ABSTRACT
MIRNA (MIR) gene origin and early evolutionary processes, such as hairpin precursor sequence origination, promoter activity acquirement and the sequence of these two processes, are fundamental and fascinating subjects. Three models, including inverted gene duplication, spontaneous evolution and transposon transposition, have been proposed for de novo origination of hairpin precursor sequence. However, these models still open to discussion. In addition, de novo origination of MIR gene promoters has not been well investigated. Here, I systematically investigated the origin of evolutionarily young polyphenol oxidase gene (PPO)-targeting MIRs, including MIR1444, MIR058 and MIR12112, and a genomic region termed AasPPO-as-hp, which contained a hairpin-forming sequence. I found that MIR058 precursors and the hairpin-forming sequence of AasPPO-as-hp originated in an ancient PPO gene through forming short inverted repeats. Palindromic-like sequences and imperfect inverted repeats in the ancient PPO gene contributed to initiate the generation of short inverted repeats probably by causing errors during DNA duplication. Analysis of MIR058 and AasPPO-as-hp promoters showed that they originated in the 3ʹ-flanking region of the ancient PPO gene. Promoter activities were gained by insertion of a CAAT-box and multiple-copper-response element (CuRE)-containing miniature inverted-repeat transposable element (MITE) in the upstream of AT-rich TATA-box-like sequence. Gain of promoter activities occurred before hairpin-forming sequence origination. Sequence comparison of MIR1444, MIR058 and MIR12112 promoters showed frequent birth and death of CuREs, indicating copper could be vital for the origination and evolution of PPO-targeting MIRs. Based on the evidence obtained, a novel model for plant MIR origination and evolution is proposed.
KEYWORDS: Gene origination, gene evolution, MIR1444, MIR058, MIR12112, polyphenol oxidase
Introduction
MicroRNAs (miRNAs) are a class of small non-coding RNAs of about 21 nucleotides in length. They are generated from primary-MIRNAs (pri-MIRs) transcribed from MIR loci [1]. Pri-MIR typically contains an imperfect hairpin structure, which is processed to aMIRNA precursor (pre-MIR). Cleavage of the pre-MIR on the stem portion generates a miRNA:miRNA* duplex, which comprises a mature miRNA on a side and a miRNA* on the other side. The duplex is then unwound to release a single-stranded mature miRNA [1]. Through direct cleavage of target transcripts, miRNAs play significant regulatory roles in plant development and stress responses. De novo origination and subsequent evolution of MIRNA genes (MIRs) is one of the most fundamental and fascinating subjects of plant biology. Origination of MIRs includes two key processes: the origination of hairpin precursor sequence and the acquirement of promoter activity. For de novo origination of MIR gene promoters, it has not been well investigated. For de novo origination of plant pre-MIRs, there are three models, including inverted gene duplication, spontaneous evolution and transposon transposition (Figure 1) [2–7].
Figure 1.
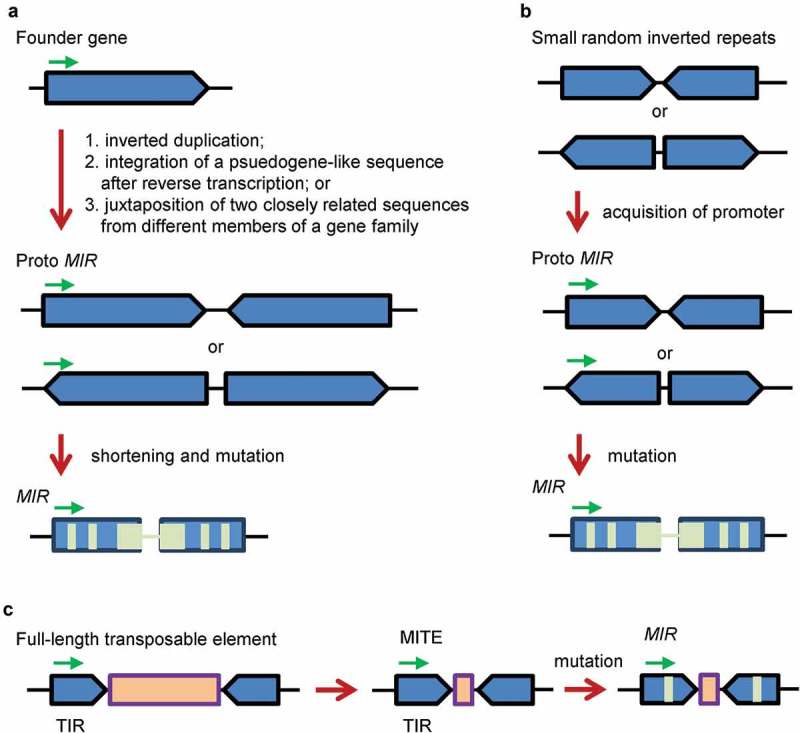
The inverted gene duplication, spontaneous evolution and transposon transposition models for de novo origination and evolution of plant MIRs. (A) The inverted gene duplication model [2]. Direct inverted duplication of a founder gene, integration of a pseudogene-like sequence after reverse transcription or juxtaposition of two closely related sequences from different members of a gene family resulted in the emergence of a proto MIR. Shortening and mutations (light green) of the proto MIR led to the generation of MIRs. (B) The spontaneous evolution model [6]. Small random inverted repeats scattered along plant genomes acquired a promoter, resulting in the emergence of a proto MIR. Mutations (light green) of the proto MIR led to the generation of MIRs. (C) The transposon transposition model [7]. A full-length transposable element with terminal inverted repeats (TIRs) and a long open reading frame (light brown) gave rise to a miniature inverted-repeat transposable element (MITE) with TIRs and a short open reading frame. Mutations (light green) of the MITE led to the generation of MIRs. Green arrows indicate transcription direction.
The inverted gene duplication model assumed that plant pre-MIRs were originated by direct inverted duplication of a target gene, integration of a psuedogene-like sequence after reverse transcription, or juxtaposition of two closely related sequences from different members of a gene family (Figure 1A) [2]. This model was originally proposed based on the observation that Arabidopsis thaliana MIR161 and MIR163 precursor hairpin sequences showed extensive similarity to their target genes [2]. Later on, it was supported by the findings that some other MIR precursor sequences, such as MIR482, MIR824, MIR846 and MIR859, contained at least one arm with similarity or complementarity to target genes [3–5]. The spontaneous evolution model assumed that the precursors of evolutionarily young MIRs were originated from high density of small-to-medium sized fold-back sequences scattered throughout the plant genome (Figure 1B) [1,6]. Evidence supporting this model is that some evolutionarily young A. thaliana MIRs, including MIR774–MIR776, MIR779, MIR823, MIR830, MIR858, MIR864, MIR865 and MIR870, have no similarity to other regions of the A. thaliana genome [6]. The transposon transposition model proposed that the precursors of some plant MIRs were derived from transposable elements (Figure 1C) [7]. This model relied on the observation that a subset of A. thaliana and rice MIR candidates was collocated with miniature inverted-repeat transposable elements (MITEs) [7]. However, it could be debatable for these candidates to be bona fide MIRs [1]. Indeed, many of them, such as A. thaliana and rice MIR416, rice MIR420, MIR445a, MIR806b, MIR806g, MIR807b, MIR807c, MIR809h, MIR811a-c, MIR813, MIR819a, MIR819d, MIR819g, MIR819h and MIR819f, have been questioned or removed from miRBase (release 22, http://www.mirbase.org/index.shtml). In addition, a pre-existing MIR precursor might evolve into a novel one through co-evolution or perhaps punctuated jumps in sequence diversity [8]. For instance, MIR390 could evolve into MIR4376, which further evolved into MIR7122 [8].
Although three models have been proposed for de novo origination of plant pre-MIRs, detailed information for early evolutionary processes and direct evidence to support the models are still lacking. In addition, there are many issues remained to be addressed, such as the origin and early evolutionary processes of MIR gene promoters, the sequence of MIR origination and promoter activity gain, and the evolutionary force driving the origination and evolution of MIRs. One of the difficulties to precisely elucidate the origin and early evolutionary processes of plant MIRs is that the majority of them have a long evolutionary history. Much sequence information required for the birth of these MIRs has largely been lost as time goes on. Therefore, identification of very recently evolved MIRs is significant for elucidation of plant MIR origination, since the sequence features acquired during origination may be well-preserved in these MIRs.
Recently, three polyphenol oxidase (PPO) gene-targeting and lineage-specific young MIR families, termed MIR1444, MIR058 and MIR12112, have been reported in Populus, Vitis and Salvia, respectively [9–14]. MIR1444 is a Salicaceae-specific MIR widely existing in Salicaceae, including Populus, Salix and Idesia [9–14]. Mature miR1444 targets a subset of PPOs in a region encoding the conserved CuB domain [12]. Precursor sequence of MIR1444 exhibits extensive sequence similarity to the PPO targets and was proposed to originate from an ancient PPO gene before the Salicoid whole-genome duplication event, which happened 60 million years ago (Ma) before the divergence of Populus, Salix and Idesia [12,15]. No sequence similarity was observed for the other parts of MIR1444 and PPO genes. It indicates that much sequence information for tracing the early evolutionary processes of MIR1444 has been lost during its evolution for more than 60 million years. MIR058 is the secondly reported PPO-targeting MIR [13]. Its mature sequence was identified through the analysis of high-throughput small RNA sequencing data from grapevines [16]. Cleavage of miR058 on PPO transcripts in a region encoding the thylakoid transfer domain has been validated through degradome sequencing and RLM-RACE analysis [13,14,16]. The expression of miR058 and PPO exhibited negative correlation in various grapevine tissues [13]. These results suggest that MIR058 is a bona fide MIR, although it has not been deposited into miRBase [17]. The third PPO-targeting MIR, termed MIR12112 [14], was identified by analysis of high-throughput small RNA sequence and the whole genome sequence of Salvia miltiorrhiza, a well-known material of traditional Chinese medicine widely used for cardiovascular and cerebrovascular disease treatment and an emerging model system for genomic and genetic studies of medicinal plants [18–21]. This miRNA targets to 15 of the 19 identified SmPPOs in a region encoding the conserved KFDV domain [14]. Except for Vitis vinifera and S. miltiorrhiza, MIR058 and MIR12112 genes in other plant species have not been analysed.
In addition to evolutionarily young, there are multiple copies of copper (Cu)-response elements (CuREs) existing in the promoter of Populus trichocarpa MIR1444 genes [11]. These sequences could be important during the origination and evolution of MIRs. CuRE contains a core sequence of GTAC and was firstly identified in the promoters of cytochrome C6 (CYC6) and coprogen oxidase (CPX1) genes in the green alga Chlamydomonas reinhardtii [22,23]. Nowadays, people have learned that CuRE is a significant cis-element widely existing in promoters of Cu-responsive protein genes and plant MIRs, such as MIR397, MIR398, MIR408, and MIR1444 [11–24]. All of these MIRs regulate the expression of genes encoding Cu-binding proteins, such as laccases (LACs), Cu-Zn superoxide dismutases (CSDs), plastocyanin-like proteins (PCLs), and PPOs. The expression of these MIRs was negatively regulated by Cu, an essential mineral required for the healthy growth and development of plants [11,25–27]. Cu-associated suppression of MIRs was in company with the up-regulation of their target genes [11]. SQUAMOSA promoter binding protein-like 7 (SPL7) could bind to this element to function as a central regulator for Cu homeostasis in A. thaliana [28]. In P. trichocarpa, PtSPL3 and PtSPL4 could be the CuRE-binding proteins controlling Cu-responsive gene expression [11], whereas in green alga, the CuRE-bing protein is known as COPPER RESPONSE REGULATOR 1 (CRR1) [29]. Through the analysis of CRR1, a model was proposed for Cu sensing in green alga [29]. In this model, the SPB domain of CRR1 binds to CuRE and activates gene expression when Cu is deficient, whereas when Cu is sufficient, Cu binds to the SPB domain, resulting in CRR1 structure transformation and CuRE-binding capability loss [28–30]. These results suggest the significance of CuREs in plant response to Cu availability. However, there is no information about the origin and variation of CuREs in gene promoters. In addition, CuREs in the promoters of MIR058 and MIR12112 have not been investigated.
With long-term interests in plant miRNAs [9–12,14,31–33], particularly those targeting Cu-binding protein genes, I performed a systematic analysis of PPO-targeting and lineage-specific young MIRs to address various fundamental questions of their origination and evolution, such as how did the hairpin sequence originate, how did the promoter activity gain, what was the sequence of pre-MIR origination and promoter activity gain, and what could be the role of Cu in the origination and evolution of PPO-targeting MIRs? Integrative sequence analysis of genome and transcriptome data allowed me to identify a total of ten MIR058 precursors (pre-MIR058s), 79 MIR12112 precursors (pre-MIR12112s), and a genomic region termed AasPPO-as-hp, which contained a hairpin-forming sequence. Sequence comparison clearly showed that pre-MIR058s and the hairpin-forming sequence of AasPPO-as-hp originated in an ancient PPO gene through generation of short inverted repeats. Promoter sequence analysis showed that promoter activities were gained by insertion of a MITE sequence in the 3ʹ-flanking region of the ancient PPO genes before the origination of hairpin-forming sequences. Analysis of CuREs showed the existence of multiple CuREs in the promoters of MIR12112, MIR058 and MIR1444, with the number of CuREs varying significantly. It suggests frequent birth and death of CuREs in promoters of these MIRs and indicates the significance of Cu in the origination and evolution of PPO-targeting MIRs. The results reveal novel mechanistic insights into MIR origination and provide first-hand information for the acquirement of gene promoter activity.
Results
Identification of 10 pre-MIR058s in vitis
In order to elucidate the origin and early evolutionary processes of MIR058, integrative sequence analysis of genome and transcriptome data from Vitaceae was carried out (Table S1). A total of 10 pre-MIR058s were identified from V. vinifera, V. aestivalis, V. rotundifolia, V. pseudoreticulata, V. quinquangularis, V. amurensis, and V. riparia x V. rupestris (Figure 2), all of which are members of the Vitis genus. No pre-MIR058 was found in the genome and transcriptome data of other related genera in Vitaceae, such as Ampelocissus, the genus phylogenetically closest to Vitis [34]. It suggests that pre-MIR058s are specific to the Vitis genus. Ampelocissus and Vitis were split around 39.4 Ma in the late Eocene [34]. Pre-MIR058s could originate in a common ancestor of Vitis plants after that time. In addition, the Vitis genus includes two subgenera split around 37.3 Ma in the late Eocene [34]. Pre-MIR058s were identified from species of both subgenera, indicating the origination of pre-MIR058s before 37.3 Ma. Taken together, pre-MIR058s could originate around 37.3–39.4 Ma. It suggests that pre-MIR058s are evolutionarily very young. It offers an opportunity to trace the early evolutionary processes of pre-MIR058s based on direct observation of the changes in DNA sequences.
Figure 2.
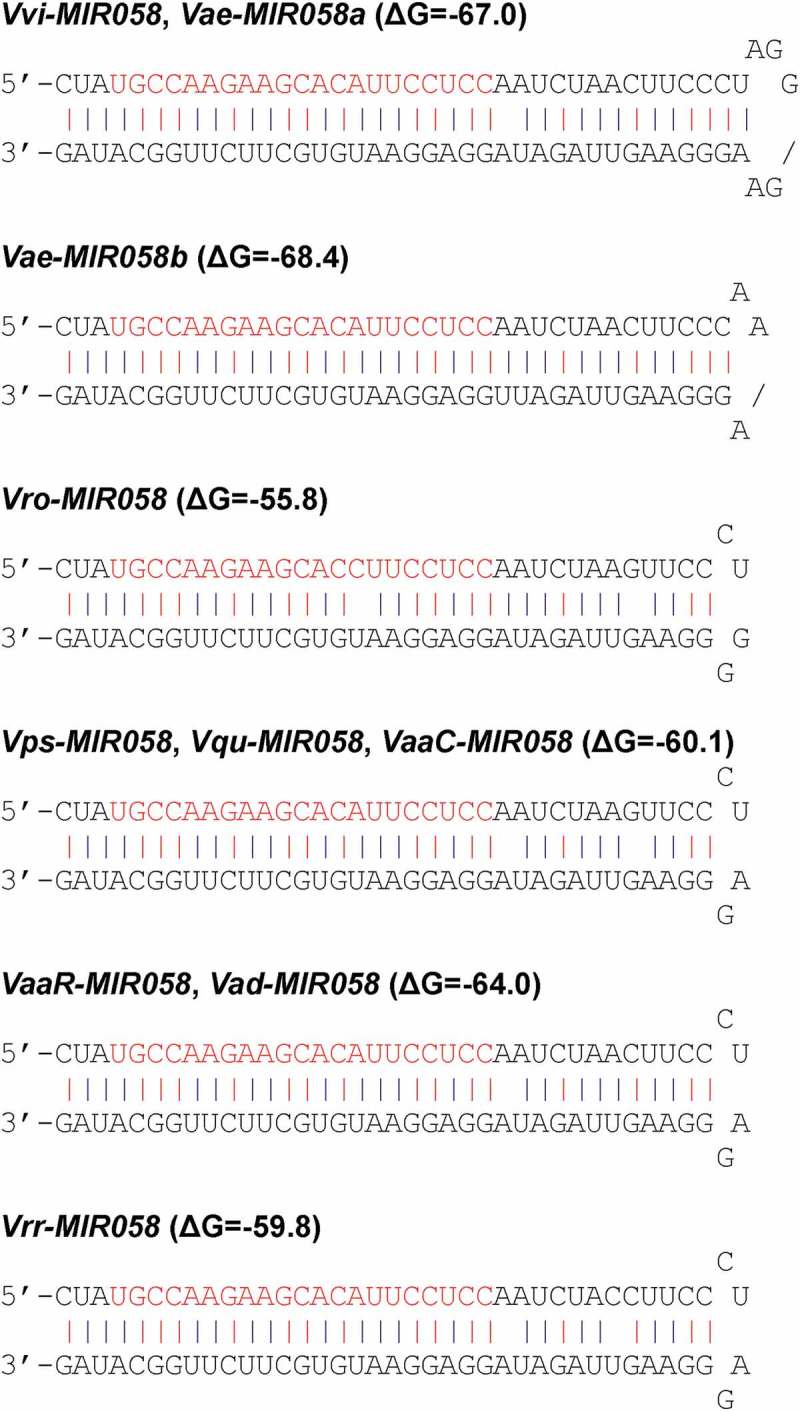
Hairpin structures of MIR058 precursors from Vitis. It includes V. vinifera (Vvi), V. aestivalis (Vae), V. rotundifolia (Vro), V. pseudoreticulata (Vps), V. quinquangularis (Vqu), V. amurensis var. amurensis from China (VaaC) and Russia (VaaR), V. amurensis var. dissecta (Vad), and V. riparia x V. rupestris (Vrr). Mature miRNA sequences are shown in red. Free energy of secondary structures calculated by the mfold program is shown as ΔG values in parentheses. Lower free energy indicates more stable secondary structure.
MIR058 genes share high similarity to the whole antisense strand of VviPPO1
In order to elucidate the origin and early evolutionary process of MIR058s, I compared the sequences of MIR058 genes and the target gene, VviPPO1 (Figure 3A). The results showed a high similarity between MIR058s and the whole antisense strand of open reading frames (ORFs) of VviPPO1. The similarity not only exists in the MIR058 precursor region but also in other parts of the gene, providing clear and convincing evidence for the origination of MIR058s from an ancient PPO. Analysis of the genomic scaffolds and the whole genome sequence of V. vinifera identified four VviPPOs, all of which are single exon genes [35]. Phylogenetic analysis of MIR058 genes and four VviPPOs showed that MIR058s had the highest similarity to the antisense strand of VviPPO1 (Figure 3B). It indicates that MIR058s and VviPPO1 share a common ancestor, or MIR058a were evolved from a copy of the ancient version of VviPPO1.
Figure 3.
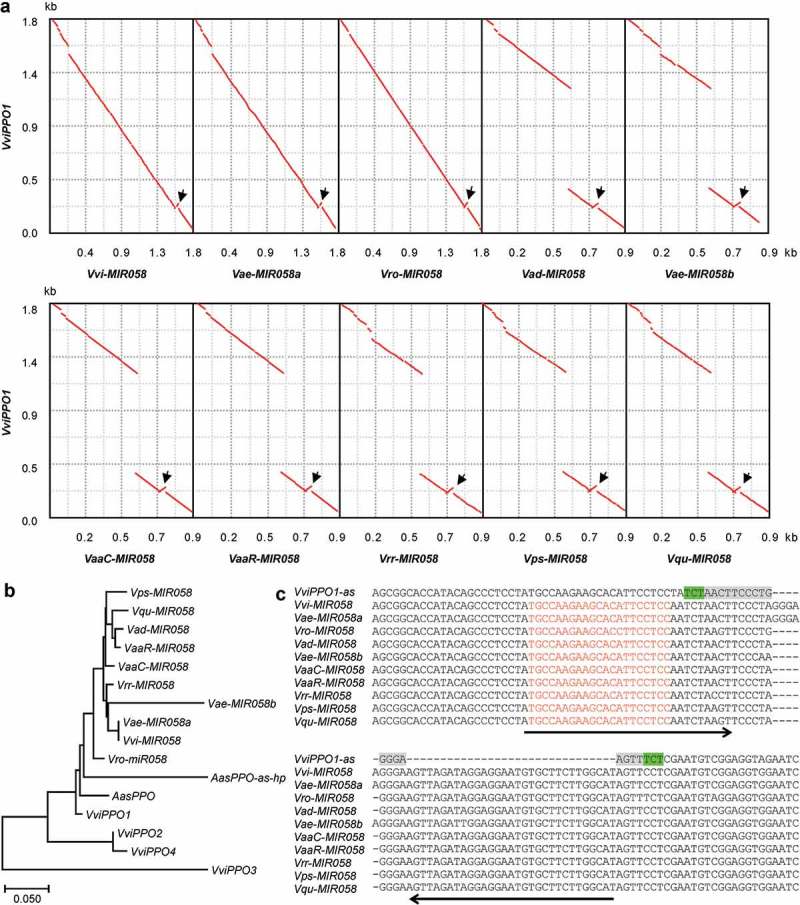
Formation of short IRs. (A) Dot-plots of VviPPO1 ORF and MIR058 sequences from V. vinifera (Vvi), V. aestivalis (Vae), V. rotundifolia (Vro), V. amurensis var. dissecta (Vad), V. amurensis var. amurensis from China (VaaC) and Russia (VaaR), V. riparia x V. rupestris (Vrr), V. pseudoreticulata (Vps), and V. quinquangularis (Vqu). The alignment and visualization were performed using zPicture (http://zpicture.dcode.org/). Arrows indicate the location of IRs formed. (B) Phylogenetic relationships of full-length MIR058s, AasPPO-as-hp, and the antisense strand of VviPPO ORFs and partial VasPPO ORF. (C) Alignment of MIR058 sequences surrounding the IRs and the corresponding antisense sequence of VviPPO1 (VviPPO1-as). Arrows indicate the IRs formed. The 18bp palindromic-like sequence (grey) flanked by 3bp DRs (green) is highlighted. Sequences for mature miR058 are shown in red.
Origination of pre-MIR058s through the generation of short inverted repeats in a 18bp palindromic-like sequence
One of the significant characteristics of MIRs is that the pri-MIR contains an imperfect hairpin structure termed MIRNA precursor (pre-MIR) [1]. To determine how the hairpin-forming sequence of MIR058s originated, multiple sequence alignment of pre-MIR058s and VviPPO1 was performed (Figure 3C). The results clearly showed that each of the pre-MIR058s has an inserted sequence with 31–36bp in length, which forms the 3ʹ-arm of MIR058 hairpin structure. The insertion is actually an inverted repeat (IR) of antisense VviPPO1 sequence that corresponds to the 5ʹ-arm of MIR058. It suggests that pre-MIR058s originated in a PPO through the generation of short IRs. Examination of the antisense strand of VviPPO1 surrounding the IR generation site showed that pre-MIR058s were generated in a 18bp palindromic-like sequence flanked by 3bp direct repeats (DRs) (Figure 3C), which could be important for of MIR058 origination.
Deletion and mutation of MIR058 gene sequences
MIRs are usually shorter than target genes and show low similarity to targets in regions other than pre-MIRs [2,12]. To investigate how it was caused, sequence comparison between MIR058 genes and the antisense strand of VviPPO1 ORF was conducted (Figure 3A). The results showed the existence of significant sequence variations in the 5ʹ- and 3ʹ-regions of MIR058 genes (Figure 3A). The variations were caused mainly by short-deletions and mutations. Additionally, a large DNA fragment with approximately 868bp in length was lost in V. aestivalis Vae-MIR058b and MIR058 genes from V. pseudoreticulata, V. quinquangularis, V. amurensis, and V. riparia x V. rupestris (Figure 3A). It suggests that, during the early evolutionary process of plant MIRs, large- and short-deletions and mutations may occur. It could be the reason that caused MIRs to be shorter than target genes and showed low similarity to targets in regions other than pre-MIRs.
Identification and characterization of AasPPO-as-hp originating from an ancient PPO gene
Integrative sequence analysis of genome and transcriptome data from Vitaceae showed that MIR058s were specific to the Vitis genus. Is there a hairpin sequence originating from a PPO in other Vitaceae species? To address this question, I analysed all of the illumina and 454 RNA-seq data available for Vitaceae plants (Table S1) (https://www.ncbi.nlm.nih.gov/sra). The results showed that, although the Vitaceae species Ampelocissus ascendiflora did not contain MIR058, it had a genomic region showing high similarity to the antisense strand of AasPPO gene and contained a hairpin-forming sequence (Figures 4A and 4B). This region is termed AasPPO-as-hp. Phylogenetic analysis and sequence alignment of AasPPO-as-hp and A. ascendiflora AAsPPO revealed that AasPPO-as-hp, similar to the case of MIR058, originated from an ancient PPO gene (Figures 3B and 4B).
Figure 4.
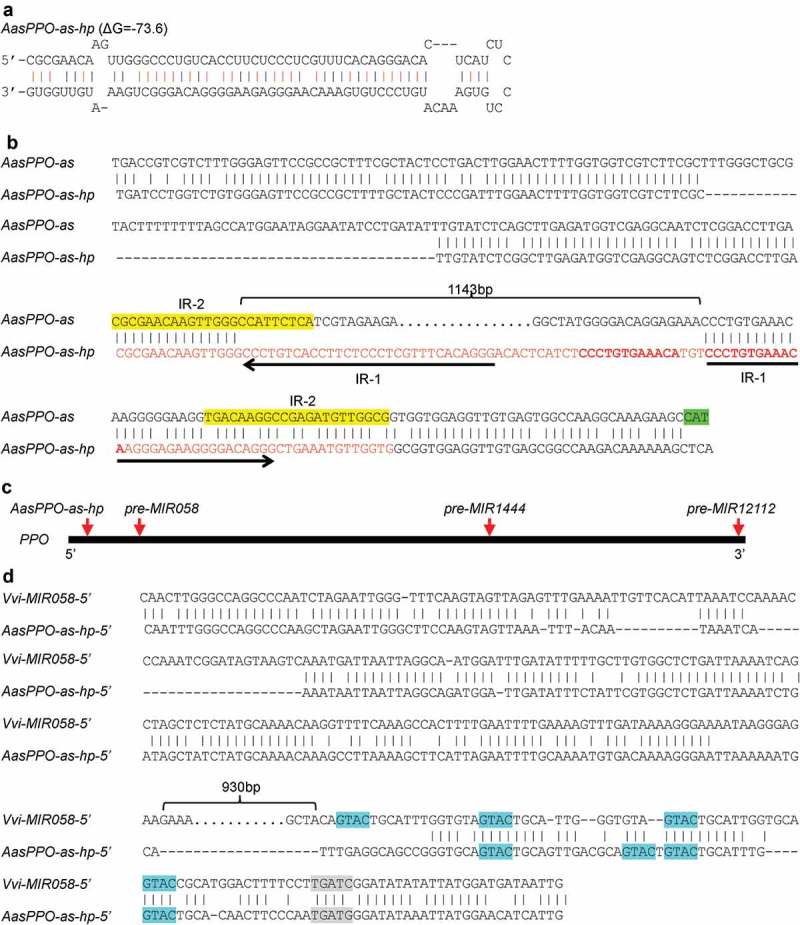
Analysis of AasPPO-as-hp from the Vitaceae species A. ascendiflora. (A) Hairpin structure of AasPPO-as-hp. (B) Sequence alignment of AasPPO-as-hp and the antisense sequence of A. ascendiflora AasPPO (AasPPO-as). Sequence being able to form hairpin structure is shown in red. Inverted repeats (IR-1) in the hairpin structure are shown by arrows. The 12bp direct repeats are shown in bold. Imperfect inverted repeats (IR-2) are highlighted in yellow. The antisense sequence of translational start site of AasPPO is highlighted in green. The 1,143bp sequence deleted in AasPPO-as-hp is indicated by an open brace. (C) Relative positions of AasPPO-as-hp, pre-MIR058, pre-MIR1444 and pre-MIR12112 originating in the open reading frame of ancient PPOs. Heavy dark line represents the open reading frame of ancient PPOs, with 5ʹ- and 3ʹ-ends shown. Red arrows show relative positions of AasPPO-as-hp, pre-MIR058, pre-miR1444 and pre-MIR12112. (D) Sequence alignment of the 5ʹ-flanking sequences of AasPPO-as-hp (AasPPO-as-hp-5ʹ) and Vvi-MIR058 (Vvi-MIR058–5ʹ). CuREs are highlighted in blue. The 930bp sequence deleted in AasPPO-as-hp-5ʹ is indicated by an open brace. The insertion site of CuRE region is highlighted in grey.
Sequence comparison showed that the generation of AasPPOas-hp hairpin sequence is more complicated than MIR058. It includes deletion of a 1,143bp fragment and insertion of a 55bp sequence at a site close to 5ʹ-end of the open reading frame of PPO (Figures 4B and 4C). The inserted sequence contains 30bp IR of antisense PPO sequence (termed IR-1 hereafter) and 12bp DR of partial that PPO sequence (Figure 4B). Examination of the antisense strand of AAsPPO sequence identified a pair of 22–23bp imperfect IRs (termed IR-2 hereafter), one of which located at the 5ʹ-junction of the deletion, whereas the other one located at the 3ʹ-region of the hairpin-forming sequence (Figure 4B). The identified IR-2 could be important for the origination of the hairpin-forming sequence of AasPPO-as-hp. A. ascendiflora is an endangered plant species native to the Malay Peninsula and Singapore [36]. The capability of AasPPO-as-hp to generate a miRNA remains to be elucidated.
Gain of promoter activity by insertion of a MITE sequence
Both MIR058s and AasPPO-as-hp are antisense to PPO genes. It indicates that their promoters did not evolve from PPO promoters. Consistently, examination of the 3ʹ- and 5ʹ-flanking sequences of MIR058s and VviPPO1 showed that no significant sequence similarity existed between the 3ʹ-flanking region of MIR058s and the antisense strand of the 5ʹ-flanking region of VviPPO1. It suggests the loss of PPO promoter activity in the 3ʹ-flanking region of MIR058s. In order to obtain information for a gain of MIR058 promoter activity, the 5ʹ-flanking regions were identified for MIR058s from V. vinifera, V. aestivalis, V. rotundifolia, V. amurensis var. dissecta, V. amurensis var. amurensis from China and Russia and V. riparia x V. rupestris. Sequence comparison showed high similarity between the 5ʹ-flanking region of MIR058s and the antisense strand of the 3ʹ-flanking region of VviPPO1 (Figure 5). It suggests that MIR058 promoters are derived from the 3ʹ-flanking region of a PPO. In addition to the conserved regions, I identified a region that existed in MIR058 promoters but not in the VviPPO1 promoter. This region is located at a site close to the corresponding stop codon of VviPPO1 and contains multiple CuREs with the core sequence of GTAC (Figure 5). The CuRE region is featured by small size (e.g. 275bp in length for Vvi-MIR058) and contains imperfect terminal inverted repeats (TIRs) and an internal AT-rich sequence (Figure 5). The insertion of this region in the 3ʹ-flanking region of PPO generates 5bp target site duplications (TSDs) (Figure 5). Small size, TIR, internal AT-rich region and TSD are characteristics of MITE transposons [37]. It indicates that the gain of CuRE region is a result of MITE transposon insertion. The CuRE region was inserted in the upstream of the AT-rich TATA-box-like sequence. An inverted CAAT-box sequence was found at the 3ʹ-end of the region (Figure 5). The capture of a multiple-CuRE-containing region in the early stage of MIR058 origination indicates the importance of Cu in the origination and evolution of Cu-responsive MIRs.
Figure 5.

Gain of the CuRE regions. The 3ʹ-flanking sequence of VviPPO1 (antisense strand, VviPPO1-t-as) and the 5ʹ-flanking sequences of MIR058s from V. vinifera (Vvi), V. aestivalis (Vae), V. rotundifolia (Vro), V. amurensis var. dissecta (Vad), V. amurensis var. amurensis from China (VaaC) and Russia (VaaR) and V. riparia x V. rupestris (Vrr) were aligned. The sequence of VviPPO1-t-as is shown in blue. CuREs are highlighted in pale blue. The antisense sequence of the stop codon of VviPPO1 is highlighted in green. The 5bp TSDs are highlighted in grey. Imperfect TIRs of the inserted region are underlined. Internal TA-rich sequences of the inserted region are shown in a red box. The inverted CAAT-box sequence is highlighted in red. The AT-rich TATA-box-like region is highlighted in pink.
Promoter gain first, then pre-MIR058 and AasPPO-as-hp origination
I next asked the sequence of pre-MIR origination and promoter activity gain. To address this question, the 5ʹ-flanking sequence of AasPPO-as-hp was identified and investigated. Sequence comparison showed that, similar to MIR058, the 5ʹ-flanking sequence of AasPPO-as-hp contained a CuRE region (Figure 4D). This region includes four CuREs and shows high sequence similarity to parts of the CuRE region existing in MIR058 promoters (Figures 4D and 5). Besides the CuRE region, high sequence similarity of MIR058 and AasPPO-as-hp was also observed in the upstream of the CuRE region (Figure 4D). The existence of a common insertion sequence in the promoters of MIR058 and AasPPO-as-hp indicates that this sequence was gained before the separation of Vitis and Ampelocissus plants. On the other hand, pre-MIR058s and the hairpin-forming sequences of AasPPO-as-hp are totally different, and the hairpin sequences of AasPP0-as-hp and MIR058 were generated at different positions of the PPO gene (Figure 4C). The most rational explanation is that pre-MIR058s and AasPPO-as-hp originated after the separation of Vitis and Ampelocissus. Thus, the gain of the CuRE-containing sequence appears to occur before the origination of pre-MIR058s and the hairpin-forming sequences of AasPPO-as-hp. However, the possibility cannot be ruled out that pre-MIR058s and AasPPO-as-hp coexisted in an ancestor of Vitis and Ampelocissus, and after separation, pre-MIR058s was lost in Ampelocissus and AasPPO-as-hp was lost in Vitis. Even so, the origination of pre-MIR058s and AasPPO-as-hp in the ancestor could also happen after the gain of the CuRE-containing sequence.
In addition to the conserved region, sequence comparison of the 5ʹ-flanking regions of MIR058 and AasPPO-as-hp showed that a 930bp DNA fragment was lost in the 5ʹ-flanking region of AasPPO-as-hp (Figure 4D). The loss of this fragment could happen in Ampelocissus after the separation of Vitis and Ampelocissus.
Origination and evolution of MIR1444 and MIR12112 genes
It has been shown that MIR1444s widely exist in Populus, Salix and Idesia [9–12]. Populus and Idesia have two MIR1444 genes, termed MIR1444a and MIR1444b, respectively. Expansion of MIR1444 genes in Populus and Idesia was through the Salicoid whole-genome duplication event [12,15]. Salix only contains MIR1444b. MIR1444a was lost in Salix through DNA segment deletion probably during chromosome rearrangements [12]. Pre-MIR1444s have extensive sequence similarity to PPO targets [12]. However, differing from MIR058s and AasPPO-as-hp, MIR1444s showed no conservation with PPO genes in DNA sequence other than the pre-MIR1444 regions. It is consistent with the origination time of pre-MIR1444s and pre-MIR058s. Pre-MIR1444s originated in an ancient PPO gene before the Salicoid whole-genome duplication event happening 60 Ma [12,15], whereas pre-MIR058 originated in an ancient PPO gene around 37.3–39.4 Ma [34]. The results suggest that other parts of a MIR underwent less selective pressure than the pre-MIR part of the MIR. During origination and evolution of a MIR, the sequence other than pre-MIR may be altered significantly.
MIR12112 is another PPO-targeting MIR. It was only reported in S. miltiorrhiza [14]. In order to know the overall situation of MIR12112 in plants, whole genome sequences and transcriptome data of S. miltiorrhiza and related plant species were systematically analysed. A total of 79 pre-MIR12112s were identified from species of the families Acanthaceae, Lentibulariaceae, Oleaceae, Orobanchaceae, Paulowniaceae, Pedaliaceae, Plantaginaceae, Verbenaceae, and Lamiaceae (Figure S1). All of these plant families belong to the order Lamiales. It suggests that MIR12112s are Lamiales-specific and relatively old MIRs. The number of MIR12112 genes in a plant species varied significantly from 1 (e.g. species of the families Acanthaceae, Lentibulariaceae, Orobanchaceae and Paulowniaceae) to 6 (Olea europaea cv. farga), suggesting significant expansion of the MIR12112 family in some plant species. The previous study had identified a MIR12112 in S. miltiorrhiza [14]. In this study, two Smi-MIR12112s were identified. Thus, the previously reported Smi-MIR12112 [14] is renamed Smi-MIR12112a, and the newly identified one is named Smi-MIR12112b.
Extensive gene number variation of MIR12112 in Lamiales species could also result from genome duplication and DNA deletion events as the case of MIR1444 [12]. A total of three Sin-MIR12112 genes exist in Sesamum indicum (Figure S1). Examining the locations of Sin-MIR12112s in the genome assembly showed that Sin-MIR12112s were located at two homologous regions resulted from the sesame lineage-specific whole genome duplication event (Figure 6A) [38]. Sin-MIR12112a was located on LG6, whereas Sin-MIR12112b and Sin-MIR12112c were located on LG14. In addition to Sin-MIR12112a, a partial sequence of Sin-MIR12112 was identified at a region close to Sin-MIR12112a (Figure 6A). The partial sequence contains partial mature miR12112 sequence and an intact miR12112* (data not shown). Similarly, there are three OeuS-MIR12112s in the O. europaea var. sylvestris genome assembly (Figure S1) [39]. OeuS-MIR12112a and OeuS-MIR12112b were located on pseudochromosome 6 at a region with homology to a pseudochromosome 21 region, where OeuS-MIR12112c was located [39]. From the draft genome of O. europaea cv. farga [40], a total of six OeuF-MIR12112s were identified (Figure S1). The reasons causing the increase of OeuF-MIR12112 gene numbers remain to be elucidated. One possibility could be assemble errors resulted from genome heterozygosity.
Figure 6.
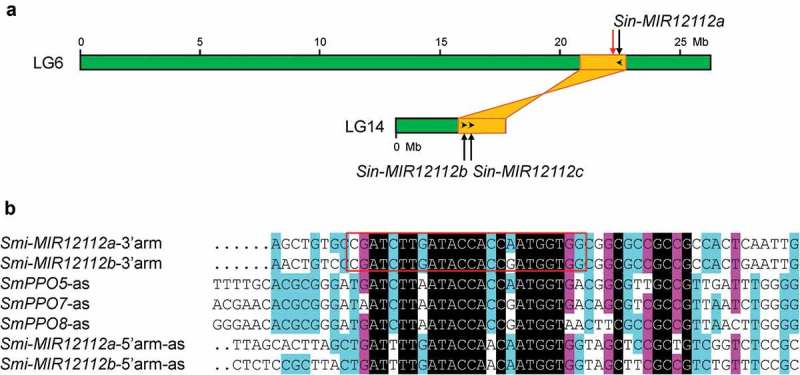
Origination and evolution of MIR12112 genes. (A) Locations of Sin-MIR12112s (black arrows) and a partial sequence of Sin-MIR12112 (red arrow) on pseudomolecules (LG6 and LG14, green boxes) of the S. indicum genome assembly. The schematic diagram of two homologous genome blocks (yellow) was adapted from Wang et al [47] . Transcription direction of Sin-MIR12112s is shown by black arrowheads. (B) Multiple sequence alignment of S. miltiorrhiza Smi-MIR12112 precursors and SmPPOs. The sense orientation of Smi-miR12112a and Smi-miR12112b foldback arms, the antisense strand of Smi-miR12112a* and Smi-miR12112b* foldback arms, and parts of the antisense strand of Smi-miR12112-targeted SmPPO5, SmPPO7 and SmPPO8 are shown. The red box indicates mature Smi-miR12112a and Smi-miR12112b sequences.
Sequence comparison showed extensive sequence similarity between the stem portions of Smi-MIR12112 precursors and partial of the antisense strand of SmPPO targets (Figure 6B). It indicated that MIR12112 also originated from an ancient PPO gene as MIR058, AasPPO-as-hp and MIR1444 did. The location of MIR12112 precursors corresponds to 3ʹ-end of the open reading frame of PPO (Figure 4C). Because of the relatively long evolutionary history of MIR12112, sequence information showing the birth of MIR12112 genes from a PPO has been lost from the up- and down-streams of pre-MIR12112s. The loop regions of MIR12112 precursors vary significantly with frequent sequence insertion, deletion and mutation occurred (Figure S1). The results further suggested that the up- and down-streams, and the loop regions of pre-MIR12112 underwent less evolutionary constraints than the stem regions.
Frequent birth and death of CuREs in promoters of MIR12112, MIR1444 and MIR058
The existence of multiple CuREs in promoters of Cu-responsive MIRs, such as A. thaliana and P. trichocarpa MIR397, MIR398 and MIR408 and P. trichocarpa MIR1444, is important for their response to Cu availability [11,24–27]. In this study, I found that CuREs in MIR058 and AasPPOas-hp promoters were gained by MITE transposon insertion (Figures 4D and 5). In order to obtain an overview of the origin and variation of CuREs in promoters of other PPO-targeting MIRs, I systematically investigated MIR12112 and MIR1444 gene promoters. A total of 34 MIR12112 gene promoters from 19 plant species of the order Lamiales and six MIR1444 gene promoters from P. trichocarpa, P. Euphratica, Salix purpurea and S. Suchowensis were identified (Figure 7). Sequence comparison showed the existence of CuREs in all of the MIR12112 and MIR1444 gene promoters investigated. The number of CuREs in MIR12112 gene promoters varies significantly from 1 to 9 (Figure 7A). Changes of CuRE number were also observed in MIR1444 promoters (Figure 7B). Taken together, the results from the investigation of MIR12112 (Figure 7A), MIR1444 (Figure 7B) and MIR058 (Figure 4) gene promoters suggest frequent birth and death of CuREs. However, I am not able to trace the source of the CuRE region in promoters of MIR12112 and MIR1444, since sequence information for the origin of CuREs in these MIR promoters has been lost. It is consistent with that MIR12112 and MIR1444 have a relatively long evolutionary history than MIR058 and AasPPOas-hp. Even though the promoter sequence has changed significantly in different plant species, the existence of CuREs in promoters of PPO-targeting MIRs suggests the importance of CuREs for PPO-targeting MIRs.
Figure 7.

Distribution of CuREs in the 5ʹ-flanking region of MIR12112 and MIR1444 genes. (A) Distribution of CuREs in the 5ʹ-flanking region of MIR12112 from S. miltiorrhiza (Smi), G. nigrocaulis (Gni), G. aurea (Gau), Forsythia viridissima (Fvi), F. excelsior (Fex), O. europaea cv. sylvestris (OeuS), O. europaea cv. farga (OeuF), Aeginetia indica (Ain), Phtheirospermum japonicum (Pja), Orobanche minor (Omi), O. cernua (Oce), Conopholis americana (Cam), Paulownia fortunei (Pfo), P. tomentosa (Pto), S. indicum (Sin), Antirrhinum majus (Ami), Plantago ovata (Pov), M. longifolia (Mlo), Pogostemon cablin (Pca), and Ocimum tenuiflorum (Ote). (B) Distribution of CuREs in the 5ʹ-flanking region of MIR1444 from P. trichocarpa (Ptr), P. euphratica (Peu), S. purpurea (Spu), and S. Suchowensis (Ssu). CuREs are indicated by pale blue vertical lines. The locations of mature miRNA sequence are shown by red boxes. The locations of miRNA* are indicated by blue boxes.
Discussion
A novel model for plant MIR origination and evolution
Although three models, including the inverted gene duplication [2,3], spontaneous evolution [6] and transposon transposition [7], have been proposed for the origination of plant MIR (Figure 1), there are many issues remained to be addressed, such as direct evidence for origin and early evolutionary process of hairpin sequences and promoters, the sequence of MIR origination and promoter activity gain, and the evolutionary force driven the origination and evolution of MIRs. Through systematic analysis of PPO-targeting MIRs, I propose, in this study, a novel model for de novo origination and evolution of plant MIRs (Figure 8). This model shows that at least some plant MIRs originated from an ancient version of targets through the generation of hairpin sequence in the ancient genes. I termed this model the short inverted repeat generation model. Although the exact mechanism of the generation of hairpin-forming sequence remains to be elucidated, a replication error occurring at pre-existing short inverted repeats in genomic sequences, known as Origin-Dependent Inverted-Repeat Amplification (ODIRA), was proposed for the formation of palindromic amplicons [41,42]. The hairpin-forming sequence in a MIR could be generated in an ancient target gene through a mechanism similar to ODIRA. Palindromic-like sequences (the 18-bp sequence in the case of MIR058 precursor, Figure 3C) and imperfect IRs (the 22–23bp IR-2 in the case of AasPPO-as-hp hairpin sequence, Figure 4B) in target genes might contribute to initiate the generation of hairpin sequences by causing errors during DNA duplication. In addition to the generation of hairpin sequence, I found that the promoter activity of MIR058 and AasPPO-as-hp could be gained by insertion of a MITE sequence in the 3ʹ-untranslated region of an ancient PPO gene. The MITE sequence contains significant cis-elements, such as CAAT-box and CuREs, and the insertion occurred in the upstream of the AT-rich TATA-box-like sequence before the generation of hairpin sequence (Figure 8). After the insertion of MITE and the generation of hairpin sequence, sequence deletion, insertion and mutation could occur during the evolution of a MIR (Figure 8).
Figure 8.
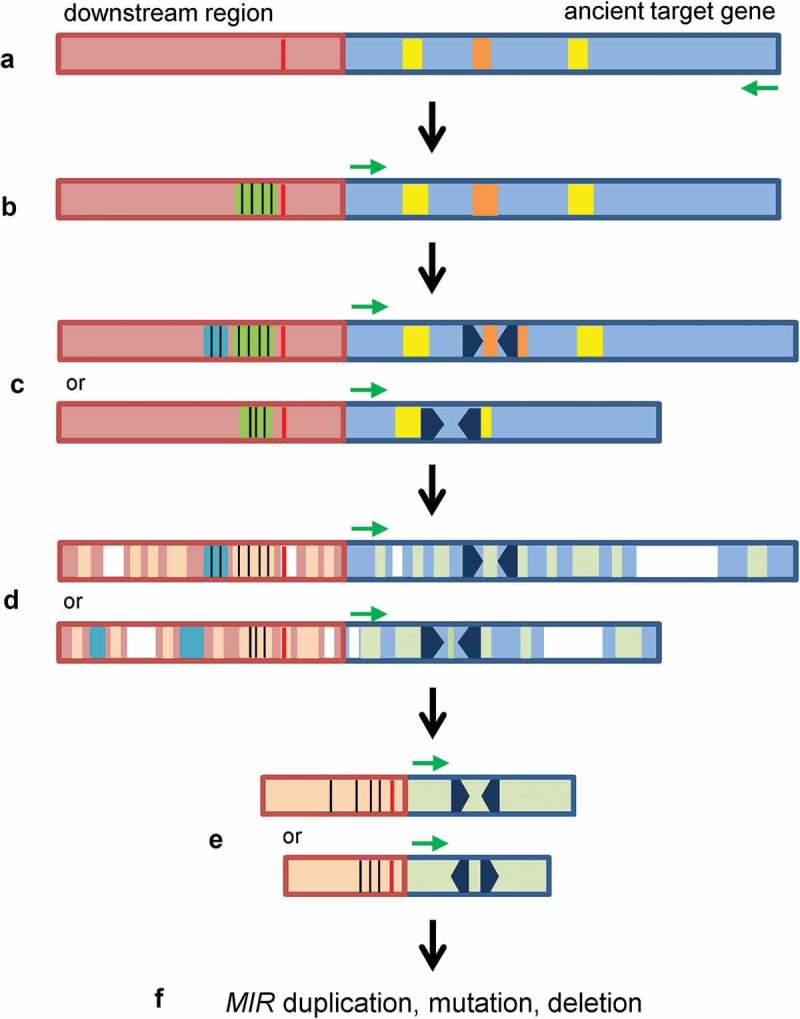
The short inverted repeat generation model for de novo origination and evolution of plant MIRs. (A) An ancient target gene (light blue) with palindromic-like (brown) and/or imperfect IR (yellow) sequences. Transcription direction is indicated by a green arrow. AT-rich TATA-box-like sequence is shown in red. (B) A MITE sequence (green) with significant cis-elements (black vertical lines), such as CAAT-box and CuREs, was inserted in the flanking region of the ancient target gene or a copy of the duplicated target gene. The insertion of MITE and subsequent evolution resulted in the gain of promoter activity. (C) Short inverted repeats (black arrowheads) were generated in the palindromic-like or imperfect IR sequences. Cis-elements could be dynamically changed (blue) in the promoters. (D) Deletion (white), insertion (blue) and mutation (light brown and light green) occurred during evolution. (E) Evolved MIRs. (F) The evolved MIRs could expand through duplication, diversify by mutation or lose by deletion during plant evolution.
Similarity and difference between the short inverted repeat generation model and the inverted gene duplication model
Both of the short inverted repeat generation model and the inverted gene duplication model show that MIRs were originated from target genes. The short inverted repeat generation model emphasizes that a MIR originated in an ancient target by generation of hairpin sequence or short inverted repeats (Figure 8), whereas the inverted gene duplication model suggests that a MIR originated by direct inverted duplication of a target gene, integration of a pseudogene-like sequence after reverse transcription, or juxtaposition of two closely related sequences from different members of a gene family (Figure 1) [1,2,43]. Generation of short inverted repeat in a target gene was proved by the results from MIR058 and AasPPO-as-hp. Solid evidence to show the origination of MIRs through the three ways mentioned in the inverted gene duplication model is still lacking, and there is not completely satisfactory explanation for how the long hairpin sequence generated through inverted gene duplication become short hairpin sequence during MIR evolution. Through this study, I cannot rule out the possibility that some plant MIRs were originated by inverted gene duplication. In addition to the generation of hairpin sequence, the short inverted repeat generation model includes the mechanism of promoter activity gain and the sequence of pre-MIR generation and promoter activity gain, which are not mentioned in the inverted gene duplication model and other two models [2,6,7].
The short inverted repeat generation model was proposed based on the results from PPO-targeting MIRs, particularly MIR058 and AasPPO-as-hp. It is very likely that this model can be extended to some other evolutionarily young MIRs with extensive similarity or complementarity to target genes, and some old MIRs losing similarity to target genes after long-term evolution. However, due to the long evolutionary history of most plant MIRs, it is very difficult to precisely elucidate the origin and early evolutionary process of each MIR and to calculate the percentage of MIRs to be explained by each proposed model in a plant species. From this point of view, short inverted repeat generation, inverted gene duplication, spontaneous evolution and transposon transposition are four possible ways for plant MIR origination.
Significance of Cu in the origination and evolution of Cu-responsive MIRs
Cu is an essential micronutrient for plant growth and development. Maintenance of Cu at appropriate concentration in plant cells is important. Cu deficiency may cause abnormal growth, whereas Cu excess is toxic [11]. In order to adapt to the changes of Cu availability in environments, plants have evolved a vital regulatory network in Cu uptake, distribution and molecular responses [11,44,45]. Cu-responsive miRNAs, such as miR397, miR398, miR408 and miR1444, are core components of this network. They are involved in the maintenance of Cu homeostasis in plant cells through regulating the expression of Cu-binding protein genes [11,25–27]. The promoters of these MIRNAs genes usually contain multiple CuREs, which sense Cu availability through SPL transcription factors [28–30]. In this study, I found that CuREs were captured by insertion of a CuRE-containing MITE sequence in the early stage of MIR origination and maintained by frequent birth and death during MIR evolution. The results indicate that Cu could be a significant force in driving the origination and evolution of MIRs targeting Cu-binding protein genes.
Materials and methods
Identification of MIR058 and AasPPO-as-hp sequences
Vvi-MIR058 sequence was identified by blast analysis of Vvi-MIR058 precursor against the whole genome sequence of V. vinifera using BLASTn [13,35,46]. MIR058 sequences in other Vitis species were identified by analysis of Vvi-MIR058 against next-generation sequence (NGS) data using BLASTn with word size set to be 7 [46]. Extension of partial MIR058 sequences was performed through blast analysis against NGS data from the species. MIR058 sequences identified from different species were cross-checked. CuRE region of A. ascendiflora AasPPO-as-hp was identified by analysis of the CuRE region of Vvi-MIR058 promoter against A. ascendiflora NGS data using BLASTn with word size set to be 7 [46]. Full-length A. ascendiflora AasPPO-as-hp was obtained by extension of the CuRE sequence through blast analysis against the NGS data. AasPPO sequence was identified by analysis of VviPPO1 against A. ascendiflora NGS data. Extension of partial AasPPO sequences was performed through blast analysis against NGS data from A. ascendiflora. The SRA accession numbers of illumina and 454 RNA-seq data used for MIR058, AasPPO-as-hp and AasPPO identification are listed in Table S1.
Identification of MIR12112 sequences
Smi-MIR12112b sequence was identified by analysis of S. miltiorrhiza Smi-MIR12112a sequence against the whole genome sequence of S. miltiorrhiza using BLASTn [14,46,47]. MIR12112 sequences in Genlisea aurea, Fraxinus excelsior, O. europaea, S. indicum and Mentha longifolia was identified by analysis of known MIR12112 sequences against their whole genome sequence and the NGS data listed in Table S2 [38–40,46,48–50]. MIR12112 sequences from plant species without the whole genome sequence available was identified and extended through analysis of known MIR12112 sequences against NGS data using BLASTn with word size set to be 7 [46]. The SRA accession numbers of illumina and 454 RNA-seq data used for MIR12112 identification are listed in Table S2.
Identification of the 5ʹ-flanking sequence of MIR1444s
The 5ʹ-flanking sequences of MIR1444s were identified through analysis of MIR1444 precursors against the current genome assemblies of P. trichocarpa (v3.0), P. euphratica (v1.0), S. purpurea (v1.0, https://phytozome.jgi.doe.gov/pz/portal.html) and S. suchowensis (v1.0) using BLASTn [12,15,46,51,52].
Bioinformatics analysis and phylogenetic tree construction
Multiple sequence alignment was carried out using DNAMAN and M-Coffee [53]. The alignment and visualization of MIR058s and VviPPO1 were performed using zPicture (http://zpicture.dcode.org/) [54]. The phylogenetic tree was constructed using MEGA7.0 by the neighbour-joining (NJ) method with 1000 bootstrap replicates [55].
RNA secondary structure prediction
Secondary structures of RNA sequence were predicted by the mfold program using the default parameters [56]. In each case, only the lowest energy structure was selected as described previously [33].
Funding Statement
This work was supported by the National Key Research and Development Program of China (grant number 2016YFD0600104), the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2016-I2M-3–016), and the National Natural Science Foundation of China (grant numbers 31570667, 81773836).
Accession numbers
Accession numbers for sequence data analysed are listed in Tables S1 and S2.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary materials
Supplementary materials for the article can be accessed here.
References
- [1].Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. [DOI] [PubMed] [Google Scholar]
- [2].Allen E, Xie Z, Gustafson AM, et al. Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nat Genet. 2004;36:1282–1290. [DOI] [PubMed] [Google Scholar]
- [3].Fahlgren N, Howell MD, Kasschau KD, et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One. 2007;2:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xia R, Xu J, Arikit S, et al. Extensive families of miRNAs and PHAS loci in Norway spruce demonstrate the origins of complex phasiRNA networks in seed plants. Mol Biol Evol. 2015;32:2905–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Xia R, Kuang H, et al. The diversification of plant NBS-LRR defense genes directs the evolution of microRNAs that target them. Mol Biol Evol. 2016;33:2692–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Felippes FF, Schneeberger K, Dezulian T, et al. Evolution of Arabidopsis thaliana microRNAs from random sequences. RNA. 2008;14:2455–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Piriyapongsa J, Jordan IK. Dual coding of siRNAs and miRNAs by plant transposable elements. RNA. 2008;14:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xia R, Meyers BC, Liu Z, et al. MicroRNA superfamilies descended from miR390 and their roles in secondary small interfering RNA biogenesis in eudicots. Plant Cell. 2013;25:1555–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu S, Sun YH, Chiang VL. Stress-responsive microRNAs in Populus. Plant J. 2008;55:131–151. [DOI] [PubMed] [Google Scholar]
- [10].Lu S, Sun YH, Chiang VL. Adenylation of plant miRNAs. Nucleic Acids Res. 2009;37:1878–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu S, Yang C, Chiang VL. Conservation and diversity of microRNA associated copper-regulatory networks in Populus trichocarpa. J Integr Plant Biol. 2011;53:879–891. [DOI] [PubMed] [Google Scholar]
- [12].Wang M, Li C, Lu S. Origin and evolution of MIR1444 genes in Salicaceae. Sci Rep. 2017;7:39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ren G, Wang B, Zhu X, et al. Cloning, expression, and characterization of miR058 and its target PPO, during the development of grapevine berry stone. Gene. 2014;548:166–173. [DOI] [PubMed] [Google Scholar]
- [14].Li C, Li D, Li J, et al. Characterization of the polyphenol oxidase gene family reveals a novel microRNA involved in posttranscriptional regulation of PPOs in Salvia miltiorrhiza. Sci Rep. 2017;7:44622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tuskan GA, Difazio S, Jansson S, et al. The genome of black cottonwood Populus trichocarpa (Torr. & Gray). Science. 2006;313:1596–1604. [DOI] [PubMed] [Google Scholar]
- [16].Wang C, Leng X, Zhang Y, et al. Transcriptome-wide analysis of dynamic variations in regulation modes of grapevine microRNAs on their target genes during grapevine development. Plant Mol Biol. 2014;84:269–285. [DOI] [PubMed] [Google Scholar]
- [17].Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(Database issue):D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ma Y, Yuan L, Wu B, et al. Genome-wide identification and characterization of novel genes involved in terpenoid biosynthesis in Salvia miltiorrhiza. J Exp Bot. 2012;63:2809–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu M, Lu S. Plastoquinone and ubiquinone in plants: biosynthesis, physiological function and metabolic engineering. Front Plant Sci. 2016;7:1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deng Y, Lu S. Biosynthesis and regulation of phenylpropanoids in plants. Crit Rev Plant Sci. 2017;36:257–290. [Google Scholar]
- [21].Zhang L, Lu S. Overview of medicinally important diterpenoids derived from plastids. Mini-Rev Med Chem. 2017;17:988–1001. [DOI] [PubMed] [Google Scholar]
- [22].Quinn JM, Merchant S. Two copper-responsive elements associated with the Chlamydomonas Cyc6 gene function as targets for transcriptional activators. Plant Cell. 1995;7:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Quinn JM, Barraco P, Eriksson M, et al. Coordinate copper- and oxygen-responsive Cyc6 and Cpx1 expression in Chlamydomonas is mediated by the same element. J Biol Chem. 2000;275:6080–6089. [DOI] [PubMed] [Google Scholar]
- [24].Nagae M, Nakata M, Takahashi Y. Identification of negative cis-acting elements in response to copper in the chloroplastic iron superoxide dismutase gene of the moss Barbula unguiculata. Plant Physiol. 2008;146:1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sunkar R, Kapoor A, Zhu JK. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell. 2006;18:2051–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yamasaki H, Abdel-Ghany SE, Cohu CM, et al. Regulation of copper homeostasis by micro-RNA in Arabidopsis. J Biol Chem. 2007;282:16369–16378. [DOI] [PubMed] [Google Scholar]
- [27].Abdel-Ghany SE, Pilon M. MicroRNA-mediated systemic downregulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem. 2008;283:15932–15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yamasaki H, Hayashi M, Fukazawa M, et al. SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell. 2009;21:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kropat J, Tottey S, Birkenbihl RP, et al. A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. Proc Natl Acad Sci USA. 2005;102:18730–18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sommer F, Kropat J, Malasarn D, et al. The CRR1 nutritional copper sensor in Chlamydomonas contains two distinct metal-responsive domains. Plant Cell. 2010;22:4098–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lu S, Li Q, Wei H, et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc Natl Acad Sci USA. 2013;110:10848–10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu S, Sun YH, Amerson H, et al. MicroRNAs in loblolly pine (Pinus taeda L.) and their association with fusiform rust gall development. Plant J. 2007;51:1077–1098. [DOI] [PubMed] [Google Scholar]
- [33].Lu S, Sun YH, Shi R, et al. Novel and mechanical stress-responsive microRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell. 2005;17:2186–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu XQ, Ickert-Bond SM, Nie ZL, et al. Phylogeny of the Ampelocissus-Vitis clade in vitaceae supports the new world origin of the grape genus. Mol Phylogenet Evol. 2016;95:217–228. [DOI] [PubMed] [Google Scholar]
- [35].Jaillon O, Aury JM, Noel B, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449:463–467. [DOI] [PubMed] [Google Scholar]
- [36].Yeo CK, Ang WF, Lok AFSL, et al. The conservation status of Ampelocissus planch. (Vitaceae) of Singapore, with a special not on Ampelocissus ascendiflora latiff. Nat Singapore. 2013;6:45–53. [Google Scholar]
- [37].Feschotte C, Jiang N, Wessler SR. Plant transposable elements: where genetics meets genomics. Nat Rev Genet. 2002;3:329–341. [DOI] [PubMed] [Google Scholar]
- [38].Wang L, Yu S, Tong C, et al. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014;15:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Unver T, Wu Z, Sterck L, et al. Genome of wild olive and the evolution of oil biosynthesis. Proc Natl Acad Sci U S A. 2017;114:E9413–9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cruz F, Julca I, Gómez-Garrido J, et al. Genome sequence of the olive tree, Olea europaea. Gigascience. 2016;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brewer BJ, Payen C, Raghuraman MK, et al. Origin-dependent inverted-repeat amplification: a replication-based model for generating palindromic amplicons. PLoS Genet. 2011;7:e1002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brewer BJ, Payen C, Di Rienzi SC, et al. Origin-dependent inverted-repeat amplification: tests of a model for inverted DNA amplification. PLoS Genet. 2015;11:e1005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cui J, You C, Chen X. The evolution of microRNAs in plants. Curr Opin Plant Biol. 2016;35:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta. 2006;1763:595–608. [DOI] [PubMed] [Google Scholar]
- [45].Puig S, Andres-Colas N, Garcia-Molina A, et al. Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ. 2007;30:271–290. [DOI] [PubMed] [Google Scholar]
- [46].Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xu H, Song J, Luo H, et al. Analysis of the genome sequence of the medicinal plant Salvia miltiorrhiza. Mol Plant. 2016;9:949–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Leushkin EV, Sutormin RA, Nabieva ER, et al. The miniature genome of a carnivorous plant Genlisea aurea contains a low number of genes and short non-coding sequences. BMC Genomics. 2013;14:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sollars ES, Harper AL, Kelly LJ, et al. Genome sequence and genetic diversity of European ash trees. Nature. 2017;541:212–216. [DOI] [PubMed] [Google Scholar]
- [50].Vining KJ, Johnson SR, Ahkami A, et al. Draft genome sequence of Mentha longifolia and development of resources for mint cultivar improvement. Mol Plant. 2017;10:323–339. [DOI] [PubMed] [Google Scholar]
- [51].Ma T, Wang J, Zhou G, et al. Genomic insights into salt adaptation in a desert poplar. Nat Commun. 2013;4:2797. [DOI] [PubMed] [Google Scholar]
- [52].Dai X, Hu Q, Cai Q, et al. The willow genome and divergent evolution from poplar after the common genome duplication. Cell Res. 2014;24:1274–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Di Tommaso P, Moretti S, Xenarios I, et al. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011;39(Web Server issue):W13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ovcharenko I, Loots GG, Hardison RC, et al. zPicture: dynamic alignment and visualization tool for analyzing conservation profiles. Genome Res. 2004;14:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


