Abstract
Growing evidence implicates altered mTORC1 signaling cascades in the pathophysiology of depression, suggesting that direct modulation of mTORC1 signaling may offer novel therapeutic potential. In this issue of the JCI, Kato and colleagues reported that administration of NV-5138, a recently developed synthetic leucine analog, has a rapid and sustained antidepressant action in rat models via activation of mTORC1 signaling. The investigators also found that the antidepressant effect of NV-5138 is mediated by upregulation of brain-derived neurotrophic factor (BDNF) signaling and that NV-5138 treatment produces rapid synaptic responses in the medial prefrontal cortex. These findings highlight the direct activation of mTORC1 signaling as a potential pharmacological intervention for the treatment of depression.
Limitations of currently available antidepressants
Major depressive disorder (MDD) is a common psychiatric disorder which imposes substantial public health burden and has a high lifetime prevalence (1). A number of clinically available antidepressants include selective serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs), which act on monoaminergic systems. These medicines, however, require chronic administration before they show treatment efficacy (in general, a few weeks or more to improve depressive symptoms), and approximately 30% to 40% of MDD patients exhibit resistance to first-line interventions, potentially due to heterogeneous etiologies (2). Thus, developing novel interventions targeting pathological mechanisms of MDD is necessary (3). Importantly, the N-methyl-d-aspartate (NMDA) glutamate receptor antagonist ketamine, a widely used anesthetic, has a rapid-acting antidepressant effect, even in treatment-resistant patients (4, 5). The US FDA has recently approved a nasal spray of esketamine for treatment-resistant adult patients with depression. This encourages researchers to explore the idea that modulation of nonmonoaminergic mechanisms may offer therapeutic potential for the treatment of depression.
mTOR signaling pathway: potential target for treatment of depression
The mTOR signaling pathway is an important homeostasis regulatory system that controls multiple intracellular functions, such as protein synthesis, metabolism, transcription, and autophagy (6). In the brain, the mTOR signaling pathway is involved, not only in developmental cellular processes, such as cellular proliferation, axon growth, and dendrite formation, but also in synaptic plasticity, contributing to the regulation of cognitive function, sleep, and mood (ref. 7 and Figure 1). mTOR-mediated synaptic regulation is mediated by two critical downstream substrates of mTOR complex 1 (mTORC1), the p70 ribosomal S6 kinases 1 and 2 (p70S6K1/2), and the eukaryotic initiation factor 4E-binding proteins (4E-BPs) (6, 7). It has been reported that the acute antidepressant action of ketamine is accompanied by elevated synaptic protein expression and upregulation of mTOR-S6K1 and 4E-BP signaling cascades in rats (8, 9). Interestingly, fast-acting antidepressant effects of ketamine are also dependent on the rapid increase of brain-derived neurotrophic factor (BDNF) production via inactivation of eukaryotic elongation factor 2 (eEF2) kinase, a substrate of S6K1 (10). Thus, although more studies are needed to understand how altered mTOR signaling is involved in the pathophysiology of depression, mTORC1 signaling is a unique molecular target that can be used to explore drugs with rapid antidepressant action, such as ketamine.
Figure 1. Antidepressant action of NV-5138 via direct activation of mTORC1 signaling.
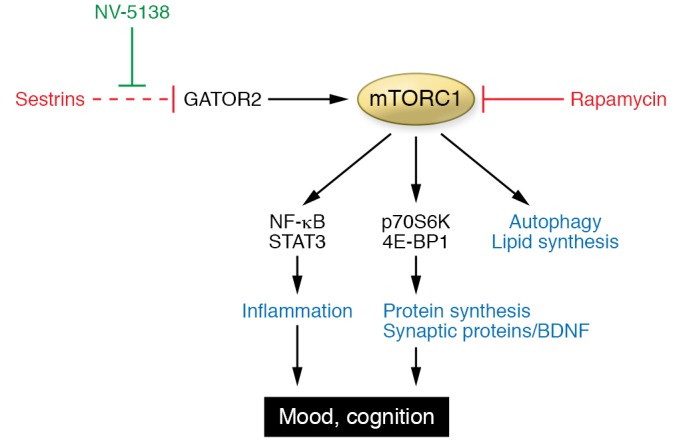
Rapamycin-sensitive mTORC1 signaling regulates the production of synaptic proteins and BDNF via p70S6K and 4E-BP1 signaling pathways. NV-5138, a synthetic leucine analog, binds sestrins and releases sestrins from GATOR2, resulting in mTORC1 activation and producing fast antidepressant effects. Note that mTORC1 signaling also regulates inflammatory machinery via NF-κB and STAT3 signaling pathways, suggesting that aberrant inflammatory mechanisms underlying depressive symptoms may also be targetable via modulation of mTORC1 signaling.
Rapid antidepressant action via direct activation of mTORC1 signaling
mTORC1 signaling is regulated by the interaction between sestrins and the GATOR2 complex (11). Leucine, a critical amino acid in mTORC1-mediated protein synthesis, can bind sestrins, resulting in release from GATOR2, leading to mTORC1 activation (12). NV-5138 is a novel synthetic analog of leucine that has sufficient oral bioavailability and brain penetration to selectively bind sestrins for the activation of mTORC1 signaling in the brain (13). In this issue of the JCI, Kato et al. report that oral administration of NV-5138 has a rapid and prolonged antidepressant action in rat models (14). They systematically demonstrate a rapid and long-lasting antidepressant effect of a single dose of NV-5138 in multiple depression-associated behavioral paradigms, including the forced swim test, the novelty suppressed feed test, and the female urine sniffing test in wild type rats. They also show that NV-5138 rapidly ameliorated chronic unpredictable stress-induced anhedonia-like behaviors in a sucrose preference test. Additionally, they confirmed that NV-5138, similarly to ketamine, rapidly upregulated phosphorylation of mTOR and increased phospho-p70S6K1 and 4E-BP1, which are downstream targets of mTORC1 in the prefrontal cortex. Importantly, intrainfusion of rapamycin, a selective mTORC1 inhibitor, blocked the antidepressant effect of NV-5138. These results suggest that activation of mTORC1 signaling is a promising approach for the treatment of depressive symptoms and that NV-5138 is a promising lead compound for the development of new drugs with rapid antidepressant action.
Rapid synaptic changes and upregulation of BDNF signaling by mTORC1 activation
Kato et al. also examined the effect of NV-5138 on synaptic function by conducting biochemical, immunohistochemical, and electrophysiological experiments. Chronic unpredictable stress exposure resulted in the reduction of GluR1 and PSD95 in the synaptosome fraction of the medial prefrontal cortex, which was rapidly reversed by a single dose of NV-5138 treatment. Morphological analysis of dendritic spine in the pyramidal neurons in layer V of the medial prefrontal cortex showed an increase in spine density in the rats subjected to acute administration of a single dose of NV-5138 compared with control groups. To examine the influence of NV-5138 treatment on synaptic function, 5-HT and hypocretin-induced excitatory postsynaptic current (EPSC) response in the layer V pyramidal neurons was measured by whole-cell electrophysiology in rat prefrontal cortex brain slices 24 hours after a single dose of NV-5138 or vehicle treatment. Consistent with the ketamine effect on synaptic function (8, 9, 15), NV-5138 treatment led to a significant increase in the frequency and amplitude of 5-HT and hypocretin-induced EPSC in neurons compared with that in controls. Expression of synaptic proteins, such as GluR1, Synapsin 1, and SV2A, was increased by NV-5138 treatment, which was similar to the effect of ketamine. These results suggest that NV-5138 and ketamine may, at least in part, share the common mechanisms underlying pharmacological effects on synaptic function, leading to a rapid antidepressant effect. This idea is supported by the results showing that intrainfusion of an antibody against BDNF in the medial prefrontal cortex 30 minutes before NV-5138 administration blocked acute antidepressant behavioral responses induced by a single dose of NV-5138. Consistent with the above, the BDNF knockin mice with a genetic variant of BDNF (Met66Met), a polymorphism reported to inhibit BDNF processing (16), suppressed the antidepressant behavioral effect of NV-5138.
Conclusions and future directions
In this study, Kato and colleagues reported, for the first time, that direct pharmacological activation of mTORC1 signaling led to a rapid and sustained antidepressant effect. Notably, the impact of NV-5138 on synaptic function and BDNF signaling is similar to that of ketamine, suggesting that NV-5138 and ketamine, at least in part, share the common mTORC1 signaling–mediated mechanisms underlying their antidepressant actions. Paradoxically, however, a recent clinical trial revealed that pretreatment with the mTORC1 inhibitor rapamycin prolonged the antidepressant effect of ketamine in MDD patients (17), underscoring the need for further investigation for understanding precise mechanisms of the modulation of mTORC1 signaling by ketamine. With the authors having shown that ketamine induces a rapid antidepressant effect in treatment-resistant patients (4), the antidepressant effect of NV-5138 reported in this study may translate to efficacy for patients, such as those with treatment-resistance MDD, who respond to ketamine. Nonetheless, considering that mTOR is a ubiquitously expressed serine/threonine kinase and plays essential roles for multiple cellular functions, future studies should be carefully conducted to determine whether NV-5138 induces any adverse effects, including the promotion of carcinogenesis. There are emerging links between CNS inflammation and glutamate signaling underlying depression (18). Furthermore, a recent study reported that pharmacological inhibition of microglial glutamate production ameliorates chronic stress-induced depressive-like behaviors (19). Considering that mTOR signaling also regulates the immune and inflammatory systems via modulation of NF-κB and STAT3 signaling pathways (ref. 20 and Figure 1), targeting mTORC1 signaling in brain immune cells also warrants future investigation.
Acknowledgments
We thank Aisa Moreno-Megui for critical reading of this Commentary. Work in the Kamiya laboratory is supported by NIH grants, including R01DA041208 (to AK) and P50MH094268 (to AK).
Version 1. 05/20/2019
Electronic publication
Version 2. 06/03/2019
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(6):2207–2209. https://doi.org/10.1172/JCI129702.
See the related article at Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation.
Contributor Information
Yuto Hasegawa, Email: yhasega3@jhmi.edu.
Xiaolei Zhu, Email: xzhu31@jhmi.edu.
Atsushi Kamiya, Email: akamiya1@jhmi.edu.
References
- 1.Hasin DS, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieblich SM, Castle DJ, Pantelis C, Hopwood M, Young AH, Everall IP. High heterogeneity and low reliability in the diagnosis of major depression will impair the development of new drugs. BJPsych Open. 2015;1(2):e5–e7. doi: 10.1192/bjpo.bp.115.000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33(9):2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 6.Bockaert J, Marin P. mTOR in brain physiology and pathologies. Physiol Rev. 2015;95(4):1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- 7.Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84(2):275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantranupong L, et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014;9(1):1–8. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfson RL, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengupta S, et al. Discovery of NV-5138, the first selective Brain mTORC1 activator. Sci Rep. 2019;9(1):4107. doi: 10.1038/s41598-019-40693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato T, et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Invest. 2019;129(6):2542–2554. doi: 10.1172/JCI126859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukumoto K, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A. 2019;116(1):297–302. doi: 10.1073/pnas.1814709116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdallah CG, et al. Rapamycin, an immunosuppressant mTORC1 inhibitor, triples theantidepressant response rate of ketamine at 2 weeks following treatment. bioRxiv Website. https://www.biorxiv.org/content/10.1101/500959v1 Published December 19, 2018. Accessed April 24, 2019.
- 18.Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42(1):193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu X, et al. JHU-083 selectively blocks glutaminase activity in brain CD11b(+) cells and prevents depression-associated behaviors induced by chronic social defeat stress. Neuropsychopharmacology. 2019;44(4):683–694. doi: 10.1038/s41386-018-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23(6):744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]


