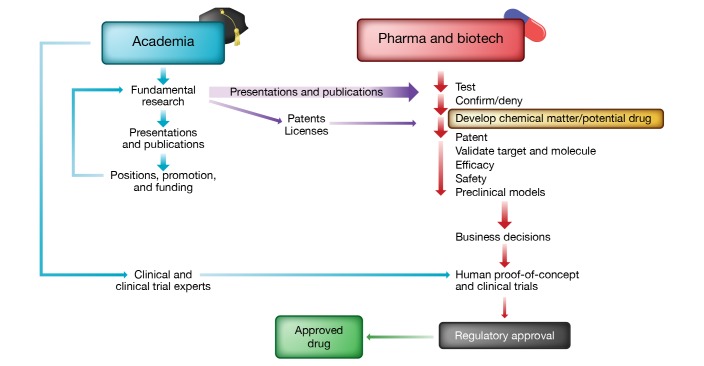
The discovery and development of new therapies involves input from academic researchers and the biopharmaceutical industry, but the nature and relative importance of their contributions has been the subject of debate. Recent concerns about drug pricing are among the factors stimulating renewed interest in this question, which has important implications for academia, industry, and society at large. To facilitate understanding and informed public discussion, this Viewpoint will offer a high-level overview of the unique, overlapping, and evolving contributions of academia and industry to drug discovery (Figure 1).
Figure 1. Schematic representation of the drug discovery and development ecosystem.
New insights into biology and pathophysiology are typically driven by fundamental research at academic institutions. Traditionally, such discoveries indirectly contribute to advances as industry seeks new projects and drug targets from basic research. The identification of new drug candidates and subsequent validation work are a strength of industry-led research. Efforts toward human proof-of-concept studies and clinical trials in patients often involve collaboration between industry partners, academic centers, clinical specialists, and clinical trial experts. Increasingly, hybrid models of drug development are being explored, wherein venture firms incubate startup companies internally to develop new approaches and patents, rather than licensing these from academic institutions, and then establish agreements with biopharma to further the development of the drug. Illustrated by Rachel Davidowitz.
A very brief history
Efforts to develop effective treatments are driven by the universal human desire to enhance health and reduce suffering from disease. For most of human history, healing traditions and folk remedies bore a limited relationship to biology or disease pathogenesis. This began to change during the 19th century, as scientists in academia studied mechanisms of biological regulation, and early pharmaceutical companies sought chemical agents to counter specific diseases.
The academic bioscience research effort began in earnest in universities, hospitals, and research institutes in the late 19th century (1). This expanded during the 20th century and was greatly accelerated when the US established the NIH as a dominant source of funding (2). The NIH stimulated growth of the research enterprise, which produced many insights into mechanistic biology and disease pathogenesis.
In parallel, pharmaceutical companies initially arose from the German chemical and dye industries, followed by similar companies in the US and elsewhere (1, 3). Early efforts involved serendipitous observations, purification of active extracts from traditional herbal remedies, and insights from chemicals tested in animals via the emerging fields of physiology and pharmacology. The path from discovery to sales was largely free of regulatory control until the early to mid-20th century, when legislation granted the FDA a key role in US drug approval (4).
The role of academia in modern drug discovery
Most fundamental biologic insights have resulted from work by academic scientists conducting research to understand how things work, rather than through applied research aiming to produce therapeutic benefits. While some characterize research as curiosity driven, many academic scientists are aware that mechanistic discoveries into fundamental biologic processes might eventually produce health benefits. Nevertheless, the academic sector long downplayed or disregarded the practical application of its discoveries and took few steps or looked askance at efforts to pursue them. Academic culture also played an important role: whether scientists were motivated by curiosity or therapeutic goals, publication and credit are the currency of the academic realm, as both are required for research funding and professional success.
This culture began to change in the 1970s, as recombinant DNA technology created new opportunities to translate basic discoveries into therapies. Genentech was founded in 1976 by a scientist and a venture capitalist. A second stimulus to change was the passage in 1980 of the Bayh–Dole Act (5), which created a path for grantee institutions to pursue commercial development of government-funded discoveries, now established as an explicit goal of federally funded research. Institutional technology transfer offices arose to identify patentable discoveries by faculty, license them to biopharmaceutical companies, and/or launch venture-funded startups in exchange for a share of equity. Previously, patents arising from federally funded academic research were not sought or were assigned to the government, which had little success developing them.
Academics publish their discoveries in scientific journals, making them available to the public. The pharmaceutical industry is a major consumer of this knowledge, which suggests new pathways and targets against which small molecules or other therapeutic agents might be developed. Although large pharma companies have had basic research programs (1), their prevalence and size have diminished in recent years, and industry-led programs were never the major source of new biologic insights. In a review of transformative medicines approved for clinical use by the FDA between 1985 and 2009, the vast majority had intellectual origins in academic research, most of which was funded by the NIH (6). In most cases, the underlying discoveries initially did not provide clear and obvious paths to therapeutic translation. Rather, decisions to launch drug development programs followed multiple discoveries that built upon one another over decades, cumulatively stimulating commercial drug discovery efforts. Most academic discoveries contribute indirectly to the development of new therapies. In only a small minority is the key patentable discovery held by academia; when this does occur, large financial rewards can accrue to institutions and inventors. The Bayh–Dole Act stipulates that institutional revenues should be reinvested in research. Despite the occasional big success, many technology transfer offices fail to cover their operating costs.
Beyond discovery research, academics facilitate drug development in other ways, such as through consultancy and service as key opinion leaders, both highly valued by industry. Such individuals play important roles in the design and implementation of clinical trials.
The role of industry
Some have argued that industry adds little value to the development of marketable drugs and largely monetizes academic insights (7). This view reflects a limited understanding of the work carried out by the pharmaceutical and biotech industries.
Pharmaceutical and biotech companies vary substantially in therapeutic focus and approaches. On the basis of their goals and the scope of their research and development organizations, companies choose new pathways and targets to explore. When a target in the published literature catches their attention, industry first seeks to confirm the academic findings, however, many high-profile academic studies are irreproducible (8–10). Companies may then establish preclinical teams to develop relevant in vitro and in vivo test systems. Critically, industry teams generate and refine chemical or biologic reagents capable of selectively perturbing the target, which then serve as leads for iterative drug development, a core industry expertise. Identification of suitable drug candidates generates patent applications for chemical matter and use that are required for programs to advance. Drug candidates are tested for efficacy and safety in preclinical models, leading to many new biological insights.
Industry has additional expertise that is critical to successful drug development, including in toxicology, drug metabolism, pharmacokinetics and pharmacodynamics, formulation and delivery, and regulatory affairs. These disciplines are poorly represented in much of academia, though such expertise does exist in schools of pharmacy.
Once a molecule demonstrates preclinical efficacy and safety, a decision to move forward requires assessment of the market, comparison with existing therapies, and prioritization against other possible programs. A decision to proceed with a “target product profile” entails plans for human clinical experimentation, developed with guidance from the FDA. Initial trials establish safety and proof of concept for target engagement. If positive, clinical trials of progressively larger scale, typically international in scope, are initiated and cost as much as hundreds of millions of dollars. In addition to clinical efficacy and safety, new drugs must be seen as superior in efficacy, safety, or some other attribute — or at least not inferior — to existing alternatives.
Regardless of initial hopes, biology is complicated, drug development is difficult, and failure is the most common result. The great majority of preclinical programs never advance to human studies, and less than 10% of drugs entering clinical trials eventually receive FDA approval. The time from preclinical program to drug approval averages more than ten years, during which much can change in the therapeutic landscape. Importantly, FDA approval does not ensure clinical adoption. Physicians may or may not prescribe an approved drug, and health insurers and formularies may or may not cover the cost of therapies, further increasing the financial risks of drug development. Counting failures and the cost of capital, the average cost of developing a new drug exceeds two billion dollars (11). While some dispute the appropriateness of counting the cost of failures, it seems hard to argue against this approach to accounting.
New types of drugs and models of development
Drug development has historically targeted common diseases, but a recent trend is to develop therapies for orphan diseases with small patient populations and defined etiologies. This path may be more rapid, requiring smaller and less expensive trials. Though less costly to develop, the small number of patients suggests that high unit prices are necessary to justify future investment in such programs. So far, many insurers have been willing to pay these charges.
In recent years, the very definition of a drug has evolved to include cellular therapies, gene therapy including use of CRISPR, mRNAs, and microbial organisms as drugs. These new modalities might benefit from modified development and regulatory paradigms and might also promote novel business models and types of academia-industry collaboration.
Venture capital plays an important role in the drug development ecosystem, raising funds for startups on the basis of technologies licensed from academia and engaging scientific founders with promising ideas and prior entrepreneurial success. After successive rounds of funding linked to progress toward clinical development, companies may go public, be acquired by larger biotech or pharmaceutical companies, or go out of business when the money runs out. In one recent variant to the drug development model, venture funds incubate companies internally by employing scientists and engaging academic advisors to generate foundational intellectual property that would otherwise be licensed from academic organizations.
Who deserves the credit?
The discovery and development of new therapies has and will likely continue to require contributions from academic institutions and the biopharmaceutical industry. Most (but not all) new insights into biology, disease, and new technologies arise in academia, funded by public grants, foundations, and institutional funds. Academic institutions identify promising discoveries and seek to initiate their development and commercialization, eventually partnering with for-profit biopharmaceutical companies, either established or venture-funded startups. To complete the path from molecular insight to approvable therapy, major involvement of industry is required, as it possesses the insights, culture, skills, and capital typically unavailable in the academic realm.
Cooperation between academia and industry takes many forms, including transfer of information, intellectual property, and reagents; consultation with industry by academic experts; and movement of people between these domains. The two cultures are different: teamwork toward shared therapeutic goals characterizes industry, and diverse paths to individual credit characterize academia. This cultural divide sometimes impedes successful interactions. A goal of public policy should be to facilitate such interchanges to enhance the success of the combined enterprise.
The cause of high pharmaceutical prices in the US is complex, including our approach to patents, regulatory approval, payment for drugs, and other factors that require attention and remediation (all of which are beyond the focus of this inquiry but described in more detail in refs. 12, 13). Productive discussion of the problem of high drug prices requires that we understand the nature of the ecosystem. A pervasive misconception is that academics use public funds to discover new therapies, which are then handed off to biopharmaceutical companies to be manufactured, packaged, and monetized. This outlook mischaracterizes and minimizes the enormous skill, effort, and time required after academic discovery to bring safe, effective, and innovative therapies to market.
We have an enormous opportunity today to develop transformative new therapies as well as better and cheaper versions of existing therapies, advances that will require enhanced interaction between academia and industry. Productive conversations on how to make the combined ecosystem more successful and efficient are urgently required.
Acknowledgments
I thank the following individuals for helpful comments on various drafts of this article: Michael Rosenblatt (Flagship Pioneering); Michael Gilman (Obsidian Therapeutics); Isaac Kohlberg (Harvard University Office of Technology Development); Chris Coburn (Partners Healthcare); Derek Lowe (Novartis); Scott Podolsky (Harvard Medical School Center for the History of Medicine); Eleftheria Maratos-Flier (Novartis); Mark Fishman (Harvard University Department of Stem Cell and Regenerative Biology); and Andrew Lo (MIT Sloan School of Management).
Version 1. 05/20/2019
Electronic publication
Version 2. 06/03/2019
Print issue publication
Footnotes
Conflict of interest: JSF declares stock ownership in and income from Scholar Rock, stock ownership in Sigilon Therapeutics, as well as stock ownership in and income from Novartis by an immediate family member.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(6):2172–2174. https://doi.org/10.1172/JCI129122.
References
- 1. Swann JP. Academic Scientists and the Pharmaceutical Industry: Cooperative Research in Twentieth Century America. Baltimore, Maryland, USA. The Johns Hopkins University Press; 1988. [Google Scholar]
- 2. Wikipedia contributors. National Institutes of Health. https://wikipedia.org/wiki/National_Institutes_of_Health Updated February 11, 2019. Accessed March 4, 2019.
- 3.Malerba F, Orsenigo L. The evolution of the pharmaceutical industry. Business History. 2015;57(5):664–687. doi: 10.1080/00076791.2014.975119. [DOI] [Google Scholar]
- 4. Junod SW. FDA And Clinical Drug Trials: A Short History. FDA Website. https://www.fda.gov/downloads/AboutFDA/History/ProductRegulation/UCM593494.pdf Accessed April 19, 2019.
- 5.Grimaldi R, Kenney M, Siegel DS, Wright M. 30 years after Bayh–Dole: Reassessing academic entrepreneurship. Research Policy. 2011;40(8):1045–1057. doi: 10.1016/j.respol.2011.04.005. [DOI] [Google Scholar]
- 6.Spector JM, Harrison RS, Fishman MC. Fundamental science behind today’s important medicines. Sci Transl Med. 2018;10(438):eaaq1787. doi: 10.1126/scitranslmed.aaq1787. [DOI] [PubMed] [Google Scholar]
- 7. Angell M. The Truth About The Drug Companies: How They Deceive Us And What To Do About It. New York, New York, USA: Random House; 2005. [Google Scholar]
- 8.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begley CG, Ioannidis JP. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res. 2015;116(1):116–126. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 10.Flier JS. Irreproducibility of published bioscience research: Diagnosis, pathogenesis and therapy. Mol Metab. 2017;6(1):2–9. doi: 10.1016/j.molmet.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Woodfield A. Augmenting reference pricing of pharmaceuticals in New Zealand with strategic cross-product agreements. Pharmacoeconomics. 2001;19(4):365–377. doi: 10.2165/00019053-200119040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139–162. doi: 10.1257/jep.29.1.139. [DOI] [PubMed] [Google Scholar]