Abstract
Rationale:
Uncontrolled growth of abdominal aortic aneurysms (AAAs) is a life-threatening vascular disease without an effective pharmaceutical treatment. AAA incidence dramatically increases with advancing age in men. However, the molecular mechanisms by which aging predisposes individuals to AAAs remain unknown.
Objective:
In this study, we investigated the role of SIRT1 (Sirtuin 1), a class III histone deacetylase, in AAA formation and the underlying mechanisms linking vascular senescence and inflammation.
Methods and Results:
The expression and activity of SIRT1 were significantly decreased in human AAA samples. SIRT1 in vascular smooth muscle cells was remarkably downregulated in the suprarenal aortas of aged mice, in which AAAs induced by angiotensin II infusion were significantly elevated. Moreover, vascular smooth muscle cell–specific knockout of SIRT1 accelerated angiotensin II–induced formation and rupture of AAAs and AAA-related pathological changes, whereas vascular smooth muscle cell–specific overexpression of SIRT1 suppressed angiotensin II–induced AAA formation and progression in Apoe−/− mice. Furthermore, the inhibitory effect of SIRT1 on AAA formation was also proved in a calcium chloride (CaCl2)–induced AAA model. Mechanistically, the reduction of SIRT1 was shown to increase vascular cell senescence and upregulate p21 expression, as well as enhance vascular inflammation. Notably, inhibition of p21-dependent vascular cell senescence by SIRT1 blocked angiotensin II–induced nuclear factor-κB binding on the promoter of monocyte chemoattractant protein-1 and inhibited its expression.
Conclusions:
These findings provide evidence that SIRT1 reduction links vascular senescence and inflammation to AAAs and that SIRT1 in vascular smooth muscle cells provides a therapeutic target for the prevention of AAA formation.
Keywords: aging, angiotensin II, inflammation, aortic aneurysm, abdominal, SIRT1 protein, human
Abdominal aortic aneurysms (AAAs), characterized by a permanent, localized dilatation (ballooning) of the abdominal aorta that exceeds the normal diameter by >50%, are the most common form of aortic aneurysm. AAA rupture and the associated catastrophic physiological insult carry an overall mortality rate in excess of 80%; ruptured AAAs are the 13th leading cause of death in the United States.1,2 Pathologically, AAAs are characterized by increased inflammatory cell infiltration, aberrant oxidant stress, medial elastin degradation, and medial collagen deposition. Apart from surgery, few medical treatments have been shown to prevent AAA development and growth,3,4 primarily as a result of the limited understanding of its pathogenic mechanisms.
AAAs are found in up to 8% of men aged >65 years. AAA incidence increases steeply by 40% every 5 years in men who are >65 years old, indicating that age is a major risk factor for AAAs.2 Although age-related alterations such as enhanced inflammatory responses, vascular stiffening, and oxidative stress make aged arteries more susceptible to vascular diseases, such as atherosclerosis,5–7 the reasons why AAAs are often observed in patients with advanced age (>65 years) and how advanced age dramatically accelerates the development and progression of aneurysms in abdominal aortas remain unknown. Furthermore, little is known about the contributions of vascular aging in AAAs and whether an alteration of age-related molecules is required for AAA initiation and progression.
Sir2 (silent information regulator 2) proteins (sirtuins), a conserved nicotinamide adenine dinucleotide-dependent protein deacetylase, play critical roles in improving metabolism and healthspan.8–10 SIRT1 (Sirtuin 1), the best characterized mammalian sirtuin, is highly expressed in the vasculature11 and is an important modulator of cardiovascular functions in health and disease. Several studies from our laboratory and others indicate that SIRT1 protects against stress-induced vascular remodeling`,12,13 aortic stiffness and dissection,14,15 and atherosclerosis in mice,16–18 suggesting a critical role of SIRT1 in vascular diseases. The activation of SIRT1 confers protective effects on atherosclerosis involving vascular endothelial and smooth muscle cell senescence.18–20 Despite that advanced age is a known risk factor for AAAs, whether advanced age accelerates AAA formation in mice and the molecular mechanisms that link vascular cell senescence and AAA formation remain elusive. Here, we report that the expression and activity of SIRT1 were significantly decreased in human AAA samples. Age-associated SIRT1 reduction in vascular smooth muscle cells (VSMCs) of abdominal aortas increases their susceptibility to AAAs in mice. The reduction of SIRT1 was shown to increase vascular cell senescence, upregulate the expression of p21, and enhance inflammatory cell recruitment for vascular inflammation, which predisposes aortas to AAAs.
Methods
An expanded Materials and Methods section is available in the Online Data Supplement.
Human Aortic Samples
All protocols using human aortic samples were approved by the Ethical Committee of Chinese Academy of Medical Sciences and Peking Union Medical College. We obtained human AAA samples from 6 patients undergoing open surgical repair. AAA was diagnosed by ultrasound scanning before the operation. The control samples were trimmed from the adjacent nonaneurysmal aortic segments from the same patients. Information on the enrolled patients is provided in Online Table I.
Animal Experiments
All animal protocols were approved by the Animal Care and Use Committee at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, and Peking Union Medical College. We used 2- to 3-month-old C57BL/6J male mice as young mice and 18- to 20-month-old C57BL/6J male mice as old mice. SIRT1-VSMC–specific transgenic (SV-Tg) Apoe−/− mice were generated by crossing SV-Tg mice12 with Apoe−/− mice. We utilized a Cre/LoxP strategy to yield SIRT1-VSMC–specific knockout (SV-KO) mice. Age-matched male mice (young and old) and 4- to 6-month-old male mice (Apoe−/−, SV-Tg Apoe−/− SV-KO, wild-type [WT]; Apoe−/−, p2 1−/−Apoe−/−) were infused with angiotensin II (Ang II) at a dosage of 1.44 mg/kg-per day or saline for 4 weeks as previously described.21 In the CaCl2-induced AAA model, we induced AAA in both SV-KO (3 weeks) and SV-Tg (6 weeks) mice by periaortic application of 0.5 mol/L CaCl2 as described previously.22
SIRT1 Deacetylase Activity Assay
SIRT1 activity was assayed using a SIRT1 deacetylase activity assay kit (Sigma) according to the manufacturer’s instructions using microplate reader (Synergy 4; BioTek).
Senescence-Associated β-Galactosidase Activity Assay
Senescence-associated β-galactosidase (SA-β-gal) activity was quantitatively measured according to the rate of conversion of 4-methylumbelliferyl-p-β-galactopyranoside to the fluorescent hydrolysis product 4-methylumbelliferone at pH 6.0, as described previously.23 The whole-aorta tissues were stained to determine SA-β-gal activity using a commercial kit (ab65351; Abcam) according to the manufacturer’s instructions, as previously reported.24
Statistical Analyses
For all statistical tests, a P<0.05 was considered statistically significant, and all tests were 2 tailed. Normality tests were assessed via the Shapiro-Wilk statistics with SPSS software package (version 19.0). All statistical analyses were performed using GraphPad Prism (version 6.01) software.
Results
Reduction of SIRT1 Expression and Activity in Human AAA Formation
To establish the impact of AAAs on SIRT1, we first examined the expression of SIRT1 in human AAA samples. Human AAA tissues and their control adjacent aortic sections without aneurysm were obtained from patients undergoing open surgery (Online Table I). As expected, proaneurysmal molecules, such as matrix metalloproteinase 2 (MMP2), human membrane type 1 MMP, and monocyte chemoattractant protein-1 (MCP-1/CCL2), were significantly elevated in human AAA sections (Figure 1A and 1B; Online Figure IA) compared with adjacent nonaneurysmal aortic sections. The activity of SIRT1 in human AAA samples was also significantly lower than that in adjacent nonaneurysmal aortic sections (Figure 1C). These results suggest that SIRT1 reduction is involved in the development of human AAA formation.
Figure 1. SIRT1 (Sirtuin 1) is decreased in human abdominal aortic aneurysm (AAA) samples and aged mouse abdominal aortas.
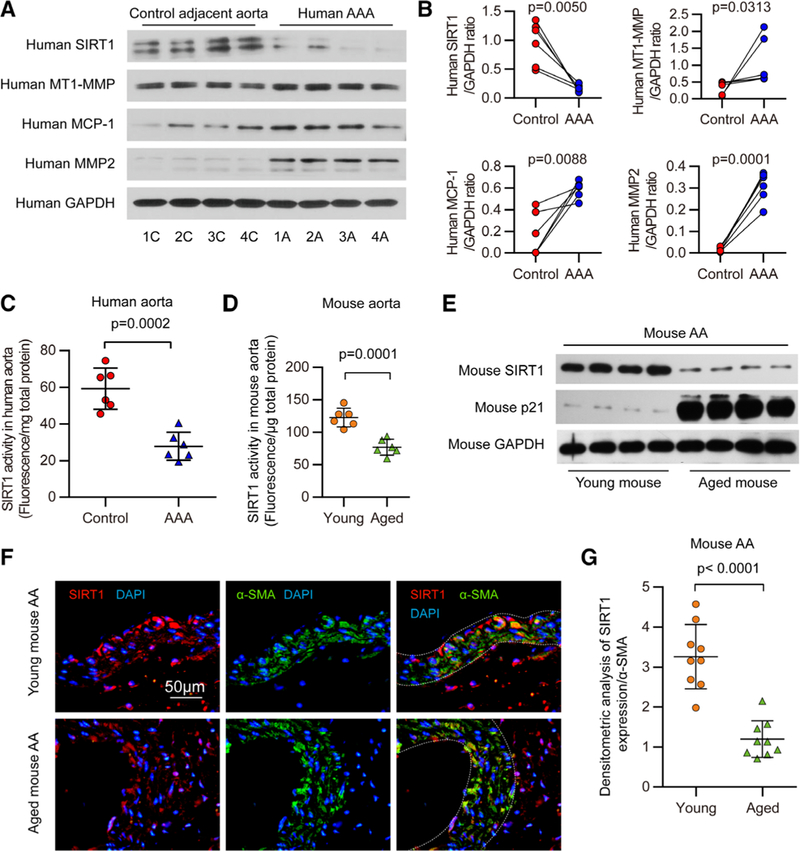
A, Representative Western blots of SIRT1, membrane type 1-matrix metalloproteinase (MT1-MMP), monocyte chemoattractant protein-1 (MCP-1/CCL2), and MMP2 in human AAA samples and adjacent control aortas. B, Densitometric analysis of the protein levels of SIRT1, MT1-MMP, MCP-1/CCL2, and MMP2 in human AAA samples and adjacent control aortas (n=6). C, Quantitative analysis of SIRT1 activity in homogenates of human AAA samples and adjacent nonaneurysmal aortic sections (n=6). D, Fluorescence intensity indicating SIRT1 activity in homogenates of young mouse compared with that of the aged mouse (n=6). E, Representative Western blots of SIRT1 and p21 in abdominal aortas (AA) from aged and young mice. Adventitial tissue was removed from the aorta as much as possible, and each sample was pooled from 3 abdominal aortas together for immunoblot analysis. F, Representative immunofluorescent staining of SIRT1, α-smooth muscle actin (SMA), and 4′,6-diamidino-2-phenylindole (DAPI) in suprarenal aortas of young and aged mice (scale bars, 50 μm). G, Densitometric analysis of the protein level of SIRT1, which is normalized to α-SMA, in suprarenal aortas of young and aged mice (n=9). Three different visual fields were captured in each slide.
Reduced SIRT1 Expression and Activity in the Aged Mouse Aortas
Because advanced age is a known risk factor for AAAs, we examined the effects of age on SIRT1 expression and activity in mouse aortas. SIRT1 activity was significantly lower in the whole aortas of aged mice than that of their young counterparts (Figure 1D). SIRT1 expression was decreased in whole aortas and substantially reduced in the abdominal aortas of aged mice compared with aortas from young mice (Figure 1E; Online Figure IB and IC). Moreover, the decrease in SIRT1 expression was mainly observed in the medial VSMCs of the suprarenal aortas (Figure 1F and 1G), where AAAs most often develop in mice with Ang II infusion.25 Therefore, the reduction of SIRT1 activity and expression in aged aortas may be linked to their susceptibility to AAAs.
Aging Increases Ang II–Induced AAA Formation in Mice
Acute infusion of Ang II recapitulates many aspects of AAAs and is widely used to study AAAs.25,26 To clarify the effects of advanced age on AAA formation, 22 C57BL/6J male mice aged 18 to 20 months (aged) and 23 C57BL/6J male mice aged 2 to 3 months (young) were infused with the same dose of Ang II for 4 weeks. In saline-infused groups, 1 in 16 aged mice displayed expanded abdominal aorta to the extent of AAA, whereas none of the young mice displayed AAA formation (Figure 2A and 2B). In Ang II–infused groups, the AAA incidence in aged mice was 86.4% (19/22), much higher than the 13.0% (3/23) observed in young mice (Figure 2A and 2B). Approximately 22.7% (5/22) of Ang II–infused aged mice died from aortic rupture, whereas only 4.3% (½3) of Ang II–infused young mice died (Figure 2C). Additionally, Ang II infusion caused a greater increase in maximal abdominal aortic diameter, the ratio of total aortic weight to body weight in aged mice than in young mice (Figure 2D and 2E), accompanied with increased expression levels of MCP-1/CCL2, a critical chemokine in inflammatory cell recruitment for vascular inflammation and AAAs,27,28 and MMP-2 as well (Figure 2F and 2G). Accordingly, inflammatory cell infiltration as determined by CD45 immunostaining was increased in the aortas of Ang II–infused aged mice compared with those of Ang II–infused young mice (Figure 2H and 2I). These results indicate that aging remarkably increases Ang II–induced AAA formation in mice.
Figure 2. Angiotensin II (Ang II) increases abdominal aortic aneurysm (AAA) formation in aged mice.

All mice were Infused with saline or Ang II for 4 wk. A, Representative photographs showing mouse aortas Infused with saline or Ang II. The arrow shows an AAA (scale bars, 5 mm). B and C, The incidence (B) and survival curve (C) of AAA in Ang II–infused young mice (n=23) compared with that in aged mice (n=22). There was no AAA formation in saline-infused young mice (n=16), and 1 of 16 saline-infused aged mice developed AAA. D and E, Maximal abdominal aortic diameter (D) and the ratio of aorta weight to body weight (E) in saline- and Ang II–infused young and aged mice. F and G, mRNA levels of monocyte chemoattractant protein-1 (MCP-1/CCL2; F) and matrix metalloproteinase 2 (MMP-2; G) detected by real-time polymerase chain reaction in aorta homogenates from young and aged mice infused with saline or Ang II for 4 wk. H, Representative immunostaining with CD45 in the suprarenal aortic wall of young and aged mice infused with saline or Ang II for 4 wk. The arrows show representative staining with CD45 antibody. I, The number of CD45-positive cells accumulating in the suprarenal aortic wall of saline- and Ang II–infused mice (n=8–23). SV-KO indicates SIRT1 (Sirtuin 1)-vascular smooth muscle cell (VSMC)–specific knockout; and WT, wild-type.
VSMC-Specific Ablation of SIRT1 Exacerbates Ang II–Induced AAA Formation and Related Pathological Changes In Vivo
To establish a causative link between the reduction of SIRT1 and AAA formation, we crossbred a conditional allele of Sirtl (Sirt1flox) mice with mice with Cre recombinase driven by the SM22α promoter to obtain SV-KO (SM22-Cre+/−; Sirt1flox/flox) mice (Online Figure IIA–IID), and their Sirt1flox/flox littermates were used as WT controls. Although SIRT1 activity and expression in the aortas of SV-KO mice were markedly reduced compared with their littermates (Online Figure IIE and IIF), there was no difference in the gross morphology of aortas between saline-infused SV-KO and WT mice (Figure 3A). However, 4 weeks of Ang II infusion caused a 64.2% (34/53) incidence of AAA in SV-KO mice compared with 19.6% (9/46) in WT mice (Figure 3B). Approximately 34.0% (18/53) of Ang II–infused SV-KO mice died because of aortic rupture, whereas only 8.7% (4/46) of Ang II–infused WT mice died (Figure 3C; Online Table II). To exclude the effect of SM22 deficiency after Cre knockin, we treated 10 SM22-Cre+/−; Sirt1+/+ mice with Ang II for 4 weeks and found that only 2 developed AAAs, suggesting that SM22 deficiency in 1 allele does not influence Ang II–induced AAA formation. In addition, compared with Ang II–treated WT mice, the maximal abdominal aortic diameter, the ratio of total aortic weight to body weight, and the elastin degradation score were remarkably higher, whereas the elastic fiber content was significantly lower in Ang II–treated SV-KO mice (Figure 3D–3F; Online Figure IIIA and IIIB). The systolic blood pressure, heart rate, and serum lipid levels after Ang II infusion did not differ between SV-KO mice and WT mice (Online Tables III and IV).
Figure 3. Vascular smooth muscle cell (VSMC)–specific SIRT1 (Sirtuin 1) ablation promotes abdominal aortic aneurysm (AAA) formation and vascular pathophysiological mechanisms induced by angiotensin II (Ang II) infusion.
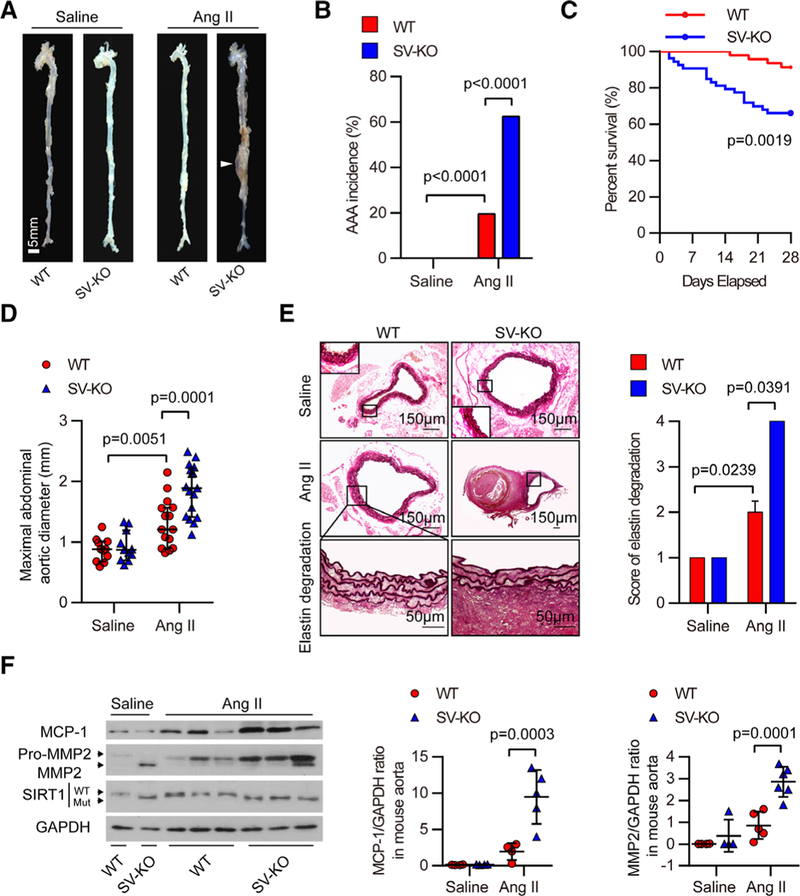
All mice were Infused with saline or Ang II for 4 wk. A, Representative photographs showing macroscopic features of aneurysms Induced by Ang II. The arrow shows a typical AAA (scale bars, 5 mm). B and C, The incidence (B) and survival curve (C) of Ang II–induced AAA in SIRT1-VSMC–specific knockout (SV-KO) mice (n=53) compared with that in wild-type (WT) mice (n=46). There was no AAA formation in saline-infused mice (n=12), and the number of mice that developed AAA included the deaths caused by abdominal aortic rupture. D, The maximal abdominal aortic diameter in saline- and Ang II–infused mice. E, Representative staining with elastin and elastin degradation score in suprarenal aortas from saline- and Ang II–infused mice. The magnified photographs were taken at the location where the most severe elastin degradation occurred (scale bars, 150 and 50 μm; magnified photographs). F, Aorta homogenates were obtained from WT and SV-KO mice infused with saline or Ang II for 4 wk. Western blot and densitometric analysis of the protein levels of monocyte chemoattractant protein-1 (MCP-1/CCL2) and matrix metalloproteinase 2 (MMP2) in aorta homogenates (n=4–6).
Aberrant levels of oxidative stress play critical roles in AAA initiation and progression.21 Therefore, we analyzed medial oxidative stress by immunostaining for 3-nitrotyrosine and 8-hydroxydeoxyguanosine (8-OH-dG), 2 oxidative stress biomarkers. Aortas from Ang II–infused SV-KO mice exhibited a significant increase in the levels of both 3-nitrotyrosine and 8-OH-dG compared with those of Ang II–infused WT mice (Online Figure IIIC and IIID). In saline-infused mice, few inflammatory cells were found in the suprarenal aortic wall (Online Figure IVA). After Ang II infusion, inflammatory cell infiltration was increased in the aortas of Ang II–infused SV-KO mice compared with those of Ang II–infused WT mice (Online Figure IVA) and was accompanied by a significantly higher expression of MCP-1/CCL2 (Figure 3F; Online Figure IVB). SV-KO mice also displayed increased MMP2 protein expression and activity after Ang II infusion (Figure 3F; Online Figure IVC), with an upregulation of membrane type 1-MMP (Online Figure IVD) and increased TIMP1 repression (Online Figure IVD). These results indicate that the VSMC-specific ablation of SIRT1 exacerbates AAA formation and related pathological changes.
VSMC-Specific Overexpression of SIRT1 Suppresses Ang II–Induced AAA Formation and Related Pathological Changes in Apoe−/− Mice
Apoe−/− mice with Ang II infusion is a widely used animal model for AAA formation,25,26 and the incidence of AAAs in Apoe−/− mice in response to Ang II is much greater than that in age-matched WT mice.21,25,26,29 Therefore, we studied whether SIRT1 VSMC-specific overexpression could reduce AAA development in vivo by examining the above-mentioned index in aortas obtained from VSMC-specific SIRT1 transgenic mice (SV-Tg mice) in the Apoe−/− background (SV-Tg Apoe−/− mice) with or without Ang II infusion for 4 weeks. In saline-infused groups, no AAAs were found among Apoe−/− and SV-Tg Apoe−/− mice. Ang II infusion for 4 weeks caused AAAs in 81.8% (27/33) of Apoe−/− mice (Figure 4A and 4B), which was similar to the incidence of Ang II–induced AAAs in aged mice. Only 27.6% (8/29) of SV-Tg Apoe−/− mice developed AAAs after Ang II infusion. Approximately 33.3% (11/33) of Apoe−/− mice died because of aortic rupture during Ang II treatment, but the death rate of Ang II–infused SV-Tg Apoe−/− mice was only 10.3% (3/29) (Figure 4A through 4C; Online Table II). Compared with Ang II-treated Apoe−/− mice, the maximal abdominal aortic diameter, the ratio of total aortic weight to body weight, and the elastin degradation score were remarkably lower, whereas the elastic fiber content was significantly higher in Ang II–treated SV-Tg Apoe−/− mice (Figure 4D and 4E; Online Figure VA and VB) without noticeable effects on hemodynamic and lipid metabolic indices (Online Tables III and IV). Moreover, the VSMC-specific SIRT1 transgene remarkably attenuated the increased levels of oxidative stress (Online Figure VC and VD), vascular inflammation, MCP-1/CCL2 expression (Figure 4F; OnlineFigure VIA and VIB) and MMP2 activation (Figure 4F; Online Figure VIC and VID) caused by Ang II infusion in Apoe−/− mice. These results indicate that SIRT1 overexpression in VSMCs ameliorates the increased AAA formation and related pathological changes.
Figure 4. Vascular smooth muscle cell (VSMC)-specific SIRT1 (Sirtuin 1) overexpression prevents abdominal aortic aneurysm (AAA) formation and vascular pathophysiological mechanisms induced by angiotensin II (Ang II) infusion.
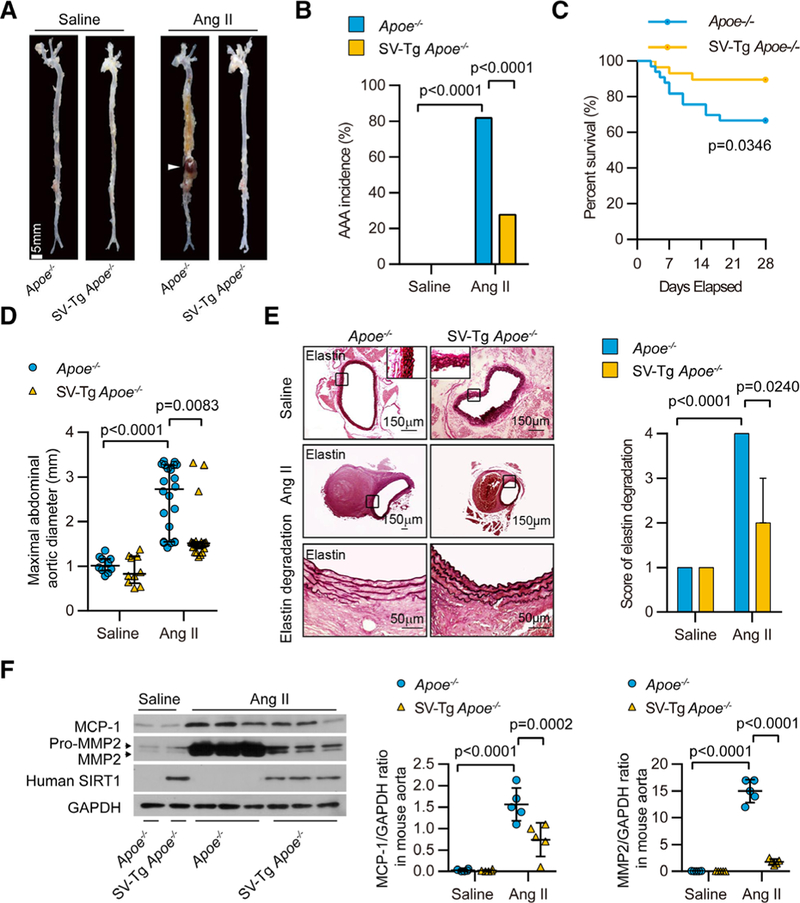
All mice were infused with saline or Ang II for 4 wk. A, Representative photographs showing macroscopic features of aneurysms induced by Ang II. The arrow shows a typical AAA (scale bars, 5 mm). B and C, The incidence (B) and survival curve (C) of Ang Il–induced AAA in SIRT1-VSMC–specific transgenic (SV-Tg) Apoe−/− mice (n=29) compared with those in Apoe−/− mice (n=33). There was no AAA formation in saline-infused mice (n=10), and the number of mice that developed AAA included the deaths caused by abdominal aortic rupture. D, The maximal abdominal aortic diameter in saline- and Ang II–infused mice. E, Representative staining with elastin and elastin degradation score in suprarenal aortas from saline- and Ang II–infused mice. The magnified photographs were taken at the location where the most severe elastin degradation occurred (scale bars, 150 and 50 μm; magnified photographs). F, Aorta homogenates were obtained from Apoe−/− and SV-Tg Apoe−/− mice infused with saline or Ang II for 4 wk. Western blot and densitometric analysis of the protein levels of monocyte chemoattractant protein-1 (MCP-1/CCL2) and matrix metalloproteinase 2 (MMP2) in aorta homogenates (n=4–6).
SIRT1 in VSMCs Inhibits Calcium Chloride–Induced AAA Formation
To further investigate whether the inhibitory effects of SIRT1 in VSMCs on AAA formation are independent of Ang II, another type of mouse AAA model, calcium chloride (CaCl2)-induced AAA22 was applied in both SV-KO and SV-Tg mice. We found that 3 weeks after CaCl2 treatment, the ratio of total aortic weight to body weight and the maximal external and internal abdominal aortic diameter were significantly increased in SV-KO mice compared with those of their littermates (Figure 5A through 5D), and a substantial increase in the elastin degradation score was also observed (Figure 5E and 5F). Moreover, in situ immunochemical staining showed that compared with WT mice, SV-KO mice displayed significantly increased MCP-1/CCL2 and MMP-2 protein expression after CaCl2 treatment (Online Figure VIIA through VIID). To determine the protective role of SIRT1 transgene in AAAs, we treated SV-Tg and their WT controls with CaCl2 for 6 weeks. In contrast, we found that the ratio of total aortic weight to body weight and the maximal external and internal abdominal aortic diameter were significantly decreased in SV-Tg mice compared with those of their littermates after CaCl2 treatment (Online Figure VIIIA through VIIIF). SIRT1 overexpression in VSMCs was also found to significantly inhibit CaCl2-induced MCP-1/CCL2 and MMP-2 protein expression (Online Figure VIIIG and VIIIH). These results indicate that SIRT1 in VSMCs plays a protective role in CaCl2-induced AAA formation.
Figure 5. Vascular smooth muscle cell (VSMC)–specific SIRT1 (Sirtuin 1) ablation promotes calcium chloride (CaCl2)–induced abdominal aortic aneurysm (AAA) formation and related vascular pathological changes.

A, Representative photographs showing macroscopic features of aneurysms induced by CaCl2 in wild-type (WT) and SIRT1-VSMC–specific knockout (SV-KO) mice for 3 wk (scale bars, 3 mm). B, The ratio of aorta weight:body weight in saline- or CaCl2-treated mice. C and D, The maximal internal (C) and external diameters (D) of infrarenal aortas in SV-KO mice (n=5–8) and WT mice (n=5–8) 3 wk after the CaCl2 treatment. There was no AAA formation in saline-treated mice (n=5–8 per group), and no death was observed. E, Representative staining with elastin in infrarenal aortas from saline- or CaCl2-treated mice (scale bars, 150, and 50 μm in magnified photographs). F, Elastin degradation score in infrarenal aortas from saline- or CaCl2-treated mice (n=5–8).
SIRT1 Is Crucial for Suppression of Vascular Cell Senescence and p21 Expression in AAAs
Ang II infusion accelerates vascular cell senescence in vivo, which can be characterized by SA-β-gal staining, as reported in the previous study,30 and we found that SA-β-gal–positive staining was mainly located in the media of the aortas (Online Figure IXA), suggesting a role of medial VSMC senescence in Ang II–induced AAAs. To investigate the role of SIRT1 in vascular cell senescence, SA-β-gal staining was performed in the aortas of Ang II- or saline-infused SV-KO mice and SV-Tg Apoe−/− mice and their respective controls. In saline-infused groups, few obvious SA-β-gal–positive areas were detected in both SV-KO mice and SV-Tg Apoe−/− mice. Ang II infusion led to the enlargement of SA-β-gal–positive regions in the aortas of WT mice, whereas the SIRT1-specific loss of function in VSMCs further increased the areas of SA-β-gal-positive staining (Figure 6A). The increase of SA-β-gal–positive staining in the aortas of SV-KO mice mainly occurred at medial VSMCs (Figure 6B). Moreover, Ang II–induced vascular cell senescence was further supported by the results of assays for SA-β-gal activity in the homogenates of whole aortas (Online Figure IXB). In contrast, SA-β-gal staining was suppressed in SV-Tg Apoe−/− mice (Figure 6C). Notably, SA-β-gal–positive staining in medial VSMCs was markedly inhibited in SV-Tg Apoe−/− mice (Figure 6D). As expected, the SA-β-gal activity assay also confirmed the antiaging effect of SIRT1 overexpression (Online Figure IXC). Furthermore, vascular aging was demonstrated by increased pulse wave velocity in the left common carotid artery in vivo.31,32 As expected, Ang II caused a modest increase in pulse wave velocity in WT mice, whereas it robustly increased the value in SV-KO mice (Online Figure IXD), and this trend was reversed in SV-Tg Apoe−/− mice (Online Figure IXE). These results indicate that the manipulation of SIRT1 expression or activity affects Ang II–induced vascular cell senescence in AAAs.
Figure 6. Vascular smooth muscle cell (VSMC)–derived SIRT1 (Sirtuin 1) is crucial for suppression of vascular cell senescence in angiotensin II (Ang II)–induced abdominal aortic aneurysms (AAAs).

All mice were infused with saline or Ang II for 4 wk. A, C, and E, Representative photographs and densitometric analysis of senescence-associated β-galactosidase (SA-β-gal)–stained aortas from wild-type (WT) and SIRT1-VSMC–specific knockout (SV-KO; A), Apoe−/− and SIRT1-vSMC–specific transgenic (Sv-Tg) Apoe−/− (C), young and aged (E) mice infused with saline or Ang II (scale bars, 5 mm; n=6 per group). B, D, and F, Representative images of SA-β-gal–stained transverse sections of suprarenal abdominal aortas from WT and SV-KO (B), Apoe−/− and SV-Tg Apoe−/− (D), and young and aged (F) mice infused with saline or Ang II. The blue region was positively stained, and nuclei were counterstained using Nuclear Fast Red (scale bars, 50 μm).
To further investigate the role of vascular cell senescence in aging-increased AAA formation, we examined SA-β-gal–positive areas in the aortas of young and aged mice with or without Ang II infusion. As shown in Figure 6E, the levels of SA-β-gal–positive areas in the aortas of saline-infused young and aged mice were low, and there was no difference between aged and young aortas; similar results were observed for SA-β-gal activity (Online Figure IXF), suggesting that vascular cell senescence was low and unaltered in aortas with advanced age. However, Ang II infusion significantly increased SA-β-gal–positive areas and SA-β-gal activity in the aortas of aged mice compared with those of young mice (Figure 6E; Online Figure IXF). The increase of SA-β-gal staining and SA-β-gal activity caused by Ang II was significantly greater in aged mice than that in young mice, indicating that aged aortas were more sensitive to Ang II–induced vascular senescence (Figure 6E; Online Figure IXF). Correspondingly, the increase of SA-β-gal–positive staining in the suprarenal aortas of aged mice mainly occurred at medial VSMCs (Figure 6F).
Cellular senescence marker p21 can be suppressed by SIRT1 in a p53-dependent and p53-independent manner.33,34 We found that acetylated p53 (Ac-p53) and p21 expression levels were higher in the whole aorta homogenates from Ang II–infused SV-KO mice (Figure 7A), and total p53 was also significantly increased (Figure 7A), whereas the SIRT1 transgene significantly reversed the increased protein levels of Ac-p53, total p53 and p21 in Ang II-infused Apoe−/− mice (Figure 7B). These results suggest that SIRT1 inhibited Ang II–induced p21 expression by deacetylation of p53 in AAAs. The increased p21 expression caused by Ang II was significantly greater in the aortas of aged mice than that in young mice (Figure 7C). Similarly, increased p21 expression was found in human AAA samples (Figure 7D). These results provide evidence that the increased vascular senescence and deregulated p21 expression caused by the reduction of SIRT1 participates in the promotional effect of aging on AAAs.
Figure 7. Vascular smooth muscle cell (VSMC)–derived SIRT1 (Sirtuin 1) is crucial for p53–p21 axis in angiotensin II (Ang II)–induced abdominal aortic aneurysms (AAAs).

All mice were infused with saline or Ang II for 4 wk. A and B, Representative Western blots and densitometric analysis of Ac-p53, p53, and p21 proteins in aorta homogenates from wild-type (WT) and SIRT1-VSMC–specific knockout (SV-KO; A), Apoe−/− and SIRTI-VSMC–specific transgenic (SV-Tg) Apoe−/− (B) mice infused with Ang II (n=6). C, Representative Western blots and densitometric analysis of p21 protein expression in aorta homogenates from young and aged mice infused with Ang II (n=4–6). D, Western blots of p21 protein expression in human AAA samples and control non-AAA adjacent aortas. The values of p21, p53, and Ac-p53 for Ang II–infused control group were set to 1.0 in each graph.
Suppression of p21 in VSMCs Abolishes Enhanced Nuclear Factor-κB Activation and MCP-1/CCL2 Expression by SIRT1 Inhibition
To further explore how SIRT1 reduction in VSMCs promotes Ang II–induced AAAs, gene expression microarray analysis was performed using RNA isolated from the aortas of Ang II–treated SV-KO mice and their Sirt1flox/flox littermates. Interestingly, analysis using ingenuity pathway analysis software revealed that the inflammatory response pathway was the pathway most affected by SIRT1 deficiency (Online Figure XA and XB and Online Table V), which was consistent with the aforementioned result of Online Figure IVA. Accordingly, nuclear factor (NF)-κB signaling was significantly activated and the mRNA levels of a series of NF-κB target genes, including MCP-1/CCL2,27,28 were significantly increased in the aortas of SV-KO mice compared with the WT mice after 4 weeks of Ang II infusion (Online Figure XIA through XID) in parallel with their protein levels as shown in Figures 3F and Online Figure IVB. Moreover, regulator effects in ingenuity pathway analysis further showed that CCR2, the receptor for MCP-1/CCL2, has the highest Consistency Score of all the regulators for cell recruitment, infiltration, and activation (Online Figure XII and Online Table VI). Furthermore, chromatin immunoprecipitation (ChIP) assays confirmed that VSMC-specific ablation of SIRT1 increased the binding activity of NF-κB on MCP-1/CCL2 promoter after Ang II infusion (Online Figure XIII), whereas a significantly lower binding level of NF-κB on MCP-1/CCL2 promoter was observed in the aortas of Ang II-infused SV-Tg Apoe−/− mice compared with their Apoe−/− littermates (Online Figure XIV), suggesting that SIRT1 reduction facilitates NF-κB–mediated transcriptional activation of MCP-1/CCL2 in AAAs. Moreover, p21 knockout mice were introduced to investigate its role in Ang II–induced transcriptional activation of MCP-1/CCL2 in AAAs. The results indicated that p21 knockout not only protects Apoe−/− mice against Ang II–induced AAA formation (Online Figure XVA through XVC) as previously reported30 but also almost completely blocked the expression of MCP-1/CCL2 and MMP-2 in Ang II–induced AAAs (Online Figure XVD and XVE).
Thus, we suspected that the promotional effect of SIRT1 reduction on Ang II–induced MCP-1/CCL2 expression might rely on the existence of p21 and VSMC cell senescence. To verify this hypothesis, adenovirus-mediated p21 RNA interference (RNAi) was introduced in VSMCs isolated from the aortas of SV-KO mice or control WT mice before treated with saline or Ang II. As expected, p21 mRNA level in both saline- and Ang II–treated VSMCs was significantly repressed by transfection of Ad-p21 RNAi (vectors for adenovirus-mediated knockdown of p21; Online Figure XVI). The results of SA-β-gal staining indicated that p21 knockdown not only blocked Ang II–induced VSMC senescence but also eradicated the promotional effect of SIRT1 knockout (Figure 8A and 8B; Online Table VII). Accordingly, Ang II–increased expression of MCP-1/CCL2 in SV-KO VSMCs was also significantly reduced by p21 knockdown (Figure 8C). Finally, we also performed ChIP assay to detect the binding level of NF-κB on MCP-1/CCL2 promoter in saline- or Ang II–treated human VSMCs infected by either Ad-SIRT1 RNAi or Ad-p21 RNAi or both of them. Three binding sites of NF-κB on human MCP-1/CCL2 promoter by ChIP assays were selected out from the 5 potential binding sites to assess the impact of p21 knockdown to the effect of SIRT1 RNAi on transcriptional activation of MCP-1/CCL2 (Online Figure XVII). The results indicated that p21 knockdown by RNAi almost completely blocked the promotional effect of SIRT1 knockdown on Ang II–increased RelA/p65 binding on the 3 potential NF-κB binding sites of MCP-1/CCL2 promoter (Figure 8D through 8F). These results support that p21-mediated VSMC cell senescence by SIRT1 reduction facilitates the transcriptional activation of MCP-1/CCL2, which may promote inflammatory cell recruitment and vascular inflammation in AAAs.
Figure 8. Suppression of p21 expression inhibits the promotional effect of SIRT1 (Sirtuin 1) reduction on angiotensin II (Ang II)–increased vascular smooth muscle cell (VSMC) senescence and transcriptional activation of monocyte chemoattractant protein-1 (MCP-1/CCL2).
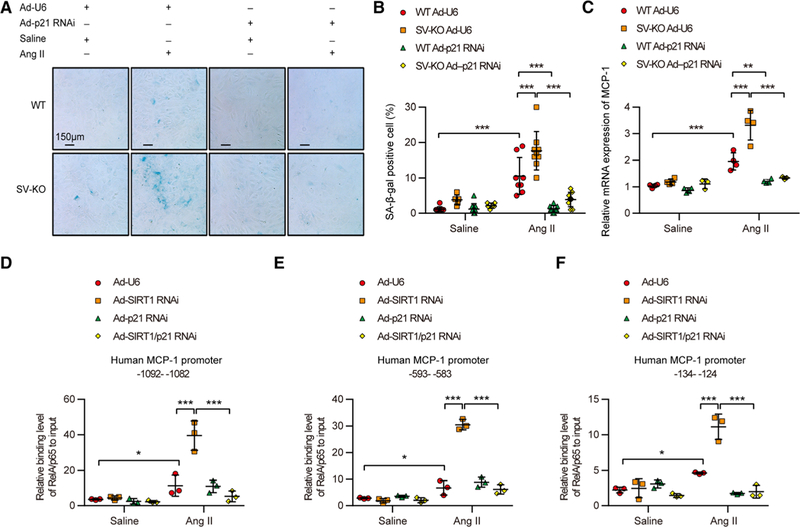
A, Senescence was evaluated through the senescence-associated β-galactosidase (SA-β-gal) staining of saline or Ang Il–treated wild-type (WT) and SIRT1-VSMC–specific knockout (SV-KO) VSMCs infected with Ad-U6 (a control RNAi vector) or Ad-p21 RNAi (vectors for adenovirus-mediated knockdown of p21) for 24 h. Blue-stained cells were considered senescent. The bar represents 150 μm. B, Statistical analysis of the percentage of sA-β-gal–positive cells. Five random fields of view were analyzed for 1 group (n=7–10). C, Relative MCP-1/CCL2 mRNA expression level detected by real-time polymerase chain reaction in saline or Ang Il–treated WT and SV-KO VSMCs infected with Ad-U6 or Ad-p21 RNAi. D–F, Relative binding level of RelA/p65 to input on indicated region of human MCP-1/CCL2 promoter. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In the present study, we demonstrated that SIRT1 acts as a novel molecular link that retards vascular senescence and inflammation to prevent AAA initiation and development. There are several major findings in this study. First, the expression and activity of SIRT1 were significantly decreased in human AAA samples. Second, SIRT1 in VSMCs was substantially downregulated in the suprarenal aortas of aged mice, in which AAAs induced by Ang II infusion were significantly elevated. Third, SIRT1 reduction in VSMCs amplified Ang II–induced vascular aging and AAA formation in mice in vivo, whereas genetic activation of SIRT1 in VSMCs displayed the opposite effect. The inhibitory effect of SIRT1 on AAA formation was also confirmed in the CaCl2–induced AAA model. Fourth, the reduction of SIRT1 was shown to increase vascular cell senescence and upregulate p21 expression and enhance vascular inflammation. Moreover, suppression of p21-dependent vascular cell senescence by SIRT1 inhibited Ang II–induced inflammation.
AAA incidence increases steeply by 40% every 5 years in men aged >65 years, indicating that age is a major risk factor for AAAs.2 Why advanced age (>65 years) precipitates AAA development and progression is unknown. In the present study, we found that SIRT1 protein and activity were significantly suppressed in the aortic VSMCs of aged mice, and vascular cell senescence in the aortas of aged mice was low and comparable to that of young mice under natural conditions. Concomitantly, only a few incidences of spontaneous AAAs were observed in the same conditions. In contrast, AAA formation and vascular cell senescence in SV-KO mice and aged mice were significantly increased by Ang II infusion, indicating that the SIRT1 reduction in advanced age creates an environment that facilitates AAA initiation and progression caused by Ang II, but does not directly instigate AAAs. This conclusion is supported by human epidemiological evidence indicating that aging is a risk factor for AAA and only a small number of men with advanced age develop AAAs. Accumulating evidence has shown that SIRT1 plays an important role in healthy aging and age-related diseases,8,9 which suggests that the SIRT1 reduction in advanced age may also predispose individuals to the aging of other tissues and to age-related cardiovascular diseases.
Systemic inflammation has been linked to multiple chronic diseases of aging and may even contribute to their causation.35–37 Although increases in chronic inflammation have been detected in the vasculature with age,5–7 the molecular mechanisms that link vascular aging and inflammation to AAA formation remain elusive. Previous studies have identified important roles for p53 in promoting aging and NF-κB in mediating inflammatory response, both of which can be targeted by SIRT1.34,38–41 In the present study, we found that SIRT1 reduction increased Ang II–induced VSMC senescence and upregulated p53 acetylation and p21 protein. Inflammatory NF-κB activation and MCP-1/CCL2 were significantly increased in parallel with increased vascular cell senescence. Accordingly, AAA formation was significantly enhanced by SIRT1 reduction in both SV-KO mice and in aged mice (Online Figure XVIIIA). Moreover, Ang II–activated NF-κB transcription of MCP-1/CCL2 expression was significantly decreased by suppression of p21 in SIRT1 knockout VSMCs. These results suggest that the deregulated SIRT1–p53–p21 axis in VSMCs in response to Ang II accelerates NF-κB–induced vascular inflammation and renders aortas susceptible to AAAs. To the best of our knowledge, this is the first time that a direct link between vascular cell senescence and vascular inflammation to AAA formation by SIRT1 reduction has been reported. Importantly, AAA formation and the related pathological and molecular changes could be effectively inhibited by VSMC-specific overexpression of SIRT1, which provides a potential therapeutic target for AAAs (Online Figure XVIIIB). However, whether SIRT1 reduction in VSMCs induces NF-κB–mediated vascular inflammation and accelerates AAA formation by downregulation of p53/p21 in vivo requires further investigation. In addition, our results highlight the importance of VSMC senescence in the development of vascular inflammation and AAAs, which is also consistent with the well-known roles of VSMCs in AAA formation.21,29,42,43
In summary, our findings indicate that the age-related reduction of SIRT1 in VSMCs predisposes aortas to AAAs by facilitating p21-dependent vascular cell senescence, secretion of inflammatory cell recruitment molecules, and vascular inflammation. These findings indicate a direct link between vascular cell senescence and AAAs through vascular inflammation that provides a deeper understanding of the relationship between aging and age-related vascular diseases.
Supplementary Material
Novelty and Significance.
What Is Known?
In humans, the incidence of abdominal aortic aneurysm (AAA) increases dramatically with age.
SIRT1 is highly expressed in the vasculature and plays a protective role in vascular remodeling, aortic stiffness and dissection, and atherosclerosis in mice.
What New Information Does This Article Contribute?
The expression and activity of SIRT1 are significantly decreased in human AAA samples.
SIRT1 in VSMCs is downregulated in the suprarenal aortas of aged mice, in which AAAs induced by Ang II infusion were significantly elevated.
VSMC-specific knockout of SIRT1 accelerates Ang II- or CaCl2-induced AAA formation, whereas VSMC-specific overexpression of SIRT1 displays the opposite effect.
SIRT1 reduction increases Ang II-induced vascular cell senescence, upregulates the expression of p21 and enhances inflammatory cell recruitment for vascular inflammation in AAAs.
Age is a major risk factor for AAAs. Uncontrolled growth of AAAs is a life-threatening vascular disease without an effective pharmaceutical treatment. Our study shows that age-associated SIRT1 reduction in VSMCs accelerates the formation and rupture of AAAs, whereas VSMC-specific overexpression of SIRT1 suppresses AAA formation and progression. SIRT1-mediated inhibition of vascular cell senescence suppresses vascular inflammation in AAAs. These findings suggest that the manipulation of SIRT1 activation may effectively inhibit vascular cell senescence, vascular inflammation, and delay or prevent AAA initiation and progression.
Acknowledgments
We wish to acknowledge the excellent technical help of De-Long Hao and Xiang Zhao in producing SIRT1-VSMC–specific transgenic and SIRT1-VSMC–specific knockout mice.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81422002, 91339201, 31571193, and 31271227) and the National Science and Technology Support Project (2013YQ0309230502 and 2014BAI02B01). Dr Zou’s laboratory was supported by the grants from the National Heart, Lung, and Blood Institute (HL080499, HL105157) and National Institute on Aging (AG047776).
Nonstandard Abbreviations and Acronyms
- AA
abdominal aortas
- AAA
abdominal aortic aneurysm
- Ang II
angiotensin II
- KO
knockout
- MCP-1/CCL2
monocyte chemoattractant protein-1
- MMP
matrix metalloproteinase
- MMP2
matrix metalloproteinase 2
- NF-κB
nuclear factor-κB
- RNAi
RNA interference
- SA-β-gal
senescence-associated β-galactosidase
- SIRT1
Sirtuin 1
- SV-KO
SIRT1-VSMC–specific knockout
- Tg
transgenic
- VSMC
vascular smooth muscle cell
- WT
wild-type
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.116.308895/-/DC1.
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 3.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golledge J, Norman PE. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis. 2011;217:57–63. doi: 10.1016/j.atherosclerosis.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 8.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Zhang HN, Chen HZ, et al. SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res. 2011;108:1180–1189. doi: 10.1161/CIRCRESAHA.110.237875. [DOI] [PubMed] [Google Scholar]
- 13.Gao P, Xu TT, Lu J, Li L, Xu J, Hao DL, Chen HZ, Liu DP. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J Mol Med (Berl). 2014;92:347–357. doi: 10.1007/s00109-013-1111-4. [DOI] [PubMed] [Google Scholar]
- 14.Fry JL, Al Sayah L, Weisbrod RM, Van Roy I, Weng X, Cohen RA, Bachschmid MM, Seta F. Vascular smooth muscle sirtuin-1 protects against diet-induced aortic stiffness. Hypertension. 2016;68:775–784. doi: 10.1161/HYPERTENSIONAHA.116.07622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry JL, Shiraishi Y, Turcotte R, Yu X, Gao YZ, Akiki R, Bachschmid M, Zhang Y, Morgan KG, Cohen RA, Seta F. Vascular smooth muscle sirtuin-1 protects against aortic dissection during angiotensin ii-induced hypertension. J Am Heart Assoc. 2015;4:e002384. doi: 10.1161/JAHA.115.002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein S, Lohmann C, Schäfer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Borén J, McBurney MW, Landmesser U, Lüscher TF, Matter CM. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J. 2010;31:2301–2309. doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, Bennett M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127:386–396. doi: 10.1161/CIRCULATIONAHA.112.124404. [DOI] [PubMed] [Google Scholar]
- 19.Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res. 2010;106:1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
- 20.Wan YZ, Gao P, Zhou S, Zhang ZQ, Hao DL, Lian LS, Li YJ, Chen HZ, Liu DP. SIRT1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell. 2014;13:890–899. doi: 10.1111/acel.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gary RK, Kindell SM. Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal Biochem. 2005;343:329–334. doi: 10.1016/j.ab.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O and et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 26.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Zhang C, Zhang M, Liang B, Zhu H, Lee J, Viollet B, Xia L, Zhang Y, Zou MH. Activation of AMP-activated protein kinase α2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nat Med. 2012;18:902–910. doi: 10.1038/nm.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 31.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. [DOI] [PubMed] [Google Scholar]
- 32.Williams R, Needles A, Cherin E, Zhou YQ, Henkelman RM, Adamson SL, Foster FS. Noninvasive ultrasonic measurement of regional and local pulse-wave velocity in mice. Ultrasound Med Biol. 2007;33:1368–1375. doi: 10.1016/j.ultrasmedbio.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, Eto M, Akishita M, Ouchi Y. Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2054–2062. doi: 10.1161/ATVBAHA.110.216739. [DOI] [PubMed] [Google Scholar]
- 34.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. [DOI] [PubMed] [Google Scholar]
- 35.Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol. 2014;29:23–28. doi: 10.1016/j.coi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R, Chen HZ, Liu DP. The four layers of aging. Cell Syst. 2015;1:180–186. doi: 10.1016/j.cels.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, Hoshii Y, Tanaka N, Ricci R, Ishihara T, Esato K, Hamano K, Matsuzaki M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 43.Salmon M, Johnston WF, Woo A, Pope NH, Su G, Upchurch GR Jr, Owens GK, Ailawadi G. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation. 2013;128:S163–S174. doi: 10.1161/CIRCULATIONAHA.112.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


