Abstract
Regenerative rehabilitation is an emerging area of investigation that seeks to integrate regenerative medicine with rehabilitation medicine. It is based on the realization that combining these two areas of medicine at an early stage of treatment will produce a better clinical outcome than the traditional, linear approach of first administering the elements of regeneration followed, after a delay, by rehabilitation. Indeed, in certain settings, a case can be made for initiating rehabilitation protocols before starting regenerative intervention. This review summarizes the contents of a workshop held during the 2018 annual meeting of the Orthopaedic Research Society. It introduced the concept of regenerative rehabilitation and then provided two orthopaedic examples drawn from the domains of cartilage repair and bone healing. Rehabilitation medicine can supply a variety of physical stimuli, including electrical stimulation, thermal stimulation and mechanical stimulation. Of these, mechanical stimulation has the most obvious relevance to orthopaedics. The mechano-responsiveness of cartilage and bone has been known for a long time, but this is poorly understood and has led to only limited clinical application. Improved bioreactor designs that allow multi-axial loading enable new insights into the responsiveness of chondrocytes and chondroprogenitor cells to specific types of load, especially shear. Recent studies on the mechanobiology of bone healing show that modulating the mechanical environment of an experimental osseous lesion by a process of “reverse dynamization” soon after injury considerably enhances healing. Future studies are needed to probe the molecular mechanisms responsible for these phenomena and to translate these findings into clinical practice.
Keywords: Chondrocyte, MSC, chondrogenesis, reverse dynamization, bone healing, regenerative rehabilitation
Introduction
Regenerative medicine and rehabilitation medicine seek to restore tissue and function when these have been lost through aging, injury, disease or congenital processes. These two approaches to therapy emerged as separate disciplines and have tended to remain this way. In most practices they are applied sequentially, with the regenerative component preceding subsequent physical therapy. During the last decade it has become apparent that this dichotomy is inappropriate. As argued by Ambrosio and Russell in a landmark editorial1, clinical outcomes are likely to be far better when the regenerative and rehabilitation medicine aspects are combined at an early stage. This integration of disciplines recognizes the importance of components in addition to the traditional mix of cells, scaffolds and growth factors that we normally think of in the context of tissue regeneration. Rehabilitation protocols deliver clinically relevant biophysical stimuli, the nature of which varies by discipline. For example, in orthopaedics important stimuli include functional loading and other types of mechanical stimulation; for neurology, electrical signals are also important (figure 1)2. Several of the most promising clinical and pre-clinical advances are to be found in the areas of nerve regrowth, muscle regeneration, cartilage repair and bone healing. Examples drawn from the last two of these areas are given in this review, which is based on a workshop presented at the annual meeting of the Orthopaedic Research Society in New Orleans, March 2018.
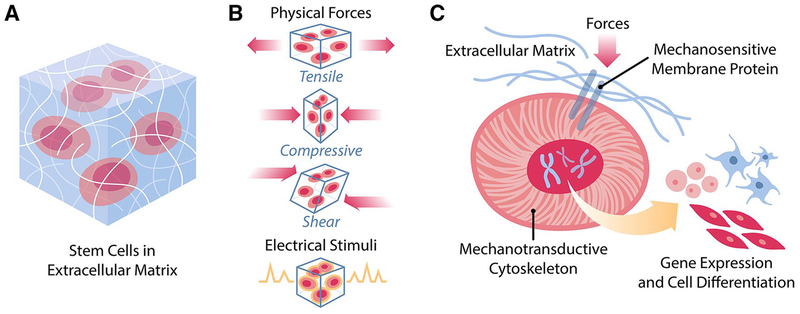
Figure 1.
Mechanotransduction of cells in vivo. A. Cells are embedded in an environment composed of extracellular matrix and local milieu that exert various biophysical pressures. B. Among the biophysical pressures are physical stimuli, such as tensile forces, compressive forces, and shear stresses, and electrical stimuli from neural cells and local fields. C. In response to biophysical stimulation, cells transduce such signals from the membrane to the nucleus through the cytoskeleton in order to influence gene expression and cell fate.
From reference 2, with permission
Chondrogenesis and cartilage repair
Cartilage is the hypocellular material that covers the ends of the long bones within diarthrodial joints and allows for low-friction, pain-free motion. Traumatic injury and osteoarthritis are two common ways through which cartilage can be damaged, leading to disability and a reduced quality of life3–5. Substantial effort has gone into developing techniques to improve cartilage regeneration, but with limited success6. Mechanical regulation of cartilage and the cells contained within, the chondrocytes, has long been appreciated, yet there have been surprisingly few attempts to correlate in vitro studies with evidence based clinical practice. The beneficial effects of continuous passive motion during cartilage regeneration have long been known7, 8 yet the underlying mechanisms are still unclear.
Bioreactor systems have been used with great effect to dissect the biological roles of various loads applied to cells and tissues9–12. This has led to a greater understanding of cell responses during the differentiation and maturation of a chondroprogenitor towards a mature chondrocyte13. A chondron responds differently from a chondrocyte without its pericellular matrix, as it has been shown that a mature pericellular matrix enhances the cells’ responses to load14,15. Alterations in mechanoresponsiveness are likely to make important contributions to various pathological states, although this has been little investigated. Aging provides one example, as one of the mechanisms by which aging plays a degenerative role in cartilage homeostasis is through a decrease in the mechanoresponsiveness of the chondrocytes16. There is also a shift in TGF-β signaling from a beneficial ALK-5/SMAD 2/3 pathway to a detrimental ALK-1/ SMAD1/5/8 signal17. Furthermore, loading regimes that are sufficient to maintain cartilage homeostasis, do not appear sufficient to trigger a chondrogenic response in mesenchymal stromal cells (MSCs)18–20. These differences, while subtle, will influence tissue quality after cell-based cartilage regenerative therapies and may need to be taken into account when considering rehabilitation protocols. For example, should microfracture patients have the same rehabilitation protocol as patients undergoing autologous chondrocyte implantation when two different cell sources are being used to restore cartilage?
Transforming growth factor beta (TGF-β) is commonly used in protocols designed to regenerate cartilage. However, the timing and dose of TGF-β exposure appear to play critical roles in this process. Classical chondrogenic culture models include 10 mg/ml TGF-β in the induction medium21. For many years, we have been investigating the mechanoregulation of human MSC chondrogenesis using a bioreactor that is able to apply compression, shear, or a combination of both using a ceramic hip ball as a counterface22. It has been shown that a combination of compression and shear can lead to a histological outcome more similar to native cartilage when using bovine articular chondrocytes23. Combining compression and shear also induces chondrogenesis of human MSCs in the absence of exogenous serum or growth factors19, 24. No response was obtained using compression alone under the same conditions. Varying the frequency and amplitude of the load modulated the response25, and this is in part due to the mechanical induction and activation of the endogenous latent TGF-β26. Activation of TGF-β was also possible in cell-free scaffolds, demonstrating that mechanical force alone is, at least in part, responsible for the activation observed. Mechanical activation of TGF-β has previously been shown by shearing of synovial fluid27, which is possible because the latency associated peptide is not covalently bound to the TGF-β peptide. Localized activation of TGF-β in response to mechanical load may be a mechanism by which local strain results in a localized biological outcome. Using this knowledge, it is possible to investigate novel materials using the activation of latent TGF-β under cyclical load as an output parameter. The activation of TGF-β appears to be related to the stiffness of the materials under loading conditions, with materials that are too soft being unable to support protein activation. As loads are applied to the scaffold and defect during patient articulation, this information can provide design criteria for novel materials intended for clinical use. Such studies would focus on the localized strain applied, while using TGF-β protein activation as an outcome parameter to assess efficacy under load.
Normal cartilage possesses a reservoir of matrix-bound, latent TGF-β. The activation of the latent TGF-β is thought to be more strongly regulated mechanically at the superficial zone28 with enzymic regulation playing a more important role in the deeper zones29, 30. In regenerating tissue, the relative contribution of mechanical activation and enzymic activation is likely to change as the tissue matures and deposits a TGF-β binding extracellular matrix. Within regenerating tissue, the strains applied will vary depending on the exact location of the cells within the tissue. During in vitro studies it has been shown that asymmetrical seeding of scaffolds leads to enhanced matrix deposition after the application of biaxial load. Seeding 10% of the cells as a monolayer on the surface of the construct, with the remaining 90% being evenly distributed, reliably led to improved tissue formation compared with uniform cell distribution, while the monolayer alone was not chondrogenic31. This has led to the following working hypothesis: the cells at the surface respond to the load by producing TGF-β that is then activated by the applied shear. Increasing the surface cell number leads to a more reproducible response as more cells are in contact with the shear. The compression then allows for fluid exchange, driving the active TGF-β into the tissue below where it stimulates chondrogenesis of the 3D encapsulated cells.
However, MSC chondrogenesis induced by mechanical stimulation is not the same as that induced by exposure to exogenous active TGF-β protein. While there are distinct similarities, mechanically induced chondrogenesis of human MSCs leads to the production of molecules not seen using exogenous TGF-β, such as angiopoietin 2 (ANG2), osteoprotegrin (OPG) and nitric oxide (NO)32 (figure 2). Such secretome studies could lead to novel therapeutic targets being identified, which may improve patient outcomes after cell-based therapies. Further work is required to see whether these observed changes lead to functional outcomes.
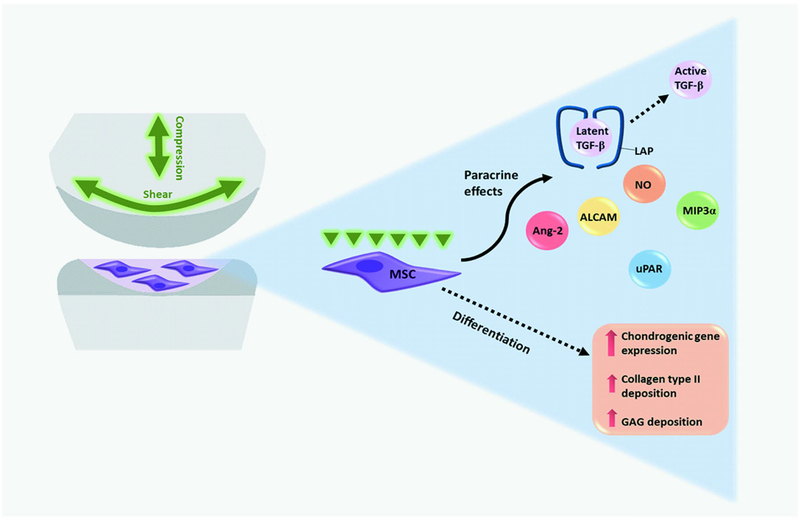
Figure 2.
Effect of joint-associated mechanical stimulation on the chondrogenic differentiation of MSCs. Diarthrodial joint-associated loading increases chondrogenic gene expression, GAG and collagen type II deposition by human MSCs in the absence of exogenous growth factors. Additionally, multiaxial loading may enhance the paracrine activity of MSCs through inducing the secretion and activation of transforming growth factor (TGF)-β as well as production of nitric oxide (NO), macrophage inflammatory protein 3α (MIP3α), urokinase receptor (uPAR), activated leukocyte cell adhesion molecule (ALCAM), and angiopoietin-2 (Ang-2).
From reference 13, with permission
A major challenge still to be overcome is how to correlate the load applied during in vitro loading studies, which are generally performed under unconfined conditions, to the mechanical forces applied in a confined cartilage defect within a patient. This will require the development of more complex in vitro culture systems, and the collaboration of cell and tissue biologists with groups working on whole joint biomechanics. Work is in progress to develop an osteochondral defect model that can be subjected to multiaxial load. This is an area where finite element analysis (FEA) models could accelerate knowledge, providing predictive outcome measures can be defined33. Models that can take into account the patient specific injury and load distribution could be used to establish the load patterns experienced by the injury site. This could then be used to optimize the rehabilitation protocol on a case-by-case basis.
Taken together, in vitro culture models incorporating complex multiaxial load offer multiple opportunities to investigate cells, materials, secretome profiles and rehabilitation protocols in a defined setting. The use of human cells from skeletally mature adults or the elderly produces data that have direct clinical relevance to specific target patient population and reduces the use of experimental animals, in line with the current 3R drive to reduce, refine and replace animal experimentation. This offers particular advantages when investigating biologics, where the response may have subtle species-specific differences. The effects of soluble molecules may be mediated by load-induced cell secretome changes and this synergistic effect can be assessed. Mechanisms being elucidated in such studies could also offer insight into the mechanical regulation of direct versus indirect bone healing during fracture repair, where the difference in healing involves the induction of a transient cartilaginous template as a response to increased motion34. The initial local strain directly influences de novo tissue development in both cartilage and bone, and the principles guiding this initial mechanobiological trigger are similar for both tissues. As described below, they are also being investigated in the context of bone healing.
Bone Healing
The management of bone loss and impaired healing is a complex clinical problem that requires innovative solutions. There are several options to treat problematic fractures and segmental bone loss, including the application of recombinant human bone morphogenetic protein-2 (rhBMP-2). However, none are reliably effective and all are associated with various complications, some of which result in a protracted course of continued medical care that is inevitably demanding and costly.
There has been much research on the biology of bone healing with the aim of improving the clinical management of recalcitrant cases. However, the mechanical environment around the fracture site, although critically important to the success of the healing process35, has received less sustained attention. Interactions between the biological and mechanical influences on bone healing in the context of regenerative rehabilitation have not yet been fully explored.
The mechanical environment itself is determined by the stiffness of the implant used to stabilize the fracture and weight-bearing; if fixation is either too flexible or too rigid a nonunion may result. The local cellular response to mechanical loading is heavily dependent upon the magnitude of interfragmentary movement (IFM), the type of loading conditions, and on the differentiation stage of the progenitor cells, collectively determining the size and quality of the callus formed36. Accordingly, stiff fixation that minimizes IFMs will result in limited callus formation, whereas flexible fixation that increases IFMs will result in the formation of a larger callus. Moreover, shear load is detrimental to fracture-healing, whereas the same amount of axial load is beneficial. Based on these observations, many authors have suggested that the delayed introduction of controlled motion (“dynamization”) as healing progresses may lead to faster maturation of bone37, but this procedure remains controversial and has not greatly influenced clinical practice. Exactly how the bone cell population is influenced by the mechanical environment when responding to mechanical signals to regenerate and remodel a successful bone structure is still uncertain. Understanding the nature of these mechanical cues, and the biological responses to them at various levels is very important as this will determine the rate of healing and the quality and nature of the newly formed tissue.
Mechanical Environment and Healing of Large Bone Defects
Appreciating that bone heals by an endochondral process led us to the hypothesis that the mechanical environment could be improved by providing higher IFMs during the first phase of healing to encourage chondrogenesis. This can be easily achieved by stabilizing the defect under conditions of low axial stiffness. Because excessive IFM prevents angiogenesis, a necessary step for endochondral ossification, the axial stiffness of fixation needs to be increased at the stage where cartilage is to be replaced by bone. We call the approach of fixing osseous defects at low initial stiffness, followed by higher stiffness once the endochondral phase of healing is starting, “Reverse Dynamization”38, 39. For those more familiar with traditional forward dynamization this is counter intuitive.
We used a rat femoral model to investigate the effects of the fixator stiffness on the healing of critical size, diaphyseal, segmental defects treated with BMP-240. Not only did the results of this study confirm that the healing of large osseous defects in response to BMP-2 treatment is strongly influenced by the local mechanical environment, but they also showed that healing can be improved by changing the stiffness of fixation to provide reverse dynamization38. Based on these observations, a subsequent study determined that a lower dose of BMP-2 could be used to heal successfully large segmental defects when reverse dynamization was applied41.
Observations made in the rat femoral defect model suggested that stiffness modulation was most effective during the early stages of healing. To refine our understanding of how the early phases of healing respond to stiffness of fixation, tissues within the defect were harvested during the inflammatory stage of healing (3 days), when soft callus had formed (7 days), and when hard callus was present (14 days) with either flexible or rigid fixation. Preliminary gene expression analyses demonstrate a substantial differential expression of genes between flexible and rigid fixation at 3 and 7 days after surgery (Glatt, unpublished data). After 3 days, there were 102 upregulated and 21 downregulated genes, mostly belonging to inflammatory pathways. In contrast, at day 7 there were only 27 significantly upregulated genes, mainly related to the endochondral ossification pathway, and 91 downregulated genes associated with inflammatory pathways. Interestingly, no differentially expressed genes were observed when comparing flexible and rigid fixation at 14 days. This strongly suggests that the critical time for modulating bone healing by altering the axial stiffness of fixation in this rat model falls during the early phases of inflammation and cartilage formation, which is precisely when reverse dynamization is implemented.
These findings suggest novel ways to improve the healing of large segmental defects while reducing the need for BMP-2 with its associated costs and potential side effects.
Mechanical Environment and Healing of sub-critical size defects
The foregoing discussion refers to effects on critical size segmental defects that require BMP-2 to heal. We wanted to assess whether reverse dynamization has the same stimulatory effect on the healing of sub-critical size defects that normally heal spontaneously. To study this we created 1 mm, middiaphyseal osteotomies in the rat femur. All defects were fixed initially at low axial stiffness, and reverse dynamization (increased stiffness) applied at various times from 3 days to 3 weeks42. The optimum time for increasing the stiffness of fixation occurred 7 days after surgery (Figure 3). Conversely, forward dynamization at 7 days was detrimental to bone healing compared to any of the other groups tested43. By 3 weeks the effect of reverse dynamization was lost and the outcome was equivalent to that occurring under standard, forward dynamization (Figure 3)42, 44. The modest gains produced by forward dynamization are not surprising considering our study demonstrating that any improvement noted when forward dynamization was implemented at the later stages of healing, was more likely a consequence of bone adaptation following Wolff’s law, rather than fixator dynamization as such (figure 3).
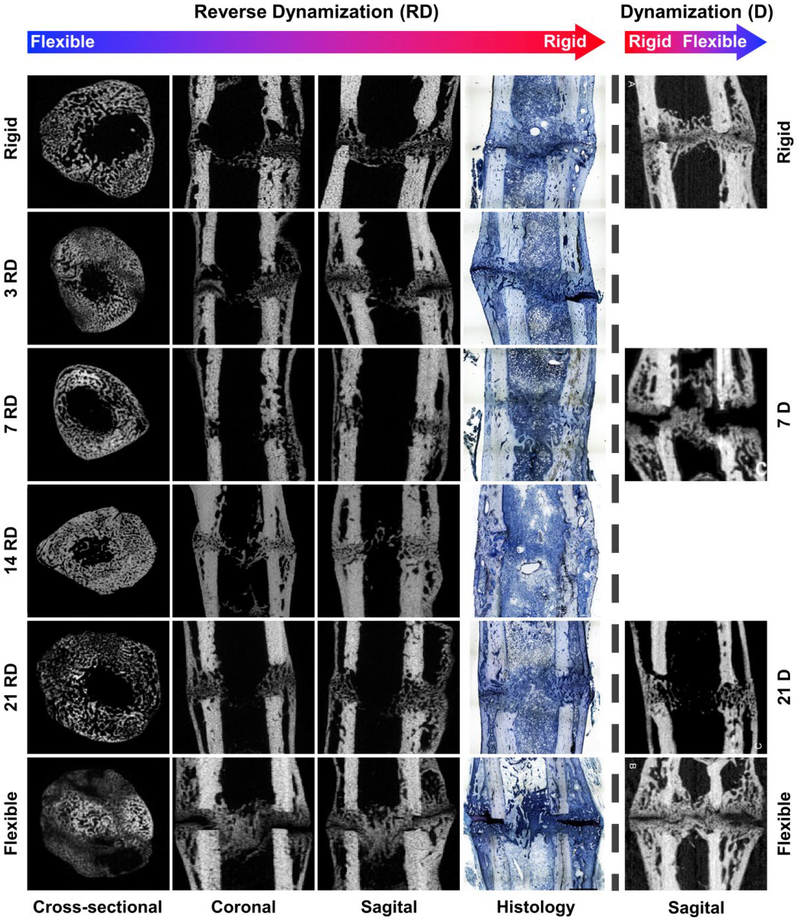
Figure 3.
Micro-computed tomography and histology images of 1 mm osteotomy defects 35 days postoperatively after reverse dynamization and standard, forward dynamization. Left hand side of dashed vertical line: reverse dynamization (flexible to rigid fixation) initiated at 3, 7, 14 and 21 days, and compared to constant stiff and constant flexible fixation control groups. Histological sections were stained with paragon: white and blue: fibrous tissue; purple: cartilage; light blue/white: bone (Images were adapted from reference 40). Right hand side of dashed vertical line: Micro-computed tomography images of 1 mm osteotomy at 35 days postoperatively after dynamization (rigid to flexible fixation) at 7 and 21 days, and compared to constant rigid and constant flexible fixation control groups.
Collectively, these pre-clinical studies in rat models confirm that early after the formation of an osseous defect there is a window of opportunity for improving healing by modulation of the mechanical environment. The counter intuitive approach of reverse dynamization shows considerable promise in this rat model, and we are optimistic the benefits will also be apparent when clinical trials are completed in the next several years.
Clinical translation
The examples described here provide strong pre-clinical evidence that modulation of the mechanical environment aids the formation of cartilage and bone at sites of injury. This generates numerous research questions with regard to possible mechanisms and optimization of the mechanical forces, which can be addressed through the use of appropriate computer modeling, ex vivo culture systems and animal models. Clinical translation is a different matter entirely.
It is difficult to impose precisely regulated forces on a cartilage lesion in situ. This is especially difficult if cells within the defect need to experience a combination of shear and dynamic loading, and if cells at different levels require different qualitative and quantitative mechanical environments. Changes in defect size and geometry will lead to variations in load distribution between individual patients that will be difficult to predict. Continuous passive motion is used clinically after certain types of joint surgery and, as indicated, this might serve as a convenient starting point. Although the quantitative and qualitative parameters of the mechanical forces cannot be controlled at the cellular level yet, there is at least partial control at the macroscopic level. Advanced computational methods, such as FEA, will be very useful in predicting the in situ mechanical environment generated by specific manipulations, and can potentially take into account the unique nature of every injury. Identifying an inexpensive and non-invasive way to monitor the progress of cartilage repair would be of great advantage to these studies.
There may be a clearer path forward for translating the mechanical stimulation of bone healing, such as by reverse dynamization, because forward dynamization has been applied to the long bones of certain patients for over 30 years. The benefits of this process have been modest, which is one reason that it is not more widely used. (Figure 3)42. Based on data with rat models, reverse dynamization promises to provide a much more dramatic improvement in bone healing. Dynamization is achieved with specially designed external fixators. These devices are already familiar in orthopaedic trauma surgery, as non-dynamizing external fixators are frequently used to stabilize complex defects. Normally, once the fracture is stabilized and the inflammation subsides, the external fixator is replaced surgically by internal fixation with intramedullary nails or plates. If reverse dynamization can accelerate healing by a sufficient amount, it could obviate the need for internal fixation, thereby reducing cost and complexity. These improvements would be especially valuable in developing countries, where cost is a major factor and tertiary medical centers are rare.
Conclusions
Regenerative rehabilitation has much to offer the field of orthopaedics. It builds on the observation that musculoskeletal tissues benefit from exposure to mechanical forces, both during development and while performing their normal physiological functions. It is thus unsurprising that such forces will also have important influences on healing and regeneration. There is much pre-clinical, experimental support for the enhancement of musculoskeletal tissue repair by combining aspects of regenerative medicine with components of rehabilitation medicine. Cartilage and bone are discussed in this review, but convincing data also exist for other tissues of orthopaedic interest, especially the repair of large volumetric muscle injuries where early phase clinical trials have taken place45. These trials have highlighted the value of “prehabilitation”, where mechanical stimulation was applied before surgery to implant a regenerative scaffold45. The clinical use of continuous passive motion after joint surgery and external fixation for bone healing suggest routes to the practical application of regenerative rehabilitation principles in orthopaedics. Regenerative rehabilitation is an emerging field and there is much work to be done, but the potential clinical benefits are enormous.
Acknowledgements
Portions of this work were supported by National Institutes of Health award numbers P2CHD086843 (PIs T. Rando and F. Ambrosio) and R01 AR050243 from NIAMS, the U.S. Department of Defense (Grant W81XWH-10–1-0888), the AO Foundation and the Vice-Chancellor’s Research Fellowship of Queensland University of Technology, Brisbane, Australia. We thank Drs. Rando and Ambrosio for permission to reproduce figure 1.
References
- 1.Ambrosio F and Russell A. 2010. Regenerative rehabilitation: a call to action. J Rehabil Res Dev. 47: xi–xv. [DOI] [PubMed] [Google Scholar]
- 2.Rando TA and Ambrosio F. 2018. Regenerative Rehabilitation: Applied Biophysics Meets Stem Cell Therapeutics. Cell Stem Cell. 22: 608. [DOI] [PubMed] [Google Scholar]
- 3.Bentley G 1975. Articular cartilage studies and osteoarthrosis. Ann R Coll Surg Engl. 57: 86–100. [PMC free article] [PubMed] [Google Scholar]
- 4.Mankin HJ. 1974. The reaction of articular cartilage to injury and osteoarthritis (first of two parts). N Engl J Med. 291: 1285–92. [DOI] [PubMed] [Google Scholar]
- 5.Mankin HJ. 1974. The reaction of articular cartilage to injury and osteoarthritis (second of two parts). N Engl J Med. 291: 1335–40. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone B, Alini M, Cucchiarini M, et al. 2013. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater. 25: 248–67. [DOI] [PubMed] [Google Scholar]
- 7.O’Driscoll SW, Keeley FW, and Salter RB. 1986. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 68: 1017–35. [PubMed] [Google Scholar]
- 8.O’Driscoll SW and Salter RB. 1986. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop Relat Res. 131–40. [PubMed] [Google Scholar]
- 9.Freed LE, Guilak F, Guo XE, et al. 2006. Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng. 12: 3285–305. [DOI] [PubMed] [Google Scholar]
- 10.Salzmann GM and Stoddart MJ. 2014. Bioreactor Tissue Engineering for Cartilage Repair, in Developing Insights in Cartilage Repair, Emans PJ, Peterson L, editor. Springer; London: p. 79–97. [Google Scholar]
- 11.Schulz RM and Bader A. 2007. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 36: 539–68. [DOI] [PubMed] [Google Scholar]
- 12.Vunjak-Novakovic G, Martin I, Obradovic B, et al. 1999. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 17: 130–8. [DOI] [PubMed] [Google Scholar]
- 13.Fahy N, Alini M, and Stoddart MJ. 2018. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J Orthop Res. 36: 52–63. [DOI] [PubMed] [Google Scholar]
- 14.Mauck RL, Soltz MA, Wang CC, et al. 2000. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 122: 252–60. [DOI] [PubMed] [Google Scholar]
- 15.Stoddart MJ, Ettinger L, and Hauselmann HJ. 2006. Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol Bioeng. 95: 1043–51. [DOI] [PubMed] [Google Scholar]
- 16.Madej W, van Caam A, Davidson EN, et al. 2016. Ageing is associated with reduction of mechanically-induced activation of Smad2/3P signaling in articular cartilage. Osteoarthritis Cartilage. 24: 146–57. [DOI] [PubMed] [Google Scholar]
- 17.van der Kraan P, Matta C, and Mobasheri A. 2017. Age-Related Alterations in Signaling Pathways in Articular Chondrocytes: Implications for the Pathogenesis and Progression of Osteoarthritis - A Mini-Review. Gerontology. 63: 29–35. [DOI] [PubMed] [Google Scholar]
- 18.Mauck RL, Byers BA, Yuan X, and Tuan RS. 2007. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 6: 113–25. [DOI] [PubMed] [Google Scholar]
- 19.Schatti O, Grad S, Goldhahn J, et al. 2011. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 22: 214–25. [DOI] [PubMed] [Google Scholar]
- 20.Thorpe SD, Buckley CT, Vinardell T, et al. 2010. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-beta3 induced chondrogenic differentiation. Ann Biomed Eng. 38: 2896–909. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone B, Hering TM, Caplan AI, et al. 1998. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 238: 265–72. [DOI] [PubMed] [Google Scholar]
- 22.Wimmer MA, Grad S, Kaup T, et al. 2004. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 10: 1436–45. [DOI] [PubMed] [Google Scholar]
- 23.Grad S, Loparic M, Peter R, et al. 2012. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoarthritis Cartilage. 20: 288–95. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Kupcsik L, Yao SJ, et al. 2010. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J Cell Mol Med. 14: 1338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Yao SJ, Alini M, and Stoddart MJ. 2010. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 16: 575–84. [DOI] [PubMed] [Google Scholar]
- 26.Gardner OFW, Fahy N, Alini M, and Stoddart MJ. 2017. Joint mimicking mechanical load activates TGFbeta1 in fibrin-poly(ester-urethane) scaffolds seeded with mesenchymal stem cells. J Tissue Eng Regen Med. 11: 2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albro MB, Cigan AD, Nims RJ, et al. 2012. Shearing of synovial fluid activates latent TGF-beta. Osteoarthritis Cartilage. 20: 1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albro MB, Nims RJ, Cigan AD, et al. 2013. Accumulation of exogenous activated TGF-beta in the superficial zone of articular cartilage. Biophys J. 104: 1794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albro MB, Nims RJ, Cigan AD, et al. 2013. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-beta. J Biomech. 46: 1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madej W, van Caam A, Blaney Davidson EN, et al. 2014. Physiological and excessive mechanical compression of articular cartilage activates Smad2/3P signaling. Osteoarthritis Cartilage. 22: 1018–25. [DOI] [PubMed] [Google Scholar]
- 31.Gardner OFW, Musumeci G, Neumann AJ, et al. 2017. Asymmetrical seeding of MSCs into fibrinpoly(ester-urethane) scaffolds and its effect on mechanically induced chondrogenesis. J Tissue Eng Regen Med. 11: 2912–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner OF, Fahy N, Alini M, and Stoddart MJ. 2016. Differences in human mesenchymal stem cell secretomes during chondrogenic induction. Eur Cell Mater. 31: 221–35. [DOI] [PubMed] [Google Scholar]
- 33.Zahedmanesh H, Stoddart M, Lezuo P, et al. 2014. Deciphering mechanical regulation of chondrogenesis in fibrin-polyurethane composite scaffolds enriched with human mesenchymal stem cells: a dual computational and experimental approach. Tissue Eng Part A. 20: 1197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claes LE, Heigele CA, Neidlinger-Wilke C, et al. 1998. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. S132–47. [DOI] [PubMed] [Google Scholar]
- 35.Glatt V, Evans CH, and Tetsworth K. 2016. A Concert between Biology and Biomechanics: The Influence of the Mechanical Environment on Bone Healing. Front Physiol. 7: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenwright J and Gardner T. 1998. Mechanical influences on tibial fracture healing. Clin Orthop Relat Res. S179–90. [DOI] [PubMed] [Google Scholar]
- 37.De Bastiani G, Aldegheri R, and Renzi Brivio L. 1984. The treatment of fractures with a dynamic axial fixator. J Bone Joint Surg Br. 66: 538–45. [DOI] [PubMed] [Google Scholar]
- 38.Glatt V, Miller M, Ivkovic A, et al. 2012. Improved healing of large segmental defects in the rat femur by reverse dynamization in the presence of bone morphogenetic protein-2. J Bone Joint Surg Am. 94: 2063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glatt V, Tepic S, and Evans C. 2016. Reverse Dynamization: A Novel Approach to Bone Healing. J Am Acad Orthop Surg. 24: e60–1. [DOI] [PubMed] [Google Scholar]
- 40.Glatt V and Matthys R. 2014. Adjustable stiffness, external fixator for the rat femur osteotomy and segmental bone defect models. J Vis Exp. e51558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glatt V, Bartnikowski N, Quirk N, et al. 2016. Reverse Dynamization: Influence of Fixator Stiffness on the Mode and Efficiency of Large-Bone-Defect Healing at Different Doses of rhBMP-2. J Bone Joint Surg Am. 98: 677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartnikowski N, Claes LE, Koval L, et al. 2017. Modulation of fixation stiffness from flexible to stiff in a rat model of bone healing. Acta Orthop. 88: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claes L, Blakytny R, Gockelmann M, et al. 2009. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J Orthop Res. 27: 22–7. [DOI] [PubMed] [Google Scholar]
- 44.Claes L, Blakytny R, Besse J, et al. 2011. Late dynamization by reduced fixation stiffness enhances fracture healing in a rat femoral osteotomy model. J Orthop Trauma. 25: 169–74. [DOI] [PubMed] [Google Scholar]
- 45.Sicari BM, Rubin JP, Dearth CL, et al. 2014. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 6: 234ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]