Abstract
Management of congenital adrenal hyperplasia (CAH) requires both glucocorticoid replacement and suppression of adrenal androgen synthesis. It is recommended that children with CAH be treated with hydrocortisone, but the appropriate glucocorticoid regimen in adults is uncertain. In order to review the outcomes of different glucocorticoid regimens in the management of CAH, a systematic search of PubMed/MEDLINE and Web of Science was conducted, including reports published up to 25 February 2019. Studies that compared at least two types of glucocorticoid preparation were included. The following information was extracted from each study: first author, year of publication, number and characteristics of patients and control subjects, types and doses of glucocorticoid regimen used, study design and outcomes [e.g., biochemical tests, weight, height, body mass index (BMI), bone mineral density (BMD)]. A total of 23 studies were included in the qualitative synthesis, with 19 included in the quantitative synthesis. Dexamethasone was associated with the greatest degree of adrenal suppression; there was no significant difference in 17-hydroxyprogesterone (17OHP) and androstenedione levels between patients treated with hydrocortisone or prednisolone. Patients treated with dexamethasone had the lowest BMD and the highest BMI. Although dexamethasone therapy is associated with significantly lower 17OHP and androstenedione levels, it is also associated with more adverse effects. There do not appear to be significant differences between hydrocortisone and prednisolone therapy, and the choice of agent should be based on individual patient factors.
Keywords: 21-hydroxylase deficiency, 11β-hydroxylase deficiency, prednisolone, hydrocortisone, dexamethasone, outcome
Congenital adrenal hyperplasia (CAH) refers to a group of heterogeneous autosomal recessive disorders characterized by defective adrenal steroidogenesis and consequent cortisol deficiency [1, 2]. The vast majority of cases (95% to 99%) are the result of a mutation in the 21-hydroxylase enzyme [3–5]. Cortisol deficiency causes a lack of inhibitory feedback to the hypothalamus and pituitary, leading to an ACTH-dependent accumulation of steroid precursors proximal to the enzyme defect, including 17-hydroxyprogesterone (17OHP) [2, 6]. These precursors are shunted into the preserved androgen pathway. Cases of CAH may be severe (classic) or mild (nonclassic). Classic forms of CAH are diagnosed in infancy on the basis of neonatal screening tests, whereas nonclassic CAH is usually diagnosed in adolescence or young adulthood [7]. Classic CAH is subdivided into salt-wasting (SW) and simple-virilizing (SV) forms, depending on whether mineralocorticoid synthesis is affected [1, 4].
Management of CAH presents unique challenges distinct from other forms of adrenal insufficiency. Higher doses of glucocorticoids are required to suppress adrenal androgen synthesis, which can lead to overtreatment and iatrogenic Cushing syndrome [3, 8, 9]. Use of long-acting glucocorticoids in children is avoided because of the risk of growth suppression; hydrocortisone is considered the preferred glucocorticoid in this population [2]. The ideal glucocorticoid for the treatment of adults with CAH is more contentious [10]. Although hydrocortisone in divided doses is a common treatment option, once- or twice-daily preparations of long-acting glucocorticoids are also used, including prednisolone and dexamethasone [1, 2, 4]. Newer preparations of hydrocortisone have also been trialed, including modified-release tablets and continuous subcutaneous hydrocortisone infusions (CSHIs) [11, 12]. Assessment of treatment adequacy remains challenging. The aim of this systematic review and meta-analysis was to review the efficacy of different glucocorticoid regimens in the management of CAH.
1. Methods
A. Search Strategy
A systematic search of PubMed/Medline and Web of Science was undertaken of articles published up to 25 February 2019. The search terms used were “congenital adrenal hyperplasia”, “CAH”, “21-hydroxylase deficiency”, “steroid regimen”, “glucocorticoid regimen”, and “glucocorticoid”. Studies of all languages and publication dates were included. Articles were screened initially by title and then by abstract for relevance. Full-text review was then undertaken for selected studies. Reference lists of identified articles were then reviewed to identify other relevant studies not retrieved in the database searches. All included and excluded articles were agreed on by all coauthors with reference to the criteria described in the following section.
B. Study Selection and Data Extraction
Studies that compared at least two different types of glucocorticoids in individuals with CAH were included, some of which used baseline glucocorticoid therapy as the comparator. Studies were excluded if only a single type of glucocorticoid was assessed or if results were combined with individuals with other forms of adrenal insufficiency and not expressed separately. Studies were also excluded if glucocorticoid preparations were compared only in terms of glucocorticoid-equivalent dose rather than type of glucocorticoid. Only original research was included (i.e., review articles and editorials were excluded). Meeting abstracts were also excluded.
The following information was extracted from each article: first author, year of publication, number and characteristics of patients and control subjects, types and doses of glucocorticoid regimen used, study design and outcomes [e.g., biochemical tests, weight, height, body mass index (BMI), bone mineral density (BMD)]. All biochemical variables were converted into International System of Units values. Glucocorticoid doses were converted into hydrocortisone-equivalent doses using growth-retarding equivalents (i.e., hydrocortisone 20 mg = cortisone acetate 25 mg = prednisolone 4 mg = dexamethasone 0.25 mg) [8]. Patients receiving cortisone acetate were included in the hydrocortisone treatment group. Any treatment combination with dexamethasone was included in the dexamethasone treatment group. Quality assessment instruments [e.g., the Newcastle-Ottawa scale (www.ohri.ca/programs/clinical_epidemiology/oxford.asp)] were unable to be applied to most of the studies by virtue of their study design, but the quality of the included studies can be generally appreciated in Table 1. The Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed [34].
Table 1.
Summary of Included Studies, Organized by Types of Compared Glucocorticoids
| Comparison | Study | Subjects | Intervention | Outcomes | Results | Comments |
|---|---|---|---|---|---|---|
| HC vs P | ||||||
| Ajish 2014 [14] | 13 subjects aged 1.9–12 y with 21OHD; all receiving MC: crossover study design; patients received each drug regimen for 2 mo | Compared HC given at different times in the evening, as well as substitution of evening HC with P | Measured 17OHP and T | No significant difference was seen in T or 17OHP levels between groups | No control group; authors concluded no clear benefit of any of the three regimens | |
| Falhammar 2014 [18]a | 30 male subjects aged 19–67 y with 21OHD | Study assessed QoL, social situation, and education with reference to current GC regimen (HC/CoAc or P); age-and sex-matched control group | Questionnaires assessing occupation and social factors; PGWB; sexual issues | No difference between control subjects and patients receiving P in terms of PGWB; patients receiving HC/CoAc had significantly lower scores than control subjects and had more anxiety than those receiving P; individuals receiving HC/CoAc also had more erectile dysfunction compared with control subjects; patients whose CAH was poorly controlled had significantly better PGWB scores than those who were over treated; patients with poorly controlled CAH were less anxious and had higher sexual satisfaction when compared with control subjects; over-treated patients had inferior QOL compared with control subjects and patients with poorly controlled disease | Results suggest positive correlation between adrenal androgen and steroid precursor levels and QoL; patients on short-acting GC had poorer QoL than those on P | |
| Falhammar 2013 [17]a | 30 male subjects aged 19–67 y with 21OHD | Study evaluated bone health in men with CAH with reference to current GC regimen (HC/CoAc, P, or D (1 subject)); age- and sex-matched control group | Measured BMD, recorded fracture history; current GC dose; T, E, 17OHP, DHEAS, PTH | Patients receiving P had lower BMD and osteocalcin levels compared with control group; patients receiving HC/CoAc were no different from control subjects; higher IGF-1 levels were measured in patients receiving HC/CoAc; 44% of those receiving P had no diurnal variation in 17OHP compared with 14% receiving HC/CoA; subject receiving D also had no diurnal variation | P therapy appeared to be associated with osteoporosis and fractures compared with HC/CoA; BMD and current GC dose did not appear to correlate | |
| Falhammar 2011[13]a | 30 male subjects aged 19–67 y with 21OHD | Cardiovascular and metabolic parameters assessed in men with CAH with reference to current GC regimen (HC/CoAc or P); age- and sex-matched control group | Measured BMI, DXA, BP, HR, OGTT, urinary catecholamines, and 17OHP | Patients receiving short-acting GCs had higher BMI, fat mass, and T levels than control subjects; patients receiving P had higher OGTT results than control subjects | Results suggested short-acting GCs led to poorer metabolic outcomes | |
| Bonfig 2007 [15] | 125 subjects with 21OHD who had reached final height (age range not specified); 68 SW, 57 SV | Study aimed to assess final height outcome and influence of GC regimen (HC vs P) | Measured height, bone age; assessed target height [(maternal height + paternal height ±13 cm)/2] | HC-treated subjects were significantly taller than P-treated subjects; P-treated subjects did not have better adrenal androgen suppression; hydrocortisone-equivalent doses at the start of puberty correlated with FH levels | No control group; P led to reduced FH levels; results expressed in terms of change in SDS, so not included in meta-analysis | |
| Leite 2007 [19] | 15 subjects (mean age, 7.2 y) with 21OHD; 14 SW, 1 SV | Compared 12 mo of treatment with HC with consecutive 12 mo of treatment with P | Measured variation of height SDS, variation of height SDS according to bone age, variation of BMI SDS and serum levels of 17OHP and A4 | No significant difference found in change in height SDS, bone age SDS, BMI SDS, 17OHP or A4 levels | No control group; authors concluded P is as efficacious as TDS HC | |
| Caldato 2004 [16] | 44 subjects aged 1.2–20 y with 21OHD randomly assigned to two groups; 27 SL, 17 SV | Compared P once daily to TDS HC | Measured T, A4, 17OHP levels; height, weight, growth velocity | Increase in height when corrected for bone age in P group; increased bone age/chronological age ratio in HC group; growth velocity was similar between groups; no significant difference in steroid levels between groups | No control group; authors favored P to HC | |
| D vs HC and/or Pa | ||||||
| Nebesio 2016 [22] | 9 subjects aged 4.8–11.6 y with 21OHD; 8 SW, 1 SV | Subjects were randomly assigned to three sequential 6-wk courses of HC, P, and D | Measured ACTH, A4, 17OHP, IGF-1, and GH levels, and BMI | ACTH, A4, and 17OHP values were all significantly lower with D; 17OHP levels were significantly lower with HC than P; no difference in GH or IGF-1; no significant difference in change in BMI | No control group; D led to significant adrenal suppression | |
| Han 2013 [33]b | 196 adults with CAH, mean age, 34.4 y | Cross-sectional study comparing different GC regimens (HC, P, D, combination) on metabolic parameters and BMD | Measured 17OHP, A4, T, SHBG levels; BMD, BP, BMI | Subjects receiving D had lower androgen and ACTH levels, higher femoral BMD, higher insulin resistance; no other differences in terms of metabolic parameters; in terms of dosing and regimen, higher doses of D when given once daily were associated with greater insulin resistance; higher HC-dose equivalents were associated with higher androgens, higher BP, and more severe enzyme defect | No control group; D led to greater suppression of androgens, but more insulin resistance, particularly when given once daily | |
| Han 2013 [20]b | 151 adults with 21OHD, aged 18–69 y | Cross-sectional study comparing different GC regimens (HC, P, D, combination) on QoL | Measured QoL using SF-36; BP, height, weight, FBGL, 17OHP, P, A4, T levels | Subjects taking HC monotherapy had higher vitality and mental health scores than those receiving combined HC and P, P, or D; QoL did not correlate with GC dose | No control group; D and P appeared to be associated with worse QoL than HC | |
| Dauber 2010 [24] | 4 subjects with 21OHD, aged 2–7 y; 4SW | Subjects’ usual HC regimen was compared with 3 d of nocturnal D therapy | Measured ACTH, 17OHP, A4 levels | Nocturnal D led to blunting of early morning ACTH, 17OHP, and A4 levels; HC was associated with sharp rise in ACTH, 17OHP, and A4 levels at 4 am; AUC was significantly lower for ACTH, but did not reach significance for 17OHP when D was compared with HC | No control group; D was associated with more effective suppression of ACTH | |
| Jääskeläinen 1996 [21] | 32 subjects aged 16–52 y with 21OHD | Study aimed to examine BMD with respect to GC regimen (HC, P, or D) | Measured DXA at left femur and lumbar spine, height, and BMI; 17OHP, A4, ACTH levels | Subjects treated with D or P had lower BMD than those receiving HC | No control group; short-acting GCs appeared to be better for BMD preservation than D or P | |
| Young 1990 [27] | 10 subjects aged 12–29 y with 21OHD; all SW | Study compared baseline HC therapy with 3-mo course of D; during first month, D was given in the morning; for the second month, D was given at night; in the final month, D was given in equal divided doses twice daily | Measured blood and saliva 17OHP levels at 8 am, 12 pm, 6 pm and 10 pm, and morning T level (in female patients only) at baseline, and then at the end of each month of D therapy (D was given at a dose of 10 μ/kg/d) | Results were expressed in terms of overtreatment, adequate treatment, undertreatment, or considerably undertreated (raw data not included, and cutoffs for each treatment category not specified); six patients were undertreated with HC; there was a tendency toward overtreatment with D, particularly when given in the evening | No control group; authors concluded D was reasonable alternative to HC to improve compliance; results expressed in terms of adequacy of treatment, but without raw data or cutoffs for each category, so data not included in meta-analysis | |
| Horrocks 1982 [25] | 7 subjects; 6 had 21OHD, 1 had 11βOHD; 1SW, 6SV | Compared 2-wk treatment courses of HC, CoAc, and D in crossover study design | Measured ACTH, 17OHP, A4 levels | Lower ACTH levels were measured in subjects taking D; significantly lower AUC for D than HC or CoAc for ACTH, 17OHP, A4 levels; ACTH morning peak was significantly lower with D compared with HC, but not compared with CoAc; 17OHP and A4 peaks were significantly lower in D group compared with both HC and CoAc | No control group; D led to better suppression of adrenal androgens than did HC or P in the short term | |
| Smith 1980 [26] | 6 subjects with 21OHD, aged 10–16 y; 3SW, 3SV | Subjects initially received 6-mo of nocturnal D (with small morning dose of HC), followed by 3-mo of BD HC | Measured cortisol, ACTH, 17OHP, PRA, GH levels | Neither HC nor D led to suppression of ACTH and 17OHP in most subjects: there was no suppression of GH in the subjects treated with D | No control group; no significant difference between D and P was observed | |
| Hansen 1976 [23] | 8 subjects aged 7.5–23 y with 21OHD; 2 SW, 6 SV | Compared subjects’ usual GC therapy (HC, CoAc, or P) to HC, CoAc, P, D, and one-half the dose of D; subjects received each GC for 1 wk | During last 2 d of each treatment week, two 24-h urine collections were undertaken to measure levels of 17ketosteroids, pregnanetriol, and creatinine | D was most effective in suppressing adrenal activity, whereas CoAc was least effective when compared in terms of HC-equivalent doses; HC, P, and the half-dose D were all equally effective; different individuals appeared to respond differently to the various GC regimens, with two patients refractory to CoAC but responsive to HC | No control group; authors concluded that choice of GC should be individualized, given variation in subject response to each GC; suggested trialing D in those patients who are refractory to HC, CoAc, and P, or who struggle with multiple daily doses; urinary 17ketosteroid and pregnanetriol levels were expressed graphically and not included in meta-analysis | |
| HC vs MR-HC | ||||||
| Mallappa 2015 [11] | 16 subjects aged 18–60 y with 21OHD; 12 SW, 4 SV | Open-label, nonrandomized study comparing usual GC regimen to 6 mo of MR-HC given twice daily | Measured 17OHP, A4, cortisol, ACTH, T levels; weight, BMI, BMD, HOMA-IR, and osteocalcin | ACTH levels were not significantly different; A4 and 17OHP levels were significantly lower; most subjects receiving MR-HC had normal 17OHP levels; no significant change in BMI; osteocalcin and HOMA-IR increased with MR-HC; no significant differences in QoL or fatigue were noted | No control group; MR-HC appeared to reduce androgen excess | |
| Verma 2010 [28] | 14 subjects with 21OHD, aged 17–55 y; 11 with SW and 3 with SV | Open-label crossover study comparing 1 wk of thrice daily HC to 1-mo MR-HC | Measured 8 am 17OHP and cortisol, ACTH and A4 profiles at drug steady state | 8 am 17OHP levels were significantly lower with MR-HC than HC; 17OHP and A4 levels were significantly higher in the afternoon in patients receiving MR-HC; cortisol levels were significantly higher with MR-HC in the morning and lower in the afternoon and evening with MR-HC | No control group; authors concluded that MR-HC was promising, but twice-daily dose needed, given the increase in 17OHP and ACTH levels seen during the day in patients receiving MR-HC | |
| CSHI vs baseline vs GC regimen | ||||||
| Nella 2016 [12] | 8 subjects aged 19–43 y with 21OHD; 2 with SV and 6 with SW | Compared subjects’ control at baseline with 6 mo of CSHI | Measured 17OHP, A4, ACTH, progesterone levels; DXA, pelvic/testicular USS; SF-36 | 7 am ACTH, 17OHP, A4, and progesterone levels decreased significantly in CSHI group, but did not reach primary efficacy end point of 7 am 17OHP < 1200 ng/dL | No control group; no direct comparison of oral preparations to CSHI, only baseline measures on range of different oral preparations; authors favored CSHI | |
| Sonnet 2011 [30] | 27-yo male subject with 21OHD, SW | Baseline biochemical parameters on D compared with 24 mo of CSHI | Measured cortisol, 17OHP, ACTH, A4, T levels; SF-36; BMI | Increase in cortisol levels; reduction in 17OHP, ACTH, A4, and T levels; reduction in BMI and improved SF-36 | Case study; rapid decrease in 17OHP and ACTH levels; well tolerated | |
| Tuli 2011 [31] | 14-yo male subject with 21OHD, SW | Compared oral HC with CSHI given for 4 mo | Measured ACTH, T, 17OHP levels | 17OHP levels normalized with CSHI; mean dose of HC was lower with CSHI | Case study; improved 17OHP levels and led to dose reduction; subject elected to cease CSHI after 4 mo | |
| Bryan 2009 [29] | 14.5-yo male subject with 21OHD, SW | Compared oral HC with CSHI given for 4 y | Measured cortisol, BMI and T, A4, 17OHP, ACTH levels | Reduction in ACTH, 17OHP, A4, and T levels; decrease in BMI, decrease in HC dose | Case study; rapid change in CAH control with 50% dose reduction in first 3 mo; feasible treatment option in children and young people with CAH | |
| IVI HC vs baseline vs GC regimen | ||||||
| Merza 2006 [32] | 2 subjects with CAH aged 21 and 39 y (along with 2 control patients and 2 with Addison disease) | Subjects’ usual HC regimen was compared with IVI HC | Measured 24-h profile of cortisol, ACTH, and 17OHP | Overall cortisol exposure was similar between conventional therapy and IVI; conventional treatment led to higher cortisol exposure during the day, whereas infusion therapy led to higher cortisol exposure during the night and early hours of the morning; 17OHP levels were elevated at the beginning and end of conventional treatment; 17OHP levels fell during the day with both conventional and infusion therapy; 17OHP levels rose during the night with oral HC, but fell with IVI HC | Circadian IVI HC appeared to improve biochemical control; study only included 24 h of therapy | |
Abbreviations: 11βOHD, 11β-hydroxylase deficiency; 21OHD, 21-hydroxylase deficiency; AUC, area under the curve; BD, twice daily; BP, blood pressure; CoAc, cortisone acetate; D, dexamethasone; DHEAS, dehydroepiandrosterone sulfate; DXA, dual-energy X-ray absorptiometry; E, estrogen; FBGL, fasting blood glucose level; FH, final height; GC, glucocorticoid; HC, hydrocortisone; HR, heart rate; IVI, IV infusion; MC, mineralocorticoid; OGTT, oral glucose tolerance test; P, prednisolone; PRA, plasma renin activity; QOL, quality of life; SDS, SD score; T, testosterone; TDS, thrice daily; USS, ultrasound; yo, years old.
Same cohort but measuring different outcomes, all data were combined in the analysis. One of the patients was on dexamethasone and was therefore excluded in the comparisons between hydrocortisone and prednisolone but was included in the meta-analysis.
Partly same cohort.
C. Statistical Analysis
Mean ± SD (if normally distributed) or median and interquartile range (25% to 75%) were used. Two groups and continuous variables were analyzed with unpaired t test or Mann-Whitney rank-sum test if not normally distributed. Three groups were analyzed with one-way ANOVA, or ANOVA on ranks when values were not normally distributed. If exact individual values could not be extracted from the articles or by contacting the authors, approximate values from figures or mean and median values were used. If several articles were from the same cohort, the data were combined. Statistical significance was defined as P < 0.05. All analyses were performed using SigmaStat, version 3.0 for Windows (Systat Software, San Jose, CA).
2. Results
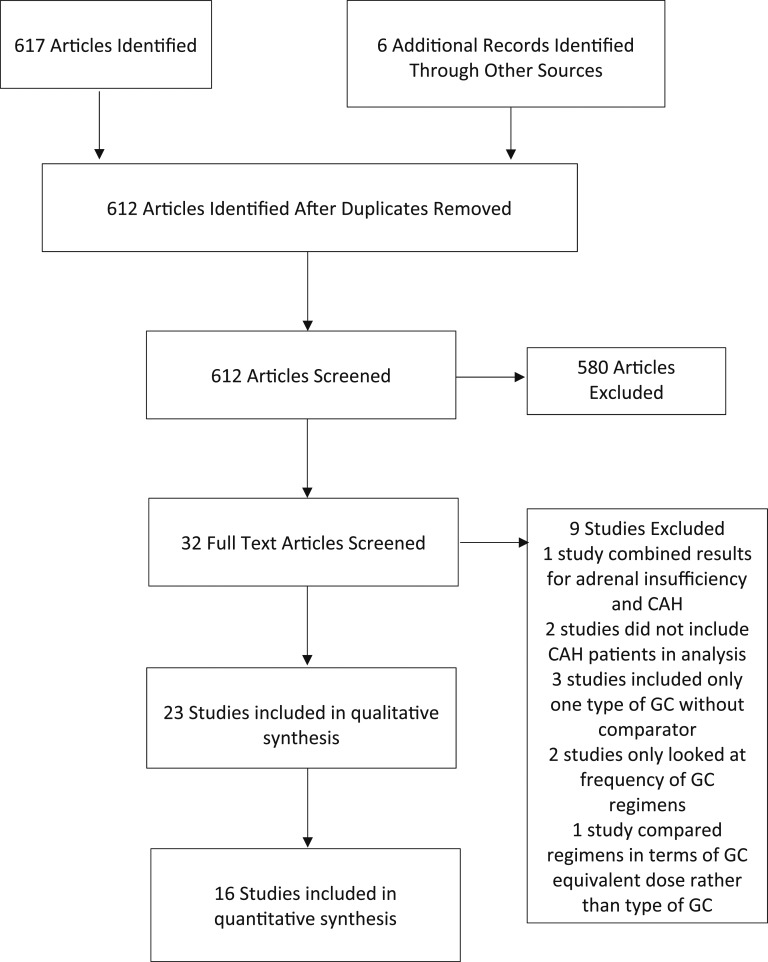
The systematic search identified 617 articles. Six additional articles were identified from review of reference lists. A total of 612 articles were identified and screened for eligibility after duplicates were removed. In total, 23 studies were included in the qualitative synthesis, with 19 included in the quantitative synthesis (Fig. 1). The included studies are summarized in Table 1. The studies assessed a variety of outcome measures. Most of the studies assessed the effect of different glucocorticoid regimens on morning 17OHP levels. Other outcome measures included testosterone and androstenedione (A4) levels, BMI, and BMD. The included studies did not report on clinical signs of hyperandrogenism. Four of the included studies assessed glucose tolerance by measuring Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) [11–13, 33].
Figure 1.
Flowchart depicting the procedure for article inclusion and exclusion in a systematic review and meta-analysis of glucocorticoid (GC) regimens in the management of congenital adrenal hyperplasia. A systematic search was conducted of PubMed/MEDLINE and Web of Science up to 25 February 2019. Including a review of reference lists, 23 relevant studies were found.
A. Hydrocortisone vs Prednisolone
Twelve of the included studies compared hydrocortisone and prednisolone therapy [13–23]. Outcomes assessed included 17OHP and A4 levels, BMI, and BMD. In terms of 17OHP levels, most studies did not find any significant differences between treatment groups. However, Nebesio et al. [22] found that mean 17OHP levels were lower in those individuals treated with hydrocortisone.
Several of the included studies assessed the impact of glucocorticoid treatment regimens on final height [15, 16, 19]. Bonfig et al. [15] found that prednisolone treatment was associated with higher hydrocortisone-equivalent doses and significantly reduced final height when compared with hydrocortisone. In contrast, Caldato et al. [16] found that the ratio of bone age to chronological age was higher in individuals treated with hydrocortisone therapy, suggesting that hydrocortisone is less effective at slowing skeletal maturation. They also found that height SD scores corrected for bone age improved significantly in those individuals treated with 12 months of prednisolone therapy when compared with those treated with hydrocortisone [16]. Leite et al. [19] did not find any difference between hydrocortisone and prednisolone therapy in terms of variation in height SD scores, variation of height SD scores according to bone age, and variation of body mass SD scores.
Falhammar et al. [13] found that hydrocortisone/cortisone acetate were associated with increased values for markers of cardiovascular and metabolic risk compared with prednisolone, including BMI, waist-to-hip ratio, serum insulin during oral glucose tolerance test, total fat mass, and trunk fat mass, despite similar doses of hydrocortisone equivalents. Prednisolone was shown in two studies to be associated with higher rates of osteoporosis and fractures [17, 21].
B. Dexamethasone vs Hydrocortisone and/or Prednisolone
Nine of the identified studies evaluated the use of dexamethasone compared with hydrocortisone and/or prednisolone [20–27], and three additional studies described only a single patient receiving dexamethasone [13, 17, 18]. As expected, dexamethasone was associated with significantly greater adrenal suppression [20, 22–25, 27]. Dexamethasone treatment was associated with lower BMD than hydrocortisone or prednisolone in one study [21], but slightly better femoral neck T-score in another study [20].
C. Hydrocortisone vs Modified-Release Hydrocortisone
Two studies compared conventional hydrocortisone tablets with modified-release hydrocortisone. Verma et al. [28] compared once-daily modified-release hydrocortisone (MR-HC) with thrice-daily hydrocortisone. The study found that 8:00 am 17OHP levels were significantly lower in the MR-HC group than in the hydrocortisone group, whereas afternoon 17OHP levels were significantly higher. Mallappa et al. [11] compared twice-daily MR-HC with subjects’ usual glucocorticoid treatment and found that 17OHP and A4 levels were significantly lower in the MR-HC group. There was no significant difference in HOMA-IR, BMI, or quality of life between groups. Because of the small number of patients treated with MR-HC, these data were not included in the meta-analysis.
D. Continuous Subcutaneous Hydrocortisone Infusion
Four studies assessed the use of CSHI in the management of CAH [12, 29–31]. Three of these studies were case reports of individual patients [29–31]; the fourth study included eight subjects and compared CSHI with subjects’ preexisting glucocorticoid regimen [12]. All four studies reported a reduction in 17OHP levels with CSHI treatment compared with baseline. Nella et al. [12] assessed changes in metabolic indices and found that weight increased with CSHI compared with individuals’ baseline glucocorticoid regimens, whereas other indices did not change (including HOMA-IR; levels of C-peptide, leptin, C-reactive protein, and lipids; and waist-to-hip ratio). As with MR-HC, the number of patients treated with CSHI was small and as such, the data were not included in the meta-analysis.
E. IV Hydrocortisone Infusion
One study of two subjects with CAH evaluated the use of IV hydrocortisone [32]. Subjects were only treated for 24 hours. Morning 17OHP levels were reduced in subjects treated with IV hydrocortisone compared with those receiving oral hydrocortisone.
E.1. Quality of life measures
Five of the 23 studies examined the influence of glucocorticoid regimens on quality of life [11, 12, 18, 20, 30]. Mallappa et al. [11] found no difference between subjects’ baseline glucocorticoid regimen and MR-HC in terms of the 36-item short-form health survey (SF-36), health-related quality of life in Addison disease, and global fatigue index scores. Falhammar et al. [18] compared psychological general well-being (PGWB) scores and found no difference between control subjects and subjects with CAH treated with prednisolone. However, individuals with CAH treated with hydrocortisone or cortisone acetate had significantly lower PGWB scores in most areas, compared with control subjects [18]. Patients with poorly controlled CAH also had significantly better PGWB scores compared with those who were over treated [18]. In contrast, SF-36 scores were significantly lower in individuals treated with prednisolone or dexamethasone compared with those in the CaHASE cohort treated with hydrocortisone [20]. CSHI was associated with improved SF-36 and global fatigue index scores in two studies [12, 30].
E.2. Bone mineral density
Four of the included studies assessed the influence of different glucocorticoid regimens on BMD [11, 17, 20, 21]. Falhammar et al. [17] reported that patients with CAH treated with prednisolone had significantly lower BMD than control subjects, whereas subjects treated with hydrocortisone or cortisone acetate did not have significantly different BMD compared with control subjects [17]. Mallappa et al. [11] found that after 6 months of treatment with MR-HC, whole-body BMD was significantly lower when compared with baseline glucocorticoid treatment. Han et al. [20] found that dexamethasone was associated with higher femoral BMD compared with hydrocortisone or prednisolone, but the number of patients who had BMD measurement was low. In contrast, Jääskeläinen et al. [21] found that subjects treated with dexamethasone or prednisolone had decreased BMD compared with subjects treated with hydrocortisone.
E.3. Meta-analysis
Of the 23 studies, 16 presented results for subjects treated with hydrocortisone and were included in the meta-analysis. In total, 172 subjects received hydrocortisone or cortisone acetate, with 192 results available (20 of the subjects were included twice, either owing to subjects receiving hydrocortisone at two different evening time points or to subjects being given hydrocortisone for a discrete period, followed by cortisone acetate [14, 25]. Ten of the 23 studies included results for subjects receiving prednisolone, with data for 178 individuals. Eleven of the 23 studies presented results for subjects taking dexamethasone therapy, with data for 79 individuals included in the meta-analysis.
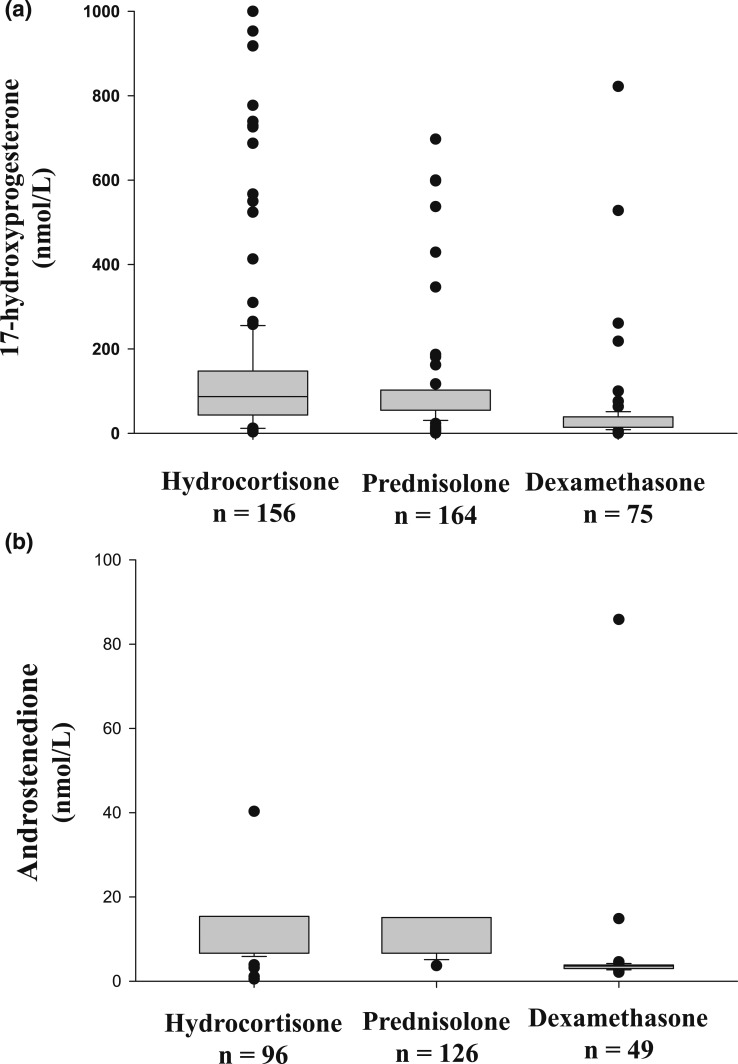
There was no significant difference in the age of subjects treated with dexamethasone, prednisolone, or hydrocortisone (P = 0.102). Subjects treated with dexamethasone had significantly higher hydrocortisone-equivalent doses compared with those treated with prednisolone or hydrocortisone [18.4 (15.0 to 24.0) vs 17.8 (15.0 to 18.8) mg/m2 vs 14.3 (12.0 to 16.0) mg/m2; P < 0.001]. 17OHP levels were significantly lower in subjects receiving dexamethasone, compared with those taking hydrocortisone or prednisolone (Fig. 2a). A4 levels were also significantly lower in individuals receiving dexamethasone, compared with prednisolone or hydrocortisone (Fig. 2b).
Figure 2.
Box plots comparing (a) 17OHP and (b) A4 levels in patients with CAH treated with hydrocortisone, prednisolone, or dexamethasone (P < 0.001 for both). The difference between hydrocortisone and prednisolone was not significant.
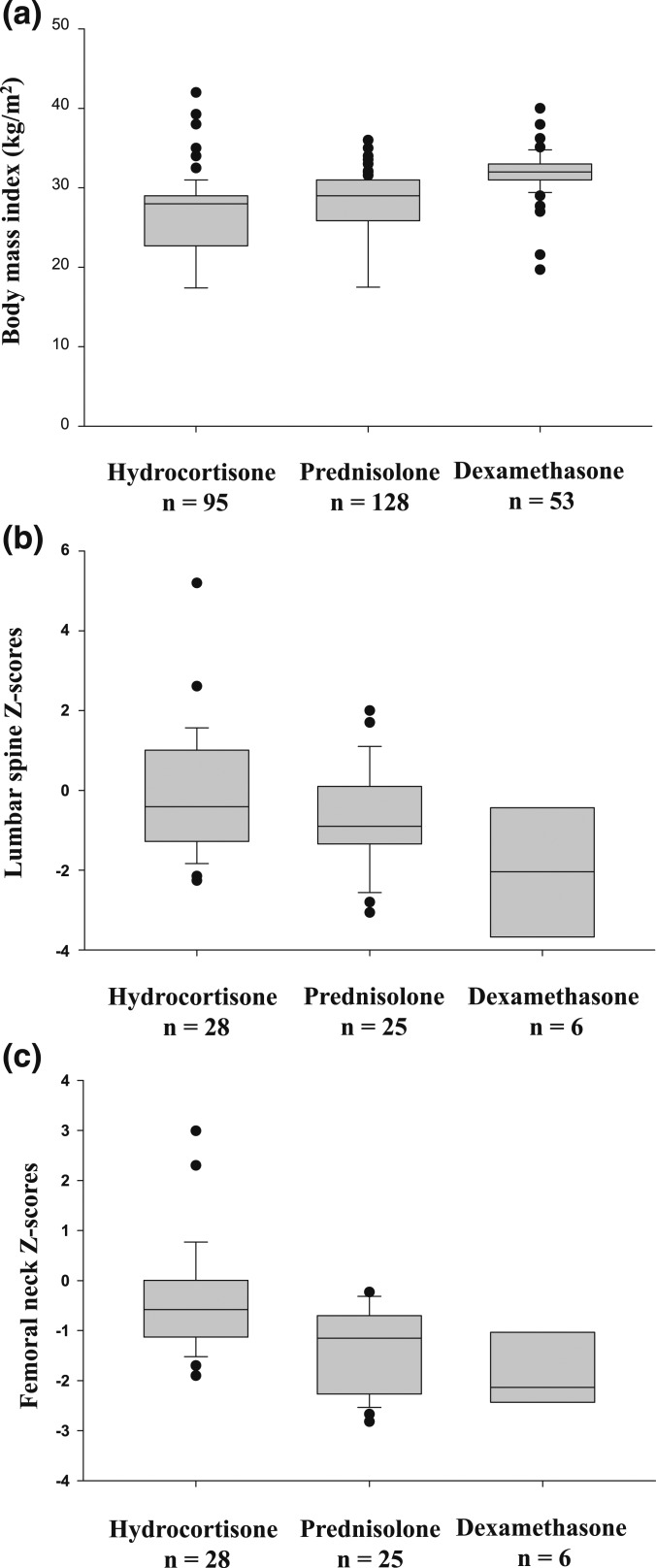
Subjects taking dexamethasone had significantly higher BMI than those taking prednisolone or hydrocortisone (Fig. 3a, only adults included). Lumbar spine Z-scores were significantly lower in subjects treated with dexamethasone compared with those treated with hydrocortisone or prednisolone (Fig. 3b). Femoral neck Z-scores were also significantly lower in subjects treated with dexamethasone (Fig. 3c).
Figure 3.
Box plots comparing (a) BMI (P < 0.001), (b) lumbar spine Z-scores in adult patients (P = 0.028), and femoral neck Z-scores in adult patients (P = 0.001) with CAH treated with hydrocortisone, prednisolone, or dexamethasone. (b) The difference between hydrocortisone and prednisolone was not significant. (c) The difference between prednisolone and dexamethasone was not significant.
3. Discussion
This is a systematic review and meta-analysis of glucocorticoid regimens in the management of CAH. Although current guidelines recommend the use of hydrocortisone in children with CAH, there is no consensus about the appropriate glucocorticoid regimen in adult patients [1, 2]. Patients are often transitioned to therapy with long-acting glucocorticoids on reaching adulthood, which allow less-frequent dosing and improved compliance [35]. Moreover, CAH poses unique management issues, distinct from other forms of adrenal insufficiency. The management of CAH requires a balance between glucocorticoid replacement and adrenal hormone suppression [2]. To achieve the latter, individuals often require supraphysiological doses of glucocorticoids. This in turn leads to detrimental effects on BMD, final height, and BMI. We found that regimens based on hydrocortisone or prednisolone have similar benefits and adverse effects, whereas a regimen based on dexamethasone was more effective in adrenal hormone suppression but also displayed more adverse effects.
The frequency of particular glucocorticoid regimens varies between cohorts [3, 8, 36]. Adult patients may be prescribed hydrocortisone, prednisolone, dexamethasone, or a combination of glucocorticoid preparations. Most of included studies were retrospective; therefore, it is important to acknowledge that the choice of therapy may have been dictated by patient factors and prone to bias. For instance, issues with compliance or difficult-to-control hyperandrogenism may have led to use of longer-acting glucocorticoids, such as prednisolone or dexamethasone. Sixteen of the included studies assessed oral hydrocortisone as a comparator, 12 of which also evaluated prednisolone therapy. Perhaps surprisingly, there was no significant difference in 17OHP levels between hydrocortisone and prednisolone treatment groups in the meta-analysis, and one of the studies actually showed lower 17OHP levels in the hydrocortisone group [22]. Caldato et al. [16] argued that the lack of difference in 17OHP levels seen in their cohort supported the use of once-daily prednisolone therapy to facilitate compliance. Moreover, Falhammar et al. [18] found that individuals treated with hydrocortisone/cortisone acetate had significantly lower PGWB scores than did control subjects, whereas scores of patients treated with prednisolone were not significantly different from those of control subjects. However, the diurnal 17OHP curves on dried blood spots were similar between those treated with hydrocortisone/cortisone acetate and those treated with prednisolone [17]. By contrast, dexamethasone therapy led to significantly greater adrenal suppression than other glucocorticoid regimens [20, 22–25, 27]. This greater adrenal suppression was at the cost of reduced BMD in the form of Z-scores in the meta-analysis, although Han et al. [20] found higher femoral neck T-scores in patients treated with dexamethasone; however, the number of individuals having dual-energy X-ray absorptiometry was relatively low.
The doses and dosing schedules of glucocorticoids also vary widely between adult patients with CAH [3]. Hydrocortisone is typically administered thrice daily, prednisolone twice daily, and dexamethasone once daily [1–4]. The adjustment of glucocorticoid doses is usually made with reference to morning 17OHP and A4 levels [2]. There are no specific treatment targets for the management of CAH, although it is recommended that morning 17OHP levels should be slightly elevated and A4 levels should be in the normal range [2, 7, 35]. Although 17OHP and androgen levels usually correlate, 17OHP is vastly more variable and may be present at 100 to 1000 times higher levels than androgens [35]. Thus, A4 levels can be normal in the setting of elevated 17OHP levels. To overcome this disparity, diurnal curves using dried blood spots measuring 17OHP can be used, but such curves are currently only available in a few centers [1, 7]. Whether the recently rediscovered 11-oxyandrogens can be used in biochemical monitoring and predicting long-term outcomes remains to be seen [37]. We found that individuals treated with dexamethasone received significantly higher hydrocortisone-equivalent doses than those treated with hydrocortisone or prednisolone, which was further supported by the lower morning 17OHP and A4 levels. It is important to note that there is uncertainty about the appropriate conversion factor of dexamethasone to hydrocortisone-equivalent doses, and a higher potency of dexamethasone has been suggested more recently [33, 38]. If prescribed, dexamethasone doses should be lower than those used in the included studies and probably lower than has been previously suggested.
Many of the long-term complications of CAH are related to excessive glucocorticoid exposure. Arlt et al. [3] found that 52% of female patients with 21-hydroxylase deficiency in a UK cohort were obese, and 28% had insulin resistance. A Swedish cohort of 588 patients with CAH had higher rates of obesity, diabetes, hypertension, and hyperlipidemia than did control subjects [9]. Thus, patients with CAH should be prescribed the lowest possible glucocorticoid dose to prevent symptoms and signs of both hyperandrogenism and adrenal crisis [39]. The choice of glucocorticoid preparation is likely to be guided by the patient and health care provider’s preferences and previous experience. However, dexamethasone should probably be avoided where possible and reserved for selected cases (e.g., patients with poor compliance).
The difficulties associated with CAH management have led to investigation of other treatment options, including MR-HC tablets and CSHI. Both regimens seem promising, but more studies are needed. However, CSHI requires extra resources and its use will probably not be widespread. Another approach is bilateral adrenalectomy, which obviates the requirement for hypothalamic–pituitary–adrenal axis suppression, such that patients require replacement doses only. A recent systematic review and meta-analysis of bilateral adrenalectomy in the management of CAH found that such surgery was a reasonable treatment option in a carefully selected cohort of patients [40]. However, the Endocrine Society Clinical Practice Guideline recommends against adrenalectomy, because of the significant surgical risk and increased risk of adrenal crisis [2].
Our study has a number of limitations. First, we did not have access to all of the individual patient data in the included studies, such that some data were approximated from figures or graphs, or from mean values. Some studies could not be included in the meta-analysis for this reason. Secondly, the studies included were heterogeneous, mostly retrospective, and included patients of varying ages with quite diverse methodology. In addition, the number of patients treated with certain regimens was somewhat limited, especially in the MR-HC and CSHI groups. Not all outcomes were analyzed in all patients. We could not separate the results for female and male patients, because in most studies, the results of different glucocorticoid regimens were not separated by sex. Individuals’ glucocorticoid doses were often not presented. To evaluate the advantages and disadvantages of the different glucocorticoid regimens more rigorously, a study would need to do the following: (i) check that baseline conditions including age, sex, BMI, BMD, and glucose tolerance, are similar in all groups of subjects; (ii) use similar hydrocortisone-equivalent doses in each regimen; (iii) investigate all positive and negative effects of each regimen. Moreover, it should be randomized. Unfortunately, such a study would be difficult to perform, owing to the low prevalence of CAH and the long follow-up needed.
In conclusion, we found in this systematic review and meta-analysis that dexamethasone therapy is associated with greater adrenal androgen suppression, as well as more adverse effects, including higher BMI and lower BMD. In contrast, we did not find significant differences between hydrocortisone and prednisolone therapy in terms of adrenal hormone levels or adverse effects. However, individuals treated with dexamethasone received higher hydrocortisone-equivalent doses than those treated with hydrocortisone or prednisolone. These results suggest that dexamethasone therapy may not be the first choice, and if used, a lower dose should be considered. The choice of therapy between hydrocortisone and prednisolone therapy in adult patients will depend on individual patient factors and physician judgement.
Acknowledgments
Financial Support: This project was supported by grants from the Magnus Bergvall Foundation (Grants 2015-00699, 2017-02138, 2018-02566 to H.F.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 17OHP
17-hydroxyprogesterone
- A4
androstenedione
- BMD
bone mineral density
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- CSHI
continuous subcutaneous hydrocortisone infusion
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- MR-HC
modified-release hydrocortisone
- PGWB
psychological general well-being
- SF-36
36-item short-form health survey
- SV
simple-virilizing
- SW
salt-wasting
References and Notes
- 1. Falhammar H, Thorén M. Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine. 2012;41(3):355–373. [DOI] [PubMed] [Google Scholar]
- 2. Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(11):4043–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, Stimson RH, Walker BR, Connell JM, Ross RJ; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE). Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95(11):5110–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194–2210. [DOI] [PubMed] [Google Scholar]
- 5. Gidlöf S, Falhammar H, Thilén A, von Döbeln U, Ritzén M, Wedell A, Nordenström A. One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2013;1(1):35–42. [DOI] [PubMed] [Google Scholar]
- 6. Falhammar H, Wedell A, Nordenström A. Biochemical and genetic diagnosis of 21-hydroxylase deficiency. Endocrine. 2015;50(2):306–314. [DOI] [PubMed] [Google Scholar]
- 7. Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2018;EJE-18–0712.R2. [DOI] [PubMed] [Google Scholar]
- 8. Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, Thorén M. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(1):110–116. [DOI] [PubMed] [Google Scholar]
- 9. Falhammar H, Frisén L, Hirschberg AL, Norrby C, Almqvist C, Nordenskjöld A, Nordenström A. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2015;100(9):3520–3528. [DOI] [PubMed] [Google Scholar]
- 10. Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol. 2014;10(2):115–124. [DOI] [PubMed] [Google Scholar]
- 11. Mallappa A, Sinaii N, Kumar P, Whitaker MJ, Daley LA, Digweed D, Eckland DJ, Van Ryzin C, Nieman LK, Arlt W, Ross RJ, Merke DP. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(3):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nella AA, Mallappa A, Perritt AF, Gounden V, Kumar P, Sinaii N, Daley LA, Ling A, Liu CY, Soldin SJ, Merke DP. A Phase 2 study of continuous subcutaneous hydrocortisone infusion in adults with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2016;101(12):4690–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falhammar H, Filipsson Nyström H, Wedell A, Thorén M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2011;164(2):285–293. [DOI] [PubMed] [Google Scholar]
- 14. Ajish TP, Praveen VP, Nisha B, Kumar H. Comparison of different glucocorticoid regimens in the management of classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Indian J Endocrinol Metab. 2014;18(6):815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. J Clin Endocrinol Metab. 2007;92(5):1635–1639. [DOI] [PubMed] [Google Scholar]
- 16. Caldato MC, Fernandes VT, Kater CE. One-year clinical evaluation of single morning dose prednisolone therapy for 21-hydroxylase deficiency. Arq Bras Endocrinol Metabol. 2004;48(5):705–712. [DOI] [PubMed] [Google Scholar]
- 17. Falhammar H, Filipsson Nyström H, Wedell A, Brismar K, Thorén M. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2013;168(3):331–341. [DOI] [PubMed] [Google Scholar]
- 18. Falhammar H, Nyström HF, Thorén M. Quality of life, social situation, and sexual satisfaction, in adult males with congenital adrenal hyperplasia. Endocrine. 2014;47(1):299–307. [DOI] [PubMed] [Google Scholar]
- 19. Leite FM, Longui CA, Kochi C, Faria CD, Borghi M, Calliari LEP, Monte O. Estudo comparativo do uso de prednisolona versus acetato de hidrocortisona no tratamento da hiperplasia adrenal congênita por deficiência da 21-hidroxilase forma clássica. Arq Bras Endocrinol Metabol. 2008;52(1):101–108. [DOI] [PubMed] [Google Scholar]
- 20. Han TS, Krone N, Willis DS, Conway GS, Hahner S, Rees DA, Stimson RH, Walker BR, Arlt W, Ross RJ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Eur J Endocrinol. 2013;168(6):887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jääskeläinen J, Voutilainen R. Bone mineral density in relation to glucocorticoid substitution therapy in adult patients with 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 1996;45(6):707–713. [DOI] [PubMed] [Google Scholar]
- 22. Nebesio TD, Renbarger JL, Nabhan ZM, Ross SE, Slaven JE, Li L, Walvoord EC, Eugster EA. Differential effects of hydrocortisone, prednisone, and dexamethasone on hormonal and pharmacokinetic profiles: a pilot study in children with congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2016;2016(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansen JW, Loriaux DL. Variable efficacy of glucocorticoids in congenital adrenal hyperplasia. Pediatrics. 1976;57(6):942–947. [PubMed] [Google Scholar]
- 24. doi: 10.1155/2010/347636. Dauber A, Feldman HA, Majzoub JA. Nocturnal dexamethasone versus hydrocortisone for the treatment of children with congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2010;2010:347636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horrocks PM, London DR. A comparison of three glucocorticoid suppressive regimes in adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 1982;17(6):547–556. [DOI] [PubMed] [Google Scholar]
- 26. Smith R, Donald RA, Espiner EA, Glatthaar C, Abbott G, Scandrett M. The effect of different treatment regimens on hormonal profiles in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1980;51(2):230–236. [DOI] [PubMed] [Google Scholar]
- 27. Young MC, Hughes IA. Dexamethasone treatment for congenital adrenal hyperplasia. Arch Dis Child. 1990;65(3):312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verma S, Vanryzin C, Sinaii N, Kim MS, Nieman LK, Ravindran S, Calis KA, Arlt W, Ross RJ, Merke DP. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2010;72(4):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009;94(9):3477–3480. [DOI] [PubMed] [Google Scholar]
- 30. Sonnet E, Roudaut N, Kerlan V. Results of the prolonged use of subcutaneous continuous infusion of hydrocortisone in a man with congenital adrenal hyperplasia. ISRN Endocrinol. 2011;2011:219494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tuli G, Rabbone I, Einaudi S, di Gianni V, Tessaris D, Gioia E, Lala R, Cerutti F. Continuous subcutaneous hydrocortisone infusion (CSHI) in a young adolescent with congenital adrenal hyperplasia (CAH). J Pediatr Endocrinol Metab. 2011;24(7-8):561–563. [DOI] [PubMed] [Google Scholar]
- 32. Merza Z, Rostami-Hodjegan A, Memmott A, Doane A, Ibbotson V, Newell-Price J, Tucker GT, Ross RJ. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2006;65(1):45–50. [DOI] [PubMed] [Google Scholar]
- 33. Han TS, Stimson RH, Rees DA, Krone N, Willis DS, Conway GS, Arlt W, Walker BR, Ross RJ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2013;78(2):197–203. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Auchus RJ, Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98(7):2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turcu AF, Mallappa A, Elman MS, Avila NA, Marko J, Rao H, Tsodikov A, Auchus RJ, Merke DP. 11-oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2017;102(8):2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: the ability to achieve normal growth. Pediatrics. 2000;106(4):767–773. [DOI] [PubMed] [Google Scholar]
- 39. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crises: perspectives and research directions. Endocrine. 2017;55(2):336–345. [DOI] [PubMed] [Google Scholar]
- 40. MacKay D, Nordenström A, Falhammar H. Bilateral adrenalectomy in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(5):1767–1778. [DOI] [PubMed] [Google Scholar]