Abstract
Acute kidney injury (AKI) is one of the most important risk factors for chronic and progressive kidney disease, leading to end-stage kidney failure. Tubule epithelial regeneration leads to the resolution of renal failure in AKI. Failure of tubule epithelial regeneration leads to concomitant hypoxia from loss of microcirculation, which serves as a critical factor leading to chronic kidney disease. In this issue of the JCI, Li et al. show that hypoxia activates the stress-responsive transcription factor FoxO3. Increased FoxO3 protein abundance leads to alterations in tubular epithelial autophagy and metabolism, highlighting an important mechanism causing permanent renal damage even after an acute injury.
Thin line between acute kidney injury and chronic kidney disease
A new categorization system for kidney diseases is long overdue. At present, we group diseases based on their temporal course, such as acute or chronic. We broadly teach that acute kidney injury (AKI) recovers, while chronic kidney disease (CKD) will likely progress to end-stage renal failure (ESRD). Recent research, however, indicates that this division is fairly artificial. AKI is an important risk factor for CKD and ESRD development. Moreover, we have difficulties distinguishing AKI from the progression of CKD, as routinely used markers, such as serum creatinine, are not sensitive enough to detect small acute injuries. Defining similarities and differences between AKI and CKD, such as those related to regeneration and fibrosis, is a clinically important question, as traditionally, we do not see recovery or regeneration in CKD.
Tubule cell injury and repair
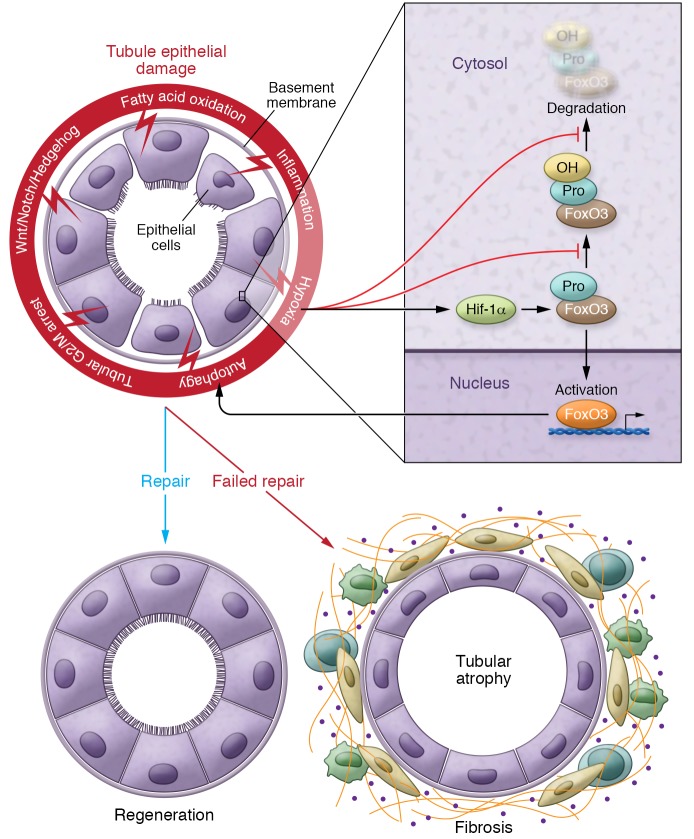
Damage to tubule epithelial cells resulting in cell death and injury is the most common causal factor in AKI and CKD. While we do not fully understand the exact molecular pathways, this damage is critical for the activation of a diverse set of repair pathways. Proliferation of epithelial cells quickly follows tubule cell injury. The exact nature of dividing epithelial cells has not been fully settled. Several markers have been shown to highlight progenitor-like cell populations in kidney tubules, including Cd133, Pax2, Pax8, Sox9, and Lgr4 (1). Lineage-tracing studies indicate that these cells expand and differentiate into multiple different epithelial cell types following injury (1). Some of the earlier reports also suggest that an incomplete cell cycle, such as G2/M-arrested proximal tubular cells, is key to CKD development (ref. 2 and Figure 1). The G2/M arrest in proximal tubule cells activates JNK signaling, which acts to upregulate profibrotic cytokine production (2). Treatment with a JNK inhibitor or bypassing the G2/M arrest by administration of a p53 inhibitor rescued fibrosis in the unilateral ischemic injured kidney (2).
Figure 1. Failed tubule repair after injury leads to fibrosis.
Schematic diagram illustrating changes following tubule injury. A series of pathways leading to either regeneration or fibrosis includes failed completion of the cell cycle (G2/M arrest), activation of developmental pathways (Wnt/Notch/Hedgehog), and altered metabolism, including a defect in fatty acid oxidation, inflammation, and hypoxia. Hypoxia plays a pivotal role and a final common pathway in fibrosis. Hif activates FoxO3 expression through Hif1-α and inhibits FoxO3 prolyl hydroxylation and FoxO3 degradation. FoxO3 activation and accumulation can trigger tubular epithelial autophagy, which also plays a role in tubule injury. In contrast to the regeneration following injury, a failed repair of tubules after injury leads to tubule atrophy, an influx of inflammatory cells, and extracellular matrix accumulation, leading to the development and progression of fibrosis. Pro, proline residue.
It is not surprising that activation of classic developmental pathways, such as Wnt, Notch, and Hedgehog, are all described in AKI and CKD (3). At a simplistic level, regeneration recapitulates features of development, such as proliferation, epithelialization, elongation, and differentiation. Notch is critical for transit amplification and binary cell–type decision making in multiple organs (Figure 1). In development, Notch regulates cadherin 6 expression and proximal tubule development (4). Notch expression and Notch-induced proliferation and transit amplification, therefore, might be important for proximal tubule repair after injury (5). Sustained expression of Notch, however, is associated with sustained proliferation and a block in terminal differentiation of tubule cells, including a defective tubule cell metabolism (6), blocking regeneration and leading to fibrosis development.
Failed tubule cell repair
Kidney tubule epithelial cells keenly depend on fatty acid oxidation and mitochondrial oxidative phosphorylation as their main energy source. A defect in fatty acid oxidation has been shown to play a key role in both AKI and CKD (7). While fatty acids are often available in excess in the circulation, cells, including kidney tubule cells, store lipids in intracellular lipid droplets (8). Autophagy, which is a mechanism of degrading cellular proteins, is important for delivering the content of intracellular lipid stores for mitochondrial oxidation (ref. 9 and Figure 1). Alterations in autophagy have been observed in both AKI and CKD and appear to contribute significantly to tubule disease pathology (10). An increase in autophagy has been described in AKI that precedes cell death (11). Genetic deletion of autophagy proteins aggravates kidney injury in the setting of AKI, indicating a protective role for delivering nutrients for metabolism by autophagy (12).
Members of the forkhead box O (FOXO) transcription factor family play an important role in regulating metabolism, proliferation, stress resistance, apoptosis, and autophagy in a variety of cell types (13). The activity of FOXO is tightly regulated by posttranslational modification, including phosphorylation, acetylation, and ubiquitylation (14). Cell survival pathways, such as PI3K and AKT, phosphorylate FOXO at different sites that regulate the nuclear localization or degradation of FOXO (15). In the current issue, Li et al. describe the prolyl hydroxylation of FoxO3 in kidney tubule cells (16).
Role of hypoxia and autophagy in kidney injury
Li et al. investigated the relationship between hypoxia and autophagy in the setting of kidney injury (16). They show that hypoxia activates the stress-responsive transcription factor FoxO3 and that FoxO3 hydroxylation depends on molecular oxygen. During renal hypoxia, prolyl hydroxylation and FoxO3 degradation are inhibited, thereby increasing FoxO3 protein abundance, leading to its activation and stimulation of stress responses, including tubular epithelial autophagy (ref. 16 and Figure 1). Deletion of FoxO3 in kidney tubule cells during the AKI-to-CKD transition reduces autophagy and aggravates oxidative damage to the kidney, causing profound fibrosis.
Hypoxia has been considered a key factor that drives the “failed” tubule cell repair in AKI and the final common pathway of fibrosis development (17). Under hypoxic conditions, HIF is not degraded by the prolyl hydroxylase domain (PHD), but accumulates in the nucleus and regulates the expression of a large number of genes, including VEGF, erythropoietin (EPO), mesenchymal genes, and cytokines (18). Reduction in tubule-specific VEGF expression has been observed in AKI, leading to peritubular rarefaction (19). It remains, however, controversial whether HIF is actually profibrotic or antifibrotic. Kapitsinou et al. inhibited PHD by a pharmacological inhibitor (GSK1002083A) to increase HIF before an injury, which ameliorated AKI-induced fibrosis in mice (20). Kobayashi et al. showed that global activation of HIF repressed fibrogenesis in mice subjected to unilateral ureteral obstruction (UUO) (21). Increasing HIF activity in interstitial cells can raise EPO levels (22). PHDs are excellent pharmacological targets. PHDs are non–heme iron–containing dioxygenases that require oxygen and 2-oxoglutarate as cosubstrates and iron and ascorbate as cofactors for their enzymatic activity (23). Several new classes of PHD inhibitors have been developed primarily for anemia management, but based on their pleiotropic effect, may also offer renal protection (24). Several PHD inhibitors are in late-stage clinical trials. The most notable of these PHD inhibitors are Roxadustat (FG-4592), Vadadustat (AKB-6548), Daprodustat (GSK1278863), Desidustat (ZYAN-1), and Molidustat (Bay 85-3934), all of which have been developed as orally acting drugs for the treatment of anemia, with the expectation that they will also improve kidney function. HIF can crosstalk with multiple profibrotic signaling pathways, including TGF-β, Notch, NF-κB, and PI3K/Akt pathways, to further regulate renal fibrosis (25). The work by Li et al. indicates that HIF works together with FOXO, regulating autophagy and metabolism, providing a potentially new therapeutic target for AKI and CKD.
Acknowledgments
Work in the Susztak lab is supported by the NIH (R01 DK076077, DK087635, DK105821, and DP3 DK108220).
Version 1. 05/06/2019
Electronic publication
Version 2. 06/03/2019
Print issue publication
Footnotes
Conflict of interest: The Susztak lab is supported by Boehringer Ingelheim, Lilly, Regeneron, GSK, Merck, ONO Pharmaceutical Co., Gilead, and Bayer for work that is not related to the current manuscript.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(6):2192–2194. https://doi.org/10.1172/JCI128985.
See the related article at FoxO3 activation in hypoxic tubules prevents chronic kidney disease.
Contributor Information
Xiangchen Gu, Email: Xiangchen.Gu@pennmedicine.upenn.edu.
Archana Raman, Email: Archana.Raman@pennmedicine.upenn.edu.
Katalin Susztak, Email: ksusztak@pennmedicine.upenn.edu.
References
- 1.Kang HM, Huang S, Reidy K, Han SH, Chinga F, Susztak K. Sox9-positive progenitor cells play a key role in renal tubule epithelial regeneration in mice. Cell Rep. 2016;14(4):861–871. doi: 10.1016/j.celrep.2015.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol. 2016;12(7):426–439. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirin Y, Susztak K. Notch in the kidney: development and disease. J Pathol. 2012;226(2):394–403. doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielesz B, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120(11):4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang S, et al. Jagged1/Notch2 controls kidney fibrosis via Tfam-mediated metabolic reprogramming. PLoS Biol. 2018;16(9):e2005233. doi: 10.1371/journal.pbio.2005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang HM, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21(1):37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stadler K, Goldberg IJ, Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15(7):40. doi: 10.1007/s11892-015-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20(1):3–11. doi: 10.1038/cdd.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takabatake Y, Kimura T, Takahashi A, Isaka Y. Autophagy and the kidney: health and disease. Nephrol Dial Transplant. 2014;29(9):1639–1647. doi: 10.1093/ndt/gft535. [DOI] [PubMed] [Google Scholar]
- 11.Havasi A, Dong Z. Autophagy and tubular cell death in the kidney. Semin Nephrol. 2016;36(3):174–188. doi: 10.1016/j.semnephrol.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82(12):1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Zhou Y, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int. 2014;2014:925350. doi: 10.1155/2014/925350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27(16):2263–2275. doi: 10.1038/onc.2008.20. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Kang H, Zhang Q, D’Agati VD, Al-Awqati Q, Lin F. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J Clin Invest. 2019;129(6):2374–2389. doi: 10.1172/JCI122256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunaratnam L, Bonventre JV. HIF in kidney disease and development. J Am Soc Nephrol. 2009;20(9):1877–1887. doi: 10.1681/ASN.2008070804. [DOI] [PubMed] [Google Scholar]
- 19.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008;294(4):F928–F936. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kapitsinou PP, et al. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol. 2012;302(9):F1172–F1179. doi: 10.1152/ajprenal.00667.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi H, et al. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol. 2012;188(10):5106–5115. doi: 10.4049/jimmunol.1103377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souma T, et al. Erythropoietin synthesis in renal myofibroblasts is restored by activation of hypoxia signaling. J Am Soc Nephrol. 2016;27(2):428–438. doi: 10.1681/ASN.2014121184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuiper C, Vissers MC. Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression. Front Oncol. 2014;4:359. doi: 10.3389/fonc.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta N, Wish JB. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69(6):815–826. doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, et al. Hypoxia, HIF, and associated signaling networks in chronic kidney disease. Int J Mol Sci. 2017;18(5):E0950. doi: 10.3390/ijms18050950. [DOI] [PMC free article] [PubMed] [Google Scholar]