Abstract
Background
Anxiety frequently coexists with depression and adding benzodiazepines to antidepressant treatment is common practice to treat people with major depression. However, more evidence is needed to determine whether this combined treatment is more effective and not any more harmful than antidepressants alone. It has been suggested that benzodiazepines may lose their efficacy with long‐term administration and their chronic use carries risks of dependence.
This is the 2019 updated version of a Cochrane Review first published in 2001, and previously updated in 2005. This update follows a new protocol to conform with the most recent Cochrane methodology guidelines, with the inclusion of 'Summary of findings' tables and GRADE evaluations for quality of evidence.
Objectives
To assess the effects of combining antidepressants with benzodiazepines compared with antidepressants alone for major depression in adults.
Search methods
We searched the Cochrane Common Mental Disorders Group's Controlled Trials Register (CCMDCTR), the Cochrane Central Register of Controlled Trials, MEDLINE, Embase and PsycINFO to May 2019. We searched the World Health Organization (WHO) trials portal and ClinicalTrials.gov to identify any additional unpublished or ongoing studies.
Selection criteria
All randomised controlled trials that compared combined antidepressant plus benzodiazepine treatment with antidepressants alone for adults with major depression. We excluded studies administering psychosocial therapies targeted at depression and anxiety disorders concurrently. Antidepressants had to be prescribed, on average, at or above the minimum effective dose as presented by Hansen 2009 or according to the North American or European regulations. The combination therapy had to last at least four weeks.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias in the included studies, according to the criteria of the Cochrane Handbook for Systematic Reviews of Interventions. We entered data into Review Manager 5. We used intention‐to‐treat data. We combined continuous outcome variables of depressive and anxiety severity using standardised mean differences (SMD) with 95% confidence intervals (CIs). For dichotomous efficacy outcomes, we calculated the risk ratio (RR) with 95% CI. Regarding the primary outcome of acceptability, only overall dropout rates were available for all studies.
Main results
We identified 10 studies published between 1978 to 2002 involving 731 participants. Six studies used tricyclic antidepressants (TCAs), two studies used selective serotonin reuptake inhibitors (SSRIs), one study used another heterocyclic antidepressant and one study used TCA or heterocyclic antidepressant.
Combined therapy of benzodiazepines plus antidepressants was more effective than antidepressants alone for depressive severity in the early phase (four weeks) (SMD –0.25, 95% CI –0.46 to –0.03; 10 studies, 598 participants; moderate‐quality evidence), but there was no difference between treatments in the acute phase (five to 12 weeks) (SMD –0.18, 95% CI –0.40 to 0.03; 7 studies, 347 participants; low‐quality evidence) or in the continuous phase (more than 12 weeks) (SMD –0.21, 95% CI –0.76 to 0.35; 1 study, 50 participants; low‐quality evidence). For acceptability of treatment, there was no difference in the dropouts due to any reason between combined therapy and antidepressants alone (RR 0.76, 95% CI 0.54 to 1.07; 10 studies, 731 participants; moderate‐quality evidence).
For response in depression, combined therapy was more effective than antidepressants alone in the early phase (RR 1.34, 95% CI 1.13 to 1.58; 10 studies, 731 participants), but there was no evidence of a difference in the acute phase (RR 1.12, 95% CI 0.93 to 1.35; 7 studies, 383 participants) or in the continuous phase (RR 0.97, 95% CI 0.73 to 1.29; 1 study, 52 participants). For remission in depression, combined therapy was more effective than antidepressants alone in the early phase (RR 1.39, 95% CI 1.03 to 1.90, 10 studies, 731 participants), but there was no evidence of a difference in the acute phase (RR 1.27, 95% CI 0.99 to 1.63; 7 studies, 383 participants) or in the continuous phase (RR 1.31, 95% CI 0.80 to 2.16; 1 study, 52 participants). There was no evidence of a difference between combined therapy and antidepressants alone for anxiety severity in the early phase (SMD –0.76, 95% CI –1.67 to 0.14; 3 studies, 129 participants) or in the acute phase (SMD –0.48, 95% CI –1.06 to 0.10; 3 studies, 129 participants). No studies measured severity of insomnia. In terms of adverse effects, the dropout rates due to adverse events were lower for combined therapy than for antidepressants alone (RR 0.54, 95% CI 0.32 to 0.90; 10 studies, 731 participants; moderate‐quality evidence). However, participants in the combined therapy group reported at least one adverse effect more often than participants who received antidepressants alone (RR 1.12, 95% CI 1.01 to 1.23; 7 studies, 510 participants; moderate‐quality evidence).
Most domains of risk of bias in the majority of the included studies were unclear. Random sequence generation, allocation concealment, blinding and selective outcome reporting were problematic due to insufficient details reported in most of the included studies and lack of availability of the study protocols. The greatest limitation in the quality of evidence was issues with attrition.
Authors' conclusions
Combined antidepressant plus benzodiazepine therapy was more effective than antidepressants alone in improving depression severity, response in depression and remission in depression in the early phase. However, these effects were not maintained in the acute or the continuous phase. Combined therapy resulted in fewer dropouts due to adverse events than antidepressants alone, but combined therapy was associated with a greater proportion of participants reporting at least one adverse effect.
The moderate quality evidence of benefits of adding a benzodiazepine to an antidepressant in the early phase must be balanced judiciously against possible harms and consideration given to other alternative treatment strategies when antidepressant monotherapy may be considered inadequate. We need long‐term, pragmatic randomised controlled trials to compare combination therapy against the monotherapy of antidepressant in major depression.
Plain language summary
Antidepressants plus benzodiazepines for major depression
Why is this review important?
Major depression is characterised by depressed mood, loss of interest or pleasure, diminished energy, fatigue, difficulties with concentration, changes in appetite, sleep disturbances and morbid thoughts of death. Depression often presents with anxiety. Depression and anxiety have negative impacts on the person and on society, often over the long term.
Who will be interested in this review?
Health professionals, including general practitioners and psychiatrists; people with major depression and the people around them.
What question does this review aim to answer?
Major depression is often treated by combining antidepressant drugs with benzodiazepines. Benzodiazepines are a family of anxiety‐reducing and hypnotic drugs. This review asked if combined antidepressant plus benzodiazepine treatment, compared with antidepressants treatment alone, had an effect on depressive symptoms, rates of recovery and the acceptability of these treatments based on the number of people who left the study early (called the dropout rate), in adults with major depression.
Which studies were included in the review?
We searched electronic databases to find all relevant studies in adults with major depression. To be included, the studies had to be randomised controlled trials (RCTs), which means adults were allocated at random (by chance alone) to receive either antidepressants plus benzodiazepines or antidepressants alone (last search date 23 May 2019).
We found 10 relevant studies involving 731 people comparing combined antidepressant plus benzodiazepine therapy with treatment with antidepressants alone. The quality of the evidence ranged from very low to moderate.
What does the evidence from the review tell us?
Combining antidepressants and benzodiazepines was more effective than antidepressants alone in improving depression and reducing symptoms in the early phase of treatment (one to four weeks), but there was no evidence of a difference at later time points. There was no evidence of a difference in acceptability (based on dropouts) between the combination treatment compared with antidepressants alone. Dropout rates due to unintended and untoward effects (side effects) were lower for antidepressants plus benzodiazepines compared with antidepressants alone, although at least one side effect was reported more frequently by those treated with a combination of antidepressants plus benzodiazepines.
What should happen next?
Due to the potential for people to become dependent on benzodiazepines, new longer‐term studies need to compare what happens when the combined treatment involves withdrawing the benzodiazepine after a short period (for example, one month).
Summary of findings
Summary of findings for the main comparison. Antidepressants plus benzodiazepines compared to antidepressants alone for major depression in adults.
| Antidepressants plus benzodiazepines compared to antidepressants alone for major depression in adults | ||||||
| Patient or population: people with major depression Setting: inpatients and outpatients Intervention: antidepressants + benzodiazepines Comparison: antidepressants alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with antidepressants alone | Risk with antidepressants plus benzodiazepines | |||||
| Depression severity: early phase (2 weeks, range 1–4 weeks) Follow‐up: range 1–4 weeks | — | The mean depression severity in the early phase in the combination group was 0.25 standard deviations lower (0.46 lower to 0.03 lower). | — | 598 (10 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Depression severity: acute phase (8 weeks, range 5–12 weeks) | — | The mean depression severity in the acute phase in the combination group was 0.18 standard deviations lower (0.40 lower to 0.03 higher). | — | 347 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Depression severity: continuous phase (> 12 weeks) | — | The mean depression severity in the continuous phase in the combination groups was 0.21 standard deviations lower (0.76 lower to 0.35 higher) | — | 50 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
| Acceptability of treatment (dropout for any reason) | Study population | RR 0.76 (0.54 to 1.07) | 731 (10 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 332 per 1000 | 253 per 1000 (180 to 356) | |||||
| Moderate | ||||||
| 200 per 1000 | 152 per 1000 (108 to 214) | |||||
| Anxiety severity: early phase (2 weeks, range 1–4 weeks) | — | The mean depression severity in early phase in the combination groups was 0.76 standard deviations lower (1.67 lower to 0.14 higher) | — | 129 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b, c | — |
| Adverse effects (dropouts) | Study population | RR 0.54 (0.32 to 0.90) | 731 (10 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 119 per 1000 | 64 per 1000 (38 to 107) | |||||
| Moderate | ||||||
| 85 per 1000 | 46 per 1000 (27 to 77) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the evidence by one level because of risk of bias. Studies were described as "double‐blind", but information on the procedure followed to guarantee the blindness, and if blinding was successful, was not reported in all randomised controlled trials. Also, information on randomisation procedures and allocation concealment was lacking in all studies. Moreover, half of the included studies had high attrition rate. bWe downgraded the evidence by one level because of low number of participants included in the analysis and 95% confidence interval included both no effect and appreciable benefit. cWe downgraded the evidence by one level because of high heterogeneity between studies.
Background
Description of the condition
Major depression is characterised by depressed mood, loss of interest or pleasure, diminished energy, fatigue, difficulties with concentration, changes in appetite, sleep disturbances and morbid thoughts of death (APA 2013). Depression is a common disorder with a lifetime prevalence of 16% (Kessler 2003), and 12‐month prevalence rate between 6% and 10% (Baumeister 2007). As the largest source of non‐fatal disease burden in the world, accounting for 12% of years lived with disability (Ustun 2004), depression is associated with marked personal, social and economic morbidity, loss of functioning and productivity; and creates significant demands on service providers in terms of workload (NICE 2009). Depression often presents with anxiety. The rate of anxiety comorbidity among people with depression varies between 33% and 85% (Murphy 1990; Wetzler 1989), is associated with a higher familial prevalence of major depression (Clayton 1991; Coryell 1992), and its presence may predict a poorer long‐term outcome.
Description of the intervention
Benzodiazepines refer to a class of psychotropic drugs whose core chemical structure is the fusion of a benzene and diazepine ring. They are mainly used for people with anxiety symptoms (anxiolytics, tranquillisers) and as hypnotic drugs for people with insomnia.
When treating people with major depression, the current guidelines recommend antidepressant monotherapy as first‐line pharmacological treatment (APA 2010; BAP 2015; Bauer 2002; NICE 2009). Some guidelines state a limited value of benzodiazepines as combination therapy and allow that benzodiazepines can be used for a short time with an antidepressant if people have symptoms of anxiety or insomnia (APA 2010; BAP 2015; NICE 2009). The WFSBP guidelines acknowledge the quick onset of action of benzodiazepines in treating agitation, anxiety and insomnia (Bauer 2002).
However, the APA guideline does not recommend benzodiazepines as primary pharmacological agents even in people with major depression with anxiety symptoms, because of the known adverse effects and toxicity profile associated with these drugs, as well as the potential for abuse and dependence (APA 2010). Some guidelines also explicitly state that benzodiazepines do not have an antidepressant effect (APA 2010; NICE 2009). In addition there are suggestions that benzodiazepines may lose their efficacy with long‐term administration (CRM 1980), and that their chronic use carries risks of dependence (Schweizer 1998).
In reality, combination prescriptions appear to be common in many parts of the world. For example, one multi‐centre study in Japan found that approximately 60% of psychiatric patients making their first presentation for treatment in a psychiatric service with major depression were prescribed benzodiazepines (excluding those used as hypnotics) in addition to antidepressants (Furukawa 2000). In Canada, the prevalence of antidepressants and benzodiazepines utilisation was 49.3% of people who had experienced an major depressive episode in the past 12 months and reported antidepressant use (Sanyal 2011). The older people tended to receive more benzodiazepines than the younger patients among Health Maintenance Organization patients with depression (49% with older and 33% with younger) (Bartels 1997). In another study, primary care physicians prescribed antidepressants to 56% of their patients diagnosed as depressed; of these, 16% were also prescribed benzodiazepines (Olfson 1992). In one survey of primary care practices in the Netherlands, 5% of the people presenting with depression and anxiety received an antidepressant only, 16% received an anxiolytic only and 5% received a combination of antidepressant and anxiolytic (van den Brink 1991). In France, a general population survey revealed that slightly less than two thirds of antidepressant users were also prescribed benzodiazepines (anxiolytic or hypnotic) concomitantly (Bouhassira 1998). At one university psychiatric clinic in Germany, monotherapy was applied in only 37% of all cases, and a combined psychopharmacotherapy in 63% of cases (Grohmann 1980). In Italy, among 281 cases treated in acute psychiatric inpatient services, only two people were prescribed an antidepressant alone while 44 received a combination of benzodiazepine plus antidepressant (de Girolamo 1987).
How the intervention might work
Benzodiazepines act through binding at, and enhancing the effect of, gamma‐aminobutyric acid (GABA)‐A receptors. Enhancement of the effect of GABA at this receptor results in sedative, anxiolytic, hypnotic and muscle relaxant properties. Combining benzodiazepines with antidepressants may lead to additive or synergistic antidepressant effect if benzodiazepines themselves have an antidepressant effect (Gomez 2000; Petty 1995), or if they act on anxiety or insomnia often comorbid with major depression.
Why it is important to do this review
Reviews of randomised controlled trials (RCTs) show that anxiolytic benzodiazepines, with the possible exception of some triazolo‐benzodiazepines for mild‐to‐moderate depression, are less effective than standard antidepressants in treating major depression (Birkenhager 1995; Schatzberg 1978). The advantages of adding benzodiazepines to antidepressants are unclear. There have been several RCTs examining the combined antidepressant plus benzodiazepine treatment in depression but the results vary (Birkenhager 1995). The first version of this systematic review was published in 2002 (with a literature searched up to 1999). The first major updated version was published in 2005 (with literature searched up to 2004). This is the second major update of this review (with literature searched up to May 2019). This update followed a revised protocol to conform with the latest edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and to provide 'Summary of findings' tables.
Objectives
Primary objective
To assess the effects of combining antidepressants with benzodiazepines compared with antidepressants alone for major depression in adults.
Secondary objectives
To determine if additional benzodiazepines benefit people with depression with high anxiety or low anxiety, and if short‐acting benzodiazepines given at bedtime influence daytime mood.
Methods
Criteria for considering studies for this review
Types of studies
All relevant RCTs meeting the inclusion criteria, including cluster randomised trials. We used only the first phase of cross‐over studies.
Types of participants
Participants
Adults (aged 18 years or older), with no restrictions in terms of gender or ethnicity.
Diagnosis
Major depression, diagnosed according to any one of the Feighner criteria, the Research Diagnostic Criteria (RDC), the Diagnostic and Statistical Manual 3rd Edition (DSM‐III), 3rd Revised Edition (DSM‐III‐R), 4th Edition (DSM‐IV), 5th Edition (DSM‐5) or the 10th Revision of the International Classification of Diseases (ICD‐10).
Comorbidities
We included studies where participants had comorbid anxiety disorders. Studies involving participants with comorbid physical or other psychological disorders were eligible for inclusion, as long as the comorbidity was not the focus of the study.
Setting
We assigned no restrictions to the type of study setting.
Types of interventions
Experimental intervention
Any combination of antidepressants and benzodiazepines. We excluded studies administering psychosocial therapies targeted at depression and anxiety disorders concurrently. Antidepressants had to be prescribed, on average, at or above the minimum effective dose as presented by Hansen and colleagues (Hansen 2009), or according to the North American (APA 2010) or European regulations. The benzodiazepines had to be prescribed in accordance with the North American (APA 2010) or European regulations. The combination therapy had to last at least four weeks.
We included the following types of antidepressants.
Tricyclic antidepressants (TCAs): amitriptyline, imipramine, trimipramine, doxepin, desipramine, protriptyline, nortriptyline, clomipramine, dothiepin, iofepramine.
Selective serotonin reuptake inhibitors (SSRIs): zimelidine (banned worlwide due to a 25‐fold increase in the risk of developing Guillain‐Barré syndrome (Fagius 1985)), fluvoxamine, fluoxetine, paroxetine, sertraline, citalopram, escitalopram.
Serotonin‐noradrenaline reuptake inhibitors: venlafaxine, milnacipran, duloxetine.
Noradrenergic and specific serotonergic antidepressants: mirtazapine.
-
Monoamine oxidase inhibitors:
irreversible: phenelzine, tranylcipromine, izocarboxazid;
reversible: brofaramine, moclobemide, tyrima.
Other antidepressants:
noradrenaline reuptake inhibitors: reboxetine, atomoxetine;
noradrenaline‐dopamine reuptake inhibitors: amineptine, bupropion;
serotonin antagonist and reuptake inhibitors: trazodone;
unclassified: agomelatine, vilazodone;
other heterocyclic antidepressants: mianserin, amoxapine, maprotiline.
For benzodiazepines, we included the following: adinazolam, alprazolam, bentazepam, bromazepam, brotizolam, camazepam, chlordiazepoxide, clobazam, clonazepam, clorazepate, clotiazepam, cloxazolam, diazepam, estazolam, etizolam, flunitrazepam, flurazepam, flutoprazepam, halazepam, ketazolam, loflazepate, lorazepam lormetazepam, medazepam, metaclazepam, mexazolam, midazolam, nitrazepam, nordazepam, oxazepam, prazepam, propazepam, quazepam, ripazepam, serazepine, temazepam, tofisopam, triazolam.
Control intervention
Antidepressants alone.
Types of outcome measures
Primary outcomes
Depressive severity: studies had to include at least one measure of depressive severity. The symptom severity could have been measured by either observer‐rating (our preference) or self‐report. The primary outcome for depressive severity was based on the observer‐rated scale, preferably the Hamilton Rating Scale for Depression (HRSD) (Hamilton 1960). We combined data on observer‐rated and self‐report outcomes, while prioritising data from observer‐rating scales in case of available data from both observer‐rating scales and self‐report questionnaire.
Acceptability of treatment: as measured by leaving study early for any reason.
Secondary outcomes
Response in depression: defined as 50% or greater reduction in depression severity measures, and global response. We distinguished between response, a relative change in depression severity from baseline, and remission, an absolute endpoint achieved through treatment (Bandelow 2006; Keller 2004). If the original authors reported several outcomes corresponding with our definition of response, we gave preference to HRSD for observer‐rating scale and Beck Depression Inventory (BDI) (Beck 1961) for self‐rating scale. If the authors reported only Clinical Global Impression (CGI) (Guy 1976), we used CGI‐Improvement to define response. If the authors used other measures and definitions of remission to indicate the relative change from baseline, we used the original authors' definition. We presented these different definitions of response used across the included studies. We examined the robustness of this outcome definition hierarchy through a sensitivity analysis limiting the included studies to those reporting on 50% reduction on HRSD. Response is one of the more consistently defined endpoints and has been widely used for defining improvement in acute treatment studies.
Remission in depression: usually defined as 7 or lower on HRSD or 11 or lower on Montgomery and Åsberg Depression Rating Scale (MADRS) (Montgomery 1979). If the original authors reported several outcomes corresponding with our definition of remission, we gave preference to the HRSD for observer‐rating scale and BDI for self‐rating scale. If the authors reported only CGI, we used CGI‐Severity to define remission. If the authors used other measures and definitions of remission to indicate the absolute endpoint achieved through treatment, we used the original authors' definition. We presented these different definitions of remission used. Remission is associated with better long‐term outcomes compared with response without remission (Lin 1998).
Anxiety severity: measured using standardised validated continuous scales, either assessor‐rated, such as the Hamilton Anxiety Scale (HAM‐A) (Hamilton 1959) or self‐report, including the Trait subscale of the Spielberger State‐Trait Anxiety Inventory (STAI‐T) (Spielberger 1983), and the Beck Anxiety Inventory (BAI) (Beck 1988).
Insomnia severity: measured using standardised validated continuous scales, either assessor‐rated or self‐report.
Adverse effects: evaluated by counting numbers of dropouts due to adverse effects and total number of participants experiencing at least one adverse effect.
Timing of outcome assessment
Outcomes were divided into early phase (in this review, defined as two weeks, ranged from one to four weeks), acute phase (defined as eight weeks, ranged five to 12 weeks) and continuous phase (defined as more than 12 weeks). Early phase was our primary time point.
Hierarchy of outcome measures
Due to the great likelihood of more than one reported eligible outcome, we included data as per the following rules:
in case of available data from both observer‐rating scales and self‐report questionnaires, we prioritised data from observer‐rating scales;
in case of several outcome measures of the same hierarchy level used in one study, we selected the outcome measure most frequently used across all studies. Therefore, availability determined the selection of the outcome measure;
in case of several outcome measures of the same hierarchy level and the same availability across studies, the outcome measures were randomly selected.
Search methods for identification of studies
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
The Cochrane Common Mental Disorders Group (CCMD) maintains two archived clinical trials registers at its editorial base in York, UK: a References register and a Studies Register. The CCMDCTR‐References Register contains over 40,000 reports of RCTs in depression, anxiety and neurosis. Approximately 50% of these references have been tagged to individual, coded trials. The coded trials are held in the CCMDCTR‐Studies Register and records are linked between the two registers using unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary (contact the CCMD Information Specialists for further details). Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 to 2016), Embase (1974 to 2016) and PsycINFO (1967 to 2016); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials were also sourced from international trial registers via the World Health Organization's (WHO) International Clinical Trials Registry Platform (ICTRP), pharmaceutical companies, the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCMD's generic search strategies (used to identify RCTs) can be found on the Group's website with an example of the core MEDLINE search used to inform the register displayed in Appendix 1.
The Group's Specialised Register became out of date with the Editorial Group’s move from Bristol to York (UK) in the summer of 2016.
Electronic searches
For the current version of this review the Cochrane Common Mental Disorders Information Specialist conducted initial searches to March 2014 on the CCMDCTR alone. Further update searches were conducted directly on Ovid MEDLINE, Embase, PsycINFO and the Cochrane Library (2014 to 23 May 2019) to account for the period when the CCMDCTR was out of date.
CCMDCTR (Studies and References Register) (all years to 28 June 2016);
Cochrane Central Register of Controlled Trials (CENTRAL) (2019, Issue 5);
Ovid MEDLINE (2014 to 23 May 2019);
Ovid Embase (2014 to 23 May 2019);
Ovid PsycINFO (2014 to 23 May 2019).
Search strategies are listed in Appendix 2.
We also searched the WHO ICTRP and ClinicalTrials.gov to identify any additional unpublished or ongoing trials to 23 May 2019.
There were no restriction on date, language (Egger 1997), or publication status applied to the searches.
We requested translations of non‐English language trial reports from contacts of the review authors or the Cochrane editorial team.
Search strategies run for the previous, published version of this review are in Appendix 3.
Searching other resources
Reference searching
We checked the references lists of all included studies for citations to additional published or unpublished research. We also conducted a forward citation search of the included studies and checked relevant review articles.
Personal communication
We contacted principal investigators, where necessary, to obtain further details of ongoing/unpublished studies, or trials reported as conference abstracts only.
Data collection and analysis
Selection of studies
In 1997 and 1999, one review author assessed every report identified by the search strategy described in Appendix 3 for relevance to this review. The criteria for selection at this stage were simple and broad so as not to miss any relevant study and were:
randomisation;
diagnosis of depression (not necessarily by operationalised criteria);
comparison between antidepressant plus benzodiazepine versus antidepressant alone.
Two review authors independently assessed the eligibility and methodological quality of the included trials.
In December 2004, we performed an updating search in the following manner. Two review authors assessed every report identified by a search of the Cochrane Group's specialised register (previously called the CCDANCTR). One review author (TAF) obtained and checked the full reports of all the studies that were rated positive by either review author according to the above mentioned approximate eligibility criteria.
For this version of the reiew, we performed study selection in the following manner. All reports of trials already included and excluded in the previous versions of this review were removed from reports identified by an update search of the Cochrane Groups' renamed specialised register (CCMDCTR) and other bibliographic database search results. Two review authors examined the titles and abstracts of all remaining reports. We obtained and inspected the full articles of all the studies identified by either of the review authors. We discussed conflicts of opinion regarding eligibility of a study with a third review author, having retrieved the full paper and consulted the authors if necessary, until we reached consensus.
Data extraction and management
Two review authors independently extracted the data from the original reports using data extraction forms. We re‐extracted data from the originally included studies as well as extracting data from the newly included studies. The data collected covered:
name and type of study setting;
diagnostic criteria used;
number of participants allocated (their diagnostic composition, age, sex, previous treatment, baseline depressive and anxiety severity, medical comorbidity);
details of intervention, duration of intervention, cointervention (if any);
their depressive and anxiety severity and response rate at one, two, four and eight weeks;
number of dropouts for any reason and later the number of dropouts due to adverse effects, and number of participants with at least one adverse effect.
We resolved any disagreements through discussion and in consultation with the principal investigators.
We note in the Characteristics of included studies table if outcome data were not reported in a usable way. One review author transferred data into the Review Manager 5 (Review Manager 2014). We double‐checked that entered data were correct by comparing the data presented in the systematic review with the study reports. A second review author spot‐checked study characteristics for accuracy against the trial report.
Main planned comparisons
The comparisons were any combination of antidepressants plus benzodiazepines versus antidepressants alone.
Assessment of risk of bias in included studies
In the original version of this review, we assessed methodological quality of included studies by criteria set out in the Cochrane Collaboration Handbook (Mulrow 1997); however, after publication of the revised and expanded Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we updated our methods accordingly. We considered the following seven domains.
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants and personnel: was knowledge of the allocated treatment adequately prevented during the study?
Blinding of outcome assessment: was knowledge of the allocated treatment adequately prevented during the study?
Incomplete outcome data for the primary outcome: were incomplete outcome data adequately addressed? (We defined dropouts of 20% or more as high risk of bias.)
Selective outcome reporting: were study reports free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias?
A judgement on the risk of bias was made for each domain, based on the following three categories: high risk of bias, low risk of bias and unclear risk of bias.
Two review authors independently assessed the risk of bias in the included studies. We resolved any disagreement through discussion and in consultation with the principal investigators. Where necessary, we contacted the authors of the studies for further information. We did not include studies where sequence generation was at high risk of bias and where allocation was clearly not concealed.
Measures of treatment effect
Continuous data
We combined continuous outcome variables of depressive and anxiety severity at approximately the same time point using standardised mean differences (SMD) as we expected that the studies would use different scales to measure the same concept. If all outcomes in a continuous meta‐analysis were sufficiently similar, we used mean differences (MDs). We reported 95% confidence intervals (CI).
Change versus endpoint data
We used endpoint data but used change data when endpoint data were not available, as empirical data support such synthesis (da Costa 2013).
Binary data
We included the dichotomous measures of response and remission in our data analysis because they were intuitively easier to understand, were more amenable to worst‐case scenario intention‐to‐treat (ITT) analysis and we expected that some studies may not have reported all the necessary data to enable meta‐analytic summary of a continuous measure but still have contained the data to enable the dichotomous analysis. We combined dichotomous outcome variables such as response, remission, dropout and presence or absence of adverse effects at approximately the same time point using risk ratios (RR) with 95% CIs using the random‐effects model, because these are more interpretable and more generalisable than risk differences, odds ratios or fixed‐effect model RRs (Furukawa 2002). Empirical data suggest that different definitions for response produce similar RRs and are therefore combinable in meta‐analyses (Furukawa 2011).
Unit of analysis issues
Cross‐over trials
For trials that had a cross‐over design, we only considered results from the first randomisation period to avoid carry‐over effects (Elbourne 2002).
Cluster randomised trials
We incorporated results from cluster RCTs into the review using generic inverse variance methods (Higgins 2011). With cluster RCTs, it is important to ensure that the data have been analysed taking into account the clustered nature of the data. We extracted the intracluster correlation coefficient (ICC) for each trial. Where no such data were reported, we requested this information from study authors. If this was not available, in line with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we used estimates from similar studies to 'correct' data for clustering, where this had not been done.
Studies with multiple treatment groups
Multiple‐arm studies contain more than two (intervention, comparison) relevant treatment arms (in addition to the control group there might be different types of interventions or different doses of medication). If data were binary, we added these and combined them in a two‐by‐two table. If data were continuous, we combined data following the formula in Section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not reproduce irrelevant additional treatment arms, but listed them in the Characteristics of included studies table.
Dealing with missing data
Missing participants
Where possible, we contacted original investigators to request missing data.
Dichotomous data
We analysed all data using the ITT principle: dropouts were always included in this analysis. Where participants were withdrawn from the trial before the endpoint, we assumed that their condition remained unchanged if they had stayed in the trial. This is conservative for outcomes related to response to treatment (because these participants were considered to have not responded to treatment). It is not conservative for adverse events but we considered that for the adverse events of interest in our review as a worst‐case scenario is clinically unlikely. When there were missing data and the method of last observation carried forward (LOCF) was used to do an ITT analysis, then the LOCF data were used with due consideration of the potential bias and uncertainty introduced.
Continuous data
The Cochrane Handbook for Systematic Reviews of Interventions recommends avoiding imputations of continuous data and suggests that the data must be used in the form presented by the original authors (Higgins 2011). Whenever ITT data were presented by the authors, we preferred them to 'per protocol or completer' data sets.
Missing data
Where studies did not report the number of responders and remitters, we imputed the values by assuming the normal distribution for the HRSD scores at each time point and calculate the number of participants according to a validated imputation method. This way of imputing response rates, especially in the case of the HRSD, has strong empirical support; when the same strategy was employed in four completed meta‐analyses of depression and anxiety, the agreement between the actually observed versus the imputed numbers of responders was almost perfect with an intraclass correlation coefficient of 0.97 (95% CI 0.95 to 0.98) (Furukawa 2005).
If the scores of continuous variables for a particular time point were missing but those for the time points before and after that particular one were reported, we interpolated the values, assuming a linear change between these time points.
Missing statistics
Where studies did not report the standard deviations of continuous measure scores and study authors were unable to provide standard deviations, we calculated the standard deviation from the standard error (SE) or P values (Altman 1996), or from CI, t‐values or P values as described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If this was not possible, we used values from all the studies examined in a previous systematic overview on the antidepressant treatment of depression (Cipriani 2009). Empirical evidence suggests that imputing missing standard deviations this way in meta‐analyses can provide quite accurate results (Furukawa 2006).
Assessment of heterogeneity
We assessed statistical heterogeneity using the Chi2 test, which provides evidence of variation in effect estimates beyond that of chance. Since the Chi2 test has low power to assess heterogeneity when there are few included trials or small numbers of participants, we set the P value conservatively at 0.1. We also quantified heterogeneity using the I2 statistic, which calculates the percentage of variability due to heterogeneity rather than chance.
We interpreted I2 values in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). An approximate guide to interpretation is as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
However, the importance of the observed I2 statistic depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (Higgins 2011). Forest plots generated using Review Manager 5 also provide an estimate of tau2, the between‐study variance in a random‐effects meta‐analysis (Review Manager 2014). Therefore, for the primary outcome, we also used tau2 to give an indication of the spread of true intervention effects.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. We investigated reporting bias by constructing funnel plots and conducting an Egger's test (Egger 1997) when 10 or more studies were pooled for the primary outcomes. We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were fewer than 10 studies or where all studies were of similar size.
Data synthesis
We analysed data using Review Manager 5 (Review Manager 2014). The included studies employed a variety of outcome measures at different time intervals. If more than 50% of the participants were lost to follow‐up in any group of the study, we considered this study of low methodological quality and examined the effects of including or excluding it in the sensitivity analysis.
We employed the random‐effects model because it incorporates an assumption that the different studies are estimating different, yet related, intervention effects. However, we examined whether use of a fixed‐effect model led to a substantial difference in the primary outcome.
We reported outcome measures for dichotomous data as RRs with 95% CIs and continuous data as SMDs as we expected that the studies would use different scales to measure the same concept. If all outcomes in a continuous meta‐analysis were sufficiently similar, we used MDs.
We calculated the number needed to treat for an additional beneficial outcome (NNTB) by taking the mean event rate among the controls and then applying RR to this rate. That is, NNTB = 1/(RR × mean – mean).
If a meta‐analysis was not possible (e.g. due to insufficient data or high levels of heterogeneity), we gave a narrative assessment of the evidence. This summarised the evidence according to intervention type.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses should be performed and interpreted with caution because multiple analyses lead to false‐positive conclusions (Oxman 1992). Nevertheless, we addressed the following a priori defined potential effect modifiers of the primary outcome.
Severity of comorbid anxiety (divided according to the median of the reported mean the HAM‐A total scores at baseline). This analysis examined effect moderation due to comorbid anxiety which may affect treatment recommendations and outcomes. We conducted analyses using the same methods as for the main analysis.
Differences between short‐acting benzodiazepines (less than 12 hour half‐life, such as triazolam, midazolam, brotizolam, oxazepam, temazepam) (Vermeeren 2004) given at bedtime and the others (such as long‐acting benzodiazepines taken at bedtime and anxiolytic benzodiazepines taken during the day). The latter may have some anxiolytic effects and affect the mood during the day, while the former may not have such effects.
Types of antidepressants, that is, SSRIs plus benzodiazepine versus SSRIs alone, TCAs plus benzodiazepine versus TCAs alone.
These analyses examined if types of drugs affected treatment recommendations and outcomes. We conducted analyses using the same methods as for the main analyses to determine if the method of data aggregation made any difference.
Sensitivity analysis
We conducted the following sensitivity analyses of the primary outcomes to test how robust our findings were to decisions made in the review process.
Fixed‐effect instead of random‐effects model. We used the random‐effects model for all main analyses. We conducted fixed‐effect analyses using the same data as the main analyses.
Exclusion of non‐double blind trials. We include open trials for main analyses. We conducted this analysis using the same data as for the main analyses.
Exclusion of trials using only self‐report. We planned to do this analysis because studies involving people with depression that use both clinician‐ and self‐report measures to assess depression severity have found that clinician and self‐reports of depression severity are not in agreement (Bailey 1976; Domken 1994; Rush 1987; Tondo 1988).
Limiting studies to those reporting on 50% reduction on HRSD. We conducted main analyses including trials in which response was not reported and imputed for all main analyses. We conducted this analysis using the same data as for the main analyses to determine if the study imputation made any differences.
Exclusion of trials with a high risk of bias because of incomplete outcome data. This analysis demonstrates the importance of the use of those trials, and the levels of confidence and caution that should be exercised in considering the analyses of all studies. We chose this domain as the one most likely to impact on our results.
Exclusion of trials where missing actual outcome data were imputed. We intended to conduct analyses including trials in which actual outcome data were not reported and imputed for all main analyses. We conducted this analysis using the same data as for the main analyses.
Giving half the weight to arms marketed by the sponsor of the trial in order to adjust for sponsorship bias. This sensitivity analysis is particularly important because repeated findings indicate that funding strongly affects outcomes of research studies (Als‐Nielsen 2003; Bhandari 2004; Lexchin 2003).
'Summary of findings' tables
We prepared 'Summary of findings' tables for all relevant comparisons. Each table included all outcomes. The quality of the body of evidence was assessed by the GRADE approach.
Results
Description of studies
Results of the search
See: Characteristics of included studies; Characteristics of excluded studies tables.
We conducted initial searches up to March 2014. After deduplication and removing reports of trials included and excluded in the previous review, we retrieved 650 references from the specialised register of the Cochrane Common Mental Disorders Group (CCMDCTR). Two review authors (YO and NT, YH or AT) independently screened 650 records and excluded 611 records based on their titles and abstracts as they evidently did not meet the inclusion criteria. We retrieved the full‐text papers for the remaining 39 reports and assessed them for eligibility. Forward and backward citation tracking of the included articles yielded no further relevant trials. One new article (Papakostas 2010) reported on a re‐analysis of data from Smith 1998. We identified no new studies from this search since the previous publication of this review in 2005. The update search in June 2015 retrieved an additional 90 references and found no additional studies. The update search in May 2018 retrieved an additional 627 references and found no additional studies. The update search in May 2019 retrieved an additional 93 references and found no additional studies (Figure 1).
1.
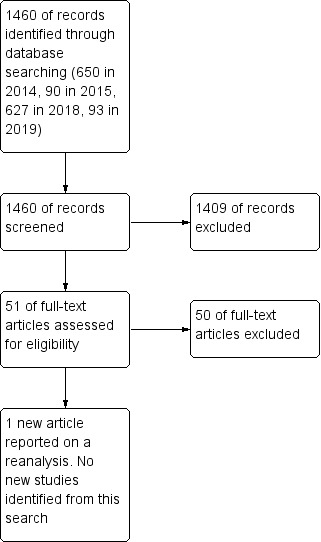
Study flow diagram for 2019 update.
Included studies
The review included 10 studies (Calcedo Ordonez 1992; Dominguez 1984; Fawcett 1987; Feet 1985; Feighner 1979; Nolen 1993; Scharf 1986; Smith 1998; Smith 2002; Yamaoka 1994). See Characteristics of included studies table.
Design
All included studies used a randomised, controlled, parallel‐group design. We identified no eligible cluster‐randomised or cross‐over trials.
Sample sizes
The study by Feighner 1979 had the largest study population with 190 participants randomised. A total of 126 participants participated in Dominguez 1984, 83 in Calcedo Ordonez 1992, 80 in Smith 1998, and 50 in Smith 2002. The total number of participants included in the review was 731.
Setting
Six studies took place in the US (Dominguez 1984; Fawcett 1987; Feighner 1979; Scharf 1986; Smith 1998; Smith 2002), with one each in the Netherlands (Nolen 1993), Spain (Calcedo Ordonez 1992), Norway (Feet 1985), and Japan (Yamaoka 1994).
Participants
All included trials enrolled people with a diagnosis of major depression based on Feighner (Feet 1985; Feighner 1979), DSM‐III (Dominguez 1984; Fawcett 1987; Scharf 1986; Yamaoka 1994), DSM‐III‐R (Calcedo Ordonez 1992; Nolen 1993) and DSM‐IV (Smith 1998; Smith 2002). Mean ages ranged from 34.8 years (Calcedo Ordonez 1992) to 48.8 years (Scharf 1986).
Interventions
Studies used a range of antidepressants including fluoxetine (Smith 1998; Smith 2002), imipramine (Dominguez 1984; Fawcett 1987), amitriptyline (Feighner 1979; Scharf 1986), clomipramine (Calcedo Ordonez 1992), desipramine (Fawcett 1987), and mianserin (Yamaoka 1994). Nolen 1993 allowed choice between maprotiline and nortriptyline. In regards to benzodiazepines, studies used clonazepam (Smith 1998; Smith 2002), triazolam (Dominguez 1984), lormetazepam (Nolen 1993), bentazepam (Calcedo Ordonez 1992), alprazolam (Fawcett 1987), diazepam (Feet 1985), flunitrazepam (Nolen 1993), mexazolam (Yamaoka 1994), and chlordiazepoxide (Feighner 1979; Scharf 1986).
Outcomes
Primary outcome assessment
The primary outcome in this review was a continuous measure of severity of depression, which all 10 studies assessed. Nine studies used the HRSD and one study used the Comprehensive Psychopathological Rating Scale (CPRS) (Feet 1985). Four RCTs reported response rates defined as at least 50% decrease in HRSD score from baseline. Three RCTs reported remission, defined as 8 or lower on HRSD (Smith 2002), much or very much improved on CGI (Smith 1998), and "global evaluation score on VAS [visual analogue scale] less than 10%" (Feet 1985). All studies reported overall dropout rates and dropout rates due to adverse effects.
Excluded studies
See Characteristics of excluded studies table.
We excluded studies for the following reasons: no operationalised diagnostic criteria (Ahmed 1988; Eckmann 1974; Johnstone 1980; Morakinyo 1970; Rickels 1970; Smith 1975), duration of the trial was shorter than four weeks (Ballinger 1974; Bowen 1978; Levin 1985; Magnus 1975; Runge 1985; Smith 1973), and dosage of the antidepressant was inadequate (Dimitriou 1982; Eckmann 1974; Otero 1994; Tsaras 1981; Yamada 2003). No studies were excluded for reasons of high risk of bias for sequence generation or allocation concealment.
Studies awaiting classification
There were no studies awaiting classification.
Ongoing studies
There were no ongoing studies.
New studies included in this update
We included one new article in this update (Papakostas 2010) reporting on a reanalysis of data from Smith 1998. The updated search identified no new studies.
Risk of bias in included studies
For details of the risk of bias judgements for each study, see Characteristics of included studies table. A graphical representation of the overall risk of bias in included studies is presented in Figure 2 and Figure 3.
2.
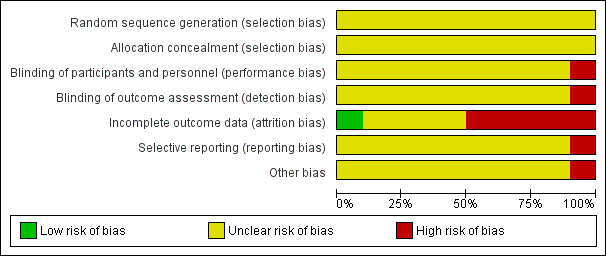
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
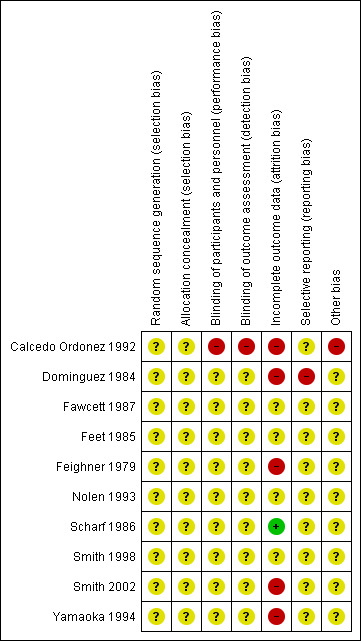
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
No included studies described methods of random sequence generation.
Allocation concealment
No included studies provided details on allocation concealment.
Blinding
Blinding of participants and personnel (performance bias and detection bias)
All studies except one open study (Calcedo Ordonez 1992) explicitly stated the double‐blind condition of their studies, but blinding itself was not adequately described in the methods section in most studies.
Blinding of outcome assessment (assessment bias)
One study was an open trial so the risk of bias for blinding of outcome assessment was high (Calcedo Ordonez 1992). No details were provided on who performed the outcome assessments in the other studies so the risk of bias for blinding of outcome assessment were unclear.
Incomplete outcome data
The greatest risk of bias in the studies included in this review came from incomplete outcome data (attrition bias), with only one study having low risk of bias in this domain (Scharf 1986). Four studies had very high risk of attrition bias, with 13 participants withdrawing from the study out of 32 participants randomised (41%) (Yamaoka 1994), 48 out of 126 (38%) (Feighner 1979), 64 out of the 190 (34%) (Dominguez 1984), and 18 out of 50 (36%) (Smith 2002). This means that the study findings must be interpreted extremely cautiously. However, reasons for dropout and numbers of dropouts were similar between treatment groups and sensitivity analyses assuming that all dropouts had either positive or negative outcomes in the trial found that combination therapy remained significantly more effective than antidepressant monotherapy in the early phase. This suggests that the primary finding was robust to a range of outcomes for dropouts. Calcedo Ordonez 1992 had high risk of bias from incomplete outcome data, because significantly more participants withdrew from the monotherapy than the combination group in the first eight weeks of the study and there was high attrition from both groups (withdrawal rates: 17/47 (36%) with monotherapy and 4/36 (11%) with combination group). Reasons for withdrawal were assessed and although "side effects" did not differ in frequency between the two groups, significantly more participants in the monotherapy group withdrew by "need to administer hypnotics", which may reflect factors associated with clinical outcomes. There was some evidence of potential risk of bias of incomplete outcome data in five studies, but we rated this as unclear owing to insufficient details in the report (Fawcett 1987; Feet 1985; Feighner 1979; Nolen 1993; Smith 1998).
Selective reporting
The protocols were unavailable in all studies. Bias from selective outcome reporting was unclear in all studies.
Other potential sources of bias
In Calcedo Ordonez 1992, more participants in the combination therapy group (11 participants) than in the control group (one participant) needed to take hypnotics and the risk of bias for other potential sources (performance bias due to cointervention) was high. Other studies provided insufficient information to assess other bias so the risk of bias for other potential sources was unclear.
Effects of interventions
See: Table 1
All included studies reported effect on depressive symptoms as their primary outcome. Five studies included response rates and three studies included remission rates.
Primary outcomes
Depressive severity
Antidepressants plus benzodiazepines therapy was more effective than the antidepressant alone in the early phase (SMD –0.25, 95% CI –0.46 to –0.03, 10 studies, 598 participants; Analysis 1.1), but there was no difference in the acute phase (SMD –0.18, 95% CI –0.40 to 0.03; 7 studies, 347 participants; Analysis 1.2) or continuous phase (SMD –0.21, 95% CI –0.76 to 0.35; 1 study, 50 participants; Analysis 1.3). There was a moderate level of heterogeneity in the overall results (I2 = 35%) in the early phase, but there was no evidence of heterogeneity (I2 = 0%) in the acute phase.
1.1. Analysis.
Comparison 1 Combination versus antidepressant (AD) alone: depressive severity, Outcome 1 Early phase (2 weeks, range 1–4 weeks).
1.2. Analysis.
Comparison 1 Combination versus antidepressant (AD) alone: depressive severity, Outcome 2 Acute phase (8 weeks, range 5–12 weeks).
1.3. Analysis.
Comparison 1 Combination versus antidepressant (AD) alone: depressive severity, Outcome 3 Continuous phase (> 12 weeks).
Acceptability of treatment
We found no difference between combination therapy and antidepressant monotherapy in terms of dropout for any reason (RR 0.76, 95% CI 0.54 to 1.07; 10 studies, 731 participants; Analysis 2.1). There was moderate level heterogeneity in the overall results (I2 = 36%).
2.1. Analysis.
Comparison 2 Combination versus antidepressant (AD) alone: acceptability of treatment, Outcome 1 Dropout for any reason.
Secondary outcomes
Response in depression
Combination therapy was more effective than monotherapy in the early phase (RR 1.34, 95% CI 1.13 to 1.58; 10 studies, 731 participants; Analysis 3.1), but there was no evidence of a difference in the acute phase (RR 1.12, 95% CI 0.93 to 1.35; 7 studies, 383 participants; Analysis 3.2) or continuous phase (RR 0.97, 95% CI 0.73 to 1.29; 1 study, 52 participants; Analysis 3.3). Taking the mean control event rate of the included RCTs, the obtained RRs could be translated into the following NNTBs. The NNT for improvement in depression was 9 (95% CI 6 to 24) in the early phase, according to the ITT analysis. There was no evidence of heterogeneity in the overall results (I2 = 0%) in the early phase, but there were moderate levels of heterogeneity (I2 = 31%) in the acute phase.
3.1. Analysis.
Comparison 3 Combination versus antidepressant (AD) alone: response in depression, Outcome 1 Early phase (2 weeks, range 1–4 weeks).
3.2. Analysis.
Comparison 3 Combination versus antidepressant (AD) alone: response in depression, Outcome 2 Acute phase (8 weeks, range 5–12 week).
3.3. Analysis.
Comparison 3 Combination versus antidepressant (AD) alone: response in depression, Outcome 3 Continuous phase (> 12 weeks).
Remission in depression
Combination therapy was more effective than monotherapy in the early phase (RR 1.39, 95% CI 1.03 to 1.90; 10 studies, 731 participants; Analysis 4.1), but there was no evidence of a difference in the acute phase (RR 1.27, 95% CI 0.99 to 1.63; 7 studies, 383 participants; Analysis 4.2) or continuous phase (RR 1.31, 95% CI 0.80 to 2.16; 1 study, 52 participants; Analysis 4.3). There was no evidence of heterogeneity in the overall results (I2 = 2%) in the early phase, but there was low‐to‐moderate heterogeneity (I2 = 25%) in the acute phase.
4.1. Analysis.
Comparison 4 Combination versus antidepressant (AD) alone: remission in depression, Outcome 1 Early phase (2 weeks, range 1–4 weeks).
4.2. Analysis.
Comparison 4 Combination versus antidepressant (AD) alone: remission in depression, Outcome 2 Acute phase (8 weeks, range 5–12 weeks).
4.3. Analysis.
Comparison 4 Combination versus antidepressant (AD) alone: remission in depression, Outcome 3 Continuous phase (> 12 weeks).
Anxiety severity
Three studies reported anxiety severity in the early and acute phases. There were no differences between combination therapy and monotherapy in the early phase (SMD –0.76, 95% CI –1.67 to 0.14, 3 studies, 129 participants; Analysis 5.1) or acute phase (SMD –0.48, 95% CI –1.06 to 0.10; 3 studies, 129 participants; Analysis 5.2). There were substantial levels of heterogeneity in the overall results in the early phase (I2 = 81%) and in the acute phase (I2 = 57%) possibly because the studies used different antidepressants and benzodiazepines. No studies reported anxiety severity in the continuous phase.
5.1. Analysis.
Comparison 5 Combination versus antidepressant (AD) alone: anxiety severity, Outcome 1 Early phase (2 weeks, range 1–4 weeks).
5.2. Analysis.
Comparison 5 Combination versus antidepressant (AD) alone: anxiety severity, Outcome 2 Acute phase (8 weeks, range 5–12 weeks).
Insomnia severity
We found no data on insomnia severity.
Adverse effects
The participants allocated to combination therapy were less likely to drop out from the treatment due to adverse effects than those receiving antidepressants monotherapy (RR 0.54, 95% CI 0.32 to 0.90; 10 studies, 731 participants; Analysis 6.1). There was no evidence of heterogeneity (I2 = 0%). However, the combination group reported at least one adverse effect more often than the monotherapy group (RR 1.12, 95% CI 1.01 to 1.23; 7 studies, 510 participants; Analysis 6.2). There was no evidence of heterogeneity (I2 = 0%).
6.1. Analysis.
Comparison 6 Combination versus antidepressant (AD) alone: adverse effects, Outcome 1 Dropouts due to adverse effects.
6.2. Analysis.
Comparison 6 Combination versus antidepressant (AD) alone: adverse effects, Outcome 2 Number of participants with ≥ 1 adverse effect.
Subgroup analyses
Severity of comorbid anxiety
Only two studies (involving 109 participants) reported HAM‐A score at baseline or screening period. Participants of one study had moderate‐to‐severe anxiety (Calcedo Ordonez 1992) and those of the other study had mild‐to‐moderate anxiety (Fawcett 1987). There was no difference in effect between these two studies in either the early or acute phases (Analysis 7.1; Analysis 7.2).
7.1. Analysis.
Comparison 7 Subgroup analysis: severity of comorbid anxiety, Outcome 1 Depression severity, early phase (2 weeks, range ranged 1–4 weeks).
7.2. Analysis.
Comparison 7 Subgroup analysis: severity of comorbid anxiety, Outcome 2 Depression severity, acute phase (8 weeks, range 5–12 weeks).
Differences between short‐acting benzodiazepines given at bedtime and the others
The two studies that used a short‐acting benzodiazepine at bedtime produced SMD of –0.66 (95% CI –1.53 to 0.20) in the early phase, and –0.07 (95% CI –0.46 to 0.32) in the acute phase (Dominguez 1984; Nolen 1993), compared with the other eight studies that did not use a short‐acting benzodiazepines at bedtime, producing an SMD of –0.28 (95% CI –0.53 to –0.03) in the early phase and –0.15 (95% CI –0.37 to 0.07) in the acute phase. There was no evidence of a difference between types of benzodiazepines in the early phase (subgroup I2 = 21%, P = 0.38; Analysis 1.1) or acute phase (subgroup I2 = 0%, P = 0.47; Analysis 1.2). In addition, no evidence of a difference in acceptability of treatment was found between types of types of benzodiazepines (subgroup I2 = 0%, P = 0.99; Analysis 2.1).
Types of antidepressants
Exploratory analysis for each class of of antidepressants (TCAs, SSRIs and other antidepressants) for our primary outcomes suggested a difference between TCA plus benzodiazepine compared with TCA alone in the acute phase (SMD –0.29, 95% CI –0.50 to –0.09; 6 RCTs, 529 participants), but no difference for SSRIs (SMD –0.30, 95% CI –0.64 to 0.05; 2 RCTs, 130 participants) or mianserin, the only compound in the 'other antidepressants' class (SMD 0.22, 95% CI –0.72 to 1.15; 1 trial, 19 participants) (Analysis 8.1). However, there were no differences between these subgroups (subgroup I2 = 0%, P = 0.58) for either severity of depression of for acceptability of treatment (subgroup I2 = 52%, P = 0.13; Analysis 8.2).
8.1. Analysis.
Comparison 8 Subgroup analysis: types of antidepressants (AD), Outcome 1 Depression severity (2 weeks, range 1–4 weeks).
8.2. Analysis.
Comparison 8 Subgroup analysis: types of antidepressants (AD), Outcome 2 Acceptability of treatment as measured by dropout for any reason.
Sensitivity analyses
Results were consistent in sensitivity analyses according to a fixed‐effect instead of a random‐effects model (Analysis 9.1), exclusion of non‐double blind trials (Analysis 10.1), response limiting the studies to those reporting 50% reduction on HRSD (Analysis 11.1), exclusion of trials with a high risk of bias because of incomplete outcome data (Analysis 12.1), or exclusion of trials where missing actual outcome data were imputed (standard deviation were imputed in excluded trials) (Analysis 13.1). We did not perform the sensitivity analysis excluding trials using self‐report because all studies reported observer‐rated scales. We did not perform the sensitivity analysis giving half the weight to arms marketed by the sponsor of the trial because we found no study without the sponsor.
9.1. Analysis.
Comparison 9 Sensitivity analysis: fixed‐effect instead of random‐effects model, Outcome 1 Depression severity (2 weeks, range 1–4 weeks).
10.1. Analysis.
Comparison 10 Sensitivity analysis: exclusion of non‐double blind trials, Outcome 1 Depression severity.
11.1. Analysis.
Comparison 11 Sensitivity analysis: limiting studies to those reporting on 50% reduction on Hamilton Rating Scale for Depression, Outcome 1 Response (2 weeks, range 1–4 weeks).
12.1. Analysis.
Comparison 12 Sensitivity analysis: exclusion of trials with a high risk of bias because of incomplete outcome data, Outcome 1 Depression severity.
13.1. Analysis.
Comparison 13 Sensitivity analysis: exclusion of trials where missing actual outcome data were imputed, Outcome 1 Depression severity (2 weeks, range 1–4 weeks).
Reporting bias
There was no evidence of possible funnel plot asymmetry for either primary outcomes: depression severity and acceptability of treatment. The graphs appeared symmetrical (see Figure 4; Figure 5) and the Egger's test for bias was not significant (depression severity: P = 0.819; acceptability of treatment: P = 0.073).
4.
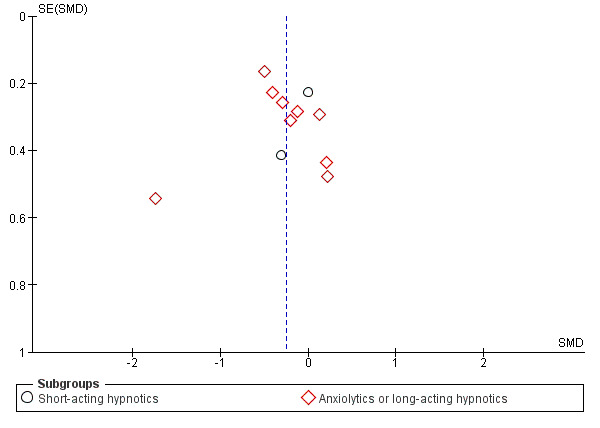
Funnel plot of comparison: 1 Depression severity, outcome: 1.1 Early phase (two weeks, range one to four weeks).
5.
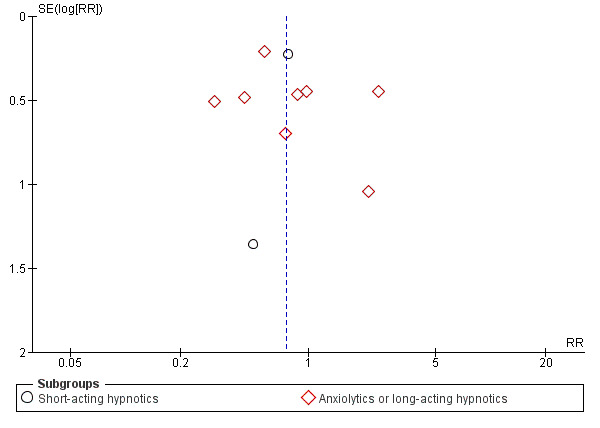
Funnel plot of comparison: 2 Acceptability of treatment, outcome: 2.1 Dropout for any reason.
Discussion
Summary of main results
See Table 1 for the main comparison.
Aggregating 10 studies involving 731 participants, antidepressant plus benzodiazepine therapy was more effective in the early phase (one to four weeks) than antidepressant monotherapy, but there was no evidence of a difference in the acute phase (five to 12 weeks) and in the continuous phase (more than 12 weeks). Participants allocated to the combination treatment were as likely to drop out from the treatment as those allocated to antidepressant alone. NNTB analysis suggested that nine participants need to be treated with an antidepressant plus a benzodiazepine in the early phase for one additional participant to show 50% or greater reduction in his or her depressive severity from baseline. Although the available studies were limited, we could find no evidence to suggest that the baseline comorbid anxiety level would alter these general findings.Six of the included studies examined TCAs, two examined SSRIs and one examined mianserin but there was no subgroup heterogeneity due to antidepressant classes (TCAs: –0.29, 95% CI –0.50 to –0.09, 6 RCTs, 529 participants; SSRIs: –0.30, 95% CI –0.71 to 0.05, 2 RCTs, 130 participants; other antidepressants (mianserin): 0.22, 95% CI –0.72 to 1.15, 1 trial, 19 participants; test for subgroup difference P = 0.58, subgroup heterogeneity I2 = 0%). The observed difference in effect between different classes of antidepressants is uncertain; whilst there may be a difference in effect, further evidence is required. In the acute and continuous phase, the relative risk for response was not statistically significant and the possibility that the combination therapy might actually lessen or might not influence the response rate could not be ruled out, although it is also possible that the corresponding NNTB could be as small as four.
These are clinically meaningful figures. For example, chlorpromazine prevents one participant out of 14 from dropping out of treatment, and promotes global improvement in one out of nine to 12 people with schizophrenia who are treated with it instead of placebo (Leucht 2013). The number needed to treat of second‐generation antipsychotics in acute mania is about four (Correll 2010) and that of antidepressants for major depression is about nine (Arroll 2009). In other words, combining a benzodiazepine with an antidepressant is as effective as chlorpromazine over placebo for acute schizophrenia, and nearly as effective as lithium over placebo for acute mania or SSRI over placebo for major depression.
Overall completeness and applicability of evidence
Our conclusions were only based 10 studies that fulfilled our inclusion criteria. We performed a subgroup analysis to compare short‐acting benzodiazepines (less than 12 hour half‐life) given at bedtime and the others (such as long‐acting benzodiazepines taken at bedtime and anxiolytic benzodiazepines taken during the day) because the latter may have some anxiolytic effects and affect the mood during the day, while the former may not have such effects. Most of the included studies used TCA and long‐acting benzodiazepines taken at bedtime or anxiolytic benzodiazepines taken during the day. Meta‐analysis of SSRIs included only two studies and there were insufficient data to conduct meta‐analyses for the other antidepressants (e.g. serotonin‐noradrenaline reuptake inhibitors). However, there was no subgroup heterogeneity noted either visually or statistically between TCAs and SSRIs, or between short‐acting benzodiazepines given at bedtime or other benzodiazepines.
Quality of the evidence
Our judgements of quality according to GRADE are given in the Table 1 for the main comparison. All included trials were RCTs and were similar in design and conduct. However, the evidence upon which the findings of this review were based was relatively poor as evaluated with the Cochrane 'Risk of bias' tool, and this was also reflected in our grading within Table 1. Most domains of risk of bias in majority of included studies were unclear. Random sequence generation, allocation concealment, blinding and selective outcome reporting were problematic due to insufficient details in most included studies and lack of availability of study protocols. Most studies included in our review were described as 'double‐blind', but none of the RCTs reported information on the procedure followed to guarantee the blindness and if blinding was successful. In addition, less than half of the studies reported most secondary outcomes (except dropout due to adverse effects), which is likely to have introduced bias for these data. The greatest limitation to the quality of evidence was issues with attrition. Some studies had high rates of dropout (the highest being 41% dropout in Yamaoka 1994). The amount of attrition was generally well described, but the timings of dropout were often insufficiently detailed to assess the likelihood of meaningful bias. We downgraded all our findings by one level for these study limitations. The quality of evidence evaluated with the GRADE methodology was moderate for depression in the early phase, acceptability of treatment and adverse effects, low for depression in the acute and continuous phases, and very low for anxiety severity in the early phase. The included participants in the outcome of anxiety severity amounted to 129 and the CIs were wide. Heterogeneity among the included studies was high, possibly because the studies used different antidepressants and benzodiazepines. We found no possible factors that would upgrade the quality of the evidence.
Potential biases in the review process
First, the most important weakness in this review was that only one trial followed the participants beyond eight weeks. Therefore, the present meta‐analysis could only provide information for early and acute phases and, with less statistical power, up to 14 weeks. Three studies examined withdrawal of acute‐phase benzodiazepines and two demonstrated some rebound (Feet 1985; Smith 1998), whereas another, which extended the use up to 12 weeks, did not (Smith 2002). Second, it is of note that all but three studies involved TCAs: two studies employed fluoxetine and one employed mianserin. However, the two studies employing fluoxetine (Smith 1998; Smith 2002) were in line with the other studies in terms of response rates according to tests of heterogeneity. Third, it could be argued that that the response in terms of HRSD may reflect changes in sleep and anxiety only and not in core depressive symptoms. By calculating anxiety subscale and insomnia subscale scores out of the total HRSD, Smith 1998 found significant superiority of the combined fluoxetine plus clonazepam treatment for all subscales. Smith 2002, employing basically identical procedures, confirmed superiority in terms of the insomnia subscale only. The one study focusing on anxious depression demonstrated superiority of the combined treatment (Feighner 1979), whereas the other study focusing on non‐anxious depression reported equivalence (Feet 1985). Therefore, three studies suggested that combination therapy outperformed antidepressant monotherapy when anxiety or insomnia were present, while such superiority for depression without these accompanying symptoms remained to be further examined (Feighner 1979; Smith 1998; Smith 2002). Fourth, we included one study with a dropout rate above 40% (Yamaoka 1994), and four studies with dropout rate above 20% (Calcedo Ordonez 1992; Dominguez 1984; Feighner 1979; Smith 2002). This may have introduced bias, but the findings of this review were robust to sensitivity analysis excluding trials with a high risk of bias because of incomplete outcome data. Fifth, some numbers had to be imputed from data reported in the RCT itself or from data of other RCTs.
At the review level, we consider the systematic review process identified all relevant trials. We attempted to identify relevant studies through database searches and citation tracking. Despite these efforts, it is possible that publication bias may have influenced the review findings. However, there was no evidence of possible funnel plot asymmetry for either primary outcome. The graphs appeared to be symmetrical and the Egger's test for bias was not significant.
For this updated review, we edited the methods section to bring it up to date with Cochrane's current methodological standards and made several updates to the protocol (e.g. the inclusion of remission as a secondary outcome, change the timing of outcome assessment and inclusion of limiting the studies to those reporting on 50% reduction on HRSD as sensitivity analysis, assessment of quality of evidence according to GRADE). All these changes in the protocol were made and appeared through peer reviews prior to the actual conduct of updating the review. Since we used the original authors' own definitions of response or remission if they used such definitions, we should consider the possibility of outcome measure reporting bias. However, there was only one study (Feet 1985) which used the authors' own definition for remission (<2.5 cm out of 10 cm Visual Analog System).
Agreements and disagreements with other studies or reviews
We edited the methods section to bring it up to date with Cochrane's current methodological standards from previous version of this review. While changes to the protocol may have introduced bias in the review process, most changes made here had no impact on the current review. We added remission to the secondary outcomes and two sensitivity analyses (exclusion of trials using only self‐report; limiting the studies to those reporting on 50% reduction on HRSD) and results were consistent in sensitivity analyses.
The benefits of adding a benzodiazepine to an antidepressant must be balanced judiciously against possible harms, including development of dependence, tolerance, accident proneness, teratogenicity and costs. While this review could not address specific downsides of benzodiazepine use, there is abundant literature suggesting them.
It is well recognised that benzodiazepines can induce dependence, defined as development of clinically meaningful symptoms upon its discontinuation. Although the reported incidence differs widely among studies, dependence is generally estimated to occur in almost one third of participants receiving regular prescription of a benzodiazepine for four weeks or longer (Noyes 1988; Schweizer 1998).
In the case of the antidepressant plus benzodiazepine combination therapy, discontinuation of benzodiazepine may cause, in addition to the above mentioned discontinuation symptoms, worsening of symptoms due to loss of synergistic treatment effects or to unmasking of adverse effects of antidepressants.
Two RCTs have addressed the effects of tapering a benzodiazepine out of the combination therapy under double‐blind, placebo‐controlled conditions. Feet 1985 followed the participants in one RCT of combination therapy (included in this review). After the participants were on imipramine (100 mg/day to 200 mg/day) in combination with diazepam (10 mg/day) or placebo for three and a half months, participants who were practically symptom free had their diazepam or placebo discontinued. The number needed to treat for an additional harmful outcome (NNTH) for impairment after discontinuation was two (95% CI 1 to 7) and that for unsuccessful discontinuation was five (not significant). Smith 1998 conducted one RCT comparing fluoxetine plus clonazepam (0.5 mg/day to 1.0 mg/day) versus fluoxetine plus placebo in major depression. The investigators tapered clonazepam and placebo after three weeks of combination therapy and over two weeks. More participants in the combination group lost the response, 50% or greater reduction, within 10 days after taper than in the fluoxetine plus placebo group and the NNTH was seven (95% CI 4 to 25). Fewer participants in the combination group complained of new or worsened adverse effects for discontinuation than monotherapy group and the NNTH was –5 (95% CI –3 to –105). The NNTH for unsuccessful discontinuation was 40 (not significant). In contrast, Smith 2002 conducted a very similarly designed study comparing fluoxetine plus clonazepam versus fluoxetine plus placebo, but this time extended over 18 weeks. In addition, this study tapered off clonazepam between 12 and 15 weeks (i.e. over three weeks), and observed no rebound phenomenon.
Benzodiazepines are also believed to be subject to development of tolerance, which is a decline in a drug's effects over time. Thus the UK Committee on the Review of Medicines concluded, "there was little convincing evidence that benzodiazepines were efficacious in the treatment of anxiety after four months' continuous treatment" (CRM 1980). However, since then, several studies have suggested that tolerance develops to motor impairments and sedative effects but not to antianxiety effects of benzodiazepines (Burrows 1993; Fabre 1981; Lucki 1986; Rickels 1983; Rickels 1985).
Benzodiazepines have been associated with accident proneness. First, one cohort study on the association between first prescription of a benzodiazepine in older people and a subsequent hospitalisation due to a fall revealed an NNTH of about 110 to 190 (Neutel 1995). This NNTH was comparable to the NNTH of SSRIs or secondary‐amine TCAs increasing hip fractures in older people (SSRIs: NNTH 220, 95% CI 180 to 300; secondary‐amine TCAs: 250, 95% CI 170 to 380) (Liu 1998). Second, some epidemiological studies hinted at an increase of motor vehicle accidents among benzodiazepine users. One cohort study on this problem suggested that approximately 2900 people need to be treated with a benzodiazepine for two months in order to cause one hospitalisation due to traffic accidents (NNTH 2860, 95% CI 2050 to 4760) (Neutel 1995). This increase in accident proneness with benzodiazepine use may be comparable to that with cyclic antidepressant use (Ray 1992). One short‐term experiment using healthy adults suggested that combining a benzodiazepine with an antidepressant might cause greater motor impairment than an antidepressant alone (Moskowitz 1988).
By contrast to the strategy of adding benzodiazepines, we also now have alternative strategies when antidepressant monotherapy is considered inadequate. They include combination with other antidepressants, antipsychotics or lithium among others (Carpenter 2002; Crossley 2007; Ferreri 2001; Licht 2002; Nelson 2009; Whale 2010). The comparison of the combined antidepressant plus benzodiazepine therapy and the other combined treatments was beyond the scope of the present review.
Authors' conclusions
Implications for practice.
This review provides moderate quality evidence that, compared to antidepressant monotherapy, combined antidepressant and benzodiazepine therapy results in a reduction in depression severity corresponding to an SMD of –0.25 (95% CI –0.46 to –0.03) in the early phase (one to four weeks) of treatment for major depression. However, this observed superiority was not maintained at the end of acute‐phase treatment (five to 12 weeks) or in the continuous phase (more than 12 weeks). These analyses suggest that patients receiving combination therapy are less likely to dropout due to adverse events than the antidepressant monotherapy, but also that they are more likely to report at least one adverse effect. The review was unable to examine details of risks associated with use of the combination therapy, especially over the longer term. Where antidepressant monotherapy may be considered inadequate, the potential benefits of the combined antidepressant plus benzodiazepine therapy must be balanced judiciously and individually against the possible harms of using a benzodiazepine (including development of dependence and accident proneness) and full consideration should be given to other alternative treatment strategies.
Implications for research.
We need long‐term, pragmatic RCTs to compare combination therapy against the monotherapy of antidepressant in major depression. Such trials should examine the addition of benzodiazepines and other combination strategies where antidepressant monotherapy may be inadequate. We also hope that such trials may elucidate if different classes of antidepressants, benzodiazepines or other psychotropics, and baseline characteristics such as comorbid anxiety may moderate treatment effects.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2019 | New search has been performed | The review has been updated |
| 31 May 2019 | New citation required and conclusions have changed | The review has been updated according to Cochrane's current methodological standards. No new studies have been added as a result of new searches. However, many sections of the review including the conclusions have been substantively updated. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 15 November 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Sarah Dawson, Information Specialist for the Cochrane Common Mental Disorders Group, for her help in developing and conducting the search strategy for the present review. We would also like to thank the Cochrane Common Mental Disorders Group editorial team, particularly Jessica Sharp, for their advice and assistance. We would also like to thank the authors of the previous version of this review (Trevor Young, Yoshihiro Kinoshita).
CRG funding acknowledgement
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer
The views and opinions expressed herein were those of the review authors and did not necessarily reflect those of the NIHR, National Health Service or the Department of Health and Social Care.
Appendices
Appendix 1. CCMDCTR (core MEDLINE search)
Core search strategy used to inform the Cochrane Common Mental Disorders Group's specialised register: Ovid MEDLINE (to June 2016) A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record. Similar weekly search alerts were also conducted on Ovid Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
The Cochrane Collaboration Depression, Anxiety and Neurosis Group (CCDAN) was renamed in 2015 to Cochrane Common Mental Disorders (CCMD) and the renaming of the Group's specialised, controlled trials register reflects this change (CCMDCTR).
Appendix 2. Search strategies (current version of the review)
1.The CCMDCTR (Studies and Reference Registers) was searched (all years to 28 June 2016) using the following terms, on the new Cochrane Register of Studies (CRS) platform:
#1. depress*:ti,ab,kw,ky,emt,mh,mc #2. (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or "monoamine oxidase inhibit*" or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*) #3. (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Bupropion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233) #4. (Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or "St John*") or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or ("Lu AA21004" or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*) #5. (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) #6. (#2 or #3 or #4 or #5) #7. (Benzodiazepin* or BZD or Abecarnil or Adinazolam or Alprazolam or Arfendazam or Bentazepam or Bretazenil or Bromazepam or Brotizolam or Camazepam or Chlordiazepoxide or Chlordesmethyldiazepam or Cinolazepam or Clobazam or Clonazepam or Clorazepate or Chlorazepate or Clotiazepam or Cloxazolam or Delorazepam or Demoxepam or Desmethyldiazepam or Desoxydemoxepam or Devazepide or Diazepam or Doxefazepam or Estazolam or "ethyl loflazepate" or "CM 6912" or CM‐6912 or Etizolam or Fludiazepam or Flunitrazepam or Flurazepam or dealkylflurazepam or Flutoprazepam or Fosazepam or Gidazepam or Girisopam or Halazepam or Haloxazolam or Ketazolam or Loflazepate or Loprazolam or Lorazepam or Lormetazepam or Meclonazepam or Medazepam or Metaclazepam or Mexazolam or Midazolam or Nerisopam or Nimetazepam or Nitrazepam or Norchlordiazepoxide or Norclobazam or Nordazepam or Norfludiazepam or Norflunitrazepam or Oxazepam or "WY 3498" or WY‐3498 or Oxazolam or Phenazepam or Pinazepam or Prazepam or Premazepam or Propazepam or Quazepam or Ripazepam or Serazepine or Sograzepide or Talampanel or Tarazepide or Temazepam or Tetrazepam or Tofisopam or Triazolam or (Zolazepam or Zaleplon or Zolpidem or Zopiclone or Eszopiclone or Z‐Drugs or "Z Drugs")) #8. (#1 and #6 and #7)
Additional searches were conducted on Ovid MEDLINE, Embase, PsycINFO and the Cochrane Library (2014 to May 2019) to account for the period when the CCMDCTR had fallen out‐of‐date with the Editorial Group’s move from Bristol to York in the summer of 2016. MEDLINE Databases: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <2014 to 23 May 2019> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 controlled clinical trial.pt. 2 randomized controlled trial.pt. 3 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf. 4 (RCT or at random or (random* adj3 (assign* or allocat* or control* or crossover or cross‐over or design* or divide* or division or number))).ti,ab,kf. 5 placebo*.ab,ti,kf. 6 trial.ab,ti,kf. 7 groups.ab. 8 (control* and (trial or study or group*) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,hw. 9 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf. 10 double‐blind method/ or random allocation/ or single‐blind method/ 11 or/1‐10 12 exp animals/ not humans.sh. 13 11 not 12 14 exp Antidepressive Agents/ 15 exp Neurotransmitter Uptake Inhibitors/ 16 exp Monoamine Oxidase Inhibitors/ 17 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic*).mp. 18 (Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin*or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine) or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Duloxetine or DVS 233 or Enilospirone or Eptapirone or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine).mp. 19 (Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin*).mp. 20 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or (Protriptylin* or Pertofrane) or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or (Setiptiline or Teciptiline) or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5‐Hydroxytryptophan or 5‐HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone or Zimeldine).mp. 21 (Alaproclate or Caroxazone or Diclofensin* or Fenfluramin*).mp. 22 or/14‐21 23 exp benzodiazepines/ 24 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or cm 6912 or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drug?).mp. 25 23 or 24 26 mood disorders/ or depressive disorder/ or depressive disorder, major/ 27 depression/ 28 (depressi* or depressed or dysthymi* or mood disorder or affective disorder? or affective symptom?).ti,ab,kw. 29 *Mental Disorders/dt 30 or/26‐29 31 13 and 22 and 25 and 30 32 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).yr,ed,dc,dp,ep. 33 (31 and 32) *************************** Ovid Embase <2014 to 2019 Week 20> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial/ 2 randomization.de. 3 controlled clinical trial/ and (Disease Management or Drug Therapy or Prevention or Rehabilitation or Therapy).fs. 4 *clinical trial/ 5 placebo.de. 6 placebo.ti,ab. 7 trial.ti. 8 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kw. 9 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. 10 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. 11 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kw,hw. 12 or/1‐11 13 ((animal or nonhuman) not (human and (animal or nonhuman))).de. 14 12 not 13 15 DEPRESSION/ or AGITATED DEPRESSION/ or ATYPICAL DEPRESSION/ or DYSTHYMIA/ or ENDOGENOUS DEPRESSION/ or INVOLUTIONAL DEPRESSION/ or MAJOR DEPRESSION/ or MASKED DEPRESSION/ or MELANCHOLIA/ or "MIXED ANXIETY and DEPRESSION”/ or "MIXED DEPRESSION AND DEMENTIA"/ or MOURNING SYNDROME/ or ORGANIC DEPRESSION/ or POSTOPERATIVE DEPRESSION/ or REACTIVE DEPRESSION/ or RECURRENT BRIEF DEPRESSION/ or SEASONAL AFFECTIVE DISORDER/ 16 (antidepress* or anti depress* or MDD or depress* or affective disorder* or affective symptom* or mood*).ti,kw. 17 (depress* adj3 (adult* or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient)).ti,ab,kw. 18 with depression.ti,ab. 19 (depress* and (Beck* or BDI* or DSM* or (Diagnostic adj Statistical Manual adj Mental Disorders) or Hamilton or HAM‐D or HAMD or MADRS or (International Classification adj Disease?) or ICD‐10)).ti,ab. 20 or/15‐19 21 ((Antidepress* or Anti‐depress* or Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine) or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Duloxetine or DVS‐233 or Enilospirone or Eptapirone or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine or Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or (Protriptylin* or Pertofrane) or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or (Setiptiline or Teciptiline) or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5 Hydroxytryptophan or 5 HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone or Zimeldine or Alaproclate or Caroxazone or Diclofensin* or Fenfluramin*) AND (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or cm 6912 or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drug? or z drug?)).ti. 22 ((Antidepress* or Anti‐depress* or Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine) or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Duloxetine or DVS 233 or Enilospirone or Eptapirone or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine or Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or (Protriptylin* or Pertofrane) or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or (Setiptiline or Teciptiline) or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5 Hydroxytryptophan or 5 HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone or Zimeldine or Alaproclate or Caroxazone or Diclofensin* or Fenfluramin*) ADJ5 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or cm 6912 or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drug? or z drug?)).ab. 23 exp *antidepressant agent/ or exp *serotonin uptake inhibitor/ or exp *serotonin noradrenalin reuptake inhibitor/ or exp *noradrenalin uptake inhibitor/ 24 exp *benzodiazepine derivative/ 25 14 and 20 and (21 or 22 or (23 and 24)) 26 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).dc,dp,yr. 27 (25 and 26) *************************** Ovid PsycINFO <2014 to May Week 3 2019> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 clinical trials.sh. 2 (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. 3 (RCT or at random or (random* adj3 (assign* or allocat* or control* or crossover or cross‐over or design* or divide* or division or number))).ti,ab,id. 4 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw. 5 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. 6 trial.ti. 7 placebo.ti,ab,id,hw. 8 treatment outcome.md. 9 treatment effectiveness evaluation.sh. 10 mental health program evaluation.sh. 11 or/1‐10 12 exp antidepressant drugs/ or exp serotonin norepinephrine reuptake inhibitors/ or exp tricyclic antidepressant drugs/ or exp monoamine oxidase inhibitors/ 13 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic*).ti,ab,id. 14 (Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin* or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine) or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Duloxetine or DVS‐233 or Enilospirone or Eptapirone or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine or Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or (Protriptylin* or Pertofrane) or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or (Setiptiline or Teciptiline) or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5 Hydroxytryptophan or 5 HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone or Zimeldine or Alaproclate or Caroxazone or Diclofensin* or Fenfluramin*).ti,ab,id,hw. 15 or/12‐14 16 exp benzodiazepines/ 17 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or cm 6912 or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drug? or z drug?).ti,ab,id,hw. 18 16 or 17 19 MAJOR DEPRESSION/ or ANACLITIC DEPRESSION/ or DYSTHYMIC DISORDER/ or ENDOGENOUS DEPRESSION/ or REACTIVE DEPRESSION/ or ATYPICAL DEPRESSION/ or "DEPRESSION (EMOTION)"/ 20 (depressi* or depressed or dysthymi* or mood disorder or affective disorder? or affective symptom?).ti,ab,id. 21 19 or 20 22 11 and 15 and 18 and 21 23 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).yr,an. 24 (22 and 23) ***************************
Cochrane Central Register of Controlled Trials, 2019, Issue 5 of 12
#1 MeSH descriptor: [Mood Disorders] this term only #2 MeSH descriptor: [Depression] this term only #3 MeSH descriptor: [Depressive Disorder] this term only #4 MeSH descriptor: [Depressive Disorder, Major] this term only #5 MeSH descriptor: [Dysthymic Disorder] this term only #6 MeSH descriptor: [Adjustment Disorders] this term only #7 MeSH descriptor: [Affective Symptoms] this term only #8 ((affective or mood or adjustment) near/2 (dis*)) #9 (depress* or dysthymi*) #10 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #11 MeSH descriptor: [Antidepressive Agents] explode all trees #12 MeSH descriptor: [Neurotransmitter Uptake Inhibitors] explode all trees #13 MeSH descriptor: [Monoamine Oxidase Inhibitors] this term only #14 (antidepress* or "anti depress*" or MAOI* or "monoamine oxidase inhibit*" or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic*) #15 (Agomelatine or Alnespirone or Amoxapine or Amersergide or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Aripiprazole or Atomoxetine or Tomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or Bupropion or Butriptylin*or Chlopoxiten or Cianopramine or Cilobamine or Cilosamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine) or Clorgyline or Clovoxamine or Dapoxetine or Deanol or Dibenzepin or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Duloxetine or DVS‐ 233 or "DVS 233" or Enilospirone or Eptapirone or Escitalopram or Esmirtazapine or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine) #16 (Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or ("Lu AA21004" or Lu‐AA21004 or Vortioxetine) or "Lu AA24530" or Lu‐AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Methylphenidate or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Monocrotophos or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin*) #17 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or (Protriptylin* or Pertofrane) or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or (Setiptiline or Teciptiline) or Tandospirone or Teniloxine or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5‐Hydroxytryptophan or 5‐HT or "5 HT" or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone or Zimeldine) #18 (Alaproclate or Caroxazone or Diclofensin* or Fenfluramin*) #19 (#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18) #20 MeSH descriptor: [Benzodiazepines] explode all trees #21 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or "cm 6912" or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or "wy 3498" or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drugs or z drugs) #22 (#20 or #21) #23 (#10 and #19 and #22) Limited 2014 to date
Appendix 3. Search strategies (previous published version)
1. The Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR) We searched the Cochrane Library and the specialised trials register of the Cochrane Depression, Anxiety and Neurosis Group (CCDANCTR) from 1972 to 1999 with an update search performed in 2004. We limited the search to studies conducted since 1972 because none of the operationalised diagnostic criteria, as listed in our inclusion criteria, existed before this date.
Search terms used: #1 antidepress* or tricycl* or heterocycl* or TCA or "serotonin reuptake inhibitor*" or SSRI or "monoamine oxidase inhibitor*" or MAOI or amitriptylin* or amoxapin* or benactyzin* or bupropion* or citalopram* or clomipramin* or clorgylin* or deanol* or dothiepin* or doxepin* or fluoxetin* or fluvoxamin* or imipramin* or iprindol* or iproniazid* or isocarboxazid* or lofepramin* or maprotilin* or mianserin* or moclobemid* or nialamid* or nortriptylin* or opipramol* or paroxetin* or phenelzin* or pizotylin* or protriptylin* or quipazin* or sulpirid* or timipramin* or tranylcypromin* or trazodon* or viloxazin* #2 benzodiazepin* or anti‐anxiety or anxiolyt* or alprazolam* or anthramycin* or bromazepam* or chlordiazepoxid* or clonazepam* or clorazepat* or diazepam* or diltiazem* or estazolam* or flumazenil* or flunitrazepam* or flurazepam* or lorazepam* or medazepam* or midazolam* or nitrazepam* or nordazepam* or oxazepam* or pirenzepin* or prazepam* or temazepam* or triazolam* #3 #1 AND #2 #4 depression* (keyword) OR depressive‐disorder* (keyword) #5 #3 AND #4
2. We searched other biomedical databases were searched using similar terms in September 1998: MEDLINE, Embase, International Pharmaceutical Abstracts, Biological Abstracts, LILACS and PsycLIT.
Search results were limited to RCTs by using the following terms: #6 randomized controlled trial* OR controlled trial* OR clinical trial* #7 random* #8 (singl* OR doubl* OR tripl* OR trebl*) AND (blind* OR mask*) #9 crossover #10 versus OR vs #11 #6 OR #7 OR #8 OR #9 OR #10
When searching the International Pharmaceutical Abstracts, line #4 was omitted as we were unsure if all relevant reports of studies would have 'depression' or 'depressive disorder' listed in either the title, abstract or keyword fields.
Data and analyses
Comparison 1. Combination versus antidepressant (AD) alone: depressive severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early phase (2 weeks, range 1–4 weeks) | 10 | 598 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.46, ‐0.03] |
| 1.1 Short‐acting hypnotics | 2 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.46, 0.32] |
| 1.2 Anxiolytics or long‐acting hypnotics | 9 | 494 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.53, ‐0.03] |
| 2 Acute phase (8 weeks, range 5–12 weeks) | 7 | 347 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.40, 0.03] |
| 2.1 Short‐acting hypnotics | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.53, 0.20] |
| 2.2 Anxiolytics or long‐acting hypnotics | 7 | 322 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.37, 0.07] |
| 3 Continuous phase (> 12 weeks) | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.76, 0.35] |
| 3.1 Anxiolytics or long‐acting hypnotics | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.76, 0.35] |
Comparison 2. Combination versus antidepressant (AD) alone: acceptability of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropout for any reason | 10 | 731 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 1.1 Short‐acting hypnotics | 2 | 153 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.49, 1.20] |
| 1.2 Anxiolytics or long‐acting hypnotics | 9 | 578 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.49, 1.22] |
Comparison 3. Combination versus antidepressant (AD) alone: response in depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early phase (2 weeks, range 1–4 weeks) | 10 | 731 | Risk Ratio (IV, Random, 95% CI) | 1.34 [1.13, 1.58] |
| 1.1 Short‐acting hypnotics | 2 | 153 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.58, 3.96] |
| 1.2 Anxiolytics or long‐acting hypnotics | 9 | 578 | Risk Ratio (IV, Random, 95% CI) | 1.34 [1.13, 1.59] |
| 2 Acute phase (8 weeks, range 5–12 week) | 7 | 383 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.93, 1.35] |
| 2.1 Short‐acting hypnotics | 1 | 27 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.42, 21.30] |
| 2.2 Anxiolytics or long‐acting hypnotics | 7 | 356 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.92, 1.34] |
| 3 Continuous phase (> 12 weeks) | 1 | 52 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.73, 1.29] |
| 3.1 Anxiolytics or long‐acting hypnotics | 1 | 52 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.73, 1.29] |
Comparison 4. Combination versus antidepressant (AD) alone: remission in depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early phase (2 weeks, range 1–4 weeks) | 10 | 731 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.03, 1.90] |
| 1.1 Short‐acting hypnotics | 2 | 153 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.47, 2.92] |
| 1.2 Anxiolytics or long‐acting hypnotics | 9 | 578 | Risk Ratio (IV, Random, 95% CI) | 1.45 [0.97, 2.18] |
| 2 Acute phase (8 weeks, range 5–12 weeks) | 7 | 383 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.99, 1.63] |
| 2.1 Short‐acting hypnotics | 1 | 27 | Risk Ratio (IV, Random, 95% CI) | 2.63 [0.14, 49.69] |
| 2.2 Anxiolytics or long‐acting hypnotics | 7 | 356 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.96, 1.65] |
| 3 Continuous phase (> 12 weeks) | 1 | 52 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.80, 2.16] |
| 3.1 Anxiolytics or long‐acting hypnotics | 1 | 52 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.80, 2.16] |
Comparison 5. Combination versus antidepressant (AD) alone: anxiety severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early phase (2 weeks, range 1–4 weeks) | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.67, 0.14] |
| 1.1 Anxiolytics or long‐acting hypnotics | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.67, 0.14] |
| 2 Acute phase (8 weeks, range 5–12 weeks) | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.06, 0.10] |
| 2.1 Anxiolytics or long‐acting hypnotics | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.06, 0.10] |
Comparison 6. Combination versus antidepressant (AD) alone: adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts due to adverse effects | 10 | 731 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.32, 0.90] |
| 1.1 Short‐acting hypnotics | 2 | 153 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.24, 1.21] |
| 1.2 Anxiolytics or long‐acting hypnotics | 9 | 578 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.28, 1.11] |
| 2 Number of participants with ≥ 1 adverse effect | 7 | 510 | Risk Ratio (IV, Random, 95% CI) | 1.12 [1.01, 1.23] |
| 2.1 Anxiolytics or long‐acting hypnotics | 7 | 510 | Risk Ratio (IV, Random, 95% CI) | 1.12 [1.01, 1.23] |
Comparison 7. Subgroup analysis: severity of comorbid anxiety.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity, early phase (2 weeks, range ranged 1–4 weeks) | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.52, 0.29] |
| 1.1 Moderate‐to‐severe anxiety | 1 | 62 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.79, 0.21] |
| 1.2 Mild‐to‐moderate anxiety | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.45, 0.70] |
| 2 Depression severity, acute phase (8 weeks, range 5–12 weeks) | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.53, 0.22] |
| 2.1 Moderate‐to‐severe anxiety | 1 | 62 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.82, 0.18] |
| 2.2 Mild‐to‐moderate anxiety | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.51, 0.64] |
Comparison 8. Subgroup analysis: types of antidepressants (AD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity (2 weeks, range 1–4 weeks) | 9 | 678 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.45, ‐0.13] |
| 1.1 Selective serotonin reuptake inhibitor (SSRI) | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.64, 0.05] |
| 1.2 Tricyclic antidepressant (TCA) | 6 | 529 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.50, ‐0.09] |
| 1.3 Other antidepressants | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.72, 1.15] |
| 2 Acceptability of treatment as measured by dropout for any reason | 9 | 678 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 2.1 SSRI | 2 | 133 | Risk Ratio (IV, Random, 95% CI) | 1.53 [0.63, 3.72] |
| 2.2 TCA | 6 | 513 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.49, 0.85] |
| 2.3 Other antidepressants | 1 | 32 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.17, 1.15] |
Comparison 9. Sensitivity analysis: fixed‐effect instead of random‐effects model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity (2 weeks, range 1–4 weeks) | 10 | 598 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.43, ‐0.10] |
| 1.1 Short‐acting hypnotics | 2 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.46, 0.32] |
| 1.2 Anxiolytics or long‐acting hypnotics | 9 | 494 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.49, ‐0.13] |
Comparison 10. Sensitivity analysis: exclusion of non‐double blind trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity | 9 | 520 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.52, ‐0.02] |
Comparison 11. Sensitivity analysis: limiting studies to those reporting on 50% reduction on Hamilton Rating Scale for Depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response (2 weeks, range 1–4 weeks) | 4 | 358 | Risk Ratio (IV, Random, 95% CI) | 1.28 [1.06, 1.55] |
Comparison 12. Sensitivity analysis: exclusion of trials with a high risk of bias because of incomplete outcome data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity | 5 | 223 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.59, ‐0.05] |
Comparison 13. Sensitivity analysis: exclusion of trials where missing actual outcome data were imputed.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity (2 weeks, range 1–4 weeks) | 5 | 190 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.78, 0.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Calcedo Ordonez 1992.
| Methods | Type of trial: parallel Randomised: yes Double‐blinding: no Duration of treatment: 6 weeks Cointervention: additional hypnotic for 1 participant in the combination and 11 participants in the antidepressant alone treatment |
|
| Participants | Setting: psychiatric OP and IP Diagnosis: major depressive episode (DSM‐III‐R) Age (mean): 48.8 (SD 13.6) years Men/women: 18/44 Baseline depressive severity (mean): 28.5 (SD 5.5) on HRSD‐21 Baseline anxiety severity: mild/moderate/severe |
|
| Interventions | Clomipramine 100–150 mg + bentazepam 75 mg | |
| Outcomes | HRSD‐21, HAM‐A up to 6 weeks | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details provided on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label design |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout was a large proportion (4/36 of intervention group and 17/47 of control group) and completer analysis was done. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available thus unsure if all of prespecified outcomes of interest. |
| Other bias | High risk | More participants in intervention group (11 participants) than in control group (1 participant) need to take administer hypnotics. |
Dominguez 1984.
| Methods | Type of trial: parallel Randomised: yes Double‐blinding: yes Duration: 4 weeks Cointervention: not available |
|
| Participants | Setting: advertisement OP Diagnosis: major depressive disorder (DSM‐III), with ≥ 1 of the 3 symptoms of insomnia Age (range): 18–65 years Men/women: not available Baseline depressive severity (mean): 26.6 (SD 5.3) on HRSD‐21 Baseline anxiety severity: not available |
|
| Interventions | Imipramine 100–145 mg + triazolam 0.5 mg hs | |
| Outcomes | HRSD‐21 up to 4 weeks | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details provided on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a four‐week, randomized, double‐blind study". Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout was a large proportion (21/63 of intervention group and 27/63 of control group) and completer analysis was done. |
| Selective reporting (reporting bias) | High risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. Only depressive severity was reported in the outcome of this review. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
Fawcett 1987.
| Methods | Type of trial: parallel Randomised: yes Duration: 6 weeks Cointervention: no supplementary psychotropic drug |
|
| Participants | Setting: psychiatric OP Diagnosis: major depression without psychotic features (DSM‐III) Age (mean): 36.7 (SD 9.1) years Men/women: 20/32 Baseline depressive severity (mean): 24.1 (SD 5.6) on HRSD‐21 Baseline anxiety severity: mild/moderate/severe |
|
| Interventions | Desipramine 100–300 mg + alprazolam 2–6 mg | |
| Outcomes | HRSD‐21, HAM‐A, CGI up to 6 weeks | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details provided on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "placebo, alprazolam (1mg), DMI [desipramine] (50mg), and the combination treatment (1mg alprazolam + 51mg DMI) were supplied in identical capsules." Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/24 of intervention group and 8/28 of control group dropped out. Quote: "last visit carried forward endpoint analyses (worst case analyses) of symptom scores were performed." |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
Feet 1985.
| Methods | Type of trial: parallel Randomised: yes Allocation concealment: unclear Double‐blinding: yes Study quality: intermediate Duration of treatment: 8 weeks Cointervention: flunitrazepam 2 mg as needed for insomnia |
|
| Participants | Setting: psychiatric OP (all participants had been previously treated in general practice without success) Diagnosis: non‐agitated primary depression (Feighner) Age (mean): 46 (range: 20–64) years Men/women: 18/24 Baseline depressive severity (mean): 4.0 (SD 1.1) on CPRS‐VAS Baseline anxiety severity: mild/moderate |
|
| Interventions | Imipramine 138–200 mg + diazepam 10 mg | |
| Outcomes | CPRS‐VAS, global VAS up to 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details provided on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A randomized double blind trial was performed." "By using matching tablets, the three groups were blindly given a fixed daily dose of either 50 mg dixyrazine, 10 mg diazepam, or placebo." Further details not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/21 of intervention group and 3/21 of control group dropped out. Quote: "Each withdrawal was assigned the least favourable status observed prior to the drop out." |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
Feighner 1979.
| Methods | Type of trial: parallel Randomised: yes Allocation concealment: yes Double‐blinding: yes Study quality: high Duration of treatment: 4 weeks Cointervention: not available |
|
| Participants | Setting: psychiatric OP Diagnosis: definite primary depression (Feighner), with moderate‐to‐severe anxiety Age (mean): 40.6 years Men/women: 66/124 Baseline depressive severity (mean): 35.2 (SD 8.8) on HRSD‐24 Baseline anxiety severity: moderate/severe |
|
| Interventions | Amitriptyline 75–150 mg + chlordiazepoxide 30–60 mg | |
| Outcomes | HRSD‐24, CGI up to 4 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details provided on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "this multicenter, double‐blind, placebo‐controlled study". Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large proportion of participants (18/97 of intervention group and 23/93 of control group) excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
Nolen 1993.
| Methods | Type of trial: parallel Randomised: yes Allocation concealment: unclear Double‐blinding: unclear Study quality: low Duration of treatment: 4 weeks Cointervention: no supplementary psychotropic drug |
|
| Participants | Setting: psychiatric IP Diagnosis: major depressive episode (DSM‐III‐R) Age (mean): 48.8 (SD 9.6) years Men/women: 19/34 Baseline depressive severity (mean): 27.5 (SD 4.7) on HRSD‐21 Baseline anxiety severity: mild/moderate/severe |
|
| Interventions | Maprotiline 160 mg or nortriptyline 150 mg + flunitrazepam 2 mg or lormetazepam 2 mg | |
| Outcomes | HRSD‐17, CGI up to 4 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details provided on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A placebo‐controlled, double‐blind comparison." Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/17 of intervention group and 2/18 of control group dropped out and missing data not imputed. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. |
| Other bias | Unclear risk | No sources of other bias identified. |
Scharf 1986.
| Methods | Type of trial: parallel Randomised: yes Allocation concealment: unclear Double‐blinding: yes Study quality: intermediate Duration of treatment: 8 weeks Cointervention: not available |
|
| Participants | Setting: psychiatric Diagnosis: "clinically depressed based on DSM‐III" Age (mean): 34.8 (SD 11.7) years Men/women: 10/10 Baseline depressive severity (mean): 24.3 (SD 5.4) on HRSD Baseline anxiety severity: not available |
|
| Interventions | Amitriptyline 50–150 mg + chlordiazepoxide 20–60 mg | |
| Outcomes | HRSD, State Anxiety Scale up to 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind, randomized comparison". Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
Smith 1998.
| Methods | Type of trial: parallel Randomised: yes Allocation concealment: yes Double‐blinding: yes Study quality: high Duration of treatment: 5 weeks Cointervention: not available |
|
| Participants | Setting: psychiatric OP Diagnosis: non‐psychotic major depressive disorder (DSM‐IV) Age (mean): 41.5 (range: 20–73) years Men/women: 38/42 Baseline depressive severity (mean): 22.1 (SD 2.9) on HRSD‐17 Baseline anxiety severity: not available |
|
| Interventions | Fluoxetine 20–40 mg + clonazepam 0.5–1 mg | |
| Outcomes | HRSD‐17, CGI up to 5 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The code could be broken in individual cases in emergencies," but the details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind treatment with clonazepam or placebo". Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/41 of intervention group and 8/40 of control group dropped out. Quote: "the most recent observation was carried forward for missing data." |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
Smith 2002.
| Methods | Type of trial: parallel Randomised: yes Allocation concealment: unclear Double‐blinding: yes Study quality: intermediate Duration of treatment: 12 weeks Cointervention: not available |
|
| Participants | Setting: psychiatric OP Diagnosis: non‐psychotic major depressive disorder (DSM‐IV), not precipitated by life stressors Age (mean): 41.1 years Men/women: 25/25 Baseline depressive severity (mean): 22.4 on HRSD‐17 Baseline anxiety severity: 58% of participants were described as "aroused." |
|
| Interventions | Fluoxetine 20–40 mg + clonazepam 0.5–1 mg | |
| Outcomes | HRSD‐17, CGI up to 18 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "using a traditional double‐blind parallel group design." Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout was a large proportion (13/27 of intervention group and 5/25 of control group) and detail of imputing method not reported. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
Yamaoka 1994.
| Methods | Type of trial: parallel Randomised: yes Allocation concealment: unclear Double‐blinding: yes Study quality: low (because of high dropout rates) Duration of treatment: 4 weeks Cointervention: not available |
|
| Participants | Setting: psychiatric OP Diagnosis: major depressive disorder (DSM‐III) Age (mean): 44.4 years Men/women: 8/11 Baseline depressive severity (mean): 26.1 (SD 8.8) on HRSD‐24 Baseline anxiety severity: NS |
|
| Interventions | Mianserin 30–60 mg + mexazolam 3 mg | |
| Outcomes | HRSD‐24 up to 4 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised, but no details on how random sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind", "placebo controlled." Further details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout was a large proportion (4/16 of intervention group and 9/16 of control group) and completer analysis was done. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; thus, unsure if all of prespecified outcomes of interest reported in prespecified way. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
CGI: Clinical Global Impression; CPRS: Comprehensive Psychopathological Rating Scale; CPRS‐VAS: Comprehensive Psychopathological Rating Scale – Visual Analogue Scale; DSM‐III: Diagnostic and Statistical Manual 3rd Edition; DSM‐III‐R: Diagnostic and Statistical Manual 3rd Revised Edition; DSM‐IV: Diagnostic and Statistical Manual 4th Edition; HRSD: Hamilton Rating Scale for Depression; IP: inpatient; OP: outpatient; SD: standard deviation; VAS: Visual Analogue Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 1988 | No operationalised diagnostic criteria used for diagnosing depression. |
| Ballinger 1974 | Duration of trial < 4 weeks. |
| Bowen 1978 | Duration of trial < 4 weeks. |
| Cohn 1983 | No operationalised criteria used for diagnosing major depression. |
| Dimitriou 1982 | Dosage of antidepressant inadequate. |
| Eckmann 1974 | No operationalised criteria used for diagnosing major depression. |
| Ferguson 1991 | Only 5/120 participants received concomitant antidepressant medication. |
| James 1985 | No operationalised diagnostic criteria used for diagnosing depression. Dosage of antidepressant inadequate. |
| Johnstone 1980 | No operationalised diagnostic criteria used for diagnosing depression. |
| Levin 1985 | Duration of trial < 4 weeks. |
| Magnus 1975 | Duration of trial < 4 weeks. |
| Morakinyo 1970 | No operationalised criteria used for diagnosing major depression. Dosage of antidepressant inadequate. |
| Otero 1994 | Dosage of antidepressant inadequate. |
| Rickels 1970 | No operationalised criteria used for diagnosing depression. |
| Runge 1985 | Duration of trial < 4 weeks. |
| Smith 1973 | No operationalised diagnostic criteria used for diagnosing depression. |
| Smith 1975 | No operationalised diagnostic criteria used for diagnosing depression. |
| Trappe 1973 | Different antidepressants used between intervention and control group. |
| Tsaras 1981 | Dosage of antidepressant inadequate. |
| Yamada 2003 | Dosage of antidepressant inadequate. |
Differences between protocol and review
We edited the methods section to bring it up to date with Cochrane's current methodological standards.
We added the "Assessment of reporting biases" and "Unit of analysis issues" sections and "How the intervention might work", "Setting", "Timing of outcome assessment", "Personal Communication", "Main planned comparisons", "change versus endpoint data", "Missing statistics" subsections.
We rewrote "Assessment of risk of bias in included studies", "Assessment of heterogeneity", "Data synthesis" and "Sensitivity analysis" sections and "Missing participants", "Missing statistics" subsections.
We added two secondary objectives: to determine if additional benzodiazepines benefit people with depression with high anxiety or low anxiety; and if short‐acting benzodiazepines given at bedtime influence daytime mood.
We added "or 5th Edition (DSM‐5)" in "Diagnosis" section.
We added the names of antidepressants and benzodiazepines, and changed the definition of minimum dosage in "Types of intervention" section.
We changed "Acceptability of treatment" from secondary outcomes to primary outcomes and added "Remission in depression" to secondary outcomes.
We added the search strategy, method of studies selection and management of data for this updating search.
We adjusted the section "Assessment of heterogeneity", to include the guide to interpreting the I2 statistic, which are provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We added sensitivity analyses (exclusion of trials using only self‐report and limiting the studies to those reporting on 50% reduction on HRSD).
In the protocol, we stated that we would impute response rates for depressive severity. However, for the review, we imputed responders and remitters.
Contributions of authors
Toshi A Furukawa and David Streiner wrote the original review. For this update, Yusuke Ogawa cowrote the protocol. Toshi A Furukawa, Norio Watanabe and David Streiner reviewed and provided feedback on the draft version of the protocol.
For the review, Yusuke Ogawa, Nozomi Takeshima, Yu Hayasaka and Aran Tajika screened all articles identified by searches, extracted data from all eligible studies and performed quality assessment. Yusuke Ogawa conducted the analysis and drafted the manuscript, which was checked and subsequently revised by all review authors.
Declarations of interest
YO: received research funds from the Japan Society for the Promotion of Science.
NT: no conflicts to declare.
YH: no conflicts to declare.
AT: received honoraria for speaking at a meeting sponsored by Eli Lilly and Tanabe‐Mitsubishi.
NW: received research funds from the Japanese Ministry of Health, Labor and Welfare and the Japanese Ministry of Education, Science, and Technology. He has also received royalties from Sogensha, Paquet and Akatsuki, and speaking fees and research funds from Asahi Kasei, Dai‐Nippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Meiji, Mochida, MSD, Otsuka, Pfizer and Tanabe‐Mitsubishi.
DS: no conflicts to declare.
TAF: received honoraria for speaking at CME meetings sponsored by Asahi Kasei, Eli Lilly, GlaxoSmithKline, Mochida, MSD, Otsuka, Pfizer, Shionogi and Tanabe‐Mitsubishi. He is diplomat of the Academy of Cognitive Therapy. He has received royalties from Igaku‐Shoin, Seiwa‐Shoten and Nihon Bunka Kagaku‐sha. He is on advisory board for Sekisui Chemicals and Takeda Science Foundation. The Japanese Ministry of Education, Science, and Technology, the Japanese Ministry of Health, Labor and Welfare, and the Japan Foundation for Neuroscience and Mental Health have funded his research projects.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Calcedo Ordonez 1992 {published data only}
- Calcedo Ordonez A, Arosamene X, Otero Perez FJ, Hernandez Herrero C, Garcia A, Moral L, et al. Clomipramine/bentazepam combination in the treatment of major depressive disorders. Human Psychopharmacology 1992;7(2):115‐22. [Google Scholar]
Dominguez 1984 {published data only}
- Dominguez RA, Jacobson AF, Goldstein BJ, Steinbook RM. Comparison of triazolam and placebo in the treatment of insomnia in depressed patients. Current Therapeutic Research 1984;36(5):856‐65. [Google Scholar]
Fawcett 1987 {published data only}
- Fawcett J, Edwards JH, Kravitz HM, Jeffriess H. Alprazolam: an antidepressant? Alprazolam, desipramine, and an alprazolam–desipramine combination in the treatment of adult depressed outpatients. Journal of Clinical Psychopharmacology 1987;7(5):295‐310. [PubMed] [Google Scholar]
- Kravitz HM, Edwards JH, Fawcett J, Fogg L. Challenging the amphetamine challenge test: report of an antidepressant treatment study. Journal of Affective Disorders 1990;20(2):121‐8. [DOI] [PubMed] [Google Scholar]
- Kravitz HM, Fogg L, Fawcett J, Edwards J. Antidepressant or antianxiety? A study of the efficacy of antidepressant medication. Psychiatry Research 1990;32(2):141‐9. [DOI] [PubMed] [Google Scholar]
Feet 1985 {published data only}
- Feet PO, Gotestam KG. Cortisol responses to imipramine combined treatments. European Journal of Psychiatry 1994;8(1):45‐52. [Google Scholar]
- Feet PO, Gotestam KG, Norman N. Gender differences in prolactin and aldosterone in primary non‐agitated depressed patients and normal controls. European Journal of Psychiatry 1993;7(2):77‐88. [Google Scholar]
- Feet PO, Larsen S, Lillevold PE, Liden A, Holm V, Robak OH. Comparison of the serum levels in primary non‐agitated depressed out‐patients treated with imipramine in combination with placebo, diazepam or dixyrazine. Acta Psychiatrica Scandinavica 1987;75(4):435‐40. [DOI] [PubMed] [Google Scholar]
- Feet PO, Larsen S, Robak OH. A double blind study in out‐patients with primary non‐agitated depression treated with imipramine in combination with placebo, diazepam or dixyrazine. Acta Psychiatrica Scandinavica 1985;72(4):334‐40. [DOI] [PubMed] [Google Scholar]
Feighner 1979 {published data only}
- Feighner JP, Brauzer B, Gelenberg AJ, Gomez E, Kiev A, Kurland ML, et al. A placebo‐controlled multicenter trial of Limbitrol versus its components (amitriptyline and chlordiazepoxide) in the symptomatic treatment of depressive illness. Psychopharmacology 1979;61(2):217‐25. [DOI] [PubMed] [Google Scholar]
- Jacobson AF. Doctor‐patient concordance in a placebo‐controlled trial of Limbitrol versus its components. Psychopharmacology Bulletin 1978;14(3):61‐3. [PubMed] [Google Scholar]
- Rickels K. Limbitrol (amitriptyline plus chlordiazepoxide) revisited. Psychopharmacology 1981;75(1):31‐3. [DOI] [PubMed] [Google Scholar]
Nolen 1993 {published data only}
- Nolen WA, Haffmans PM, Bouvy PF, Duivenvoorden HJ. Hypnotics as concurrent medication in depression. A placebo‐controlled, double‐blind comparison of flunitrazepam and lormetazepam in patients with major depression, treated with a (tri)cyclic antidepressant. Journal of Affective Disorders 1993;28(3):179‐88. [DOI] [PubMed] [Google Scholar]
Scharf 1986 {published data only}
- Scharf MB, Hirschowitz J, Zemlan FP, Lichstein M, Woods M. Comparative effects of Limbitrol and amitriptyline on sleep efficiency and architecture. Journal of Clinical Psychiatry 1986;47(12):587‐91. [PubMed] [Google Scholar]
Smith 1998 {published data only}
- Londborg PD, Smith WT, Glaudin V, Painter JR. Short‐term cotherapy with clonazepam and fluoxetine: anxiety, sleep disturbance and core symptoms of depression. Journal of Affective Disorders 2000;61(1‐2):73‐9. [DOI] [PubMed] [Google Scholar]
- Smith W, Londborg PD, Glaudin V, Painter JR. Treating anxiety and sleep disturbance as symptoms of depression and as side effects of specific serotonin reuptake inhibitors: low‐dose clonazepam augmentation of fluoxetine. XXIst Collegium Internationale Neuro psychopharmacologicum; 1998 Jul 12‐16; Glasgow, UK. 1998.
- Smith WT, Londborg PD, Glaudin V, Painter JR. Short‐term augmentation of fluoxetine with clonazepam in the treatment of depression: a double‐blind study. American Journal of Psychiatry 1998;155(10):1339‐45. [DOI] [PubMed] [Google Scholar]
Smith 2002 {published data only}
- Smith WT, Londborg PD, Glaudin V, Painter JR. Is extended clonazepam cotherapy of fluoxetine effective for outpatients with major depression?. Journal of Affective Disorders 2002;70:251‐9. [DOI] [PubMed] [Google Scholar]
- Smith WT, Londborg V, Glaudin V, Painter JR. Extended low‐dose clonazepam cotherapy of fluoxetine in the treatment of depressed outpatients. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):231. [Google Scholar]
Yamaoka 1994 {published data only}
- Yamaoka K. Augmentation therapy of antidepressant and benzodiazepine in treatment of depression. Seishinka‐Chiryo‐Gaku 1994;9:1349‐55. [Google Scholar]
References to studies excluded from this review
Ahmed 1988 {published data only}
- Ahmed MH, Onyemelukwe GC, Onyewotu II. A double‐blind controlled clinical trial of benzoctamin (Tacitin) and imipramine (Tofranil) in the treatment of 'internal heat' and its associated symptoms. East African Medical Journal 1988;65(4):230‐7. [PubMed] [Google Scholar]
Ballinger 1974 {published data only}
- Ballinger BR, Presly A, Reid AH, Stevenson IH. The effects of hypnotics on imipramine treatment. Psychopharmacology 1974;39(3):267‐74. [DOI] [PubMed] [Google Scholar]
Bowen 1978 {published data only}
- Bowen RC. The effect of diazepam on the recovery of endogenously depressed patients. Journal of Clinical Pharmacology 1978;18(5‐6):270‐4. [DOI] [PubMed] [Google Scholar]
Cohn 1983 {published data only}
- Cohn JB. Triazolam treatment of insomnia in depressed patients taking tricyclics. Journal of Clinical Psychiatry 1983;44(11):401‐6. [PubMed] [Google Scholar]
Dimitriou 1982 {published data only}
- Dimitriou EC, Logothetis JA, Paschalidou M. A double blind comparative study of mianserin and a fixed combination of amitriptyline plus chlordiazepoxide. In: Costa E, Racagni G editor(s). Advances in Biochemical Psychopharmacology: Typical and Atypical Antidepressants: Clinical Practice. Vol. 32, New York (NY): Raven Press, 1982:213‐22. [PubMed] [Google Scholar]
Eckmann 1974 {published data only}
- Eckmann F. Clinical studies with nomifensin [Klinische Untersuchungen mit dem Antidepressivum Nomifensin]. In: Walcher W editor(s). Zur Systematik, Provokation und Therapie depressiver Psychosen. Graz: Brüder Hollinek, 1974:199‐204. [Google Scholar]
Ferguson 1991 {published data only}
- Ferguson JM, Bielski RJ, Houston J, Post GL, Crowder J, Bailey L, et al. Comparison of estazolam and placebo in the outpatient treatment of insomnia associated with major depression. Current Therapeutic Research 1991;49(5):898‐907. [Google Scholar]
James 1985 {published data only}
- James RT, Dean BC. Comparison of Limbitrol (chlordiazepoxide/amitriptyline) and amitriptyline alone as a single night‐time dose for the treatment of depression with anxiety. Journal of International Medical Research 1985;13(2):84‐7. [DOI] [PubMed] [Google Scholar]
Johnstone 1980 {published data only}
- Frith CD, Stevens M, Johnstone EC, Owens DG. The effects of chronic treatment with amitriptyline and diazepam on electrodermal activity in neurotic outpatients. Physiological Psychology 1984;12(3):247‐52. [Google Scholar]
- Johnstone EC, Bourne RC, Crow TJ, Frith C, Gamble S, Lofthouse R, et al. The relationship between clinical response, psychophysiological variables and plasma levels of amitriptyline and diazepam in neurotic outpatients. Psychopharmacology 1981;72(3):233‐40. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Owens DG, Frith CD, McPherson K, Dowie C, Riley G, et al. Neurotic illness and its response to anxiolytic and antidepressant treatment. Psychological Medicine 1980;10(2):321‐28. [DOI] [PubMed] [Google Scholar]
Levin 1985 {published data only}
- Levin A, Schlebusch L. Mianserin is better tolerated and more effective in depression than a nomifensin‐clobazam combination: a double‐blind study. Acta Psychiatrica Scandinavica. Supplementum 1985;320:75‐80. [DOI] [PubMed] [Google Scholar]
Magnus 1975 {published data only}
- Magnus RV. A placebo controlled trial of viloxazine with and without tranquillizers in depressive illness. Journal of International Medical Research 1975;3(3):207‐13. [DOI] [PubMed] [Google Scholar]
Morakinyo 1970 {published data only}
- Morakinyo VO. Amytriptyline and chlordiazepoxide (Limbitrol) in depressive states in Nigerians: a double‐blind study. African Journal of Medical Sciences 1970;1(4):409‐14. [PubMed] [Google Scholar]
Otero 1994 {published data only}
- Otero FJ, Hernadez‐Herrero C, Martinze‐Arevalo MJ, Garrido J, Armenteros S, Velasco J. Fluoxetine/bentazepam combination in the treatment of dysthymic disorders. Current Therapeutic Research 1994;55(5):519‐31. [Google Scholar]
Rickels 1970 {published data only}
- Rickels K, Gordon PE, Jenkins BW, Perloff M, Sach T, Stepansky W. Drug treatment in depressive illness: amitriptyline and chlordiazepoxide in two neurotic populations. Disease of Nervous System 1970;31:30‐42. [PubMed] [Google Scholar]
- Rickels K, Hesbacher P, Downing RW. Differential drug effects in neurotic depression. Diseases of Nervous System 1970;31:468‐475. [PubMed] [Google Scholar]
Runge 1985 {published data only}
- Runge I, Sastre‐Y‐Hernadez M, Horowski R, Fichte K. Use of lormetazepam (Noctamid (R)) as a hypnotic in depressed patients: double‐blind study on interactions with anti‐depressant medication. Current Therapeutic Research 1985;38(6):953‐9. [Google Scholar]
Smith 1973 {published data only}
- Smith M. Clinical response to amitriptyline and chlordiazepoxide‐amitriptyline (Limbitrol) in anxiety‐depressive states. A double‐blind study. Psychosomatics 1973;14(3):168‐75. [DOI] [PubMed] [Google Scholar]
Smith 1975 {published data only}
- Smith JA, Renshaw DC. A clinical study of insomnia. American Family Physician 1975;11(3):140‐1. [PubMed] [Google Scholar]
Trappe 1973 {published data only}
- Trappe B. Doxepin and amitriptyline‐chlordiazepoxide combination in neurotic states. Psychiatrica Fennica 1973;4:269‐75. [Google Scholar]
Tsaras 1981 {published data only}
- Tsaras G, Ambrus J. Comparative clinical trial of chlordiazepoxide plus amitriptyline against maprotiline in depression with a component of anxiety [Etude clinique comparative de l'association chlordiazepoxide amitriptyline et de la maprotiline chez des patients souffrant de depression a composante anxieuse]. Revue Medicale de la Suisse Romande 1981;101(6):483‐6. [PubMed] [Google Scholar]
Yamada 2003 {published data only}
- Yamada K, Yagi G, Kanba S. Clinical efficacy of tandospirone augmentation in patients with major depressive disorder: a randomized controlled trial. Psychiatry and Clinical Neurosciences 2003;57:183‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. [PUBMED: 12928469] [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2010
- American Psychiatric Association. American Psychiatric Association Practice Guideline for the Treatment of Major Depressive Disorder. Washington (DC): American Psychiatric Publishing, 2010. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Arroll 2009
- Arroll B, Elley CR, Fishman T, Goodyear‐Smith FA, Kenealy T, Blashki G, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 1976
- Bailey J, Coppen A. A comparison between the Hamilton Rating Scale and the Beck Inventory in the measurement of depression. British Journal of Psychiatry 1976;128:486‐9. [PUBMED: 1276553] [DOI] [PubMed] [Google Scholar]
Bandelow 2006
- Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder?. Journal of Clinical Psychiatry 2006;67:1428‐34. [DOI] [PubMed] [Google Scholar]
BAP 2015
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al. Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology 2015;29:459‐525. [DOI] [PubMed] [Google Scholar]
Bartels 1997
- Bartels SJ, Horn S, Sharkey P, Levine K. Treatment of depression in older primary care patients in health maintenance organizations. International Journal of Psychiatry in Medicine 1997;27(3):215‐31. [DOI] [PubMed] [Google Scholar]
Bauer 2002
- Bauer M, Whybrow PC, Angst J, Versiani M, Möller H‐J. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 1: acute and continuation treatment of major depressive disorder. World Journal of Biological Psychiatry 2002;3:5‐43. [DOI] [PubMed] [Google Scholar]
Baumeister 2007
- Baumeister H, Härter M. Prevalence of mental disorders based on general population surveys. Social Psychiatry and Psychiatric Epidemiology 2007;42:537‐46. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893‐7. [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170(4):477‐80. [PUBMED: 14970094] [PMC free article] [PubMed] [Google Scholar]
Birkenhager 1995
- Birkenhager TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. International Clinical Psychopharmacology 1995;10(3):181‐95. [DOI] [PubMed] [Google Scholar]
Bouhassira 1998
- Bouhassira M, Allicar MP, Blachier C, Nouveau A, Rouillon F. Which patients receive antidepressants? A 'real world' telephone study. Journal of Affective Disorders 1998;49(1):19‐26. [DOI] [PubMed] [Google Scholar]
Burrows 1993
- Burrows GD, Judd FK, Norman TR. Long‐term drug treatment of panic disorder. Journal of Psychiatric Research 1993;27 Suppl 1:111‐25. [PUBMED: 7908330] [DOI] [PubMed] [Google Scholar]
Carpenter 2002
- Carpenter LL, Yasmin S, Price LH. A double‐blind, placebo‐controlled study of antidepressant augmentation with mirtazapine. Biological Psychiatry 2002;51:183‐8. [DOI] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373:746‐58. [DOI] [PubMed] [Google Scholar]
Clayton 1991
- Clayton PJ, Grove WM, Coryell W, Keller M, Hirschfield R, Fawcett J. Follow‐up and family study of anxious depression. American Journal of Psychiatry 1991;148:1512‐7. [DOI] [PubMed] [Google Scholar]
Correll 2010
- Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo‐controlled trials. Bipolar Disorders 2010;12(2):116‐41. [DOI] [PubMed] [Google Scholar]
Coryell 1992
- Coryell W, Endicott J, Winokur G. Anxiety syndromes as epiphenomena of primary major depression: outcome and familial psychopathology. American Journal of Psychiatry 1992;149:100‐7. [DOI] [PubMed] [Google Scholar]
CRM 1980
- Committee on the Review of Medicines. Systematic review of the benzodiazepines: guidelines for data sheets on diazepam, chlordiazepoxide, medazepam, clorazepate, lorazepam, oxazepam, temazepam, triazolam, nitrazepam, and flurazepam. BMJ 1980;280(6218):910‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crossley 2007
- Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta‐analyses of randomized, placebo‐controlled trials. Journal of Clinical Psychiatry 2007;68:935‐40. [DOI] [PubMed] [Google Scholar]
da Costa 2013
- Costa BR, Nuesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, et al. Combining follow‐up and change data is valid in meta‐analyses of continuous outcomes: a meta‐epidemiological study. Journal of Clinical Epidemiology 2013;66:847‐55. [DOI] [PubMed] [Google Scholar]
de Girolamo 1987
- Girolamo G, Williams P, Cappiello V. Psychotropic drug utilization and audit in two Italian psychiatric services. Psychological Medicine 1987;17(4):989‐97. [DOI] [PubMed] [Google Scholar]
Domken 1994
- Domken M, Scott J, Kelly P. What factors predict discrepancies between self and observer ratings of depression?. Journal of Affective Disorders 1994;31(4):253‐9. [PUBMED: 7989640] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Zellweger‐Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350(9074):326‐9. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Fabre 1981
- Fabre LF, McLendon DM, Stephens AG. Comparison of the therapeutic effect, tolerance and safety of ketazolam and diazepam administered for six months to out‐patients with chronic anxiety neurosis. Journal of International Medical Research 1981;9(3):191‐8. [PUBMED: 6113174] [DOI] [PubMed] [Google Scholar]
Fagius 1985
- Fagius J, Osterman PO, Sidén Å, Wiholm BE. Guillain‐Barré syndrome following zimeldine treatment. Journal of Neurology, Neurosurgery and Psychiatry 1985;48:65‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreri 2001
- Ferreri M, Lavergne F, Berlin I, Payan C, Puech AJ. Benefits from mianserin augmentation of fluoxetine in patients with major depression nonresponders to fluoxetine alone. Acta Psychiatrica Scandinavica 2001;103:66‐72. [DOI] [PubMed] [Google Scholar]
Furukawa 2000
- Furukawa T, Kitamura T, Takahashi K. Treatment received by depressed patients in Japan and its determinants: naturalistic observation from a multi‐center collaborative follow‐up study. Journal of Affective Disorders 2000;60(3):173‐9. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31:72‐6. [DOI] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20:49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Furukawa 2011
- Furukawa TA, Akechi T, Wagenpfeil S, Leucht S. Relative indices of treatment effect may be constant across different definitions of response in schizophrenia trials. Schizophrenia Research 2011;126:212‐9. [DOI] [PubMed] [Google Scholar]
Gomez 2000
- Gomez R, Barros HM. Ethopharmacology of the antidepressant effect of clonazepam in diabetic rats. Pharmacology Biochemistry and Behavior 2000;66:329‐35. [DOI] [PubMed] [Google Scholar]
Grohmann 1980
- Grohmann R, Strauss A, Gehr Ch, Ruther E, Hippius H. On the practice of clinical therapy with psychotropic drugs – retrospective investigation of physicians prescribing practices in a psychiatric hospital [Zur Praxis der klinischen Therapie mit Psychopharmaka. Retrospektive Untersuchung der Verordnungsgewohnheiten in einer Psychiatrischen Universitatsklinik]. Pharmacopsychiatry 1980;13(1):1‐19. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, 1976. [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32(1):50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2009
- Hansen RA, Moore CG, Dusetzina SB, Leinwand BI, Gartlehner G, Gaynes BN. Controlling for drug dose in systematic review and meta‐analysis: a case study of the effect of antidepressant dose. Medical Decision Making 2009;29:91‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (update March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Keller 2004
- Keller MB. Remission versus response: the new gold standard of antidepressant care. Journal of Clinical Psychiatry 2004;65 Suppl 4:53‐9. [PubMed] [Google Scholar]
Kessler 2003
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS‐R). JAMA 2003;289:3095‐105. [DOI] [PubMed] [Google Scholar]
Leucht 2013
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple‐treatments meta‐analysis. Lancet 2013;382(9896):951‐62. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ (Clinical Research Ed.) 2003;326(7400):1167‐70. [PUBMED: 12775614] [DOI] [PMC free article] [PubMed] [Google Scholar]
Licht 2002
- Licht RW, Qvitzau S. Treatment strategies in patients with major depression not responding to first‐line sertraline treatment. A randomised study of extended duration of treatment, dose increase ormianserin augmentation. Psychopharmacology 2002;161:143‐51. [DOI] [PubMed] [Google Scholar]
Lin 1998
- Lin EH, Katon WJ, VonKorff M, Russo JE, Simon GE, Bush TM, et al. Relapse of depression in primary care. Rate and clinical predictors. Archives of Family Medicine 1998;7(5):443‐9. [DOI] [PubMed] [Google Scholar]
Liu 1998
- Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N. Use of selective serotonin‐reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998;351(9112):1303‐7. [PUBMED: 9643791] [DOI] [PubMed] [Google Scholar]
Lucki 1986
- Lucki I, Rickels K, Geller AM. Chronic use of benzodiazepines and psychomotor and cognitive test performance. Psychopharmacology 1986;88(4):426‐33. [PUBMED: 2871579] [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134(4):382‐9. [DOI] [PubMed] [Google Scholar]
Moskowitz 1988
- Moskowitz H, Burns M. The effects on performance of two antidepressants, alone and in combination with diazepam. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1988;12(5):783‐92. [PUBMED: 3265525] [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. Oxford: Cochrane Library, 1997. [Google Scholar]
Murphy 1990
- Murphy JM. Diagnostic comorbidity and symptom co‐occurrence: the Stirling County Study. In: Maser JD, Cloninger CR editor(s). Comorbidity of Mood and Anxiety Disorders. Washington (DC): American Psychiatric Press, 1990:153‐76. [Google Scholar]
Nelson 2009
- Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta‐analysis of placebo‐controlled randomized trials. American Journal of Psychiatry 2009;166:980‐91. [DOI] [PubMed] [Google Scholar]
Neutel 1995
- Neutel CI. Risk of traffic accident injury after a prescription for a benzodiazepine. Annals of Epidemiology 1995;5(3):239‐44. [PUBMED: 7606314] [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: treatment and management, 2009. www.nice.org.uk/guidance/CG90. [PubMed]
Noyes 1988
- Noyes R Jr, Garvey MJ, Cook BL, Perry PJ. Benzodiazepine withdrawal: a review of the evidence. Journal of Clinical Psychiatry 1988;49(10):382‐9. [PUBMED: 2902071] [PubMed] [Google Scholar]
Olfson 1992
- Olfson M, Klerman GL. Th treatment of depression: prescribing practices of primary care physicians and psychiatrists. Journal of Family Practice 1992;35(6):627‐35. [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI] [PubMed] [Google Scholar]
Papakostas 2010
- Papakostas GI, Clain A, Ameral VE, Baer L, Brintz C, Smith WT, et al. Fluoxetine‐clonazepam cotherapy for anxious depression: an exploratory, post‐hoc analysis of a randomized, double blind study. International Clinical Psychopharmacology 2010;25(1):17‐21. [PUBMED: 19898245] [DOI] [PubMed] [Google Scholar]
Petty 1995
- Petty F, Trivedi MH, Fulton M, Rush AJ. Benzodiazepines as antidepressants ‐ does GABA play a role in depression. Biological Psychiatry 1995;38:578‐91. [DOI] [PubMed] [Google Scholar]
Ray 1992
- Ray WA, Fought RL, Decker MD. Psychoactive drugs and the risk of injurious motor vehicle crashes in elderly drivers. American Journal of Epidemiology 1992;136(7):873‐83. [PUBMED: 1442753] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rickels 1983
- Rickels K, Case WG, Downing RW, Winokur A. Long‐term diazepam therapy and clinical outcome. JAMA 1983;250(6):767‐71. [PUBMED: 6348314] [PubMed] [Google Scholar]
Rickels 1985
- Rickels K, Case WG, Downing RW, Winokur A. Indications and contraindications for chronic anxiolytic treatment: is there tolerance to the anxiolytic effect?. Advances in Biochemical Psychopharmacology 1985;40:193‐204. [PUBMED: 3895840] [PubMed] [Google Scholar]
Rush 1987
- Rush AJ, Hiser W, Giles DE. A comparison of self‐reported versus clinician‐related symptoms in depression. Journal of Clinical Psychiatry 1987;48(6):246‐8. [PUBMED: 3584081] [PubMed] [Google Scholar]
Sanyal 2011
- Sanyal C, Asbridge M, Kisely S, Sketris I, Andreou P. The utilization of antidepressants and benzodiazepines among people with major depression in Canada. Canadian Journal of Psychiatry 2011;56(11):667‐76. [DOI] [PubMed] [Google Scholar]
Schatzberg 1978
- Schatzberg AF, Cole JO. Benzodiazepines in depressive disorders. Archives of General Psychiatry 1978;35(11):1359‐65. [DOI] [PubMed] [Google Scholar]
Schweizer 1998
- Schweizer E, Rickels K. Benzodiazepine dependence and withdrawal: a review of the syndrome and its clinical management. Acta Psychiatrica Scandinavica. Supplementum 1998;393:95‐101. [DOI] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD. Manual for the State‐Trait Anxiety Inventory STAI (form Y) ("Self‐Evaluation Questionnaire"). Sunnyvale (CA): Consulting Psychology Press, 1983. [Google Scholar]
Tondo 1988
- Tondo L, Burrai C, Scamonatti L, Weissenburger J, Rush J. Comparison between clinician‐rated and self‐reported depressive symptoms in Italian psychiatric patients. Neuropsychobiology 1988;19(1):1‐5. [PUBMED: 3185892] [DOI] [PubMed] [Google Scholar]
Ustun 2004
- Ustun TB, Ayuso‐Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. British Journal of Psychiatry 2004;184:386‐92. [DOI] [PubMed] [Google Scholar]
van den Brink 1991
- Brink W, Leenstra A, Ormel J, Willige G. Mental health intervention programs in primary care: their scientific basis. Journal of Affective Disorders 1991;21(4):273‐84. [DOI] [PubMed] [Google Scholar]
Vermeeren 2004
- Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs 2004;18(5):297‐328. [DOI] [PubMed] [Google Scholar]
Wetzler 1989
- Wetzler S, Katz MM. Problems with the differentiation of anxiety and depression. Journal of Psychiatric Research 1989;23(1):1‐12. [DOI] [PubMed] [Google Scholar]
Whale 2010
- Whale R, Terao T, Cowen P, Freemantle N, Geddes J. Pindolol augmentation of serotonin reuptake inhibitors for the treatment of depressive disorder: a systematic review. Journal of Psychopharmacology 2010;24:513‐20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Furukawa 2001
- Furukawa TA, Streiner DL, Young LT. Is antidepressant‐benzodiazepine combination therapy clinically more useful? A meta‐analytic study. Journal of Affective Disorders 2001;65:173‐7. [DOI] [PubMed] [Google Scholar]


