Abstract
JAK/STAT pathway is one among the several oxidative stress-responsive signaling pathways that play a critical role in facilitating cisplatin-induced ototoxicity. Cisplatin treatment decreases the levels of cochlear LMO4, which acts as a scaffold for IL6-GP130 protein complex. Cisplatin-induced nitration and degradation of LMO4 could destabilize this protein complex, which in turn could compromise the downstream STAT3-mediated cellular defense mechanism. Here, we investigated the link between cisplatin-induced nitrative stress and STAT3-mediated apoptosis by using organ of Corti cell cultures. SRI110, a peroxynitrite decomposition catalyst that prevented cisplatin-induced decrease in LMO4 levels and ototoxicity, was used to inhibit nitrative stress. Immunoblotting and immunostaining indicated that cisplatin treatment decreased the expression levels, phosphorylation, and nuclear localization of STAT3 in UB/OC1 cells. Inhibition of nitration by SRI110 co-treatment prevented cisplatin-induced inactivation of STAT3 and promoted its nuclear localization. SRI110 co-treatment reversed the cisplatin-induced changes in the expression levels of Bcl2l1, Ccnd1, Jak2, Jak3, and Src and significantly attenuated the changes in the expression levels of Cdkn1a, Egfr, Fas, Il6st, Jak1, Stat3, and Tyk2. Collectively, these results suggest that the inhibition of cisplatin-induced nitration prevents the inactivation of STAT3, which in turn enables the transcription of anti-apoptotic genes and thereby helps to mitigate cisplatin-induced toxicity.
Keywords: STAT3, Cochlea, Cisplatin, Ototoxicity, LMO4, Nitrative stress
INTRODUCTION
Cisplatin, a potent chemotherapeutic drug, induces cellular apoptosis to kill cancer cells. The cochlear, renal, and neuronal cells are also highly susceptible to cisplatin-induced apoptosis (Rathinam et al., 2015) and therefore, ototoxicity, nephrotoxicity, and neurotoxicity are important side effects of cisplatin treatment (Maccio and Madeddu, 2013). Cisplatin-induced toxicity in the cochlea is partly attributed to the imbalance between anti-apoptotic and pro-apoptotic proteins, which result in the activation of cell death pathways (Sheth et al., 2017). Alterations in the expression of BCL-2 family of proteins (Rybak et al., 2012; Waissbluth et al., 2012), activation of several caspases (Borse et al., 2017; Devarajan et al., 2002), and increased levels of tumor suppressor protein p53 (Zhang et al., 2003; Benkafadar et al., 2017) have been implicated in cisplatin-induced ototoxicity. More specifically, elevated ratio of pro-apoptotic BAX to anti-apoptotic BCL-2 was observed after cisplatin treatment (Wang et al., 2004). The transcription of BCL-2 family of anti-apoptotic genes is regulated by STAT3, whose expression levels in the cochlea were decreased by cisplatin treatment (Jamesdaniel, 2014; Levano and Bodmer, 2015). Collectively, these findings suggest that the STAT3-mediated anti-apoptotic machinery is a potential cochlear target of cisplatin. However, the upstream events that contribute to cisplatin-induced downregulation of STAT3 and downstream effects on STAT3-related signaling molecules, in the inner ear, are yet to be fully understood.
The generation of reactive oxygen species usually precedes the induction of apoptotic cell death in cisplatin ototoxicity (Sheth et al., 2017; Mohan et al., 2014; Kim et al., 2010). JAK/STAT pathway is one among the several oxidative stress-responsive signaling pathways that are activated by cisplatin (Martindale and Holbrook, 2002; Levano and Bodmer, 2015). The Janus kinases, such as JAK1 and TYK2, are part of the IL6-GP130 molecular complex, and are usually activated by the binding of membrane receptors to several hormones, cytokines, and growth factors. Upon activation, JAK1 enables the dimerization and phosphorylation of STAT3 and facilitates signal transduction to the nucleus where the STATs bind specific regulatory sequences allowing activation or repression of target genes involved in cell growth, differentiation and survival (Rawlings et al., 2004; Nicolas et al., 2013). Several studies have shown that STAT3 facilitates the inhibition of programmed cell death (Yu and Jove 2004). However, cisplatin treatment could compromise this STAT3-mediated cellular defense mechanism by destabilizing the IL6-GP130 complex, because cisplatin decreases the levels of cochlear LMO4, which acts as a scaffold for this protein complex. Our studies indicated that cisplatin induces nitration of LMO4 and the consequent decrease in LMO4 levels facilitates ototoxicity (Jamesdaniel et al., 2012; Jamesdaniel et al., 2016). Based on these findings, we hypothesize that the destabilization of IL6-GP130 complex, due to cisplatin-induced nitration and downregulation of LMO4, impedes JAK1 dependent recruitment/activation of STAT3 and thereby compromises the transcription of anti-apoptotic genes and facilitates cisplatin-induced ototoxicity.
Protein nitration and its signaling have been effectively targeted to prevent apoptosis in many other models (Kim et al., 2010; Abdelsaid et al., 2010; Zou et al., 2011). Selective inhibition of protein nitration by using SRI110, a peroxynitrite decomposition catalyst, prevented cisplatin-induced decrease in LMO4 levels and ototoxicity (Jamesdaniel et al., 2016). SRI110 is a manganese pyrrole compound, which has also been employed to inhibit paclitaxel induced protein nitration and to ameliorate its neuropathic side effects (Doyle et al., 2012). Targeting peroxynitrite appears to be an attractive interventional approach because peroxynitrite does not have any known beneficial role and selective inhibition of protein nitration did not interfere with the anti-cancer activity of drugs such as paclitaxel. In this study, we used UB/OC1 cells, an immortal cell line derived from the mouse embryonic organ of Corti, to investigate the effects of inhibition of nitration on STAT3-mediated anti-apoptotic signaling in cisplatin-induced toxicity.
MATERIALS AND METHODS
Cell culture:
Fully differentiated UB/OC1 cells were used for all experiments (Rivolta et al., 2002). The UB/OC1 cells are immortal cells derived from the mouse organ of Corti and were provided by Dr. Mathew C Holley (University of Sheffield, UK). Initially, the cells were proliferated by culturing in minimum essential medium (GlutaMAX, catalog no.41090–036, Thermo Fisher Scientific, Rockford, IL) with 10% fetal bovine serum (Gibco BRL, Gaithersburg, MD) and 50 U/ml γIF (# 315–05, PeproTech, Rocky Hill, NJ, USA). To facilitate differentiation, the cells were cultured in the same medium but without γIF and incubated in a humidified atmosphere at 37°C with 5%CO2.
Drug treatment:
Cisplatin (P4394) was purchased from Sigma-Aldrich (St. Louis, MO) and SRI110 was synthesized as reported previously (Rausaria et al., 2011). The UB/OC1 cells were seeded in 6-well plates at the rate of 220,000 cells/well and were cultured for 24 hours. Then the cells were treated with either cisplatin (20 μM) or SRI110 (10 μM) or both and cultured for another 24 hours. SRI110 treatment was performed 1 hour before cisplatin treatment, for cells that received both drugs. Both cisplatin and SRI110 were dissolved in sterile culture media and their dose was determined based on our previous study (Rathinam et al., 2015). For controls, the cells were treated with equal volume of the culture media.
Protein extraction:
UB/OC1 cells were washed with ice-cold 1× PBS and lysed in 100 μL of radio-immunoprecipitation assay buffer containing protease and phosphatase inhibitors (Thermo Fisher Scientific, Rockford, IL). The concentration of the proteins was determined by Bradford protein assay (# 1856209, Thermo Fisher Scientific, Rockford, IL).
Immunoblotting:
Thirty micrograms of protein was loaded on 10% acrylamide/bis gel. The separated proteins were transferred on to a polyvinylidene difluoride membrane, blocked with 5% fat-free milk in phosphate-buffered saline containing 0.05% Tween 20 (Sigma-Aldrich, St. Louis, MO), and incubated overnight with mouse monoclonal STAT3 (# 9139, Cell Signaling Technology, Danvers, MA; 1:1000) or rabbit polyclonal pSTAT3(# 9131, Cell Signaling Technology, Danvers, MA; 1 : 500). Then the blots were washed thrice with TBS-T buffer and incubated with peroxidase-conjugated anti-mouse (# sc-2005, Santa Cruz Biotechnology Inc., Dallas, TX) or anti-rabbit secondary antibody (# sc-2030, Santa Cruz Biotechnology Inc., Dallas, TX) for an hour at room temperature. Then the blots were washed thrice with TBS-T buffer and developed using chemiluminescence detection reagent (# 34076, Thermo Fisher Scientific), and the bands were visualized using FluorChem E System (ProteinSimple, San Jose, CA, USA). Background-corrected bands were normalized against actin detected using HRP-conjugated actin antibody (# sc-1615, Santa Cruz Biotechnology Inc., Dallas, TX) and their intensity was measured using ImageJ software (NIH, Bethesda, MD).
Immunocytochemistry:
UB/OC1 Cells were plated on two-well chamber slides (# 154461, Nunc Lab-Tek II Chamber Slide system, Fisher Scientific, Pittsburgh, PA) at the rate of 25,000 cells/well and incubated for 24 hours. Then the cells were treated with 20 μM cisplatin or 20 μM cisplatin plus 10 μM SRI110 or SRI110 alone, for 24 hours. The cells were fixed with pre-cold methanol/acetone (1:1) mixture at −20 °C for 20 min, permeabilized with 0.2% Triton X-100 in PBS for 30 min, blocked (5% goat serum, 2% (w/v) bovine serum albumin in 1× PBS) for 1 hour, and incubated with primary antibodies (1:600) for 2 hours at room temperature. STAT3 (# 9139S) and pSTAT3 (# 9145S) antibodies were from Cell Signaling Technology (Danvers, MA) while nitrotyrosine antibody (# sc-32757) was from Santa Cruz Biotechnology Inc, (Dallas, TX). The cells were washed thrice with 1× PBS and then incubated with Alexa Fluor 568 donkey anti-mouse (# A100037, Life Technologies, Carlsbad, CA; 1:400), Alexa Fluor 647 goat anti-rabbit (# A21244, Life Technologies, Carlsbad, CA; 1:500), and fluorescein phalloidin (# F432, Life Technologies, Carlsbad, CA) for 1 hour at room temperature. ProLong Gold antifade reagent containing DAPI (# P36935, Life Technologies, Carlsbad, CA) was used for mounting the cells and Carl Zeiss Laser Scanning Systems (Zeiss LSM 780, Jena, Germany) was used to capture the images. The intensity of the staining was quantified by measuring the pixel values using ImageJ software (version IJ 1.49).
Co-immunoprecipitation:
Lysate containing 1 mg of protein was pre-cleared for 1 hour and then incubated overnight at 4 °C with anti-LMO4 (1:20; # ab 131030, abcam, Cambridge, MA). Then the lysate was incubated with 50 μl of magnetic dynabeads G (# 10007D, Life Technologies, Carlsbad, CA) for 2 hours at 4 °C. The supernatant was removed and the beads were washed and eluted following manufacturer’s directions. The eluate was loaded on a 7.5% acrylamide/bis gel and the separated proteins were transferred on to a polyvinylidene difluoride membrane. The blot was developed as described before after overnight incubation with anti-JAK1 (1:1000; # 50996, Cell Signaling Technology, Danvers, MA).
RNA isolation and cDNA synthesis:
UB/OC1 cells were washed once with 1× PBS, and lysed in 500 μL Qiazol. The lysate was centrifuged and the RNA was extracted from the supernatant using RNeasy Microarray Tissue Mini kit (# 73304, Qiagen, Valencia, CA). The quality of the RNA and its concentration were verified using NanoDrop 8000 (Thermo Fisher Scientific, Rockford, IL). The purity of RNA was determined from A260: A230 and A260: A280 ratios. First strand cDNA was synthesized from 0.5 μg RNA using the RT2 First Strand kit (# 330401, Qiagen, Valencia, CA), following the manufacturer’s protocol.
Reverse-Transcription PCR:
The first strand cDNA was mixed with RT2 SYBR Green qPCR Mastermix (# 330529, Qiagen, Valencia, CA) and 25 μL of the mixture was loaded in each well of the Jak/Stat pathway RT2 Profiler PCR Array (# PAMM-039Y, Qiagen, Valencia, CA), following the manufacturer’s instructions. Step One RT-PCR system (Applied Biosystems, Foster City, CA) was used for real-time PCR amplification. The system was programed to include a 10 min Hot-Start at 95 °C, followed by 40 cycles of 15 s denaturation at 95 °C and 30 s annealing at 60 °C. Actb, B2m, Gapdh, Gusb and Hsp90ab1 were used as housekeeping genes and fold changes of mRNA levels were calculated from Ct values of individual genes. Fold regulation of 2 or above was considered as upregulation while fold regulation of −2 or below was considered as downregulation.
Data analysis:
All experiments were repeated at least three times using different cell passages. The results are expressed as mean ± standard deviation or standard error mean. All data were statistically analyzed using the GraphPad Prism 6 software (La Jolla, CA). Significant differences between the control and drug treated groups were determined by using unpaired t tests. P value of < 0.05 was considered significant.
RESULTS
Cisplatin treatment decreased the expression as well as phosphorylation of STAT3.
Immunoblots with anti-STAT3 (Figure 1 A,B) indicated that cisplatin treatment significantly decreased the levels of STAT3 (p<0.05) in the UB/OC1 cells. This is consistent with our previous findings indicating cisplatin-induced decrease in STAT3 levels in rat cochlea (Jamesdaniel, 2014). In addition, immunoblots with anti-pSTAT3 (Figure 1 A,B) indicated that cisplatin treatment significantly decreased the levels of phosphorylated STAT3 (p<0.05) in UB/OC1 cells. These findings collectively suggest that cisplatin treatment not only decreases the expression of STAT3 but also results in the inactivation of STAT3.
Figure 1. Cisplatin-induced decrease in the expression of STAT3 and PSTAT3 was prevented by SRI110 co-treatment.
Immunoblots with anti-STAT3 and anti-pSTAT3 show that cisplatin-induced decrease in the expression and phosphorylation of STAT3 were reversed by SRI110 co-treatment (*p=0.030 and *p=0.0411, respectively), in UB/OC1 cells. STAT3 and pSTAT3 expression were normalized with that of actin. Results are expressed as mean ± standard deviation (n=3).
Cisplatin-induced changes in STAT3 levels were inversely correlated with nitrotyrosine levels.
Immunostaining of UB/OC1 cells indicated that cisplatin treatment decreased the expression of STAT3, while immunostaining with anti-nitrotyrosine indicated that cisplatin treatment increased the nitrotyrosine levels in UB/OC1 cells (Figure 2). These results are consistent with our previous findings in the rat cochlea (Jamesdaniel, 2014, Jamesdaniel et al, 2012). Together, these findings suggest an inverse correlation between cisplatin-induced changes in the levels of STAT3 and nitrotyrosine.
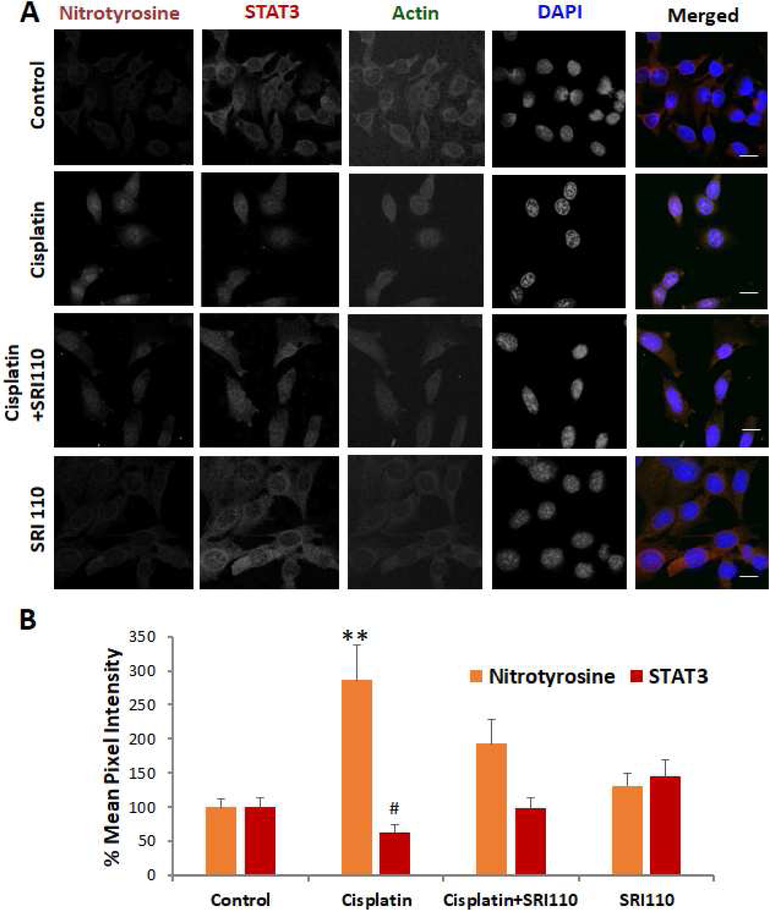
Figure 2. Cisplatin-induced alterations in STAT3 as well as nitrotyrosine levels were attenuated by SRI110 co-treatment.
A) Immunostaining indicated that cisplatin treatment decreased the expression levels of STAT3 (#p=0.027) and increased the levels of nitrotyrosine (**p=0.003), in UB/OC1 cells. SRI110 co-treatment reversed the cisplatin-induced changes. Orange staining indicates nitrotyrosine, red staining indicates STAT3, green indicates actin staining by phalloidin, and blue indicated nuclear staining by DAPI. The images are representative of three replicates. Scale bar = 20 μm. B) Quantification of the changes in the expression levels suggest that cisplatin-induced changes in the expression of STAT3 were inversely correlated with that of nitrotyrosine and attenuated by SRI110 co-treatment. The results are expressed as mean ± standard error mean (n=3).
Inhibition of nitration attenuated cisplatin-induced decrease in the expression and phosphorylation of STAT3.
SRI110 was used to inhibit cisplatin-induced nitration. Co-treatment of UB/OC1 cells with SRI110 reversed the cisplatin-induced decrease in the level of STAT3 (p<0.05) as well as the phosphorylated STAT3 (p<0.05), as indicated by the immunoblots (Figure 1 A,B). Similarly, immunostaining with anti-STAT3 indicated that SRI110 co-treatment restored the expression of STAT3 in the cisplatin-treated UB/OC1 cells (Figure 2). Together, these findings suggest a potential link between cisplatin-induced nitrative stress and the inactivation of STAT3 proteins in UB/OC1 cells.
Cisplatin treatment decreased nuclear pSTAT3 levels while SRI110 co-treatment promoted nuclear localization of pSTAT3.
Sub-cellular localization of pSTAT3 was evaluated in UB/OC1 cells by immunostaining with anti-pSTAT3 (Figure 3 A,B). Cisplatin treatment decreased the levels of phosphorylated STAT3 in the nucleus (*p<0.05), suggesting potential compromise of STAT3-mediated transcription by cisplatin. However, co-treatment with SRI110 increased the levels of phosphorylated STAT3 in the nucleus (*p<0.05), suggesting that inhibition of nitration promotes the nuclear localization of phosphorylated STAT3.
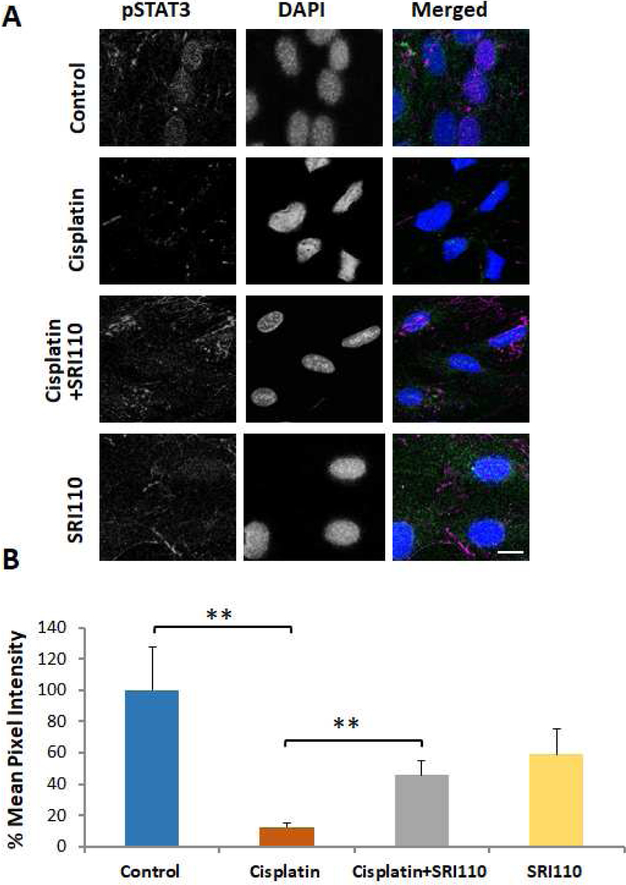
Figure 3. Cisplatin treatment decreased the nuclear localization of pSTAT3, which was attenuated by SRI110 co-treatment.
A) Immunostaining of UB/OC1 cells indicated that cisplatin decreased the levels of pSTAT3 in the nucleus while SRI110 co-treatment restored the nuclear localization of pSTAT3. Violet staining indicates pSTAT3, green indicates actin staining by phalloidin, and blue indicated nuclear staining by DAPI. The images are representative of three replicates. Scale bar = 10 μm. B) The quantification of the immunostaining indicated that the cisplatin-induced significant decrease in the levels of phosphorylated STAT3 in the nucleus (**p=0.003) was reversed by SRI110 co-treatment (**p=0.001). The results are expressed as mean ± standard error mean (n=3).
Inhibition of nitration attenuated cisplatin induced decrease in the expression of JAK1.
Co-immunoprecipitation of JAK1 with LMO4 suggested the direct interaction between JAK1 and LMO4 in UB/OC1 cells (Figure 4 A), which is consistent with previous reports using other cell lines (Novotny-Diermayr et al., 2005). JAK1 was not detected in negative controls that were immunoprecipitated with anti-GAPDH. Moreover, the JAK1 levels were significantly decreased by cisplatin treatment while co-treatment with SRI110 attenuated the cisplatin-induced decrease in JAK1 (Figure 4 B). Together, these findings support the hypothesis that JAK/STAT signaling is altered by cisplatin-induced nitrative stress.
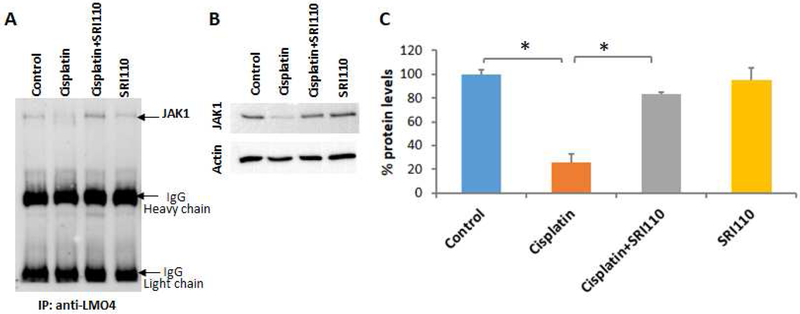
Figure 4. JAK1 interacts with LMO4 and is downregulated by cisplatin treatment.
A) Immunoprecipitation with anti-LMO4 followed by immunoblotting with anti-JAK1 indicated that JAK1 interacts with LMO4 in UB/OC1 cells. B & C) Immunoblots indicated that treatment with 20 μM Cisplatin significantly decreased the protein levels of JAK1 in UB/OC1 cells and co-treatment with 10 μM of SRI110 significantly attenuated the cisplatin-induced decrease in JAK1 (*p<0.01). The expression of JAK1 were normalized with that of actin. Results are expressed as mean ± standard deviation (n=3).
Cisplatin treatment altered the expression levels of STAT3 signaling genes.
Treatment of UB/OC1 cells with cisplatin altered the expression levels of 13 genes associated with the IL6/STAT3 signaling pathway (Figure 5). Among these, the expression of 2 genes (Cdkn1a and Cebpd) was up regulated while the expression of 11 genes (Bcl2l1, Ccnd1, Egfr, Fas, Il6st, Jak1, Jak2, Jak3, Src, Stat3, and Tyk2) was down regulated by cisplatin treatment. Together, the cisplatin-induced changes in STAT3 signaling genes suggest a potential role of IL6/STAT3 signaling pathway in cisplatin-induced cytotoxicity.
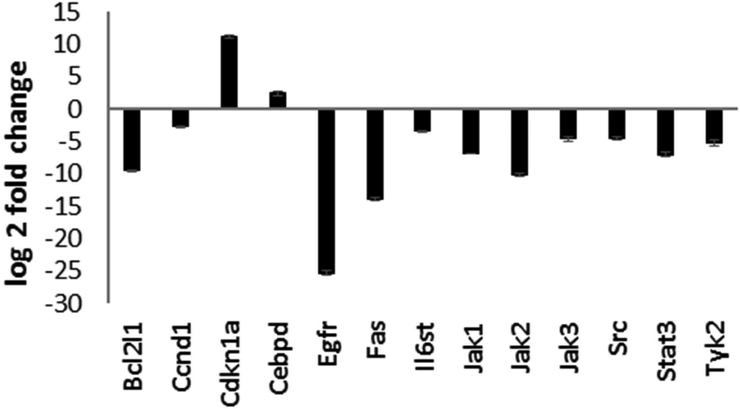
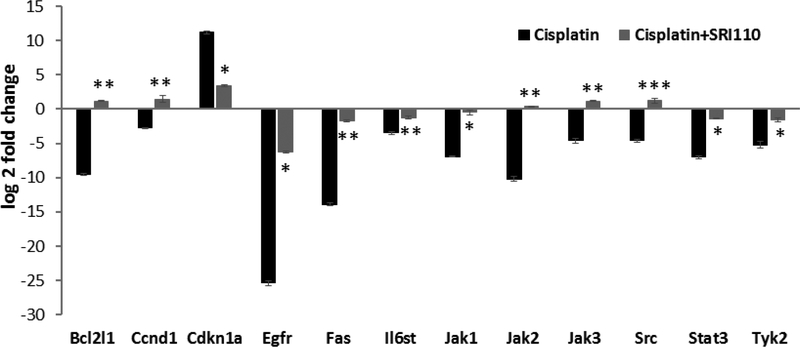
Figure 5. Cisplatin treatment modulated JAK/STAT signaling genes in UB/OC1 cells.
Treatment of UB/OC1 cells with cisplatin significantly up regulated the expression of Cdkn1a and Cebpd and down regulated the expression of Bcl2l1, Ccnd1, Egfr, Fas, Il6st, Jak1, Jak2, Jak3, Src, Stat3, and Tyk2, which are genes associated with the IL6/STAT3 signaling pathway. P value of < 0.01 was considered significant. Results are expressed as mean ± standard deviation (n=3).
Inhibition of nitration attenuated cisplatin-induced alterations in the expression of STAT3-related anti-apoptotic genes.
Inhibition of cisplatin-induced nitrative stress by SRI110 co-treatment reversed the cisplatin-induced changes in the expression levels of 5 genes (Bcl2l1, Ccnd1, Jak2, Jak3, and Src) and significantly attenuated the changes in the expression levels of 7 genes (Cdkn1a, Egfr, Fas, Il6st, Jak1, Stat3, and Tyk2). This suggests that cisplatin-induced nitration alters the STAT3 mediated anti-apoptotic signaling in cisplatin toxicity (Figure 6).
Figure 6. SRI110 co-treatment attenuated cisplatin-induced modulation of JAK/STAT signaling genes.
Inhibition of cisplatin-induced nitrative stress by SRI110 co-treatment reversed the cisplatin-induced changes in the expression levels of Bcl2l1, Ccnd1, Jak2, Jak3, and Src and significantly attenuated the changes in the expression levels of Cdkn1a, Egfr, Fas, Il6st, Jak1, Stat3, and Tyk2 in UB/OC 1 cells. Results are expressed as mean ± standard deviation, * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, (n=3).
DISCUSSION
STAT3 is an important downstream target of LMO4, whose nitration and consequent degradation play a central role in cisplatin ototoxicity (Jamesdaniel et al., 2012; Jamesdaniel 2014). Although targeting cisplatin-induced nitrative stress mitigated ototoxicity (Jamesdaniel et al., 2016) it is not clear whether the therapeutic effects are mediated via the activation of STAT3-associated anti-apoptotic machinery. To clarify this, cisplatin-induced changes in the expression, activation, and localization of STAT3 as well the modulation of IL6/STAT3 signaling genes were investigated. Cisplatin treatment decreased the expression as well as phosphorylation of STAT3 in organ of Corti cell cultures. Inhibition of cisplatin-induced nitration by SRI110 co-treatment promoted the phosphorylation as well as nuclear localization of STAT3. Moreover, cisplatin treatment modulated several genes in the IL6/STAT3 signaling pathway while SRI110 co-treatment attenuated the cisplatin-induced up/down-regulation of these genes. Together, these findings provided critical insights on STAT3 signaling in cisplatin toxicity and its potential link with cisplatin-induced nitrative stress.
A role of STAT3 in facilitating cisplatin-induced ototoxicity has been reported in recent studies. Repression or loss of STAT1 (Kaur et al., 2011; Levano and Bodmer, 2015), which is cross-regulated by STAT3 (Stephanou and Latchman, 2005; Hu and Ivashkiv, 2009), protected the hair cells from ototoxicity while attenuation of cisplatin-induced decrease in cochlear STAT3 by co-treatment with antioxidant Trolox mitigated associated hearing loss (Jamesdaniel, 2014). Our observations indicated that cisplatin treatment not only decreased the expression levels of STAT3 but also its phosphorylation and nuclear localization, in UB/OC1 cells. The inactivation of STAT3 is probably mediated by cisplatin-induced decrease in JAK1 as well as changes in the expression levels of several upstream signaling genes in the IL6/STAT3 pathway. Cisplatin treatment decreased the expression levels of Egfr, Fas, and Il6st, which encode receptors that trigger the activation of STAT3. In addition, the expression levels of genes that facilitate the recruitment and activation of STAT3, such as Jak1, Jak2, Jak3, Src, and Tyk2, were also decreased.
The cisplatin-induced inactivation of STAT3 could compromise the transcription of anti-apoptotic genes and thereby enhance the susceptibility of the cells to the toxic effects of cisplatin. This notion is supported by cisplatin-induced modulation of target genes in the IL6/STAT3 signaling pathway, such as down regulation of Bcl2l1 and Ccnd1, and upregulation of Cdkn1a and Cebpd. Bcl2l1 encodes Bcl-xL, which acts as an inhibitor of apoptosis while Ccnd1 encodes cyclin, which promotes the progression of cell cycle. Cisplatin-induced downregulation of both these genes can facilitate cellular apoptosis. Similarly, the upregulation of Cdkn1a, which encodes a cyclin-dependent kinase inhibitor that mediates p53 dependent cell cycle arrest, and Cebpd, which acts as a tumor suppressor can result in enhanced apoptosis. Thus, the cisplatin-induced apoptosis could be attributed to the up or down regulation of STAT3-dependent genes and their signaling.
In previous studies, cisplatin treatment increased nitrotyrosine levels and decreased LMO4 protein levels not only in the auditory cells, but also in the renal and neuronal cells, which are susceptible to the toxic side-effects of cisplatin (Jamesdaniel et al., 2012; Rathinam et al., 2015). As a molecular adaptor for protein-protein interactions, LMO4 forms transcriptional complexes and thereby regulates cell survival and cell death (Novotny-Diermayr et al., 2005; Schock et al., 2008; Setogawa et al., 2006; Wang et al., 2007). Particularly, it associates with IL-6 receptor glycoprotein 130 and stabilizes this protein complex, which in turn enables the activation of JAK1, TYK2, and STAT3 (Novotny-Diermayr et al., 2005). However, cisplatin-induced nitration and degradation of LMO4 could destabilize the GP130 complex and compromise the STAT3-mediated anti-apoptotic machinery. The attenuation of cisplatin-induced decrease in the expression of JAK1 as well as the expression, phosphorylation, and nuclear localization of STAT3 by SRI110 co-treatment implicates nitrative stress as a critical regulator of STAT3 in cisplatin cytotoxicity. Furthermore, the cisplatin-induced modulation of IL6/STAT3 signaling genes was reversed by SRI110 co-treatment. Collectively, these results suggest that the inhibition of cisplatin-induced nitration prevents the inactivation of STAT3 probably due to the stabilization of the IL6-GP130 complex, which in turn enables the transcription of anti-apoptotic genes and thereby helps to mitigate cisplatin-induced toxicity.
Highlights.
Cisplatin treatment decreased the expression levels and phosphorylation of STAT3
Inhibition of nitration by SRI110 prevented cisplatin-induced inactivation of STAT3
SRI110 co-treatment enables the transcription of STAT3-related anti-apoptotic genes
ACKNOWLEDGEMENTS
This work was supported by WSU faculty start-up funds to SJ and NIEHS P30 Grant (P30 ES020957) to Center for Urban Responses to Environmental Stressors (CURES).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, and El-Remessy AB (2010). Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther 332, 125–134. [DOI] [PubMed] [Google Scholar]
- Benkafadar N, Menardo J, Bourien J, Nouvian R, Francois F, Decaudin D, et al. (2017). Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol Med 9, 7–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borse V, Al Aameri RFH, Sheehan K, Sheth S, Kaur T, Mukherjea D, et al. (2017). Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis 8, e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, et al. (2002). Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 21, 8843–8851. [DOI] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Muscoli C, Bryant L, Esposito E, Cuzzocrea S, et al. (2012). Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci 32, 6149–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, and Ivashkiv LB (2009). Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 31, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S (2014). Downstream targets of Lmo4 are modulated by cisplatin in the inner ear of Wistar rats. PLoS One 9, e115263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S, Coling D, Hinduja S, Ding D, Li J, Cassidy L, et al. (2012). Cisplatin-induced ototoxicity is mediated by nitroxidative modification of cochlear proteins characterized by nitration of Lmo4. J Biol Chem 287, 18674–18686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S, Rathinam R, and Neumann WL (2016). Targeting nitrative stress for attenuating cisplatin-induced downregulation of cochlear LIM domain only 4 and ototoxicity. Redox Biol 10, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, and Ramkumar V (2011). Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis 2, e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Sahm H, You J, and Wang M (2010). Knock-down of superoxide dismutase 1 sensitizes cisplatin-resistant human ovarian cancer cells. Anticancer Res 30, 2577–2581. [PubMed] [Google Scholar]
- Kim M, Morales LD, Jang IS, Cho YY, and Kim DJ (2018). Protein Tyrosine Phosphatases as Potential Regulators of STAT3 Signaling. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levano S, and Bodmer D (2015). Loss of STAT1 protects hair cells from ototoxicity through modulation of STAT3, c-Jun, Akt, and autophagy factors. Cell Death Dis 6, e2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccio A, and Madeddu C (2013). The role of interleukin-6 in the evolution of ovarian cancer: clinical and prognostic implications--a review. J Mol Med (Berl) 91, 1355–1368. [DOI] [PubMed] [Google Scholar]
- Martindale JL, and Holbrook NJ (2002). Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192, 1–15. [DOI] [PubMed] [Google Scholar]
- Mohan S, Smyth BJ, Namin A, Phillips G, and Gratton MA (2014). Targeted amelioration of cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck Surg 151, 836–839. [DOI] [PubMed] [Google Scholar]
- Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, et al. (2013). The role of JAK-STAT signaling within the CNS. JAKSTAT 2, e22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny-Diermayr V, Lin B, Gu L, and Cao X (2005). Modulation of the interleukin-6 receptor subunit glycoprotein 130 complex and its signaling by LMO4 interaction. J Biol Chem 280, 12747–12757. [DOI] [PubMed] [Google Scholar]
- Rathinam R, Ghosh S, Neumann WL, and Jamesdaniel S (2015). Cisplatin-induced apoptosis in auditory, renal, and neuronal cells is associated with nitration and downregulation of LMO4. Cell Death Discov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings JS, Rosler KM, and Harrison DA (2004). The JAK/STAT signaling pathway. J Cell Sci 117, 1281–1283. [DOI] [PubMed] [Google Scholar]
- Rausaria S, Kamadulski A, Rath NP, Bryant L, Chen Z, Salvemini D, et al. (2011). Manganese(III) complexes of bis(hydroxyphenyl)dipyrromethenes are potent orally active peroxynitrite scavengers. J Am Chem Soc 133, 4200–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta MN, and Holley MC (2002). Cell lines in inner ear research. J Neurobiol 53, 306–318. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Mukherjea D, Jajoo S, Kaur T, and Ramkumar V (2012). siRNA-mediated knock-down of NOX3: therapy for hearing loss? Cell Mol Life Sci 69, 2429–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock SC, Xu J, Duquette PM, Qin Z, Lewandowski AJ, Rai PS, et al. (2008). Rescue of neurons from ischemic injury by peroxisome proliferator-activated receptor-gamma requires a novel essential cofactor LMO4. J Neurosci 28, 12433–12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setogawa T, Shinozaki-Yabana S, Masuda T, Matsuura K, and Akiyama T (2006). The tumor suppressor LKB1 induces p21 expression in collaboration with LMO4, GATA-6, and Ldb1. Biochem Biophys Res Commun 343, 1186–1190. [DOI] [PubMed] [Google Scholar]
- Sheth S, Mukherjea D, Rybak LP, and Ramkumar V (2017). Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front Cell Neurosci 11, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou A, and Latchman DS (2005). Opposing actions of STAT-1 and STAT-3. Growth Factors 23, 177–182. [DOI] [PubMed] [Google Scholar]
- Wagner M, and Siddiqui MA (2009). Signaling networks regulating cardiac myocyte survival and death. Curr Opin Investig Drugs 10, 928–937. [PubMed] [Google Scholar]
- Waissbluth S, Pitaro J, and Daniel SJ (2012). Gene therapy for cisplatin-induced ototoxicity: a systematic review of in vitro and experimental animal studies. Otol Neurotol 33, 302–310. [DOI] [PubMed] [Google Scholar]
- Wang G, Reed E, and Li QQ (2004). Molecular basis of cellular response to cisplatin chemotherapy in non-small cell lung cancer (Review). Oncol Rep 12, 955–965. [PubMed] [Google Scholar]
- Wang N, Lin KK, Lu Z, Lam KS, Newton R, Xu X, et al. (2007). The LIM-only factor LMO4 regulates expression of the BMP7 gene through an HDAC2-dependent mechanism, and controls cell proliferation and apoptosis of mammary epithelial cells. Oncogene 26, 6431–6441. [DOI] [PubMed] [Google Scholar]
- Yu H, and Jove R (2004). The STATs of cancer--new molecular targets come of age. Nat Rev Cancer 4, 97–105. [DOI] [PubMed] [Google Scholar]
- Yuan J, Zhang F, and Niu R (2015). Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep 5, 17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Li C, Halfter H, and Liu J (2003). Delineating an oncostatin M-activated STAT3 signaling pathway that coordinates the expression of genes involved in cell cycle regulation and extracellular matrix deposition of MCF-7 cells. Oncogene 22, 894–905. [DOI] [PubMed] [Google Scholar]
- Zou MH, Li H, He C, Lin M, Lyons TJ, and Xie Z (2011). Tyrosine nitration of prostacyclin synthase is associated with enhanced retinal cell apoptosis in diabetes. Am J Pathol 179, 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]