role of enterobactin as an oxidative stress protector allows escherichia coli colony development. PLoS One. 2014;9: e84734. doi: 10.1371/journal.pone.0084734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N, Joe B, Vijay-Kumar M. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun. 2015;6: 7113. doi: 10.1038/ncomms8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruenheid S, Finlay BB. Microbial pathogenesis and cytoskeletal function. Nature. 2003;422: 775–781. doi: 10.1038/nature01603. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287: 1655–1658. [DOI] [PubMed] [Google Scholar]
- 39.Chakravortty D, Hansen-Wester I, Hensel M. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med. 2002;195: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunology. 2001;166: 5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- 41.Yancey RJ, Breeding SA, Lankford CE. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979;24: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chieppa M, Galleggiante V, Serino G, Massaro M, Santino A. Iron chelators dictate immune cells inflammatory ability: potential adjuvant therapy for ibd. Curr Pharm Des. 2017. doi: 10.2174/1381612823666170215143541. [DOI] [PubMed] [Google Scholar]
- 43.Pan X, Tamilselvam B, Hansen EJ, Daefler S. Modulation of iron homeostasis in macrophages by bacterial intracellular pathogens. BMC Microbiol. 2010;10: 64. doi: 10.1186/1471-2180-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutz JM, Abdullah T, Singh SP, Kalve VI, Klebba PE. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J Bacteriol. 1991;173: 5964–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grandchamp GM, Caro L, Shank EA. Pirated siderophores promote sporulation in bacillus subtilis. Appl Environ Microbiol. 2017;83. doi: 10.1128/AEM.03293-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keogh D, Tay WH, Ho YY, Dale JL, Chen S, Umashankar S, Williams RH, Chen S, Dunny G, Kline K. Enterococcal metabolite cues facilitate interspecies niche modulation and polymicrobial infection. Cell Host Microbe. 2016;20: 493–503. doi: 10.1016/j.chom.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adler C, Corbalan NS, Seyedsayamdost MR, Pomares MF, de Cristobal RE, Clardy J, Kolter R, Vincent PA, Liles MR. Catecholate siderophores protect bacteria from pyochelin toxicity. PLoS One. 2012;7: e46754. doi: 10.1371/journal.pone.0046754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knaus UG, Hertzberger R, Pircalabioru GG, Yousefi SP. Branco Dos Santos F. Pathogen control at the intestinal mucosa - H2O2 to the rescue. Gut Microbes. 2017;8: 67–74. doi: 10.1080/19490976.2017.1279378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14: 82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Gart EV, Suchodolski JS, Welsh TH Jr., Alaniz RC, Randel RD, Lawhon SD. Salmonella typhimurium and multidirectional communication in the gut. Front Microbiol. 2016;7: 1827. doi: 10.3389/fmicb.2016.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q, Jin B, Shi Z, Wang X, Liu Q, Lei S, Peng R. Novel enterobactin analogues as potential therapeutic chelating agents: synthesis, thermodynamic and antioxidant studies. Sci Rep. 2016;6: 34024. doi: 10.1038/srep34024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages In: Coligan John E, et al editor. Current protocols in immunology. 2008;Chapter 14:Unit 14 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanley ER. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116: 564–587. [DOI] [PubMed] [Google Scholar]
- 54.Thakur BK, Saha P, Banik G, Saha DR, Grover S, Batish VK, Das S. Live and heat-killed probiotic lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int Immunopharmacol. 2016;36: 39–50. doi: 10.1016/j.intimp.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 55.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160: 47–56. [DOI] [PubMed] [Google Scholar]
- 56.Prus E, Fibach E. Flow cytometry measurement of the labile iron pool in human hematopoietic cells. Cytometry A. 2008;73: 22–27. doi: 10.1002/cyto.a.20491. [DOI] [PubMed] [Google Scholar]
- 57.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7: e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masaratana P, Patel N, Latunde-Dada GO, Vaulont S, Simpson RJ, McKie AT. Regulation of iron metabolism in hamp (-/-) mice in response to iron-deficient diet. Eur J Nutr. 2013;52: 135–143. doi: 10.1007/s00394-011-0295-z. [DOI] [PubMed] [Google Scholar]
- 59.Dupic F, Fruchon S, Bensaid M, Loreal O, Brissot P, Borot N, Roth MP, Coppin H. Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut. 2002;51: 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


