Abstract
In children, cancers are the deadliest of diseases and second only to accidents as the leading cause of death. The deadliest of the brain cancers are the malignant gliomas. Approximately two-thirds of children can survive less malignant types of brain cancers, however, in ~67% of these survivors recurs under the current regimes of surgery followed by administration of high doses toxic drugs and exposure to high doses of radiation. Even more distressing is that fortunate survivors are generally left with life-long cognitive disabilities. A new medical approach is desperately needed. Stem cells, with their natural ability to seek out brain tumors, could be used to accurately deliver therapy directly to the cancer sparing normal tissues for suppression of tumor growth. Despite exciting initial reports, clinical potency of stem cell therapy in animal brain tumor models has to date proven disappointing. Attempts to extrapolate the animal study results to humans are stymied by the fact that stem cells are heterogeneous, resulting in differences in their efficacy. Indeed, therapeutic success relies on an effective strategy to select for a stem cell sub-population within some particular stage of the development at which they are competitive and capable of targeting brain tumors. To improve this during developmental path, concept of a ‘therapeutic window’ is proposed. The “therapeutic window” for stem cells or more specifically a “biochemical therapeutic window” can be determined from biochemical assays and a “biological therapeutic window” from biological assays or even a molecular window from genetic description. Taken together, we can use selective processes to generate more effective stem cells to treat cancers as is clearly needed today.
Keywords: Pediatric brain tumor, stem cells, therapy, tumor-targeting
MALIGNANT BRAIN TUMOR IS THE LEADING CAUSE OF CANCER-RELATED DEATH IN CHILDREN
As estimated 18,820 new cases of brain and other CNS cancers will be diagnosed in the United States each year, and more than 12,000 will die from the diseases (data from the US National Cancer Institute). Brain tumors are now the leading cause of cancer-related deaths in children under age 15 [1]. In contrast to pediatric hematological malignancies, meaningful improvements in survival statistics for patients with malignant brain tumors have not been realized in over 30 years of clinical research [2, 3]. The median survival after diagnosis of glioblastoma multiforme, the most aggressive type of brain tumors, remains less than one year, with a two-year survival rate near zero. A significant challenge to treating gliomas arises from the fact that high grade gliomas diffusely migrate and disseminate tumor microsatellites deeply into distant regions of the normal central nervous system. Surgical removal combined with adjuvant therapies including chemotherapy and radiation therapy proves insufficient to eliminate the neoplastic disease. Despite gross total surgical resection, chemotherapy and radiation therapy, neoplastic cells persist and subsequently give rise to recurrent tumor.
As a heterogeneous group of neoplasia with diverse histological, molecular, and genetic spectra and widely variable clinical prognoses, brain tumors remain difficult to cure. Early treatment response may be transient, not necessarily translating into long-term responses or favorable clinical outcomes. This may reflect a small window of opportunity, i.e., therapeutic window, during which time tumor cells can be most effectively addressed by therapeutic interventions [4].
“TIME IS LIFE” FOR THE RESECTION OF THE PRIMARY TREATMENT MODALITIES FOR PEDIATRIC MALIGNANT BRAIN TUMORS
“Time is life” comes from the idea that neurosurgeons must treat patients within a time window of severe neurological attack in order to have a measurable effect. The primary treatment of malignant brain tumors is resection followed by chemotherapy and radiation therapy, however; these regimes carry consequences. Evidence accumulated shows that the effects of tumor removal may activate minimal residual tumor growth, suggesting that therapeutic approaches are needed to protect patients against the oncologically adverse effects after the tumor removal [5]. As such, certain postoperative periods can offer a window of opportunity during which the patient may be further protected against the minimal residual tumor activation by the resection.
For example, surgical intervention coupled with postradiation chemotherapeutic regimens within a window of opportunity as defined clinically is the most effective treatment for pediatric medulloblastoma as pharmacokinetically guided by topotecan dosing for assessment of the antitumor efficacy [6, 7]. To maximize delivery of chemotherapy in the treatment of brain tumor patients, osmotic breaching of the blood-brain barrier (BBB) allows the spatial and temporal distribution of molecular liposolubility, enabling a window of BBB permeabilization, promoting therapeutic agents across the BBB and into the CNS [8].
Rakesh Jain has pointed out that there is a “normalization window,” in which cancer cells may be more vulnerable to traditional cytotoxic therapies and to novel targeted therapies (such as anti-angiogenic antibody to VEGF receptor-2) [9–11]. The degree of “normalization” is dependent upon the spatial and temporary regulation of these targeted molecules. This window was reported to be short-lived (about 6 days) and is characterized by an increase in tumor oxygenation, which enhances radiation therapy by increasing the concentration of reactive oxygen species created by the radiation. In animals, targeted toxins for treatment of malignant astrocytoma, have shown prolongation of survival and complete tumor regression without significant neurological toxicity. These studies have confirmed the existence of a therapeutic window between normal brain tissue and malignant cells that can be exploited with targeted therapy directed against the transferrin receptor [12]. In children with medulloblastoma, the theoretical possibility of a therapeutic window immediately after surgery has led to neoadjuvant treatments, improving the therapeutic effects of multimodality therapy [13].
CURRENT KNOWLEDGE AND UNANSWERED QUESTIONS ABOUT EMERGING STEM CELL THERAPY OF BRAIN TUMORS
The lack of efficacy for conventional treatments of malignant brain tumors underscores the need for new strategies which circumvent the limitations of conventional brain tumor treatments. One such emerging strategy is to use the tumor-tracking capacity apparently inherent in many stem cell populations to “seek out” dispersed tumor cells in the brain and delivers anti-neoplastic agents to these infiltrative cancer satellite foci [14–17]. Stem cells possess the capacity of self-renewal and the potential to differentiate into different types of cells. Stem cells transplanted in the tumor bed may directly modulate the tumor microenvironment via the therapeutic effects of their regenerative potential, neurotrophic and neuroprotective properties, and immune regulatory functions (e.g., inhibit the cellular inflammatory process in the tumor) [18, 19]. Human bone marrow derived mesenchymal stem cells (MSC) transcriptome analyses reveal that MSC transplanted at sites of nerve injury promote functional recovery by producing trophic factors that induce survival and regeneration of host neurons, including BDNF and β-NGF, various neuriteinducing factors, axon guidance and neural cell adhesion molecules [20–23].
However, the clinical potentials for stem cell therapy have resulted in controversial efficacies including stem cell-driven tumor formation in human recipients [24]. Many factors influence efficacy including the number of effective stem cells in a sub-population, the differentiation status, the age of the stem cells, the occurrence of graft versus host reaction, etc. [25]. Certain stages of stem cell developmental cycles may be useful for transplantation as stem cells in other stages lose their capacity for migration and integration into the injured tissues, or may generate tumor formation (Fig. 1). Stem cell migratory capacity is quintessential to their successful use in therapy as stem cells lacking migratory ability may induce new tumor formation at the injection site [26, 27].
Fig. (1).
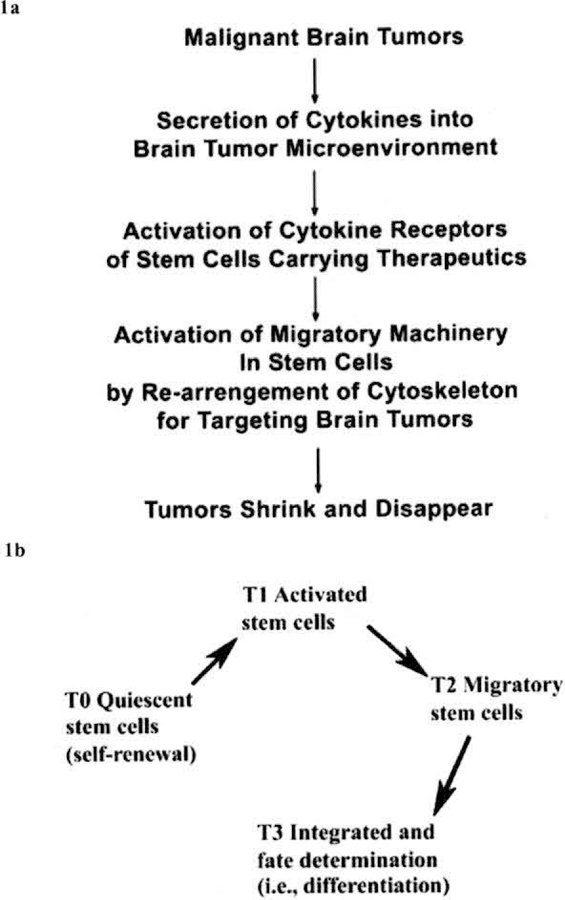
Stem cell developmental stages, a: Schematic diagram for stem cell migration toward cytokines produced by the tumor microenvironment (modified from Li and Loudon, 2008). b: Stem cell developmental cycle can be defined into four stages: quiescent (T0), activation/proliferation (T1), migration (T2), and integration/fate determination (T3). In T1 activated stem cells, target-related molecules are upregulated, which drive stem cells toward injured tissues. Upon reaching the injury tissues, stem cells lose expression of the targeting molecules and integrate into the injured tissue by differentiation (fate determination) in response to microenvironmental cues in the injured tissue. T0, T1, T2, and T3 represent different times during the stem cell development.
For migratory behavior of stem cells, two properties of stem cell functional activity are critical: the ability of the stem cell to detect a target (homing) and the ability of the stem cell to move through the tissue (migration) to its target (ECM-remodeling). These functions have been discussed interchangeably but they are distinct and equally important. Homing appears to be mediated to a large extent by the secretion of chemokines into the tumor microenvironment and the parallel expression of chemokine receptors on the stem cells [28, 29]. ECM remodeling appears to be mediated, by the secretion of matrix metalloproteinases by the stem cell as well as the tumor [30]. Independent, but coordinated, regulation of these two functional behaviors (therapeutic activation) must occur for stem cells to be therapeutically useful. Little is known about how we can effectively activate and program stem cells for transplantation. For example, quiescent stem cell populations minimally express chemokine receptors [31,32].
Variations in stem cell efficacies may arise from their activation heterogeneous. A therapeutic state of stem cells must be clearly defined and utilized in a clinical setting. We postulate to identify and manipulate the “therapeutic activation state or therapeutic window” to address a potential window of opportunity when stem cells are most effective at targeting brain tumors (Fig. 2).
Fig. (2).

The “Therapeutic window” of stem cells with cancertargeting capability can be defined in the developmental path of stem cells at the molecular level of expressing cancer-targeting molecules (e.g., CXCR4 and MMP-9) in an in vivo mimic three-dimensional, extracellular microenvironment model (Tl, T2, T3, and T4 represent different times during the stem cell development). Panel a: The rectangles (in pink color) represent different therapeutic windows, or by “biochemical therapeutic windows”, expression of different levels of biomarker and “biological therapeutic window” defined by temporal biological functions (e.g., migratory behavior). Panel b: The “biochemical therapeutic windows” can be determined by expression levels of molecules responsible for migration. Left: The time course of MMP9 expression zymography-Lane 1: 16 h, lane 2: 1 day, lane 3: 3 d, lane 4: 1 week, lane 5: 2 wk, lane 6: 3 wk, lane 7: 4 wk (Lanes were loaded with equivalent amounts of total lysate); Right: CXCR4 mRNA expression. Panel c: “biological therapeutic windows” are defined by the capacity of stem cells for migration, showing that cultivated mesenchymal stem cells of 3-week in 3D/ cytokines increased the migratory capacity compared with that of 1-day culture and control cells of NIH3T3.
THE HYPOTHESIS
Understanding of the functional events during different stages of the stem cell development is essential for development of effective stem cell therapy. The therapeutic efficacy of stem cells can be defined by the percentage of a given number of stem cells competent to target brain tumors.
The therapeutic window (or pharmaceutical window) is an estimate of the drug dosage which can effectively treat a disease condition within the safe range. More specifically, it is formally determined as the range between the ED50 and the starting point of LD50 curve, i.e. over that which starts to produce adverse effects. It also indicates a usually short time interval during which a particular therapy can be given safely and effectively.
This concept is also highly relevant to stem cell therapy. Specifically, a “therapeutic activation state or therapeutic window”—a critical stage of stem cell development can be defined as a time when stem cells acquire ability to migrate, target and integrate when grafted into a targeting injured tissue for regeneration (Fig. 2). The defined parameters of a “therapeutic window” can be specifically determined by using molecular, biochemical and biological studies.
Optimizing targeting capacity is an essential requirement of stem cells used in the therapy of brain tumors. Little is known, however, about the mechanisms by which SC target malignant brain tumors. Growing evidence shows that chemokine signaling axes (homing) and matrix remodeling (enhancement of migration) represent major pathways for driving SC trafficking and migration towards the microenvironment of a brain tumor. Strategies that optimize the chemokine responsiveness (expressed as chemokine receptors-CR) and upregulate matrix-remodeling (as matrix metalloproteinase-MMP) arc seen as creating a “Biochemical Therapeutic Window” in stem cells that enhance the biological behavior (e.g., targeted migration)—hereby defined as a “biological therapeutic window” and subsequently their therapeutic potential. To determine the migratory behavior of stem cells, (1) the “biochemical therapeutic window” can be modulated through biochemical events such as matrix remodeling enzymatic activities (e.g., MMPs) and chemokine receptor expression (e.g., CXCR4) in stem cells in vitro; and (2) the “biological therapeutic window” can be confirmed by using ex vivo and in vivo models [1].
In parallel to the “therapeutic window” state of normal stem cell development, there may be also a ‘therapeutic window’ for targeting brain tumor stem cells (BTSC) (Fig. 3). To establish a “Therapeutic Window” for the optimal treatment of inherited and acquired malignant brain tumors, we have to identify a period of time in the life cycle of the BTSC in which intervention (which may include chemotherapy, radiation therapy, and stem cell therapy) is likely to be most effective. To understand the life cycle of cancer cells, a great deal of information has been gained through molecular profiling of cancer cells. However, these profiles, patterns of gene or protein expression have been identified by analysis of purified components. Little is known about the context and timing of the expression of these molecules. Lack of information on spatial and temporal changes in protein and other molecules in the development of human cancers requires new methods for real-time visualization of gene expression, proteins or other molecules in normal and cancer cells to define a therapeutic window at the molecular level for treatment of human cancers.
Fig. (3).
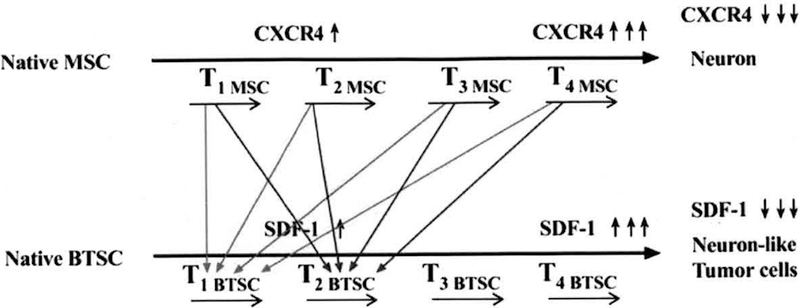
Matching the “Therapeutic Window” of the normal stem cell (e.g., mesenchymal stem cells (MSC)) with the “Therapeutic Window” of the brain tumor stem cell (BTSC) for the target therapy. Arrows in red or blue color illustrate that different matches between MSC and BTSC in their developmental stages can be tried out to determine a crossing point at which an optimized stem cell state (i.e., the therapeutic window of stem cells) is acclimatized to a brain tumor in its most receptive state (i.e., the therapeutic window of brain tumors). Tl, T2, T3, and T4 represent different times (horizontal black arrows) during the stem cell development (See text for details).
TESTING THE HYPOTHESIS: SUPPORTING EVIDENCE
Our hypothesis of the “Therapeutic Window” for SC for transplantation is modeled after the clinically established concept of a “therapeutic window” for embryonic organ transplantation [33, 34]. Previous study by Thomson et al. (1998) suggests that embryonic stem cells (ESC) can be derived from human blastocysts and maintained in culture [35]. Human ESC, after 4–5 months of culture, were injected into four-week-old severe combined immunodeficient (SCID) mice resulting in teratomas composed of endoderm, cartilage, bone, smooth muscle and mesoderm. These results led to a new effort to define the optimal gestational age at which to grow functional tissues with minimal risk for teratoma formation [33]. This goal prompted Eventov-Friedman et al. [36] to expand the scope of the “narrow window”, defined previously [33], by examining various embryonic pig organ precursors at different gestational stages. They reported that maximal liver growth and function were achieved at age (E28), the earliest teratoma-free gestational age. Growth and functional ability of the pancreas increased toward E42 and E56 and insulin-secreting capacity declined at E80 and El00. Mature lung tissue development, with essential respiratory system elements, was observed at E56. Determining the optimal window for pig embryonic pancreas transplantation improves the chances for the successful treatment of diabetes [37]. Our preliminary studies indicate that if a distinct “Therapeutic Window” for expression of SC targeting related molecules is determined, the chances for successful implementation of SC transplantation in the treatment of human diseases is enhanced. “Therapeutic Windows” in brain tumor work can be determined by the use of our proprietary platform technology: living slices derived from rodent brain, which now needs to be translated into improved clinical treatment for the pediatric gliomas [1].
This novel concept of an optimal window might partially explain the failures in previous transplantation trials. Examining the relative amount of committed stem cells and pluripotent cells found in the specific developing organ might explain why some organs are more prone to formation of teratoma while others are not. As suggested by Eventov-Friedman et al. (2005), further studies using phenotypic analysis as well as in vitro molecular and cellular assays are needed to define the levels of pluripotential as opposed to committed stem cells in the early embryonic tissue [36]. No further characterization of these “therapeutic windows” is reported. For example, it has been proposed that there is a limited “therapeutic window” following myocardial infarction during which stem cells can home in on and integrate within the heart tissue for myocardial regeneration. In the “therapeutic window,” “healing pathways” are activated [38]. Conversely, some initial attempts to use embryonic stem cell therapy in human diseases have proved dangerous to the subject, e.g., the formation of teratomas and inflammation responses [39,40].
Little is known about characterizing the “therapeutic window” of stem cells at the molecular level. Some of the cellular, molecular and biochemical mechanisms underlying the therapeutic window have now been elucidated. In a range of laboratory assays certain cancers-malignant glioma in particular-are intrinsically sensitive to stem cell therapy. We and others are characterizing the cellular, molecular and biochemical mechanisms underlying this therapeutic window.
The potential complexity associated with generating adequate populations of SC with targeting capacity requires therapeutic reprogramming (directed activation steps for optimization of: activation, mobilization, tracking, and targeting) prior to delivery of SC-mediated anti-tumor treatment. The mechanisms by which SC target glioblastoma multiforme (GBM) are slowly being elucidated with growing evidence demonstrating that chemokine signaling axes (homing) and matrix remodeling (enhancement of migration) providing much of the driving force in SC trafficking and migration towards GBM microenvironment. Strategies to optimize the chemokine responsiveness and to upregulate matrix-remodeling should enhance the biological behavior of targeted migration and improve the potential of cultured SC.
We have taken the following approaches for proof-of-concept experiments: 1) an in vitro three dimensional extracellular milieu model (3D) for controlled expression of chemokine receptors (CRs) and MMPs; 2) an ex vivo organotypic brain slice model (Li and Loudon, 2008); and 3) an in vivo intracranial brain tumor model. We should be able to confirm that the expression levels of chemokine receptors and MMPs of the stem cell correlate quantitatively with their capacity for migration toward brain tumors.
Our data support the fact that a three dimensional (3D) system can induce up-regulation of chemokine receptors and matrix remodeling capacity in a time-dependent manner, effectively creating a “therapeutic window for stem cells” (Fig. 2a). Stem cells in a 3D system remain quiescent in the absence of chemokine, as assessed by morphology and expression markers (data not shown). In the presence of chemokines in a 3D environment, stem cells undergo neurite formation and produce matrix remodeling enzymes. In addition, these cells home on the chemokine source (publication in preparation).
The functional gelatinolytic activity of SDF-1-induced MMP-9 in MSCs grown in a 3D matrix is demonstrated by zymography (Fig. 2b). As we did not see such induction when the cells were grown in monolayer, it appears that dual signals from SDF-1 and the 3D matrix are required for induction of MMP-9 (Fig. 2). We have seen similar results with several types of stem cells, including human neural stem cells. We have also tested synthetic polymers such as PLA/PGLA. The polymers can keep the stem cell in a quiescent state without chemokine or cytokine stimulation. However, the level of MMP-9 production is higher in 3D collagen I than in synthetic polymers, suggesting that when collagen I is degraded by induced MMP-9, it acts synergistically with chemokines to enhance MMP-9 production.
As whether chemokine receptor expression correlates with stem cell migration toward chemokines by experiments in microfluidic chambers, it was found that homing of neural stem cells (NSC) could be enhanced by an intermediate concentration of SDF-1 but while inhibited at a high concentration, suggesting the SDF-1 signals both the start and stop of stem cell migration. Interestingly, NSCs also migrate toward fresh tumors [1]. In clinical, high-dose melphalan (HDM) followed by autologous stem cell transplantation (ASCT) for treatment of patients with multiple myeloma show that the early lymphodepletion period, occurring 4–11 d post-HDM and ASCT, is associated with an increase of circulating immune cytokines and could be an optimal window to enhance the survival and proliferation of polyclonal T cells present in the ASC autograft and also of specific antimyeloma T cells previously expanded in vitro [41]. Alternative supporting evidence of the therapeutic window concept has been derived from increasing the therapeutic potency of anti-BCR-ABL drugs by selective temporarily blocking of the SDF-1/CXCR4 axis by the CXCR4 antagonist plerixafor in treatment of BCR-ABL(+) leukemia [42]. This antagonist significantly inhibits SDF-1 alpha-mediated chemotaxis and cell migration toward the bone marrow environment that provides survival-enhancing effects to myeloid progenitor cells. Such a temporal inhibition of myeloid cell migration provide a potential mechanism to overcome the protective effect of the bone marrow environment.
IMPLICATIONS OF THE HYPOTHESIS FOR CLINICIANS
Mirroring a therapeutic window in stem cells, is a therapeutic window for treatment of brain tumors (Fig. 3). A major challenge in identifying this latter therapeutic window is to develop imaging technology that will determine the optimal time for treatment with stem cells. Measurement of tumor growth in situ currently requires tissue biopsy, which may provide information on brain tumor staging. Stem cell transplant and tumor imaging have been reviewed by us (in press) and others. Imaging can provide data about tumor viability, growth and invasion. Imaging techniques can also provide serial measures of stem cell migration, permeability, and differentiation and can therefore be used to monitor the window of therapeutic efficacy in patients. PET with 18-fluoromisonidzole and MRI can provide some indication of tumor oxygenation and might be useful for checking the efficacy of stem cell therapy [43].
We propose that understanding the unique characteristics of stem cells will help determine the best therapeutic windows, (i.e., time) in which cancer-specific targeted therapy ought to be introduced for optimal and ultimate destruction of cancers. Research results will likely lead to the identification of novel targets for therapy, better clinical diagnostic and prognostic tools, and ultimately, to better treatments and cures for CSC-related malignancies.
N euro-oncology argues for a window of opportunity to attack a brain cancer early phase of cancer development, in which treatments are usually more successful in inhibiting tumor progression. It is worth considering stem cell treatment as an effective alternative to conventional therapies that fail.
Research from stem cell clinical trials, has shown inconsistent results possibly due to the heterogeneity of stem cells as well as tumor cells. The production of cytokines after tumor resection reveals an Achilles heel, which opens a window of opportunity for combating cancers using stem cell therapy. To take full advantage of the therapeutic window of opportunity, we need to develop suitable screening tests that are clinically relevant, rapid, simple, inexpensive, and impose minimal additional risk to patients as we discussed previously [1]. This test system can be further enhanced by implanting a global positioning system imaging to track the stem cell behaviors in the whole body [44].
ACKNOWLEDGEMENTS
Supports are from CHOC Children’s Hospital Foundation, Neuroscience Institute, Heart Institute, Austin Ford Tribute Fund and WM Keck Foundation (to S.C.L.) and the US National Institutes of Health R01 DK069418–05 and R01 AR051558–5 (to Y.-P.H.). Many thanks to the Li lab members: Long Vu, Vic Keschrumrus, Michael Ho, Lisa Tachiki, Shi (Sherrie) Yu, and Tiffany Dao for their helpful discussions. We thank Henry J Klassen, MD-PhD; Philip H Schwartz, PhD; Maria Minon, MD; Hector W Ho, MD; Saul Puszkin, PhD; Michael P Lisanti, MD-PhD; Richard G Pestell, MD-PhD; Joan S Brugge, PhD; and Robert A Koch, PhD, for their support and enthusiasm.
ABBREVIATIONS
- BTSC
Brain tumor stem cells
- ALCAM
Activated leukocyte cell adhesion molecule
- CNS
Central nerve system
- CXCR-4
Chemokine (C-X-C motif) receptor 4, a.k.a., SDF-1 receptor or CXCL 12 receptor
- DCX
Doublecortin
- ECM
Extra cellular matrix
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- G-CSF
Granulocyte colony-stimulating factor
- GFAP
Glial fibril acidic protein
- MMP
Matrix metalloproteinase
- MSC
Bone marrow derived mesenchymal stem cells
- NRCAM
Neuronal cell adhesion molecule
- NSC
Neural stem cells
- PROM1
Prominin 1
- 3D
Three dimensional extra-cellular matrix microenvironment
- SCF
Stem cell factor
- SDF-1
Stromal derived factor-1 (chemokine), a.k.a., CXCL 12
- SOX2
SRY (sex determining region Y)-box 2
- VCAM1
Vascular cell adhesion molecule 1
REFERENCES
- [1].Li SC, Loudon WG. A novel and generalizable organotypic slice platform to evaluate stem cell potential for targeting pediatric brain tumors. Cancer Cell Int 2008; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Legler JM, Ries LAG, Smith MA, et al. Brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst 1999; 91(16): 1382–90. [DOI] [PubMed] [Google Scholar]
- [3].Surawicz TS, Davis F, Freels S, Laws ER, Menck HR. Brain tumor survival: Results from the National Cancer Data Base. J Neurooncol 1998; 40(2): 151–60. [DOI] [PubMed] [Google Scholar]
- [4].Reiman JM, Knutson KL, Radisky DC. Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res 2010; 70(8): 3005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Coffey JC, Wang JH, Smith MJF, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol 2003; 4(12): 760–8. [DOI] [PubMed] [Google Scholar]
- [6].Stewart CF, lacono LC, Chintagumpala M, et al. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. J Clin Oncol 2004; 22(16): 3357. [DOI] [PubMed] [Google Scholar]
- [7].Amann M, Brischwein K, Lutterbuese P, et al. Therapeutic window of MuSl10, a single-chain antibody construct bispecific for murine EpCAM and murine CD3. Cancer Res 2008; 68(1): 143–51. [DOI] [PubMed] [Google Scholar]
- [8].Bellavance MA, Blanchette M, Fortin D. Recent advances in blood–brain barrier disruption as a CNS delivery strategy. AAPS J 2008; 10(1): 166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307(5706): 58–62. [DOI] [PubMed] [Google Scholar]
- [10].Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev 2007; 8(8): 610–22. [DOI] [PubMed] [Google Scholar]
- [11].Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res 2007; 67(6): 2729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hall WA. Targeted toxin therapy for malignant astrocytoma. Neurosurgery 2000;46(3):544. [DOI] [PubMed] [Google Scholar]
- [13].Attard-Montalto S, Plowman N, Breatnach F, Saha V, Eden OB. Is there a danger in delaying radiotherapy in childhood medulloblastoma? Br J Radiol 1993; 66(789): 807. [DOI] [PubMed] [Google Scholar]
- [14].Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther 2008; 15(10): 739–52. [DOI] [PubMed] [Google Scholar]
- [15].Li SC, Loudon WG. Stem cell therapy for paediatric malignant brain tumours: the silver bullet? ONcology News (UK), June-July 2008, ISSN 1751–4975. 2008; 3(1): 10–4. http://www.oncologynews.biz/pdf/jun_jul_08/ONJJ_stemcell.pdf (Accessed May 4, 2008). [Google Scholar]
- [16].Mapara KY, Stevenson CB, Thompson RC, Ehtesham M. Stem cells as vehicles for the treatment of brain cancer. Neurosurg Clin N Am 2007; 18(1): 71–80, ix. [DOI] [PubMed] [Google Scholar]
- [17].Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci 2006. 01; 7(1): 75–84. [DOI] [PubMed] [Google Scholar]
- [18].Ben-Hur T Human embryonic stem cells for neuronal repair. Isr Med Assoc J 2006; 8(2): 122–6. [PubMed] [Google Scholar]
- [19].Chen HI, Bakshi A, Royo NC, Magge SN, Watson DJ. Neural stem cells as biological minipumps: a faster route to cell therapy for the CNS? Curr Stem Cell Res Ther 2007; 2(1): 13–22. [DOI] [PubMed] [Google Scholar]
- [20].Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle 2007; 6(23): 2884–9. [DOI] [PubMed] [Google Scholar]
- [21].Phinney DG, Baddoo M, Dutreil M, Gaupp D, Lai WT, Isakova IA. Murine mesenchymal stem cells transplanted to the central nervous system of neonatal versus adult mice exhibit distinct engraftment kinetics and express receptors that guide neuronal cell migration. Stem Cells Dev 2006; 15(3): 437–47. [DOI] [PubMed] [Google Scholar]
- [22].Phinney DG, Hill K, Michelson C, et al. Biological activities encoded by the murine mesenchymal stem cell transcriptome provide a basis for their developmental potential and broad therapeutic efficacy. Stem Cells 2006; 24(1): 186–98. [DOI] [PubMed] [Google Scholar]
- [23].Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 2007; 25(11): 2896–902. [DOI] [PubMed] [Google Scholar]
- [24].Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 2009; 6(2): el000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int 2007; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Weidt C, Niggemann B, Kasenda B, Drell TL, Zanker KS, Dittmar T. Stem cell migration: a quintessential stepping stone to successful therapy. Curr Stem Cell Res Ther 2007; 2(1): 89–103. [DOI] [PubMed] [Google Scholar]
- [27].Weidt C, Niggemann B, Hatzmann W, Zanker KS, Dittmar T. Differential effects of culture conditions on the migration pattern of stromal cell-derived factor-stimulated hematopoietic stem cells. Stem Cells 2004; 22(6): 890–6. [DOI] [PubMed] [Google Scholar]
- [28].Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1 alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA 2004; 101(52): 18117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA 2000; 97(23): 12846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 2007; 109(9): 4055–63. [DOI] [PubMed] [Google Scholar]
- [31].Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005; 106(2): 419–27. [DOI] [PubMed] [Google Scholar]
- [32].Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004; 104(9): 2643–5. [DOI] [PubMed] [Google Scholar]
- [33].Dekel B, Burakova T, Arditti FD, et al. Human and porcine early kidney precursors as a new source for transplantation. Nat Med 2003; 9(1): 53–60. [DOI] [PubMed] [Google Scholar]
- [34].Dekel B, Reisner Y. Embryonic committed stem cells as a solution to kidney donor shortage. Expert Opin Biol Ther 2004; 4(4): 443–54. [DOI] [PubMed] [Google Scholar]
- [35].Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282(5391): 1145–7. [DOI] [PubMed] [Google Scholar]
- [36].Eventov-Friedman S, Katchman H, Shezen E, et al. Embryonic pig liver, pancreas, and lung as a source for transplantation: optimal organogenesis without teratoma depends on distinct time windows. Proc Natl Acad Sci USA 2005; 102(8): 2928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eventov-Friedman S, Tchorsh D, Katchman H, et al. Embryonic pig pancreatic tissue transplantation for the treatment of diabetes. PLoS Med 2006; 3(7): e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Penn MS, Zhang M, Deglurkar I, Topol EJ. Role of stem cell homing in myocardial regeneration. Int J Cardiol 2004; 95 (Suppl 1): S23–S5. [DOI] [PubMed] [Google Scholar]
- [39].Brederlau A, Correia AS, Anisimov SV, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkin-son’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 2006; 24(6): 1433–40. [DOI] [PubMed] [Google Scholar]
- [40].Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007; [43] 25(8): 1940–53. [DOI] [PubMed] [Google Scholar]
- [41].Condomines M, Veyrune JL., Larroque M, et al. Increased plasma-immune cytokines throughout the high-dose melphalan-induced lymphodepletion in patients with multiple myeloma: a window for adoptive immunotherapy. J Immunol 2010; 184 (2): 1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dillmann F, Veldwijk MR, Laufs S, et al. Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and Nilotinib. Leuk Lymphoma 2009; 50(10): 1676–86. [DOI] [PubMed] [Google Scholar]
- [43].Munson S, Schroth E, Ernst M. The Role of Functional Neuroimaging in Pediatric Brain Injury. Pediatrics 2006; 117(4): 1372–81. [DOI] [PubMed] [Google Scholar]
- [44].Li SC, Tachiki LM, Luo J, Dethlefs BA, Chen Z, Loudon WG. A biological global positioning system: considerations for tracking stem cell behaviors in the whole body. Stem Cell Rev 2010; 6(2): 317–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


