ABSTRACT
miR-1246 is considered an oncomiR in various cancer types. However, the origin and biogenesis of miR-1246 remain controversial which often leads to misinterpretation of its detection and biological function, and inevitably masking its mechanisms of action. Using next generation small RNA sequencing, CRISPR-Cas9 knockout, siRNA knockdown and the poly-A tailing SYBR qRT-PCR, we examined the biogenesis of exosomal miR-1246 in human cancer cell model systems. We found that miR-1246 is highly enriched in exosomes derived from human cancer cells and that it originates from RNU2-1, a small nuclear RNA and essential component of the U2 complex of the spliceosome. Knockdown of Drosha and Dicer did not reduce exosomal miR-1246 levels, indicating that exosomal miR-1246 is generated in a Drosha- and Dicer-independent manner. Direct digestion of cellular lysate by RNase A and knockdown of the RNU2-1 binding protein SmB/B’ demonstrated that exosomal miR-1246 is a RNU2-1 degradation product. Furthermore, the GCAG motif present in the RUN2-1 transcript was shown to mediate miR-1246 enrichment in cancer exosomes. We conclude that exosome miR-1246 is derived from RNU2-1 degradation through a non-canonical microRNA biogenesis process. These findings reveal the origin of an oncomiR in human cancer cells, providing guidance in understanding miR-1246 detection and biological function.
Abbreviations: CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats; miRNA, microRNA; PDAC, pancreatic ductal adenocarcinoma; RNU2-1, U2 small nuclear RNA; RT-PCR, Reverse transcription polymerase chain reaction; sgRNA, single-guide RNA.
KEYWORDS: Exosome, microRNA, RNU2-1, biogenesis, cancer
Introduction
miR-1246 is a microRNA (miRNA) that was identified in 2008 by sequencing of small RNAs in human embryonic stem cells [1]. The gene encoding miR-1246, MIR1246, was mapped to human chromosome 2q31.1 and its expression was found to be regulated by p53 [2]. Several miR-1246 target transcripts have since then been experimentally identified, such as SLC12A2 [3], RTKN2 [4], CCNG2 [5] and DENND2D [6]. In the context of cancer, miR-1246 was found to act as an oncomiR to promote tumor angiogenesis [7], growth and metastasis [8], migration and invasion [9], and cancer stemness in various malignancies [10–12], including pancreatic ductal adenocarcinoma (PDAC) [5]. We recently reported that miR-1246 is highly enriched in PDAC exosomes and that plasma exosome miR-1246, along with miR-196a, is elevated in patients with localized PDAC [13]. These previous findings indicate the biological significance of miR-1246 in human cancer, and the need for a better understanding of this miRNA species in cancer biology.
Intriguingly, the mature miR-1246 sequence fully overlaps with the central region of the RNU2-1 transcript, a small nuclear RNA serving as a scaffold for the U2 complex in the spliceosome [14]. This RNU2-1 fragment was detected in patient plasmas and described as a potential diagnostic biomarker for pancreatic and colorectal adenocarcinoma [15]. However, the concept of miR-1246 being derived from RNU2-1 has not been widely accepted and recent reports continue to reveal new insight of miR-1246 biology in various model systems without distinguishing miR-1246 and RNU2-1 detection and function [6,12,16–20]. This controversial scenario is primarily due to two reasons: first, there has been no decisive evidence to show that cellular miR-1246 is derived from RNU2-1. No knockdown or knockout experiments have been performed on either RNU2-1 or miR-1246 to prove that miR-1246 is derived from RNU2-1. Second, the methods used for miRNA detection have compounded this issue. Current miRNA detection mainly relies on two qRT-PCR strategies: the stem-loop TaqMan method and the poly-A tailing SYBR method [21–23] (Figure 1). To our knowledge, most recent studies on miR-1246 detection applied the stem-loop TaqMan method, which would theoretically detect both miR-1246 and RNU2-1 with the same primer set as depicted in Figure 1. On the other hand, the poly-A tailing SYBR method, by design, could differentiate miR-1246 and RNU2-1 based on the length of the detected nucleotide fragments indicated by their meting curves.
Figure 1.
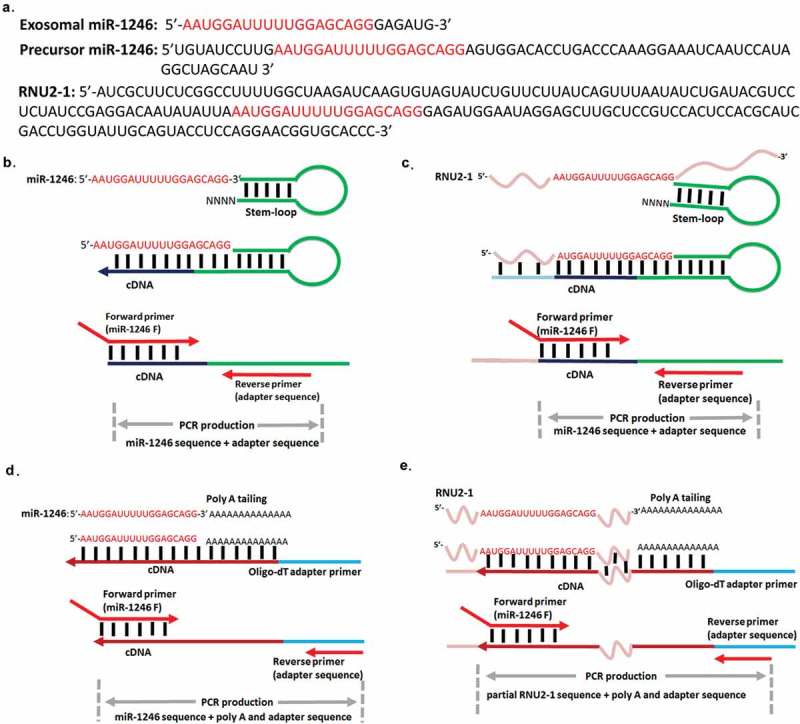
The sequence overlap of the precursor miR-1246, RNU2-1 and exosomal miR-1246, and comparison of two qRT-PCR detection strategies. (a). Exosomal miR-1246, precursor miR-1246, and RNU2-1 sequences. Mature miR-1246 as registered in miRBase (miRBase.org) is red colored. Schematic illustration of miR-1246 and RNU2-1 detection using the stem-loop qRT-PCR method (b and c). This method cannot qualitatively differentiate the detection of miR-1246 and RNU2-1. Schematic illustration of miR-1246 and RNU2-1 detection using the poly A tailing qRT-PCR method (d and e). This method can differentiate the detection of miR-1246 and RNU2-1.
Considering the fact that both miR-1246 [5,7,9–12,24] and RNU2-1 [25–27] are significant small RNA molecules in cancer biology, we sought to clarify the issue by applying the poly-A tailing SYBR method to analyze miR-1246 expression in wild type and MIR1246 knockout PDAC cells and their exosomes. We found that a variant of the mature miR-1246 is detected in PDAC cell-derived exosomes (Figure 1(a)) that is originated from cellular RNU2-1 in a Drosha- and Dicer-independent manner. Our results provide guidance on understanding of miR-1246 detection and biological function in human cancer cells.
Results
miR-1246 is highly enriched in exosomes derived from PANC-1 cells
Next generation small RNA sequencing was performed using RNA from PANC-1 cells and exosomes, as we described [13]. We found that a great number of reads mapped to miR-1246, but frequently contained additional nucleotides at the 3ʹ end. The addition of GAGA to the miR-1246 sequence read would indicate that the origin of the sequence was from RNU2-1, while the addition of AGTG would indicate that the origin of the sequence was from the pre-miR-1246 (Figure 1(a)). Majority of the miR-1246 sequences detected in both PANC-1 cells and PANC-1 exosomes contained the GAGA, and none of them contain AGTG, indicating that the miR-1246 sequence detected is derived from RNU2-1 and not from the precursor miR-1246 (Table 1). qRT-PCR using the poly-A tailing SYBR method was then used to amplify miR-1246 from cellular and exosome RNA of PANC-1, MIA-PaCa-2, and hTERT-HPNE cells (Figure 2((a and b)). Melting curve analysis showed that, with the same primer set, the miR-1246 amplicon from cellular RNA melted at a higher temperature when compared to the miR-1246 amplicon from exosome RNA in all cell lines analyzed (Figure 2(a and b)), indicating that different fragments are amplified in cellular RNA versus exosome RNA by the qRT-PCR technology. The amplified fragments were then ligated into a cloning vector and sequenced. The sequencing results, from three individual clones, confirmed the amplification of an RNU2-1 fragment from cellular RNA and that of a miR-1246 variant sequence from exosomal RNA (Figure 2(c)). The results thus show that the miR-1246 variant sequences are enriched in PANC-1 exosomes but undetectable in cellular RNA preparations by the poly-A tailing SYBR qRT-PCR method. Instead the same primers amplified RNU2-1 from cellular RNA. As a control, when miR-1246 mimics were pre-mixed with the PANC-1 cell lysate, the miR-1246 sequence was detected by the poly-A tailing SYBR qRT-PCR (Supplement Figure 1). We also performed qRT-PCR using the stem-loop TaqMan method, which shows no difference between the amplicons from cellular and exosome RNAs (supplement Figure 2). Sequencing of the cellular amplicon by this method confirmed that the stem-loop TaqMan method cannot distinguish between miR-1246 and RNU2-1 sequence detection (data not shown). To determine whether the detected exosomal miR-1246 variant sequence is functional, a miR-1246 reporter gene plasmid was constructed and introduced to PANC-1 cells. As shown in Figure 2(d), mimics of both mature miR-1246 and the miR-1246 variant significantly suppressed luciferase activity in our model system, indicating that the highly enriched exosomal miR-1246 variant sequence is biologically active. We also performed northern blot analysis using cellular RNAs from different human cancer cell lines (Figure 2(e)). It turned out that in all cell lines tested, there was no miR-1246 being detected. Instead, a prominent band was detected at the size of RNU2-1.
Table 1.
Analysis of the detected miR-1246 sequences in PANC-1 cells and exosomes. miR-1246 sequence+GAGA resembles the sequence of RNU2-1, while the miR-1246 sequence+AGTG resembles the sequence of precursor miR-1246.
| Raw sequence from NGS | Percentage of miR-1246-like sequences detected |
|---|---|
| miR-1246 sequence+GAGA in PANC-1 cells | 75.4 |
| miR-1246 sequence+GAGA in PANC-1 exosomes | 64.6 |
| miR-1246 sequence+AGTG in PANC-1 cells | 0 |
| miR-1246 sequence+AGTG in PANC-1 exosomes | 0 |
Figure 2.
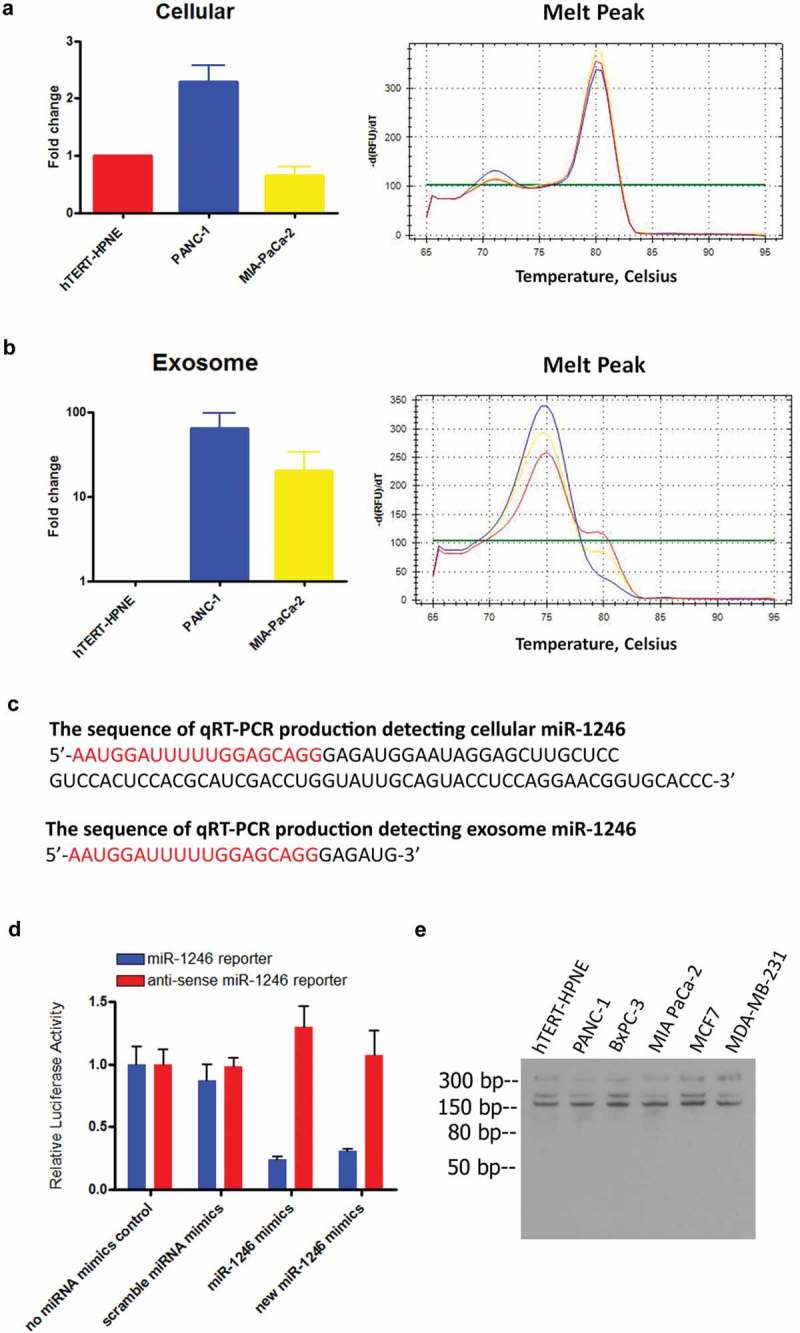
miR-1246-like sequences are highly enriched in PANC-1 exosomes. (a and b), qRT-PCR analysis using the poly A tailing method on miR-1246 expression in hTERT-HPNE, PANC-1 and MIA-PaCa-2 cells and their exosomes (means ± SEM, n = 3). The melting curves showed detection of different fragments in cells versus exosomes with the same set of primers. (c), Representative sequencing results from three individual clones of the detected exosomal miR-1246 and cellular RUN2-1. (d), miR-1246 target gene reporter assay. Mimics of mature miR-1246 and the exosomal miR-1246 variant suppress reporter gene activity as compared to scramble mimics (means ± SEM, n = 3). * p < 0.05, one-way ANOVA, followed by Dunnett analysis. (e), Northern blot analysis using a labeled miR-1246 probe against RNAs from several human cancer cell lines. There was no precursor miR-1246 and mature miR-1246 being detected and the primary band migrating at a size of RNU2-1 in all cell lines examined.
Exosome miR-1246 is derived from RNU2-1
The exosome miR-1246 qRT-PCR amplicon sequence contains six extra nucleotides that match to the RNU2-1 sequence (Figure 2(c)), supporting the notion that it originates from RNU2-1. To definitively clarify the origin of exosomal miR-1246 in our model system, a knockout or knockdown approach was applied. First, we used the CRISPR-Cas9 HDR method [28], as shown in Figure 3(a), to replace the precursor sequence of miR-1246 on 2q31.1 with GFP in PANC-1 cells. GFP-positive genetically engineered cells were sorted by flow cytometry; and verified by fluorescence microscopy (Figure 3(b)). Homozygous replacement of the precursor miR-1246 sequence by the GFP-HDR fragment was verified by PCR on cellular DNA using miR-1246-up-F and miR-1246-down-R, covering upstream and downstream sequences of the precursor miR-1246 (Figure 3(c)). As shown in Figure 3(c), only the GFP-HDR fragment, and not the precursor miR-1246 sequence, was detected in the MIR1246KO cells. This was further confirmed by DNA sequencing of the PCR product (Supplement-sequence). Knockout of MIR1246 significantly slowed down cell growth, as evidenced by the MTS assay (Supplement Figure 3). However, knockout of MIR1246 did not alter PANC-1 exosomal miR-1246 levels (Figure 3(d)) as detected by poly-A tailing SYBR qRT-PCR, strongly indicating that exosomal miR-1246 is not derived from the precursor miR-1246. Stem-loop TaqMan qRT-PCR analysis of miR-1246 expression in exosomes derived from wild type and MIR1246KO PANC-1 cells confirmed that no differences of exosomal miR-1246 expression were detected between the two groups (Supplemental Figure 4). Next, since RNU2-1 is a component of the U2 complex in the spliceosomes and regarded as an essential small nuclear RNA in eukaryotic cells [29], we performed siRNA knockdown of RNU2-1 in PANC-1 cells. Transfection of PANC-1 cells with siRNAs targeting RNU2-1 (Supplement Table 1) did not reduce cellular RNU2-1 levels (Figure 4(a)), however a dramatic increase in exosomal miR-1246 expression was observed (Figure 4(b)), suggesting that exosomal miR-1246 is originated from RNU2-1 degradation. It is known that mature U2 snRNA is abundant and stable in eukaryotic cells, and that its levels often do not reflect rates of production [30,31]. The inability of the siRNA to significantly reduce cellular RNU2-1 levels is consistent with previous reports showing that RNU2-1 levels cannot be altered by siRNA likely due to its essentiality to cell viability and its well-balanced rate of turnover and production [29,32]. It is conceivable that RNU2-1 is being rapidly produced to offset siRNA-induced degradation, therefore cellular RNU2-1 level remains unchanged yet exosomal miR-1246 expression is enhanced due to siRNA-induced RNU2-1 degradation. Taken together, these results indicate that exosomal miR-1246 is derived from RNU2-1.
Figure 3.
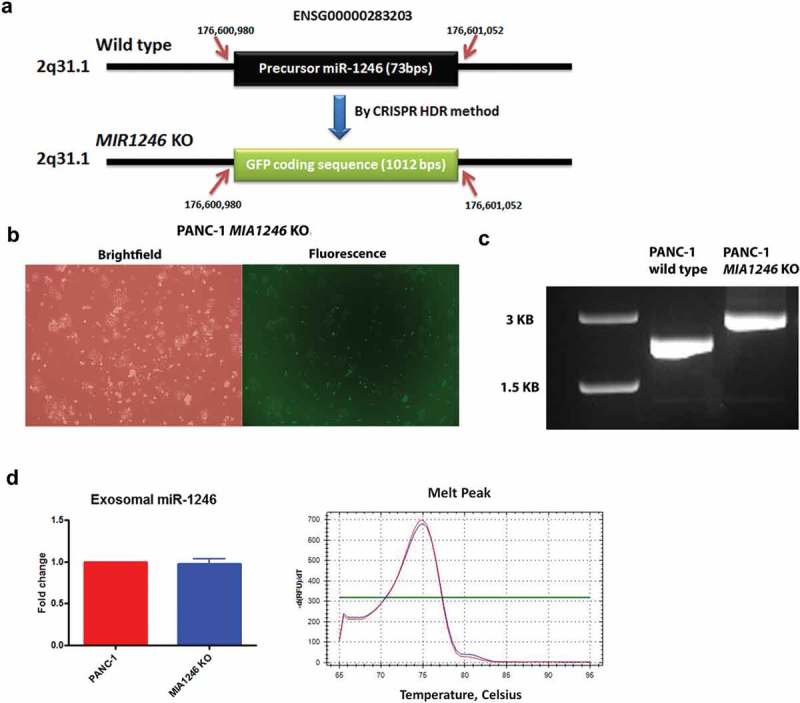
Effects of the MIR1246 gene knockout on exosomal miR-1246 expression. (a), The strategy of CRISPR-Cas9 HDR knockout of the MIR1246 gene. Ensembl gene ID and the precise sequence location are indicated. (b), GFP detection of the homozygous MIR1246 knockout PANC-1 cells by fluorescence microscopy. (c), Agarose gel electrophoresis of the PCR products of DNA from wild type PANC-1 and the MIR1246 knockout PANC-1 cells, using primers covering the DNA regions upstream and downstream of the MIR1246 gene. (d), qRT-PCR analysis using the poly A tailing method detecting miR-1246 in exosomes derived from wild type and MIR1246 knockout PANC-1 cells (means ± SEM, n = 3).
Figure 4.
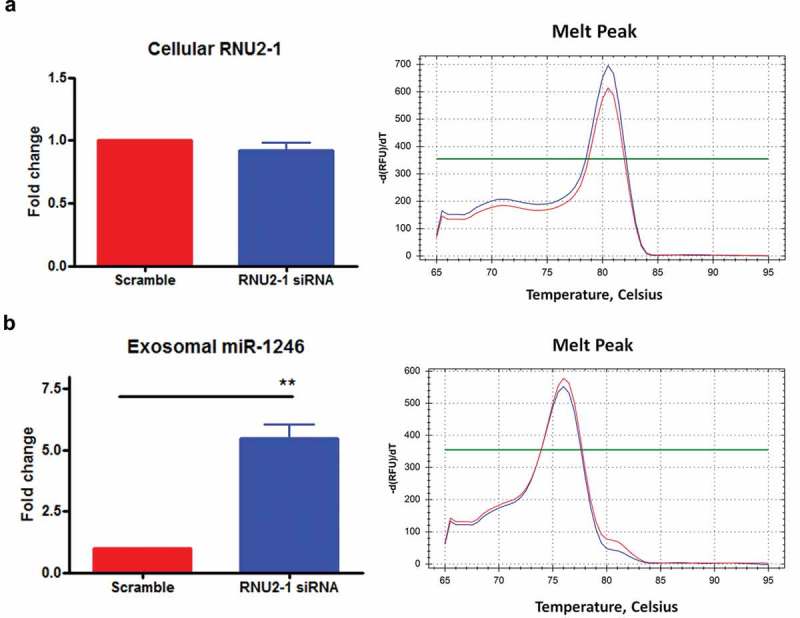
Effects of RNU2-1 knockdown on exosomal miR-1246 expression. (a), qRT-PCR analysis of RNU2-1 expression in scramble siRNA or RNU2-1 siRNA transfected PANC-1 cells. (b), qRT-PCR analysis of miR-1246 expression in exosomes derived from scramble siRNA or RNU2-1 siRNA transfected PANC-1 cells (means ± SEM, n = 3). ** p < 0.01, Student t-test, performed using ∆CT values.
We also applied a forced expression approach to address the origin of exosomal miR-1246 in PANC-1 cells. The precursor miR-1246 was cloned into the pcDNA3.1 vector and transfected to PANC-1 cells. Interestingly, the precursor miR-1246 expression was only detected in the transfected PANC-1 cells and not in wild type PANC-1 cells (Figure 5(a)). In addition, no mature miR-1246 sequence was detected in the precursor miR-1246 transfected PANC-1 cells (Figure 5(b)). No significant difference of miR-1246 expression was detected in exosomes derived from the precursor miR-1246 transfected PANC-1 cells versus those from the empty vector transfected PANC-1 cells (Figure 5(c)), suggesting that the precursor miR-1246 is not processed to generate the mature miR-1246 in PANC-1 cells. In contrast, when RNU2-1 was cloned into pcDNA3.1 vector and transfected to PANC-1 cells, miR-1246 expression was dramatically increased in exosomes derived from the RNU2-1 transfected PANC-1 cells compared to those derived from the empty vector transfected PANC-1 cells (Figure 5(e)), further indicating that exosomal miR-1246 is derived from RNU2-1. Consistent with the siRNA knockdown experiment and previous reports [29–32], we did not detect the increase in cellular RNU2-1 levels under the forced expression (Figure 5(d)).
Figure 5.
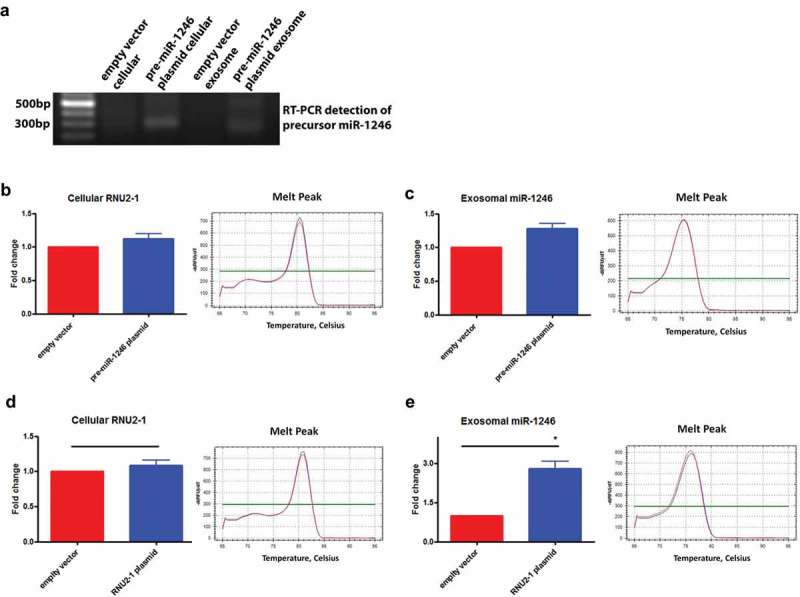
Effects of forced expression of the precursor miR-1246 or RNU2-1 on exosomal miR-1246 expression. (a), Agarose gel electrophoresis of the RT-PCR products detecting the precursor miR-1246 in PANC-1 exosomes and cells transfected with the precursor miR-1246 expression plasmid. (b), qRT-PCR detection of RNU2-1 in wild type and the precursor miR-1246 expression plasmid transfected PANC-1 cells. (c), qRT-PCR detection of miR-1246 in exosomes derived from wild type and the precursor miR-1246 expression plasmid transfected PANC-1 cells. (d), qRT-PCR detection of RNU2-1 in wild type and the RNU2-1 expression plasmid transfected PANC-1 cells. e, qRT-PCR detection of miR-1246 in exosomes derived from wild type and RNU2-1 expression plasmid transfected PANC-1 cells (n = 3, means ± SEM, for b. c, d, and e). * p < 0.05, Student t-test, performed using ∆CT values.
Exosomal miR-1246 is generated through a non-canonical miRNA biogenesis process
It is well-established that the majority of human mature miRNAs are processed through the canonical miRNA biogenesis pathway, involving processing of the preliminary miRNA transcripts to the precursor miRNAs in the nucleus by Drosha and further processing of the precursor miRNAs to mature miRNAs by Dicer in the cytoplasm [33]. To determine whether exosomal miR-1246 is processed through the canonical miRNA biogenesis pathway, we knocked down expression of Drosha and Dicer in PANC-1 cells using shRNA technology. Transfection of the shRNA constructs to PANC-1 cells significantly reduced Drosha and Dicer expression as evidenced by western blot analysis (Figure 6(a)). Knockdown of Drosha or Dicer slightly reduced cellular RNU2-1 levels (Figure 6(b)). However, the knockdowns did not reduce exosomal miR-1246 expression; and in fact, knockdown of Drosha even increased exosome miR-1246 levels (Figure 6(c)), suggesting that exosome miR-1246 is not generated through the canonical miRNA biogenesis pathway, and a non-canonical miRNA biogenesis process, likely a RNU2-1 degradation pathway, is involved in this process. In a control experiment, knockdown of Drosha or Dicer significantly reduced miR-21 expression (Supplement Figure 5).
Figure 6.
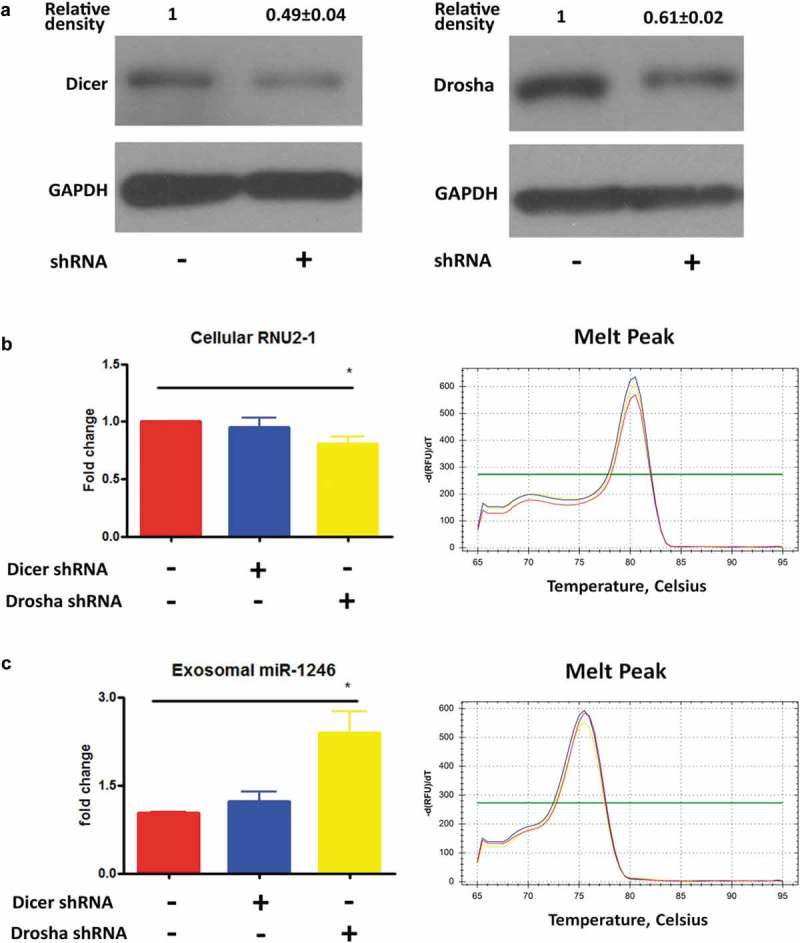
Effects of knockdown of Drosha and Dicer in PANC-1 cells on exosomal miR-1246 expression. (a), Western blot analysis confirming Dicer (left) and Drosha (right) knockdown in PANC-1 cells. (b), qRT-PCR analysis of RNU2-1 expression in Dicer and Drosha knockdown PANC-1 cells. (c), qRT-PCR analysis of miR-1246 expression in exosomes derived from Dicer and Drosha knockdown PANC-1 cells (means ± SEM, n = 3).
Exosomal miR-1246 is generated through RNU2-1 transcript degradation
To determine whether exosomal miR-1246 is generated from RNU2-1 transcript degradation, RNase A (12.5ug/mL, 50ug/mL or 250ug/mL) was incubated with PANC-1 cell lysate for 3 min, and the digestion was stopped by adding TRIzol reagent for RNA isolation. The Bioanalyzer analysis confirmed that RNase A treatment effectively degrades total cellular RNA (Figure 7(a and b)), and qRT-PCR analysis showed that miR-1246 is undetectable in control lysate but detected in RNase A-treated lysate in a concentration-dependent manner (Figure 7(c)). These observations strongly indicated that exosomal miR-1246 is derived from RNU2-1 transcript degradation. To further understand how the miR1246 sequence fragment was preserved during RNU2-1 degradation, we knocked down the expression of SmB/B’, a protein known to bind to the miR-1246 fragment in the RNU2-1 transcript [34], in PANC-1 cells. As shown in Figure 7(d and e), knockdown of SmB/B’ expression dramatically reduced exosomal miR-1246 levels (Figure 7(d and e)), indicating that the SmB/B’ protein binding protects the miR-1246 sequence during RNU2-1 degradation.
Figure 7.
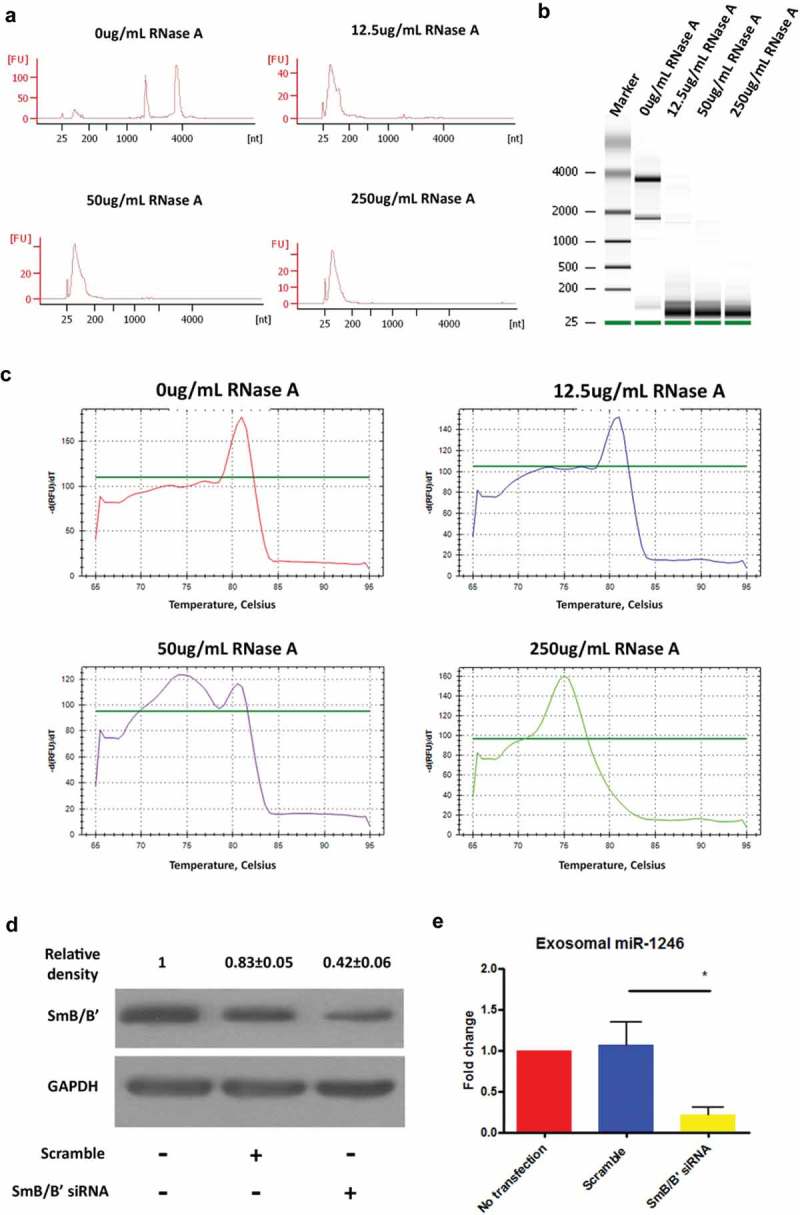
Exosomal miR-1246 is generated through RNU2-1 transcript degradation. (a and b), PANC-1 cell lysate was digested with RNase A at various concentrations for 3 min and the RNA isolated from the lysate was analyzed using Agilent 2100 Bioanalyzer. (c), The melting curves of qRT-PCR analysis showing the detection of RNU2-1 in control and 12.5 μg/ml RNase A-treated lysate, detection of miR-1246 in 250 μg/ml RNase A-treated lysate, and detection of both RNU2-1 and miR-1246 in 50 μg/ml RNase A-treated lysate. (d), Western blot analysis confirming SmB/B’ knockdown in PANC-1 cells. (e), qRT-PCR analysis of miR-1246 expression in exosomes derived from control and SmB/B’ knockdown PANC-1 cells (means ± SEM, n = 3). * p < 0.05, one-way ANOVA, followed by Dunnett analysis.
The GCAG motif mediates miR-1246 enrichment in PANC-1 exosomes
Both RNU2-1 and RNU2-2 are expressed in eukaryotic cells [35,36]. The RNU2-2 sequence is highly similar to that of RNU2-1, with a major difference in the 5ʹ primary end of the transcripts. With regard to the miR-1246 fragment, the two snRNAs differ in 4 nucleotides at the 3ʹ end of the miR-1246 fragment of RNU2-1 (GCAG in RNU2-1 and AAUA in RNU2-2, Figure 8(a)). However, next generation sequencing analysis showed that only the miR-1246 fragment generated from RNU2-1 is highly enriched in PANC-1 exosomes (Figure 8(b)). We therefore hypothesized that the GCAG motif in RNU2-1 mediates exosomal miR-1246 enrichment in PANC-1 cells. To test this hypothesis, several mutants were generated using the pcDNA3.1-RNU2-1 construct and transfected to PANC-1 cells (Figure 8(c)). The qRT-PCR analysis of the exosomes isolated from the transfected cells showed that miR-1246 expression in exosomes derived from the GCAG to AATA mutant-transfected cells was significantly lower than that in exosomes derived from RNU2-1 wild type and other mutants transfected PANC-1 cells (Figure 8(c)), demonstrating that the GCAG motif mediates exosomal miR-1246 enrichment in PANC-1 cells.
Figure 8.
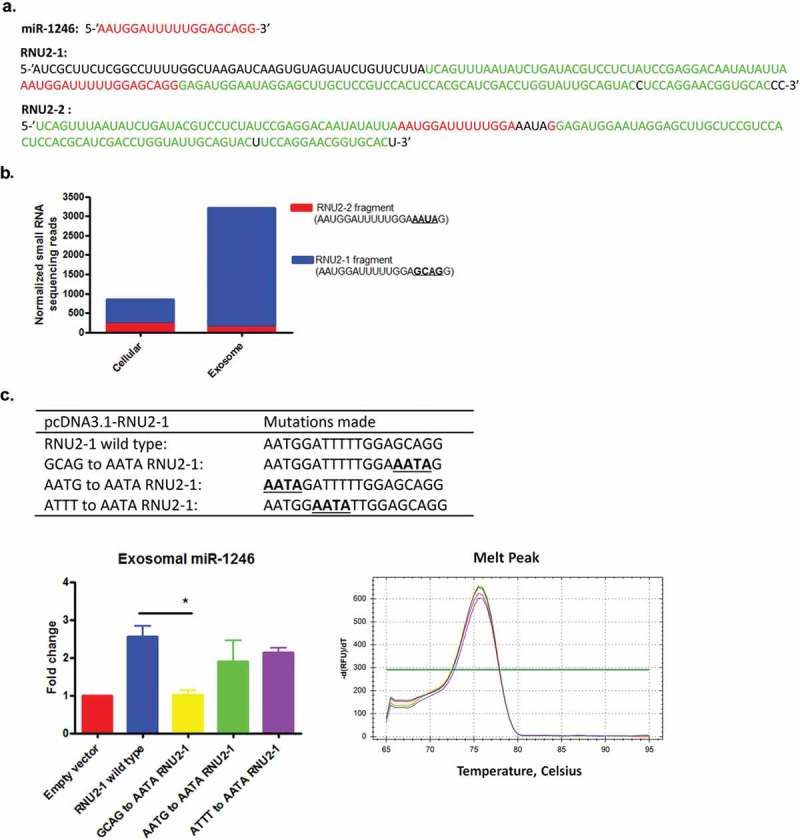
The GCAG motif in the RNU2-1 transcript is responsible for miR-1246 enrichment in PANC-1 exosomes. (a), Sequences of RNU2-1 and RNU2-2 with homology (green), miR-1246 fragment (red), and differences (black) highlighted. (b), small RNA sequencing reads match to RNU2-1 and RNU2-2 in PANC-1 cells and exosomes. (c), RNA motif mutants constructed using RNU2-1 expression plasmid. (d), qRT-PCR analysis of miR-1246 expression in exosomes derived from wild type and RUN2-1 mutants transfected PANC-1 cells (means ± SEM, n = 3). ** p < 0.05, one-way ANOVA, followed by Dunnett analysis.
Discussion
The origin and biogenesis of miR-1246 in eukaryotic cells remains controversial [15,37]. Using the human PANC-1 cell line as a model system, the present study provided evidence demonstrating that exosomal miR-1246 is derived from RNU2-1. The same was true in other human cells, including MIA-PaCa-2, and hTERT-HPNE lines. We further revealed that exosomal miR-1246 is processed via RUN2-1 transcript degradation in a Drosha- and Dicer-independent manner, and that the GCAG motif present in the miR-1246 fragment of the RNU2-1 transcript mediates exosomal miR-1246 enrichment in PANC-1 cells.
The evidence supporting our conclusion that exosomal miR-1246 originates from RNU2-1 includes the following: first, our small RNA next generation sequencing analysis of a cDNA library made from PANC-1 cells and their exosomes indicates that the majority of the miR-1246 reads detected are identical to fragments of the RNU2-1 transcript (Table 1), but not the precursor miR-1246 transcript, similar to a previous report [15]. The detection of various miR-1246 variant sequences in cells and exosomes suggest that miR-1246 is likely generated during RNU2-1 degradation. Second, the qRT-PCR analysis using the poly-A tailing SYBR method detected different fragments in cellular RNA versus exosomal RNA, and sequencing of the fragments confirmed that the RNU2-1 sequence is detected in PANC-1 cells and a variant of miR-1246 resembling a RNU2-1 fragment was detected in exosomes. The inability to detect cellular miR-1246-like sequences by the poly-A tailing SYBR qRT-PCR is likely due to a very low copy number of this sequence in the cells or that the miR-1246-like sequences are rapidly exported via exosome secretion. Our northern blot analysis indicated that there is no mature miR-1246 being detected in several human cancer cell lines, supporting our observations from the poly A+ tailing SYBR qRT-PCR analysis. The miR-1246-like sequences are highly enriched in exosomes compared to its cellular content in other types of cancer cells as well [38]. Third, the most convincing evidence to demonstrate the origin of exosome miR-1246 was obtained from the MIR1246 knockout cells. Knockout of the precursor miR-1246 sequence did not alter exosomal miR-1246 levels, assayed by both the poly-A tailing SYBR qRT-PCR and the Stem-loop TaqMan qRT-PCR, clearly showing that exosomal miR-1246 is not derived from the precursor miR-1246. In contrast, transfection of siRNAs targeting RNU2-1 to PANC-1 cells led to a significant increase in exosomal miR-1246 expression, indicating that the degradation of RNU2-1 produces miR-1246-like sequences that are rapidly encapsulated by and secreted through exosomes. This was further supported by the observations that direct digestion of PANC-1 lysate with RNase A led to a concentration-dependent production of miR-1246 and that knockdown of the RNU2-1 binding protein SmB/B’ dramatically reduced exosomal miR-1246 levels in PANC-1 cells. The specific endogenous nuclease that degrades RNU2-1 transcript merits further investigation.
Our findings support the concept that cellular miR-1246 is not processed from the precursor miR-1246, and is rarely detectable in cellular RNAs. Forced expression of the precursor miR-1246 in PANC-1 cells led to the detection of the precursor transcript, but not the mature miR-1246, indicating a lack of a cellular mechanism to process the precursor miR-1246 into the mature form. Moreover, we were unable to detect the precursor miR-1246 in wild type PANC-1 cells and their exosomes by next generation sequencing or by qRT-PCR using the poly-A tailing SYBR method, bringing into question whether the miR-1246 gene is transcribed and its transcript processed. These findings are significant as new studies have demonstrated the importance of miR-1246 in cancer biology [4,6,11,12,24,39], yet in these studies miR-1246 has been detected primarily by qRT-PCR using the stem-loop TaqMan method, which cannot qualitatively distinguish the detection of miR-1246 from RNU2-1. Given the fact that RNU2-1 is an essential component of the U2 complex of the spliceosome [14], which has been recognized to play significant roles in cancer biology [40–42], the inability to distinguish the detection of miR-1246 from RNU2-1 will likely lead to misinterpretation of experimental results, and impede the advancement of cancer biology involving in RNU2-1 and miR-1246.
The identification of the GCAG motif present in the RNU2-1 transcript that mediates exosome enrichment of miR-1246 sequences supports the concept that RNA sequence motifs are significant determinants of selective sorting of cellular RNAs into exosomes. This is consistent with recent reports showing the importance of certain RNA motifs present in selectively encapsulated exosomal miRNAs [43,44]. In addition, our findings that exosomal miR-1246 is derived from RNU2-1 through a non-canonical miRNA biogenesis process is also in line with recent reports showing that several miRNAs are derived from small nucleolar RNAs [45–47], and that expression of some miRNAs are independent of the canonical miRNA biogenesis pathway [48]. Furthermore, the conclusion that exosomal miR-1246 is derived from RNU2-1 transcript may have a significant impact on improving our understanding of the biology of RNU2-1. It is well known that RNU2-1 is stably expressed and can serve as endogenous control for RNA detection [49], and as such RNU2-1 expression levels are difficult to alter in eukaryotic cells [29]. Thus, exosomal miR-1246 may serve as an indicator of RNU2-1 turnover or degradation in eukaryotic cells.
In conclusion, we have demonstrated that exosomal miR-1246 originates from RNU2-1 transcript degradation in human PDAC cells, and that the GCAG motif mediates miR-1246 enrichment in PDAC exosomes. These findings reveal the origin of an oncomiR in human cancer cells and suggest that current reports regarding miR-1246 detection or biological function in cancer cells should be interpreted with caution.
Methods
Cell culture
The human pancreatic epithelial cell line hTERT-HPNE, pancreatic cancer cell lines PANC-1, MIA-PaCa-2 and BxPC-3, and breast cancer cell lines MCF7 and MDA-MB-231 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured according to ATCC’s instructions except that exosome-depleted fetal bovine serum (FBS) and horse serum were used when appropriate. Exosome-depleted FBS and horse serum were prepared by pelleting the serum exosomes by ultracentrifugation at 100,000 × g for 2 hours at 4°C. Cells were routinely maintained in a humidified chamber at 37°C and 5% CO2.
Exosome isolation
Exosomes were isolated from the culture medium utilizing a combination of centrifugation, ultracentrifugation, and filtration as we recently described [38,50].
Western blot analysis
Total protein was prepared by resuspending cells in RIPA Buffer (50mM Tris-HCl pH 7.4, 150mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, and 0.1% sodium dodecyl sulfate) containing 1 mM phenlymethylsulfonyl fluoride, 5μg/ml leupeptin, 2μg/ml aprotinin, and 1μg/ml pepstatin A [38]. Approximately 30-40μg of protein from each sample was separated under reducing conditions on a 10% SDS-PAGE gel, transferred to a polyvinylidene fluoride membrane, blotted with a primary antibody against Dicer (Cell Signaling Technology, 3363S), Drosha (Cell Signaling Technology, 3364S), SmB/B’ (Santa Cruz Biotechnology, sc-130670), and GAPDH (ProMab, 20035).
Small RNA library preparation and next generation sequencing
Total RNA was extracted from cells and exosome pellets using the TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, California) following the manufacturer’s protocol [38]. RNA concentration was quantitated using the NanoDrop ND-100 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Small RNA libraries were constructed using the New England Biolabs (NEB) NEBNext Multiplex Small RNA Library Prep Set for Illumina sequencers and the NEB standard protocol [38]. Each library was indexed to multiplex four samples per sequencing run on the Illumina MiSeq platform using MiSeq 50 cycle Reagent Kits v2. A minimum of 17 million 50-bp sequencing reads were collected from each sample and data were analyzed using the Genesifter software (formerly Geospiza, PerkinElmer, Santa Clara, CA, USA). The raw data of next generation small RNA sequencing was analyzed using TxetWrangler software (BARE BONES, North Chelmsford, MA, USA) to identify miR-1246 sequence.
Quantitative real-time reverse transcription polymerase chain reaction
For quantitative miRNA expression analysis, cDNA was synthesized from 80 ng of total RNA using the qScript miRNA cDNA Synthesis Kit (Quanta BioSciences, Inc., Beverly, MA, USA). An aliquot of cDNA, equivalent of 4 ng of the original RNA, was mixed with Perfecta SYBR Green SuperMix, Quanta Universal PCR Reverse Primer (Quanta, lot#015003), and a sequence specific forward primer (Table 2) or positive control forward primer (Quanta, Lot# 015644) in 20 μl PCR reactions. A synthetic Caenorhabditis elegans miR-54 (cel-miR-54) RNA oligonucleotide (Integrated DNA Technologies, Coralville, IA, USA) was spiked-into RNA samples as a control [51]. PCR reactions were run on a Bio-Rad CFX 96 Real-Time PCR (Bio-Rad, Hercules, CA, USA) instrument under following conditions: 95°C for 2 min, 40 cycles of 95°C for 5 sec, 60°C for 15 sec and 70°C for 15 sec. Changes in gene expression were calculated using the ΔΔCT method: ΔCT = CT (target RNA) − CT (PerfeCTa Human Positive Control for cell or cel-miR-54 for exosome); ΔΔCT = ΔCT (experimental group)- ΔCT (control group); the fold change = 2−ΔΔCT. The specific primers used for RT-PCR reactions were listed in Table 2.
Table 2.
List of primers used in this study.
| Primer name | Primer sequence (all 5ʹ to 3ʹ) |
|---|---|
| miR-1246-F | GCGCGATGGATTTTTGGAGCAG |
| miR1246-up-F | TGCGTAACAGCTCCCTTTTT |
| miR1246-up-R | AAGTCCCGTTGATTTTGGTGTCAAAGGGCTATTGCAAACA |
| GFP-F | TGTTTGCAATAGCCCTTTGACACCAAAATCAACGGGACTT |
| GFP-R | GAGACATTGCATTTGGAGGCGGGAGGTGTGGGAGGTTTT |
| miR-1246-down-F | AAAACCTCCCACACCTCCCGCCTCCAAATGCAATGTCTC |
| miR-1246-down-R | GAGGACAGCAACGTCACAGA |
| pre-miR-1246-clone-F | CCGCTCGAGTGTATCCTTGAATGGATTTTTGGAGC |
| pre-miR-1246-clone-R | CCGCTCGAGATTGCTAGCCTATGGATTGATTTCC |
| miR-1246-precursor-F | GTGGACACCTGACCCAAAGGAAA |
| RNU2-1-clone-F | CCGCTCGAGATCGCTTCTCGGCCTTTTGG |
| RNU2-1-clone-R | CCGCTCGAGGGGTGCACCGTTCCTGGA |
| GCAG-AATA-L | GCAAGCTCCTATTCCATCTCCTATTTCCAAAAATCCATTTAATATATTG |
| TCCTCGGATAGA | |
| GCAG-AATA-R | TCTATCCGAGGACAATATATTAAATGGATTTTTGGAAATAGGAGATGG |
| AATAGGAGCTTGC | |
| AATG-AATA-L | TCCCTGCTCCAAAAATCTATTTAATATATTGTCCTCGGATAGAGG |
| AATG-AATA-R | CCTCTATCCGAGGACAATATATTAAATAGATTTTTGGAGCAGGGA |
| ATTT-AATA-L | TCCGAGGACAATATATTAAATGGAATATTGGAGCAGGGAGATGGAAT |
| ATTT-AATA-R | ATTCCATCTCCCTGCTCCAATATTCCATTTAATATATTGTCCTCGGA |
Sanger sequencing
cDNA was prepared from PANC-1 cellular and exosome RNA using the qScript miRNA cDNA Synthesis Kit (Quanta BioSciences, Inc., Beverly, MA, USA). The cDNA was amplified using the miR-1246-F (Table 2) and the Quanta Universal PCR Reverse Primer (Quanta, lot#015003) in a standard PCR reaction. The PCR products were separated on a 1.5% agarose gel, and purified using the DNA gel purification kit (EZNA, D2500-01). The purified PCR products were cloned to the pCR4-TOPO vector (Invitrogen, lot#1734930) using the TOPO TA Cloning Kit (Invitrogen, lot#1747966). The cloned constructs were transformed to NEB® 5-alpha Competent E. coli cells (C2987I) and the cells were selected by an ampicillin containing LB agar plate at 37°C. Three random individual colonies were further amplified in ampicillin containing LB broth, and the plasmid DNA were prepared and sequenced.
Northern blot analysis
Northern blot was performed following a previous protocol [52] with a synthesized DIG labeled LNA probe for miR-1245. 15 μg total RNA were separated in a SequaGel (National diagnostics, cat. no. EC-833) and transferred to positively charged nylon membranes (Roche, cat. no. 11 209 299 001). The membranes were blocked, hybridized to the miR-1246 probe (QIAGEN, miRCURY LNATM miRNA Detection Probe), and washed [52]. After incubating with anti-Digoxigenin-AP, Fab fragments (Roche, cat. no. 11 093 274 910) and CSPD (Roche, cat. no. 11 655 884 001), the membranes were exposed to X-ray film. The film was scanned and images cropped using Photoshop software.
Luciferase reporter gene assay
A luciferase reporter plasmid was constructed using the pGL3-Promoter vector (Promega, Madison, WI, USA) by inserting the miR-1246 target sequence (synthesized by IDT) at the XbaI site according to a well-established strategy for miRNA target gene study (Promega, pmirGLO Dual-Luciferase miRNA Target Expression). The antisense of the target sequence was also inserted into the XbaI site serving as a control plasmid. Mimics of mature miR-1246 and our newly identified miR-1246 like sequence (Figure 2(c)) were synthesized by Ambion (Life Technologies, Carlsbad, CA, USA). Cells were transfected using Lipofectamine 3000 (Invitrogen) with 1 µg of each constructed vector and 300 nM of the miR-1246, miR-1246 variant mimics or scramble controls in each well of the 96-well plate. In each transfection, 100ng of pRL-TK (Promega, Madison, WI) was included to serve as transfection controls. 72 hours after transfection, luciferase activity was analyzed using the Dual-Luciferase Reporter reagent (Promega, Madison, WI) with the Perkin Elmer EnVision® Multilabel Reader.
Knockout of MiR1246 in PANC-1 cells
The gene encoding miR-1246 (MIR1246) on 2q31.1 was knocked out using the CRISPR-Cas-9 Homology Directed Repair (HDR) method [28]. Guide RNA (gRNA) was designed using a software program from the http://crispr.mit.edu website, and the gRNA oligonucleotides (CACCGGCCTATGGATTGATTTCCTT, AAACAAGGAAATCAATCCATAGGCC) were ordered from IDT. The oligonucleotides were annealed and subcloned into the BsmBI sites of the lentiviral vector (Lenti CRISPR V2 from addgene, plasmid # 52961). The GFP-HDR fragment was constructed by connecting the DNA sequences upstream and downstream of MIR1246 and the GFP coding sequence using overlap PCR. Briefly, the Phusion® High-Fidelity DNA Polymerase (NEB M0530S) was used to amplify the upstream and downstream sequences from PANC-1 chromosome DNA and the GFP coding sequence from the pEGFP-C1 vector, using respective primers (miR1246-up-F, miR1246-up-R, GFP-F, GFP-R, miR1246-down-F, miR1246-down-R) as shown in Table 2. An overlap PCR was performed to connect the three DNA fragments using a protocol previously described [53]. The Lenti CRISPR V2 vector and the HDR fragment were co-transfected to PANC-1 cells using the FuGENE® HD Transfection Reagent (E2311, Promega, Madison, WI, USA). Forty-eight hours post transfection, cells were selected by puromycin (1ug/ml) treatment for six days. The GFP positive cells were then sorted using the FACSJazz Cell Sorter (BD Bioscience, Sparks, MD, USA). The homozygous knockout of MIR1246 was verified by PCR amplification of the genomic DNA and DNA sequencing of the amplified DNA production confirming the insertion of the GFP-HDR fragment. The selected MIR1246 knockout cells were used for the downstream experiments.
Construction of precursor miR-1246 and RNU2-1 expression plasmids
The precursor miR-1246 and RNU2-1 fragments were PCR amplified using sequence specific primers (pre-miR-1246-clone-F/R, RNU2-1-clone F/R, see Table 2) from PANC-1 DNA samples. The purified PCR fragments and the pcDNA3.1(+) (Invitrogen) vector were digested with Xho1 (NEB, R0146L) respectively and purified. The cut pcDNA3.1(+) vector was dephosphorylated and ligated to the precursor miR-1246 or RNU2-1 fragments. The ligation was transformed to DH5α competent cell (NEB C2987H) and single colonies were obtained under ampicillin selection. The selected colonies were amplified, the plasmids were extracted and DNA sequencing was performed to verify a successful cloning. Based on the pcDNA3-RNU2-1 plasmid, the mutant RNU2-1 expression plasmids were made using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, 210518), using primers (GCAG-AATA-L/R, AATG-AATA-L/R, ATTT-AATA-L/R) listed in Table 2. Plasmid transfection of PANC-1 cells was performed using FuGENE® HD Transfection Reagent according to the manufacturer’s protocol (Promega, E2311).
siRNA targeting RNU2-1 and SmB/B’
siRNAs targeting RNU2-1 sequence (shown in Supplemental Table 1) (IDT, catalog number: hs.Ri.RNU2-1.13.1, hs.Ri.RNU2-1.13.2) or SmB/B’ mRNA (Santa Cruz, sc-36503) were transfected to PANC-1 cells (100nM, final concentration) using the FuGENE® HD Transfection Reagent (Promega, E2311). Forty-eight hours post transfection the cells were harvested and exosomes were isolated from the medium.
Dicer or drosha knockdown
The pSicoR human Dicer1 and pSicoR human Drosha1 shRNA plasmids were a gift from Tyler Jacks (Addgene plasmids # 14763 and # 14766). Plasmid DNA was transfected to PANC-1 cells using the FuGENE® HD Transfection Reagent. Seventy-two hours post transfection the cells were harvested and exosomes were isolated. Dicer and Drosha1 knockdown was confirmed by western blot analysis.
RNase a treatment of PANC-1 lysate
PANC-1 cell lysate was prepared using the passive lysis buffer (Promega, Madison, WI). The lysate was treated with various concentrations of RNase A (Omega Bio-tek, Norcross, GA) for 3 minutes at room temperature. Total RNA was extracted using the TRIzol reagent following the manufacturer’s protocol. The quality of isolated RNA was analyzed by Agilent 2100 Bioanalyzer.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA, USA). Student’s t test or one-way ANOVA was applied to determine significant differences among control and experimental groups.
Funding Statement
This study was supported in part by grants from the National Institute of General Medical Sciences of the National Institutes of Health (U54GM104938, P20GM103640); the Oklahoma Center for the Advancement of Science and Technology (HR14-147, HR17-052); and Presbyterian Health Foundation.
Acknowledgments
We thank the Stephenson Cancer Center at the University of Oklahoma, Oklahoma City, OK for providing core facility support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethics approval and consent to participate
NA
Consent for publication
All authors have read this manuscript and approved for the submission.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article and its additional file.
Authors’ contributions
XY carried out the study and drafted the manuscript. BNH supervised the experiments and helped drafting the manuscript. UK and AG assisted with cellular assays. WQD conceived of the study and participated in its design and coordination and finalized the manuscript. All authors read and approved the final manuscript.
Supplementary material
Supplementary material for this article can be accessed here.
References
- [1].Morin RD, O’Connor MD, Griffith M, et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008. April;18(4):610–621. PubMed PMID: 18285502; PubMed Central PMCID: PMCPMC2279248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang Y, Liao JM, Zeng SX, et al. p53 downregulates Down syndrome-associated DYRK1A through miR-1246 [research support, N.I.H., Extramural]. EMBO Rep. 2011. June 03;12(8):811–817. PubMed PMID: 21637297; PubMed Central PMCID: PMC3147276. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gillen AE, Gosalia N, Leir SH, et al. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem J. 2011. August 15;438(1):25–32. PubMed PMID: 21689072; PubMed Central PMCID: PMCPMC4323381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li W, Wu YF, Xu RH, et al. miR-1246 releases RTKN2-dependent resistance to UVB-induced apoptosis in HaCaT cells. Mol Cell Biochem. 2014. September;394(1–2):299–306. PubMed PMID: 24880483. [DOI] [PubMed] [Google Scholar]
- [5].Hasegawa S, Eguchi H, Nagano H, et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer [research support, Non-U.S. Gov’t]. Br J Cancer. 2014. October 14;111(8):1572–1580. PubMed PMID: 25117811; PubMed Central PMCID: PMC4200094. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sakha S, Muramatsu T, Ueda K, et al. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep. 2016. December 08;6:38750 PubMed PMID: 27929118; PubMed Central PMCID: PMCPMC5144099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yamada N, Tsujimura N, Kumazaki M, et al. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells [research support, Non-U.S. Gov’t]. Biochim Biophys Acta. 2014. November;1839(11):1256–1272. PubMed PMID: 25218966; eng. [DOI] [PubMed] [Google Scholar]
- [8].Wang S, Zeng Y, Zhou JM, et al. MicroRNA-1246 promotes growth and metastasis of colorectal cancer cells involving CCNG2 reduction. Mol Med Rep. 2016. January;13(1):273–280. PubMed PMID: 26573378; eng. [DOI] [PubMed] [Google Scholar]
- [9].Sun Z, Meng C, Wang S, et al. MicroRNA-1246 enhances migration and invasion through CADM1 in hepatocellular carcinoma [research support, Non-U.S. Gov’t]. BMC Cancer. 2014;14:616 PubMed PMID: 25159494; PubMed Central PMCID: PMC4150976. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chai S, Ng KY, Tong M, et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/beta-catenin activation in liver cancer stem cells. Hepatology. 2016. December;64(6):2062–2076. PubMed PMID: 27639189. [DOI] [PubMed] [Google Scholar]
- [11].Kim G, An HJ, Lee MJ, et al. Hsa-miR-1246 and hsa-miR-1290 are associated with stemness and invasiveness of non-small cell lung cancer. Lung Cancer. 2016. January;91:15–22. PubMed PMID: 26711929; eng. [DOI] [PubMed] [Google Scholar]
- [12].Zhang WC, Chin TM, Yang H, et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat Commun. 2016. June;21(7):11702 PubMed PMID: 27325363; PubMed Central PMCID: PMC4919505. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu YF, Hannafon BN, Zhao YD, et al. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget. 2017. September 29;8(44):77028–77040. PubMed PMID: ISI:000412066700100; English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Patel SB, Bellini M.. The assembly of a spliceosomal small nuclear ribonucleoprotein particle. Nucleic Acids Res. 2008. November;36(20):6482–6493. PubMed PMID: 18854356; PubMed Central PMCID: PMCPMC2582628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baraniskin A, Nopel-Dunnebacke S, Ahrens M, et al. Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for pancreatic and colorectal adenocarcinoma [research support, Non-U.S. Gov’t]. Int J Cancer. 2013. January 15;132(2):E48–57. PubMed PMID: 22907602; Eng. [DOI] [PubMed] [Google Scholar]
- [16].Fang Y, Gao F, Hao J, et al. microRNA-1246 mediates lipopolysaccharide-induced pulmonary endothelial cell apoptosis and acute lung injury by targeting angiotensin-converting enzyme 2. Am J Transl Res. 2017;9(3):1287–1296. PubMed PMID: 28386354; PubMed Central PMCID: PMCPMC5376019. [PMC free article] [PubMed] [Google Scholar]
- [17].Jia HL, Liu CW, Zhang L, et al. Sets of serum exosomal microRNAs as candidate diagnostic biomarkers for Kawasaki disease. Sci Rep. 2017. March;20(7):44706 PubMed PMID: 28317854; PubMed Central PMCID: PMCPMC5357789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bott A, Erdem N, Lerrer S, et al. miRNA-1246 induces pro-inflammatory responses in mesenchymal stem/stromal cells by regulating PKA and PP2A. Oncotarget. 2017. July 04;8(27):43897–43914. PubMed PMID: 28159925; PubMed Central PMCID: PMCPMC5546423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim JH, Ahn JH, Lee M. Upregulation of microRNA-1246 is associated with BRAF inhibitor resistance in melanoma cells with mutant BRAF. Cancer Res Treat. 2017. January 03;49:947–959 PubMed PMID: 28052651; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Todeschini P, Salviato E, Paracchini L, et al. Circulating miRNA landscape identifies miR-1246 as promising diagnostic biomarker in high-grade serous ovarian carcinoma: A validation across two independent cohorts. Cancer Lett. 2017. March;01(388):320–327. PubMed PMID: 28017893. [DOI] [PubMed] [Google Scholar]
- [21].Wark AW, Lee HJ, Corn RM. Multiplexed detection methods for profiling microRNA expression in biological samples. Angew Chem Int Ed Engl. 2008;47(4):644–652. PubMed PMID: 17994653; PubMed Central PMCID: PMCPMC2739110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen C, Ridzon DA, Broomer AJ, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005. November 27;33(20):e179 PubMed PMID: 16314309; PubMed Central PMCID: PMCPMC1292995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dunnett H, van der Meer D, Williams GA. Evaluation of stem-loop reverse transcription and poly-A tail extension in microRNA analysis of body fluids. Microrna. 2014;3(3):150–154. PubMed PMID: 25612781. [DOI] [PubMed] [Google Scholar]
- [24].Yuan D, Xu J, Wang J, et al. Extracellular miR-1246 promotes lung cancer cell proliferation and enhances radioresistance by directly targeting DR5. Oncotarget. 2016. May 31;7(22):32707–32722. PubMed PMID: 27129166; PubMed Central PMCID: PMC5078045. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tessereau C, Buisson M, Monnet N, et al. Direct visualization of the highly polymorphic RNU2 locus in proximity to the BRCA1 gene [research support, Non-U.S. Gov’t]. PLoS one. 2013;8(10):e76054 PubMed PMID: 24146815; PubMed Central PMCID: PMC3795722. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jiang C, Liao D. Striking bimodal methylation of the repeat unit of the tandem array encoding human U2 snRNA (the RNU2 locus) [Research Support, Non-U.S. Gov’t]. Genomics. 1999. December 15;62(3):508–518. PubMed PMID: 10644450; eng. [DOI] [PubMed] [Google Scholar]
- [27].Schertzer M, Jouravleva K, Perderiset M, et al. Human regulator of telomere elongation helicase 1 (RTEL1) is required for the nuclear and cytoplasmic trafficking of pre-U2 RNA [research support, Non-U.S. Gov’t]. Nucleic Acids Res. 2015. February 18;43(3):1834–1847. PubMed PMID: 25628358; PubMed Central PMCID: PMC4330364. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ran FA, Hsu PD, Wright J, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013. November;8(11):2281–2308. PubMed PMID: 24157548; PubMed Central PMCID: PMCPMC3969860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jawdekar GW, Henry RW. Transcriptional regulation of human small nuclear RNA genes. Biochim Biophys Acta. 2008. May;1779(5):295–305. PubMed PMID: 18442490; PubMed Central PMCID: PMCPMC2684849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zieve G, Penman S. Small RNA species of the HeLa cell: metabolism and subcellular localization [research support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.]. Cell. 1976. May;8(1):19–31. PubMed PMID: 954090; eng. [DOI] [PubMed] [Google Scholar]
- [31].Weinberg RA, Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968. December;38(3):289–304. PubMed PMID: 5718554; eng. [DOI] [PubMed] [Google Scholar]
- [32].Liang XH, Vickers TA, Guo S, et al. Efficient and specific knockdown of small non-coding RNAs in mammalian cells and in mice. Nucleic Acids Res. 2011. February;39(3):e13 PubMed PMID: 21062825; PubMed Central PMCID: PMCPMC3035437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014. August;15(8):509–524. PubMed PMID: 25027649. [DOI] [PubMed] [Google Scholar]
- [34].Ishikawa H, Nobe Y, Izumikawa K, et al. Truncated forms of U2 snRNA (U2-tfs) are shunted toward a novel uridylylation pathway that differs from the degradation pathway for U1-tfs. RNA Biol. 2018. February 1;15(2):261–268. PubMed PMID: 29168419; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reveillaud I, Lelay-Taha MN, Sri-Widada J, et al. Mg2+ induces a sharp and reversible transition in U1 and U2 small nuclear ribonucleoprotein configurations. Mol Cell Biol. 1984. September;4(9):1890–1899. PubMed PMID: 6238232; PubMed Central PMCID: PMCPMC368999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Htun H, Lund E, Westin G, et al. Nuclease S1-sensitive sites in multigene families: human U2 small nuclear RNA genes. Embo J. 1985. July;4(7):1839–1845. PubMed PMID: 2411549; PubMed Central PMCID: PMCPMC554425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mazieres J, Catherinne C, Delfour O, et al. Alternative processing of the U2 small nuclear RNA produces a 19-22nt fragment with relevance for the detection of non-small cell lung cancer in human serum [comparative study research support, Non-U.S. Gov’t]. PLoS one. 2013;8(3):e60134 PubMed PMID: 23527303; PubMed Central PMCID: PMC3603938. eng. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hannafon BN, Trigoso YD, Calloway CL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18(1):90 10.1186/s13058-016-0753-x. PubMed PMID: 27608715; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu LJ, Jiang T, Zhao W, et al. Parallel mRNA and microRNA profiling of HEV71-infected human neuroblastoma cells reveal the up-regulation of miR-1246 in association with DLG3 repression. PLoS One. 2014;9(4):e95272 PubMed PMID: 24739954; PubMed Central PMCID: PMCPMC3989279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xiping Z, Qingshan W, Shuai Z, et al. A summary of relationships between alternative splicing and breast cancer. Oncotarget. 2017. August 01;8(31):51986–51993. PubMed PMID: 28881705; PubMed Central PMCID: PMCPMC5584306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Y, Guo H, Jin C, et al. Spliceosome-associated factor CTNNBL1 promotes proliferation and invasion in ovarian cancer. Exp Cell Res. 2017. August 01;357(1):124–134. PubMed PMID: 28501461. [DOI] [PubMed] [Google Scholar]
- [42].Takayama KI, Suzuki T, Fujimura T, et al. Dysregulation of spliceosome gene expression in advanced prostate cancer by RNA-binding protein PSF. Proc Natl Acad Sci U S A. 2017. September 26;114(39):10461–10466. PubMed PMID: 28893982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs [research support, Non-U.S. Gov’t]. Nat Commun. 2013;4:2980 PubMed PMID: 24356509; PubMed Central PMCID: PMC3905700. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Santangelo L, Giurato G, Cicchini C, et al. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep. 2016. October 11;17(3):799–808. PubMed PMID: 27732855. [DOI] [PubMed] [Google Scholar]
- [45].Saraiya AA, Wang CC. snoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathog. 2008. November;4(11):e1000224 PubMed PMID: 19043559; PubMed Central PMCID: PMCPMC2583053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li W, Saraiya AA, Wang CC. Gene regulation in Giardia lambia involves a putative microRNA derived from a small nucleolar RNA. PLoS Negl Trop Dis. 2011. October;5(10):e1338 PubMed PMID: 22028939; PubMed Central PMCID: PMCPMC3196473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li W, Saraiya AA, Wang CC. The profile of snoRNA-derived microRNAs that regulate expression of variant surface proteins in Giardia lamblia. Cell Microbiol. 2012. September;14(9):1455–1473. PubMed PMID: 22568619; PubMed Central PMCID: PMCPMC3422372.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim YK, Kim B, Kim VN. Re-evaluation of the roles of DROSHA, export in 5, and DICER in microRNA biogenesis. Proc Natl Acad Sci U S A. 2016. March 29;113(13):E1881–9. PubMed PMID: 26976605; PubMed Central PMCID: PMCPMC4822641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dingle KE, Crook D, Jeffery K. Stable and noncompetitive RNA internal control for routine clinical diagnostic reverse transcription-PCR. J Clin Microbiol. 2004. March;42(3):1003–1011. PubMed PMID: 15004045; PubMed Central PMCID: PMCPMC356891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hannafon BN, Carpenter KJ, Berry WL, et al. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer. 2015. July;16(14):133 PubMed PMID: 26178901; PubMed Central PMCID: PMCPMC4504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection [Research Support, N.I.H., extramural research support, Non-U.S. Gov’t]. Proc Natl Acad Sci U S A. 2008. July 29;105(30):10513–10518. PubMed PMID: 18663219; PubMed Central PMCID: PMC2492472. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kim SW, Li Z, Moore PS, et al. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic Acids Res. 2010. April;38(7):e98 PubMed PMID: 20081203; PubMed Central PMCID: PMCPMC2853138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xu Y, Itzek A, Kreth J. Comparison of genes required for H2O2 resistance in Streptococcus gordonii and Streptococcus sanguinis. Microbiology. 2014. December;160(Pt 12):2627–2638. PubMed PMID: 25280752; PubMed Central PMCID: PMCPMC4252910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional file.


