Abstract
There is a strong relationship between tissue factor (TF) and cancer. Many cancer cells express high levels of both full-length TF and alternatively-spliced (as) TF. TF expression in cancer is associated with poor prognosis. In this review, we summarize the regulation of TF expression in cancer cells and the roles of TF and asTF in tumor growth and metastasis. A variety of different signaling pathways, transcription factors and microRNAs regulate TF gene expression in cancer cells. The TF/factor VIIa complex enhances tumor growth by activating protease-activated receptor (PAR) 2 signaling and by increasing the expression of angiogenic factors, such as VEGF. AsTF increases tumor growth by enhancing integrin β1 signaling. TF and asTF also contribute to metastasis via multiple thrombin-dependent and independent mechanisms that include protecting tumor cells from natural killer cells. Finally, a novel anti-cancer therapy is using tumor TF as a target to deliver cytotoxic drugs to the tumor. TF may be useful in diagnosis, prognosis and treatment of cancer.
Keywords: Cancer, metastasis, thrombosis, tissue factor, tumor
Introduction
Full-length tissue factor (TF) is a transmembrane receptor and cofactor for factor (F)VII/FVIIa1. In addition to full-length TF, an alternative spliced (as) form of TF can be generated that lacks the transmembrane domain and is released from cells2. In contrast to TF, asTF has low procoagulant activity because it lacks the transmembrane domain3,4.
TF is expressed by cells around blood vessels, such as adventitial fibroblasts, and body surfaces, such as epithelial cells, and plays a critical role in hemostasis5. TF also contributes to various forms of thrombosis6. Many cancers, particularly adenocarcinomas, express high levels of TF7. A high level of tumor TF expression is associated with poor prognosis in many types of cancers, including breast, prostate, colorectal and pancreatic cancer7–15. The TF/factor (F)VIIa complex and downstream coagulation proteases, such as FXa and thrombin, activate protease-activated receptors (PARs) on a variety of cells16. This signaling requires the procoagulant activity of the extracellular domain of TF but not its cytoplasmic domain17. In addition, the TF cytoplasmic domain contributes to signaling via interaction with integrins17.
In this review, we summarize the current knowledge on the regulation of TF in cancer cells and the contributions of TF to tumor growth and metastasis (Figure 1).
Figure 1.
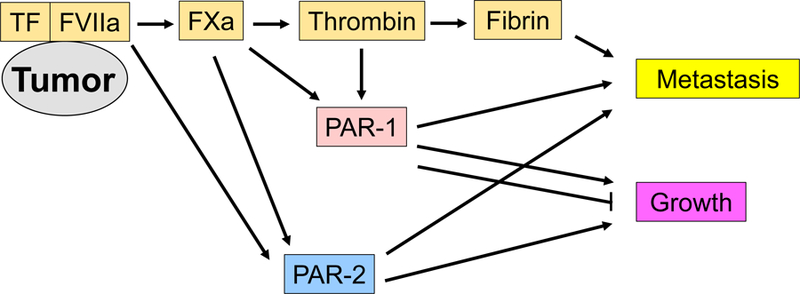
Contributions of tumor tissue factor (TF) to tumor growth and metastasis. The tumor TF and factor (F) VIIa complex contributes to tumor growth and metastasis by activating coagulation cascade generating fibrin and by directly and indirectly activating protease activated receptors (PARs). There are negative and positive roles of PAR-1 in tumor growth.
Regulation of Tissue Factor Expression in Cancer Cells
Tumor cells have genetic and epigenetic alterations that constitutively activate signaling pathways involved in tumorigenesis18. These pathways control cell growth, cell motility, cell metabolism, cell death and the tumor microenvironment, such as angiogenesis and inflammation18–20.
Many studies have assessed the regulation of TF in different types of cancer, including brain21–25, breast26–31, and colorectal32–34 cancer. These studies demonstrated that multiple signaling pathways, transcription factors and microRNAs (miRNAs) regulate TF gene expression in cancer cells (Tables 1 and 2) (Figure 2). For instance, the Raf-MEK-ERK signaling pathway and the transcription factors activator protein 1 (AP-1) and nuclear factor-κB (NF-κB) induce TF gene expression in the human breast cancer cell line MDA-MB-23126. Another study found that hepatocyte growth factor activation of the c-Met kinase pathway induced TF expression via Src family kinases in the human brain tumor cell line DAOY22. More recently, the mammalian target of rapamycin (mTOR) kinase pathway was shown to induce TF gene expression in the human pancreatic neuroendocrine tumor cell line BON35.
Table 1.
Tissue factor expression regulators and pathways in human cancer
| Regulator | Pathway | Type of human cancer (cell line) | Refs |
|---|---|---|---|
| PI3K | PI3K/AKT/mTOR | glioma (23.11, U87MG) | 21, 23 |
| Akt | PI3K/AKT/mTOR | glioma (U87MG) | 23 |
| mTOR | PI3K/AKT/mTOR | glioma (23.11, U87MG), pancreatic (BON) | 21, 23, 35 |
| KRAS | RAS/MAP kinase | colorectal (379.2), colorectal and NSCLC primary tumor | 32, 33, 38 |
| Raf | RAS/MAP kinase | breast (MDA-MB-231) | 26 |
| MEK | RAS/MAP kinase | glioma (23.11), breast (MDA-MB-231) | 21, 26 |
| ERK | RAS/MAP kinase | glioma (U87MG), breast (MDA-MB-231) | 23, 26 |
| c-Met | MET receptor | brain (DAOY) | 22 |
| HGF | MET receptor | brain (DAOY) | 22 |
| Src | kinase | brain (DAOY) | 22 |
| EGF | EGFR family tyrosine kinase | breast (MDA-MB-468), endometrial (Ishikawa) | 29, 37 |
| TGFα | EGFR family tyrosine kinase | squamous cell carcinoma (A431) | 36 |
| EGFR | EGFR family tyrosine kinase | squamous cell carcinoma (A431), glioma (U87MG) | 23, 27, 36 |
| EGFRvIII | EGFR family tyrosine kinase | glioma (U373, U87MG) | 23, 36 |
| HER2 | EGFR family tyrosine kinase | breast (SKBR3) | 27 |
| KSR1 | EGFR family tyrosine kinase | squamous cell carcinoma (A431) | 27 |
| JNK1 | JNK pathway | glioma (U87MG) | 23 |
| AP-1 | transcription factor | glioma (U87MG), breast (MDA-MB-231) | 23, 26 |
| Egr-1 | transcription factor | glioma (U87MG), breast (MDA-MB-231, MDA-MB-435) | 23, 28 |
| NF-kB | transcription factor | glioma (U87MG), breast (MDA-MB-231) | 23, 26 |
| SP-1 | transcription factor | glioma (U87MG) | 23 |
| HIF-1α | transcription factor | breast (MDA-MB-231, MDA-MB-435) | 28 |
| p53 | transcription factor | colorectal (379.2) | 32, 33 |
| Snail | transcription factor | breast (MDA-MB-468) | 29 |
| ZEB1 | transcription factor | breast (MDA-MB-468) | 29 |
| PTEN | phosphatase | glioma (23.11, U87MG) | 21, 23 |
| IDH1 | metabolic enzyme | primary glioma | 24 |
PI3K: phosphatidylinositol-3 kinase; mTOR: mammalian target of rapamycin; HGF: hepatocyte growth factor; EGF: epidermal growth factor; TGFα: transforming growth factor alpha; EGFR: epidermal growth factor receptor; HER2: human epidermal growth factor receptor 2; KSR1: kinase suppressor of Ras 1; JNK1: c-Jun N-terminal kinase 1; AP-1: activator protein-1; Egr-1: early growth response protein-1; NF-κB: Nuclear factor-κB; HIF-1α: hypoxia induced factor-1α; PTEN: phosphatase and tensin homolog; IDH1: isocitrate dehydrogenase 1
Table 2.
MicroRNAs regulating tissue factor expression in human cancer
| miRNA | Type of human cancer (cell line) | Refs |
|---|---|---|
| miR-19a | breast (MCF7, MDA-MB-231), colorectal (LoVo, DLD1, HT29, SW 480), early stage colorectal primary tumor | 30, 34 |
| miR-19b | breast (MDA-MB-231) | 31 |
| miR-20a | breast (MDA-MB-231) | 31 |
| miR-93 | leiomyosarcoma (SKLMS-1), primary cells from leiomyoma tumor | 43 |
| miR-106b | breast (MDA-MB-231), leiomyosarcoma (SKLMS-1), primary cells from leiomyoma tumor | 31, 43 |
| miR-520g | brain cancer (DAOY, UW228) | 25 |
Figure 2.
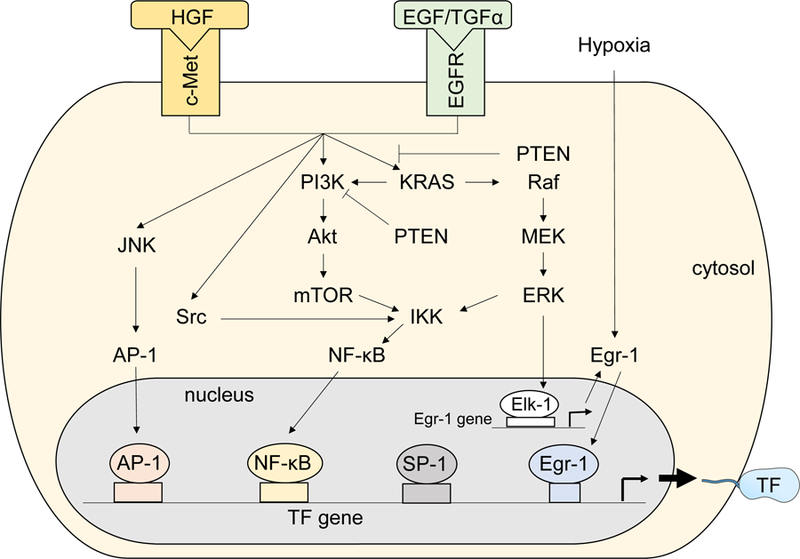
Proposed regulation of tissue factor (TF) expression in cancer cells. Hepatocyte growth factor (HGF)/c-Met and EGFR pathways activate multiple kinase pathways, including c-Jun N-terminal kinase (JNK), Src, phosphatidylinositol-3 kinase (PI3k)/Akt/mammalian target of rapamycin (mTOR), and KRAS/Raf/MEK/ERK. These kinase pathways enhance TF gene expression by expressing transcription factors, such as activator protein-1 (AP-1), nuclear factor-κB (NF-κB), and early growth response protein-1 (Egr-1). TF protein was modified from Servier Medical Art, licensed under Creative Common Attribution 3.0 Unported License. (http://www.servier.fr/servier-medical-art)
Activation of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases, including EGFR and human epidermal growth factor receptor 2 (HER2), induces TF gene expression in glioma cells23,36. The EGFR ligands EGF and TGFα increased TF expression in the human endometrial adenocarcinoma cell line Ishikawa37, and the human squamous cell carcinoma line A43127,36. In addition, expression of a constitutively activated EGFRvIII variant induced TF gene expression in the human glioma cell lines U373 and U87MG23,36. Furthermore, TF expression was reduced by the neutralizing anti-HER2 antibody trastuzumab in the breast cancer cell line SKBR327.
TF expression was increased in human colorectal cancer cell lines by activation of the KRAS oncoprotein and by inactivation of the p53 tumor suppressor32. These changes led to activation of the MEK/mitogen-activated protein kinase and phosphatidylinositol-3-kinase (PI3K) signaling pathways. In addition, a correlation between mutations of KRAS and p53, and TF expression was shown in patients with colorectal and lung cancer33,38.
Rong and colleagues showed that hypoxia coupled with the loss of the tumor suppressor PTEN led to the induction of TF gene expression via the Akt-mTOR and RAS-MEK-ERK pathways in the human glioma cell line 23.1121. Furthermore, increased TF expression was observed in cells that form the hypoxic pseudopalisades surrounding necrotic areas in human glioblastoma specimens21. Another study showed that the transcription factors early growth response gene-1 and hypoxia-inducible factor-1α independently induce TF gene expression under hypoxic conditions in the human breast cancer cell lines MDA-MB-231 and MDA-MB-43528.
Epithelial-mesenchymal transition (EMT) is a process whereby epithelial cells lose cell-cell adhesion and polarization and gain the mesenchymal traits of motility and invasion39. One study found that induction of an EMT-like phenotype in vitro induced TF expression in human squamous cell carcinoma A431 cells40. In addition, the EMT transcription factors Snail and zinc finger E-box-binding homeobox 1 (ZEB1) induced TF gene expression in the breast cancer cell line MDA-MB-46829. These studies indicated that several cancer-dependent phenotypes, such as oncogenic mutations, tumor hypoxia and EMT, induce TF expression in cancer cells.
Alterations in tumor cell metabolism also regulates TF expression. Isocitrate dehydrogenase 1 (IDH1) is a metabolic enzyme and the mutant form of IDH1 is associated with increased levels of 2-hydroxyglutarate41. Accumulation of 2-hydroxyglutarate competitively inhibits demethylation of α-ketoglutarate-dependent enzymes, such as TET2, resulting in hypermethylation of a select group of promoters42. Importantly, there was increased methylation of the TF promoter and decreased TF expression in gliomas expressing mutant IDH1 compared with gliomas expressing wild-type IDH124.
Recent studies have demonstrated that miRNAs regulate TF expression in cancer cells. For instance, transfection of a miR-19a mimic reduced TF expression in the breast cancer cell line MDA-MB-23130 and colorectal cancer cell lines34. Furthermore, Teruel and colleagues showed that miR-19b, miR-20a and miR-106b reduced TF expression by 20–60% in MDA-MB-23131. MiR-93 and miR-106b reduced TF expression in leiomyoma cell line TF324 and leiosarcoma cell line SKLM-S143. However, there was no inverse correlation between TF gene expression and these miRNAs in samples from patients with leiomyoma43. Expression of miR-520g reduced TF expression in the human medulloblastoma tumor cell lines DAOY and UW22825. More importantly, oncogenic amplification of the chromosome 19 miRNA cluster, C19MC, which includes miR-520g, was associated with reduced TF expression in pediatric embryonal brain tumors, providing a link between oncomirs and TF expression25.
TF also regulates miRNAs. D’Asti and colleagues showed that administration an anti-TF monoclonal antibody (clone CNTO2559) to mice led to upregulation of 20 miRs and downregulation of 55 miRs in MDA-MB-231 subcutaneous tumors44. This antibody selectively inhibits signaling but not coagulation. These TF-regulated miRs are associated with the regulation of pathways that are activated in cancer, such as ErbB and PI3K/Akt44.
Glioblastoma multiforme (GBM) can be subdivided into 4 subtypes: proneural, neural, classic and mesenchymal45. One study found different levels of TF expression among subtypes of GBM with the classic subtype and the proneural subtype exhibiting the highest and lowest levels of TF expression, respectively45. More recently, Tawil and colleague found that single cells from proneural and classical subtypes of GBM showed different levels of TF expression46. Importantly, TF expression reversed the dormant phenotype of the non-tumorigenic human GBM cell line U373 by driving permanent changes in the gene expression profile, DNA copy number and DNA methylation state47. These data indicate that TF expression affects tumor characteristics and malignancy in GBM.
Tissue factor and tumor growth
There are several different tumor mouse models that use immunodeficient or immunocompetent mice, different sites of tumor growth (orthotopic or subcutaneous) and different cancer types48. Orthotopic models are superior to subcutaneous models but may require reporters to measure tumor growth in internal organs such as the pancreas. Genetically engineered spontaneous tumor models are more clinically relevant but the appearance of tumors can be variable48. Cancer cell lines are often used but may not fully reproduce the pathophysiology of human tumors. The choice of cell line is also very important. For instance, the human breast cancer cell line MDA-MB-231 is widely used in TF studies. However, it should be noted that this cell line expresses much higher levels of TF than a large number of primary breast tumor samples of varying stages and grades49. We have observed a wide range of TF expression in human pancreatic cancer cell lines50,51. Similarly, pancreatic patient-derived xenografts (PDXs) express different levels of TF (Hisada and Mackman, unpublished data). PDXs are considered a superior model compared to cell line-derived xenografts because tumors of PDX maintain the pathological features52,53, gene expression patterns54 and single nucleotide polymorphisms55 of primary tumors. However, PDXs are more difficult to maintain.
TF has been described as a strong tumor growth enhancer56. Studies have shown that TF expression from plasmid vectors introduced into the murine sarcoma cell line Meth-A and the TF-negative human pancreatic cancer cell line MIA PaCa-2 enhances tumor growth in mice57,58. Conversely, silencing TF expression in Meth-A cells and the human colorectal cancer cell line HCT-119 with siRNA was associated with reduced tumor growth in mice32,57. An ovarian cancer cell line was shown to express FVII59. In addition, coagulation factors, such as FX, can readily enter the tumor from the blood due to the leaky tumor vasculature. This suggests that the TF/FVIIa and TF/FVIIa/FXa complex can be assembled on the surface of tumors cells. Consistent with this notion, subcutaneous growth of murine melanoma B16 tumors was also inhibited by tissue factor pathway inhibitor (TFPI)60. Similarly, the endogenous inhibitor of the TF/FVIIa complex, and a nematode factor X-dependent inhibitor of TF/FVIIa called NAPc2 but not by the FXa inhibitor NAP5, inhibited the growth of B16 tumors60. NAPc2 also inhibited tumor growth of HCT-119 colorectal tumors in nude mice61. An antibody that inhibits TF/FVIIa signaling (Mab-10H10) but not an antibody that inhibits TF-dependent coagulation (Mab-5G9) inhibited tumor growth of the MDA-MB-231 tumors in severe combined immunodeficent (SCID) mice62. In contrast to these studies, the absence of TF did not affect the growth of murine tumor lines in mice that express the strong oncogenic driver Ha-Ras (C12V)63. These data indicate that the TF/FVIIa complex contributes to growth of a variety of tumor types but not all tumor types in preclinical models.
TF appears to enhance tumor growth via several mechanisms, including increasing angiogenesis via expression of VEGF32. TF/FVIIa-dependent activation of PAR2 induced another angiogenic factor interleukin-8 in the MDA-MB-231 in vitro64. In addition, growth of mouse mammary tumors in the virus-polyoma middle T (MMTV-PyMT) model was reduced in PAR2 deficient mice but not in PAR1 deficient mice65. Interestingly, phosphorylation of the TF cytoplasmic domain was shown to contribute to TF/FVIIa-PAR2 signaling and tumor growth in the MMTV-PyMT mouse model66. Further, there was an association between phosphorylation of the TF cytoplasmic domain and PAR2 expression in patients with recurrent breast cancer67. Together, these studies suggest that TF/FVIIa-PAR2 signaling contributes to tumor growth in mouse models of breast cancer.
The MAB-10H10 antibody not only blocked TF/FVIIa-PAR2 signaling in MDA-MB-231 cells but also disrupted the association of TF with β1 integrin62. An integrin binding motif (KGE) in the FVIIa protease domain was identified that is required for binding of the TF/FVIIa complex with the active conformer of integrin β168. A point mutation in this binding motif inhibited TF/FVIIa binding with integrin β1 and integrin signaling but not PAR2 signaling. This study indicates that there is crosstalk between the TF/FVIIa complex and integrin trafficking in cancer cells (Figure 3A).
Figure 3.
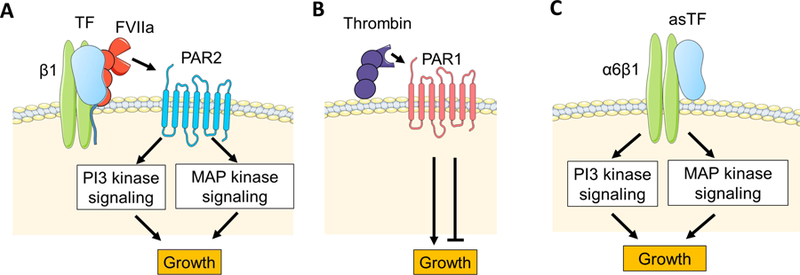
Proposed mechanisms of tumor tissue factor (TF) and thrombin-dependent tumor growth. (A) The full-length TF/factor (F) VIIa complex binds to integrin β1 and the TF/FVIIa/ integrin β1 complex activate protease activated receptor (PAR)2. PAR2 activates phosphatidylinositol- 3-kinase (PI3 kinase) and mitogen activated protease (MAP) kinase signalings resulting in tumor growth. (B) Thrombin activates PAR1 that has both positive and negative role in tumor growth. (C) Alternative spliced (as) TF is released from tumor cells and binds to integrin α6β1. This asTF/integrin α6β1complex contributes to tumor growth via PI3 and MAP kinases. Cells and proteins were modified from Servier Medical Art, licensed under Creative Common Attribution 3.0 Unported License. (http://www.servier.fr/servier-medical-art)
One study investigated the contribution of host TF to tumor growth. Low levels of host TF did not affect subcutaneous growth of tumors of murine lung carcinoma cell line Lewis Lung Carcinoma (LLC) and murine melanoma cell line B16F169. However, low levels of host TF was associated with reduced blood vessel size in the B16F1 tumors69.
The role of tumor and host PAR1 in tumor growth is complex (Figure 3B). Tumor PAR1 has been shown to positively and negatively regulate tumor growth. In vitro growth of a variety of PAR1 expressing human colon cancer cell lines (HT29, CI.19A, LoVo, and HCT116) but not the PAR1-negative cell line LS174T was enhanced by either thrombin or a PAR1 agonist peptide70. In addition, silencing PAR1 expression with shRNA in the human malignant pleural mesothelioma cell line REN reduced growth in nude mice71. Other studies have examined the role of PAR1 in murine pancreatic cancer cell lines, including Panc02 and KPC lines derived from genetically engineered mice. Silencing of either TF or PAR1 in a KPC line derived from LSL-KRASG12D/+, LSL-P53R172H/+, ElasCreER/+ mice significantly reduced growth of subcutaneous and orthotopic tumors (Dr. Matthew Flick, Cincinnati Children’s Hospital, personal communication). Surprisingly, another study found that reducing PAR1 expression in either Panc02 cells or KPC cells derived from LSL-KRASG12D/+, LSL-P53R172H/+, p48Cre mice resulted in increased growth in mice72. At present, it is not clear why these two groups observe opposite results.
Similar to tumor PAR1, host PAR1 has been shown to positively and negatively regulate tumor growth. One study observed reduced growth of murine pancreatic Panc02 tumors in PAR1 deficient mice compared with to the growth of tumors in wild-type mice73. In contrast, Adams and colleagues showed that growth of prostate tumors in the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model and colon tumors in the adenomatous polyposis coli Min (APCMin/+) mouse model was increased in PAR1 deficient mice74. Further studies are required to elucidate the roles of tumor and host PAR1 in tumor growth in different models.
Several studies have shown that asTF enhances tumor growth. One study showed that expression of asTF in human pancreatic cancer cell line MIA PaCa-2 enhanced tumor growth in nude mice75. Another study found that expression of asTF in the TF-negative human breast cancer cell line MCF7 enhanced cell growth in vitro, and this was inhibited by an anti-asTF antibody (clone Rb1)76. AsTF-dependent cell growth was inhibited by silencing β1 integrin gene expression in MCF7 cells76. Moreover, orthotopic growth of MCF7 tumors was increased in cells expressing asTF76. A recent study found an association between asTF-dependent gene expression and estrogen receptor (ER)-dependent gene expression profiles77. Furthermore, growth of MCF7 cells expressing asTF but not the parental MCF7 cells was significantly increased when cells were treated with an ER agonist, estradiol, both in vitro and in vivo (Non-Obese Diabetic (NOD) /SCID)77. In addition, asTF-ER-dependent growth of MCF7 was inhibited by silencing β1 integrin expression in vitro, which suggests that asTF requires β1 integrin to enhance tumor growth77 (Figure 3C).
Similar to the studies with breast cancer, expression of asTF in the human pancreatic ductal adenocarcinoma cell line Pt45P1 enhanced orthotopic growth in nude mice78. Furthermore, growth of wild-type Pt45P1 tumors were inhibited when cancer cells were pre-incubated with an anti-asTF antibody (clone RabMab-1)78. RNA sequence analysis revealed that Pt45P1 expressing asTF showed significantly higher expression of genes associated with MAPK signaling than parental Pt45P1 cells79 In addition, phosphorylation of Akt was observed in Pt45P1 cells expressing asTF79. These studies indicate that asTF contributes to growth of breast and pancreatic tumors in preclinical mouse models.
Preclinical studies have led to clinical trials that inhibit either the TF/FVIIa complex or downstream proteases. One trial investigated the anti-cancer effect of a small molecule FVIIa inhibitor (PCI-27483) combined with gemcitabine in patients with metastatic or locally advanced pancreatic cancer (ClinicalTrials.gov identifier: NCT01020006). In the trial 16 patients received gemcitabine and 18 patients received gemcitabine plus PCI-27483 (1.2 mg/kg BID). Patients receiving PCI-27483 had increased anemia, coagulopathy, gastric bleeding and upper gastrointestinal bleeding compared with the controls, which suggested that inhibition of the TF/FVIIa complex may increase the risk of hemorrhage. Another trial investigated if administration of the FXa inhibitor rivaroxaban before surgery or chemotherapy has an anti-cancer effect in patients with early stage breast cancer (EU Clinical Trials Register Eudract No: 2014-004909-33). At present, no results have been reported.
Tissue factor and metastasis
Metastasis involves cancer cells spreading from a primary site to a secondary site80. Treatment of metastatic tumors is much less successful compared with the treatment of localized tumors because metastasis is often a marker of advanced disease. Different cancers exhibit an organ-specific pattern of metastasis. For instance, breast and prostate cancers often metastasize to bone81. One of the most popular models of metastasis is the experimental metastasis model (also known as the hematogenous metastasis model) where cancer cells are injected via the tail vein of mice and form tumors in the lung. There are also some “spontaneous metastasis” models where primary tumors in different organs spontaneously metastasize to other organs, such as the lung. Detection of metastasis is facilitated by expression of reporters in the cancer cells.
Clinical studies have shown that a high level of TF expression in tumors is associated with metastasis in a variety of cancer types, including colorectal cancer, gastric cancer, and pancreatic cancer7,9,13,82.
One study used different antibodies to demonstrate a role for TF procoagulant activity (extracellular domain) but not TF signaling in metastasis of human melanoma cell lines M24 and C8161 cells into SCID mice83. Expression of TF in the TF-negative human melanoma cell line A7 increased clot formation and tumor cell survival in mice compared to the parental cells84. Another study found that injection of recombinant murine TFPI reduced metastasis of TF-expressing B16F10 cells85. Similarly, expression of TFPI in B16F10 cells reduced metastasis84.
The role of the TF cytoplasmic domain in metastasis is controversial. An early study found that expression of different TF mutants in the low TF expressing human melanoma cell line YU-SIT1 showed a role for the TF cytoplasmic domain but not TF procoagulant activity in metastasis86. An independent study found both TF procoagulant activity and the cytoplasmic domain contributed to metastasis of Chinese hamster ovary cells in SCID mice87. However, two recent studies found no role of the TF cytoplasmic domain in metastasis63,84. One study generated three C57BL/6-derived tumor lines that either lacked TF, expressed wild-type murine TF or expressed a mutant TF lacking the cytoplasmic domain63. This study found that TF supports metastasis via its procoagulant activity and independently of the cytoplasmic domain63. Another study expressed either wild-type TF or a TF mutant lacking the cytoplasmic domain in the TF-negative human melanoma cell line A7 and found increased metastasis with both lines compared with the parental cells84. Finally, expression of TF in TF-negative PyMT breast cancer cells enhanced metastasis88. These data indicate that the procoagulant activity of the TF/FVIIa complex plays a role in metastasis in mice.
TF may enhance metastasis by increasing fibrin deposition or by facilitating generation of coagulation proteases that activate PARs (Figure 4). Many studies have investigated the role of the TF-thrombin-fibrin pathway in experimental metastasis. Palumbo and colleagues showed a significant reduction in experimental metastasis of murine melanoma B16-BL6 and LLC cells in fibrinogen-deficient mice compared with wild-type controls89. In addition, inhibition of thrombin with hirudin significantly reduced metastasis of B16-BL6 in both wild-type C57BL/6 and fibrinogen-deficient mice89. The same group showed that spontaneous metastasis from subcutaneous LLC tumors to the lung and regional lymph nodes was reduced in fibrinogen-deficient mice compared with wild-type controls90.
Figure 4.
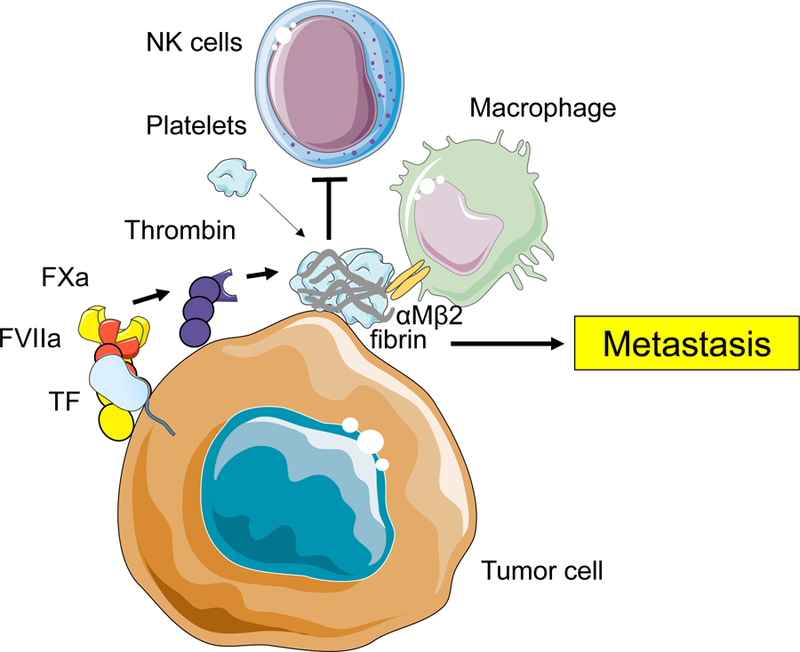
Proposed mechanisms of tumor tissue factor (TF)-dependent metastasis. The full-length TF/factor (F)VIIa/FXa complex generates thrombin that activates platelets and generate fibrin. These activated platelets and fibrin inhibit the function of natural killer (NK) cells and attract monocytes/macrophage that helps establishment of premetastatic niche and tumor cell survival in metastatic niche. Cells and proteins were modified from Servier Medical Art, licensed under Creative Common Attribution 3.0 Unported License. (http://www.servier.fr/servier-medical-art)
One study examined the roles of platelets, PAR4 and fibrin(ogen) in experimental metastasis of B16-F10 cells in both syngeneic C57BL/6 and SCID mice. Metastasis was significantly reduced in mice with low platelets (NF-E2 deficient mice), in mice lacking PAR4, which is the main thrombin receptor on platelets in mice, and in fibrinogen-deficient mice91. Another study observed reduced metastasis of LLC and spontaneous metastasis from subcutaneous LLC tumors to lung in mice lacking Gα, which is required for platelet activation92. Interestingly, immunologic and genetic depletion of natural killer (NK) cells abolished the reduction of metastasis of LLC cells in Gα-deficient and fibrinogen-deficient mice, which suggest that fibrin and platelets impeded NK cell elimination of tumor cells92. A study with TF expressing C57BL/6-derived tumor cells demonstrated that TF contributes to metastasis by restricting NK cell-mediated clearance of micrometastases in a fibrin(ogen)-dependent and platelet-dependent manner63. In addition, TF plays a role in metastasis in a thrombin-dependent mechanism independent of NK cells63. A recent study demonstrated that the TF-thrombin pathway enhanced metastasis by recruiting macrophages84. Furthermore, macrophage function was essential for tumor cell survival independent of NK cells because protection was lost by either ablating CD11b-positive cells or by using Mac1 (αM/β2)-deficient mice (Figure 4).
TF-dependent generation of downstream coagulation proteases, such as thrombin, may enhance metastasis. Shi and colleagues found that thrombin but not a PAR1 agonist peptide increased the migration of M24 cells in vitro93. Interestingly, a combination of PAR1 and PAR2 agonist peptides reproduced the enhancement of migration observed with thrombin. PAR2 agonist peptide alone did not increase cell migration. Furthermore, stimulation of M24 cells with the PAR2 agonists trypsin or PAR2 agonist peptide increased metastasis of these cells in CB-17 SCID/Beige mice93. Another study analyzed the role of PAR1 in the metastasis of the human melanoma cells lines A375SM and C816194. Silencing PAR1 in the tumor cells led to an increase in the expression of the tumor suppressor Maspin and decreased the metastasis94. These data indicate that tumor PAR1 and PAR2 contribute to metastasis.
Host PAR2 was shown to contribute to spontaneous metastasis in the MMTV-PyMT model but not experimental metastasis of murine melanoma cell line B16-F1065,91. Host PAR1 did not contribute to metastasis in either the spontaneous or the experimental metastasis (B16-F10 cells) models65,91. However, a study using hyperthrombotic mice found that deletion of PAR1 in both the tumor and the host but not either alone reduced metastasis of PyMT breast cancer cells88. These studies indicate that the roles of PAR1 and PAR2 in metastasis are complex.
A study by Unruh and colleagues showed that overexpressing of asTF in orthotopic pancreatic Pt45P1 tumors increased metastasis in nude mice compared with parental Pt45P1 tumors79. AsTF expression significantly increased gene expression of components of various oncogenic pathways, including MAPK signaling cascade, Rho signaling, cell migration, and EMT79. In contrast, expression of asTF in TF-negative PyMT breast cancer cells did not affect metastasis88. These data suggest that asTF can activate pathways involved in metastasis.
Targeting tissue factor as a novel anti-cancer therapy
TF expression by tumors and possibly tumor-associated endothelial cells has been used as a target in a novel approach to anti-cancer therapy. The rationale for using TF as a tumor target is that it is expressed at high levels on many tumors but is not expressed within the vasculature. This should allow targeted deliver to the tumor. There are two strategies that have been employed: 1. target TF to induce infarction within the tumor; 2. target TF to deliver cytotoxic drugs to the tumor. However, targeting TF may increase the risk of bleeding because TF is essential for hemostasis. In addition, TF expression in tumors is variable and it is unclear if there is a minimal amount of TF that is required for these approaches to be effective.
One group developed two different bispecific antibodies where one arm of the antibody targets the tumor vasculature and the other binds a truncated form of TF (tTF)95,96. The antibody-tTF conjugate is referred to as a ‘coaguligand’. The tTF form has very low procoagulant activity in the circulation but its activity is increased when it is localized to a phospholipid surface and this allows local thrombosis of tumor vessels and infarction of the tumor95,96. One study showed that one coaguligand induced regression of murine neuroblastoma C1300 subcutaneous tumors in nude mice95. Another study targeting VCAM-1 expression on the tumor vasculature reduced growth of human Hodgkin’s L540 tumors in CB17 SCID mice95,96. Other studies used conjugates consisting of tTF fused to proteins that bind to the tumor vasculature97,98. A prostate specific membrane antigen (PSMA)-directed/tTF fusion protein reduced the size of human prostate LuCap subcutaneous tumors in WEHI nude mice97. In addition, a heparin-binding domain (HBDt)-directed/tTF fusion proteins inhibited growth of breast carcinoma N202 orthotopic tumors in mice98. However, this approach has not been used clinically possibly because of concerns about thrombosis associated with tTF.
Hu and colleagues were the first to develop immunoconjugates that use TF to target the Fc effector domain of IgG to tumors. The Fc domain induces a cytolytic immune response by NK cells and complement activation to kill the tumor99–101. A mutant version of FVII (mFVIIasm) was used that had a point mutation in the active site that dramatically reduced it procoagulant activity but did not affect its high-affinity binding to TF. Intravenous injection of adenoviral vectors expressing a conjugate expressing human Fc inhibited the growth of human melanoma TF2 subcutaneous tumors in SCID mice99. In addition, mFVIIasm-humanFc reduced the growth of human melanoma LXSN and human prostate C4–2 subcutaneous tumors, and experimental metastasis of TF2 subcutaneous tumors to lung in SCID mice100,101. Similarly, mFVIIasm-mouseFc reduced the growth of B16F10 and murine prostate RM-1 subcutaneous tumors and spontaneous metastasis of RM-1 subcutaneous tumors to bone in C57BL/6 mice100,101.
More recently, antibody drug conjugates (ADCs) have been developed that target TF. One group developed an anti-TF antibody-conjugated epirubicin incorporated in micelle (NC6300). This conjugate reduced the growth of TF high human pancreatic BxPc-3 and TF high human gastric 44As3 subcutaneous tumors but not TF low human pancreatic SUIT2 subcutaneous tumors in nude mice102. In the second study, an anti-human TF antibody (clone 1859) that does not inhibit TF activity was used because this would reduce the risk of bleeding. This anti-TF antibody-conjugate reduced the growth of BxPc-3 subcutaneous tumors but not SUIT2 subcutaneous tumors103. Three groups developed different anti-TF antibody conjugates with monomethyl auristatin E (MMAE)104–106. MMAE inhibits cell division of actively dividing cells by blocking the polymerization of tubulin and shows an anti-tumor effect107. Koga and colleagues showed that both anti-human TF antibody-MMAE and anti-mouse TF antibody-MMAE conjugates inhibited the growth of TF high BxPc-3 and TF low human pancreatic Capan1 subcutaneous tumors. ADCs were shown to accumulate into tumors both actively and passively105. In a second study, the same group showed that different anti-human TF antibody-MMAE conjugates inhibited growth of BxPc-3 subcutaneous tumors in nude mice108. A study by Zhang and colleagues showed that their anti-TF antibody-MMAE conjugate reduced growth of both BxPc-3 and human liver HCC1806 subcutaneous tumors in a dose-dependent manner106. Finally, Breij and colleagues showed their anti-human TF antibody-MMAE conjugate that uses a non-inhibitory anti-TF antibody called Tisotumab vedotin inhibited growth of TF high human pancreatic HPAF-II subcutaneous tumors in SCID mice104. Moreover, the authors showed that their anti-human TF antibody-MMAE conjugate significantly reduced growth of several types of subcutaneous PDX tumors in PDX, including pancreatic tumors104.
There are 6 clinical trials that aimed at investigating the effect of Tisotumab vedotin in patients with different types of cancer, including colorectal, pancreatic, and lung cancer (ClinicalTrials.gov identifier: NCT03245736, NCT03485209, NCT03438396, NCT03657043, NCT02552121, NCT02001623). It will be interesting to see if the anti-tumorigenic activity of Tisotumab vedotin observed in mouse studies translates to the clinical setting.
Conclusions
Many basic studies have investigated the regulation of TF in cancer cells, the roles of TF and asTF in tumor growth and metastasis, and the effect of anti-TF antibody/drug conjugate on tumor growth. However, the results are highly dependent on the selection of cancer cells, mice, tumor models and one must be careful with making generalizations. Directly targeting TF with antibodies may cause an increase in bleeding. Thus, ADCs that target TF using a non-inhibitory antibody may be a safer approach to reduce tumor growth without increasing bleeding.
Acknowledgement
The work of the authors has been supported by grants from the NIH (Y.H. T32 HL007149 and N.M. CA190717) and the John C. Parker Professorship (N.M.). We thank Drs. S. P. Grover, J. Rak, Vladimir Bogdanov, and Matthew Flick for helpful comments.
Footnotes
Addendum
Y. Hisada and N. Mackman cowrote the manuscript.
Disclosure of Conflict of Interests.
Nigel Mackman is a consultant for Seattle Genetics. Yohei Hisada has no conflict of interest.
References:
- 1.Grover SP, Mackman N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol 2018 [DOI] [PubMed]
- 2.Bogdanov VY, Balasubramanian V, Hathcock J, et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med 2003;9(4):458–462 [DOI] [PubMed] [Google Scholar]
- 3.Censarek P, Bobbe A, Grandoch M, Schror K, Weber AA. Alternatively spliced human tissue factor (asHTF) is not pro-coagulant. Thromb Haemost 2007;97(1):11–14 [PubMed] [Google Scholar]
- 4.Bogdanov VY, Versteeg HH. “Soluble Tissue Factor” in the 21st Century: Definitions, Biochemistry, and Pathophysiological Role in Thrombus Formation. Semin Thromb Hemost 2015;41(7):700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol 1989;134(5):1087–1097 [PMC free article] [PubMed] [Google Scholar]
- 6.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 2013;122(11):1873–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitori N, Ino Y, Nakanishi Y, et al. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin Cancer Res 2005;11(7):2531–2539 [DOI] [PubMed] [Google Scholar]
- 8.Kaido T, Oe H, Yoshikawa A, et al. Tissue factor is a useful prognostic factor of recurrence in hepatocellular carcinoma in 5-year survivors. Hepatogastroenterology 2005;52(65):1383–1387 [PubMed] [Google Scholar]
- 9.Seto S, Onodera H, Kaido T, et al. Tissue factor expression in human colorectal carcinoma: correlation with hepatic metastasis and impact on prognosis. Cancer 2000;88(2):295–301 [DOI] [PubMed] [Google Scholar]
- 10.Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br J Cancer 2000;83(2):164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akashi T, Furuya Y, Ohta S, Fuse H. Tissue factor expression and prognosis in patients with metastatic prostate cancer. Urology 2003;62(6):1078–1082 [DOI] [PubMed] [Google Scholar]
- 12.Patry G, Hovington H, Larue H, et al. Tissue factor expression correlates with disease-specific survival in patients with node-negative muscle-invasive bladder cancer. Int J Cancer 2008;122(7):1592–1597 [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H, Kitayama J, Ishikawa M, Nagawa H. Tissue factor expression is a clinical indicator of lymphatic metastasis and poor prognosis in gastric cancer with intestinal phenotype. J Surg Oncol 2007;95(4):324–331 [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Luo G, Tan Y, et al. Immunolocalisation of tissue factor in esophageal cancer is correlated with intratumoral angiogenesis and prognosis of the patient. Acta Histochem 2010;112(3):233–239 [DOI] [PubMed] [Google Scholar]
- 15.Poon RT, Lau CP, Ho JW, et al. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res 2003;9(14):5339–5345 [PubMed] [Google Scholar]
- 16.Zelaya H, Rothmeier AS, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost 2018;16(10):1941–1952 [DOI] [PubMed] [Google Scholar]
- 17.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol 2005;25(8):1545–1550 [DOI] [PubMed] [Google Scholar]
- 18.Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med 2015;5(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rak J, Mitsuhashi Y, Bayko L, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995;55(20):4575–4580 [PubMed] [Google Scholar]
- 20.Rak J, Mitsuhashi Y, Sheehan C, et al. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res 2000;60(2):490–498 [PubMed] [Google Scholar]
- 21.Rong Y, Post DE, Pieper RO, et al. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res 2005;65(4):1406–1413 [DOI] [PubMed] [Google Scholar]
- 22.Provencal M, Labbe D, Veitch R, et al. c-Met activation in medulloblastoma induces tissue factor expression and activity: effects on cell migration. Carcinogenesis 2009;30(7):1089–1096 [DOI] [PubMed] [Google Scholar]
- 23.Rong Y, Belozerov VE, Tucker-Burden C, et al. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res 2009;69(6):2540–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol 2016;132(6):917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Asti E, Huang A, Kool M, et al. Tissue Factor Regulation by miR-520g in Primitive Neuronal Brain Tumor Cells: A Possible Link between Oncomirs and the Vascular Tumor Microenvironment. Am J Pathol 2016;186(2):446–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou JN, Ljungdahl S, Shoshan MC, Swedenborg J, Linder S. Activation of tissue-factor gene expression in breast carcinoma cells by stimulation of the RAF-ERK signaling pathway. Mol Carcinog 1998;21(4):234–243 [PubMed] [Google Scholar]
- 27.Yu JL, Xing R, Milsom C, Rak J. Modulation of the oncogene-dependent tissue factor expression by kinase suppressor of ras 1. Thromb Res 2010;126(1):e6–10 [DOI] [PubMed] [Google Scholar]
- 28.Sun L, Liu Y, Lin S, et al. Early growth response gene-1 and hypoxia-inducible factor-1alpha affect tumor metastasis via regulation of tissue factor. Acta Oncol 2013;52(4):842–851 [DOI] [PubMed] [Google Scholar]
- 29.Bourcy M, Suarez-Carmona M, Lambert J, et al. Tissue Factor Induced by Epithelial-Mesenchymal Transition Triggers a Procoagulant State That Drives Metastasis of Circulating Tumor Cells. Cancer Res 2016;76(14):4270–4282 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Yu H, Lou JR, et al. MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells. J Biol Chem 2011;286(2):1429–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teruel R, Perez-Sanchez C, Corral J, et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J Thromb Haemost 2011;9(10):1985–1992 [DOI] [PubMed] [Google Scholar]
- 32.Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 2005;105(4):1734–1741 [DOI] [PubMed] [Google Scholar]
- 33.Rao B, Gao Y, Huang J, et al. Mutations of p53 and K-ras correlate TF expression in human colorectal carcinomas: TF downregulation as a marker of poor prognosis. Int J Colorectal Dis 2011;26(5):593–601 [DOI] [PubMed] [Google Scholar]
- 34.Yu G, Li H, Wang X, et al. MicroRNA-19a targets tissue factor to inhibit colon cancer cells migration and invasion. Mol Cell Biochem 2013;380(1–2):239–247 [DOI] [PubMed] [Google Scholar]
- 35.Lewis CS, Elnakat Thomas H, Orr-Asman M, et al. mTOR kinase inhibition reduces tissue factor expression and growth of pancreatic neuroendocrine tumors. J Thromb Haemost 2018 [DOI] [PMC free article] [PubMed]
- 36.Milsom CC, Yu JL, Mackman N, et al. Tissue factor regulation by epidermal growth factor receptor and epithelial-to-mesenchymal transitions: effect on tumor initiation and angiogenesis. Cancer Res 2008;68(24):10068–10076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato S, Pinto M, Carvajal A, et al. Tissue factor is regulated by epidermal growth factor in normal and malignant human endometrial epithelial cells. Thromb Haemost 2005;94(2):444–453 [DOI] [PubMed] [Google Scholar]
- 38.Regina S, Rollin J, Blechet C, et al. Tissue factor expression in non-small cell lung cancer: relationship with vascular endothelial growth factor expression, microvascular density, and K-ras mutation. J Thorac Oncol 2008;3(7):689–697 [DOI] [PubMed] [Google Scholar]
- 39.Jolly MK, Ware KE, Gilja S, Somarelli JA, Levine H. EMT and MET: necessary or permissive for metastasis? Mol Oncol 2017;11(7):755–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garnier D, Magnus N, Lee TH, et al. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem 2012;287(52):43565–43572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462(7274):739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012;483(7390):479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuang TD, Luo X, Panda H, Chegini N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol 2012;26(6):1028–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Asti E, Anderson GM, Rak J. Inhibition of tissue factor signaling in breast tumour xenografts induces widespread changes in the microRNA expression profile. Biochem Biophys Res Commun 2017;494(3–4):700–705 [DOI] [PubMed] [Google Scholar]
- 45.Magnus N, Gerges N, Jabado N, Rak J. Coagulation-related gene expression profile in glioblastoma is defined by molecular disease subtype. J Thromb Haemost 2013;11(6):1197–1200 [DOI] [PubMed] [Google Scholar]
- 46.Tawil N, Chennakrishnaiah S, Bassawon R, et al. Single cell coagulomes as constituents of the oncogene-driven coagulant phenotype in brain tumours. Thromb Res 2018;164 Suppl 1:S136–S142 [DOI] [PubMed] [Google Scholar]
- 47.Magnus N, Garnier D, Meehan B, et al. Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations. Proc Natl Acad Sci U S A 2014;111(9):3544–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hisada Y, Mackman N. Mouse models of cancer-associated thrombosis. Thromb Res 2018;164 Suppl 1:S48–S53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEachron T, Mackman N. Tumors, ticks and tissue factor. J Thromb Haemost 2009;7(11):1852–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JG, Geddings JE, Aleman MM, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 2012;119(23):5543–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geddings JE, Hisada Y, Boulaftali Y, et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J Thromb Haemost 2016;14(1):153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loukopoulos P, Kanetaka K, Takamura M, et al. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas 2004;29(3):193–203 [DOI] [PubMed] [Google Scholar]
- 53.DeRose YS, Wang G, Lin YC, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 2011;17(11):1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao X, Liu Z, Yu L, et al. Global gene expression profiling confirms the molecular fidelity of primary tumor-based orthotopic xenograft mouse models of medulloblastoma. Neuro Oncol 2012;14(5):574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEvoy J, Ulyanov A, Brennan R, et al. Analysis of MDM2 and MDM4 single nucleotide polymorphisms, mRNA splicing and protein expression in retinoblastoma. PLoS One 2012;7(8):e42739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruf W Tissue factor and cancer. Thromb Res 2012;130 Suppl 1:S84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Deng Y, Luther T, et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest 1994;94(3):1320–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kakkar AK, Chinswangwatanakul V, Lemoine NR, Tebbutt S, Williamson RC. Role of tissue factor expression on tumour cell invasion and growth of experimental pancreatic adenocarcinoma. Br J Surg 1999;86(7):890–894 [DOI] [PubMed] [Google Scholar]
- 59.Koizume S, Jin MS, Miyagi E, et al. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res 2006;66(19):9453–9460 [DOI] [PubMed] [Google Scholar]
- 60.Hembrough TA, Swartz GM, Papathanassiu A, et al. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res 2003;63(11):2997–3000 [PubMed] [Google Scholar]
- 61.Zhao J, Aguilar G, Palencia S, Newton E, Abo A. rNAPc2 inhibits colorectal cancer in mice through tissue factor. Clin Cancer Res 2009;15(1):208–216 [DOI] [PubMed] [Google Scholar]
- 62.Versteeg HH, Schaffner F, Kerver M, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood 2008;111(1):190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood 2007;110(1):133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hjortoe GM, Petersen LC, Albrektsen T, et al. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood 2004;103(8):3029–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Versteeg HH, Schaffner F, Kerver M, et al. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res 2008;68(17):7219–7227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaffner F, Versteeg HH, Schillert A, et al. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood 2010;116(26):6106–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryden L, Grabau D, Schaffner F, et al. Evidence for tissue factor phosphorylation and its correlation with protease-activated receptor expression and the prognosis of primary breast cancer. Int J Cancer 2010;126(10):2330–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothmeier AS, Liu E, Chakrabarty S, et al. Identification of the integrin-binding site on coagulation factor VIIa required for proangiogenic PAR2 signaling. Blood 2018;131(6):674–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu J, May L, Milsom C, et al. Contribution of host-derived tissue factor to tumor neovascularization. Arterioscler Thromb Vasc Biol 2008;28(11):1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darmoul D, Gratio V, Devaud H, Lehy T, Laburthe M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol 2003;162(5):1503–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keshava S, Sahoo S, Tucker TA, et al. Endothelial cell protein C receptor opposes mesothelioma growth driven by tissue factor. Cancer Res 2013;73(13):3963–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tekin C, Shi K, Daalhuisen JB, et al. PAR1 signaling on tumor cells limits tumor growth by maintaining a mesenchymal phenotype in pancreatic cancer. Oncotarget 2018;9(62):32010–32023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Queiroz KC, Shi K, Duitman J, et al. Protease-activated receptor-1 drives pancreatic cancer progression and chemoresistance. Int J Cancer 2014;135(10):2294–2304 [DOI] [PubMed] [Google Scholar]
- 74.Adams GN, Sharma BK, Rosenfeldt L, et al. Protease-activated receptor-1 impedes prostate and intestinal tumor progression in mice. J Thromb Haemost 2018 [DOI] [PMC free article] [PubMed]
- 75.Hobbs JE, Zakarija A, Cundiff DL, et al. Alternatively spliced human tissue factor promotes tumor growth and angiogenesis in a pancreatic cancer tumor model. Thromb Res 2007;120 Suppl 2:S13–21 [DOI] [PubMed] [Google Scholar]
- 76.Kocaturk B, Van den Berg YW, Tieken C, et al. Alternatively spliced tissue factor promotes breast cancer growth in a beta1 integrin-dependent manner. Proc Natl Acad Sci U S A 2013;110(28):11517–11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kocaturk B, Tieken C, Vreeken D, et al. Alternatively spliced tissue factor synergizes with the estrogen receptor pathway in promoting breast cancer progression. J Thromb Haemost 2015;13(9):1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Unruh D, Unlu B, Lewis CS, et al. Antibody-based targeting of alternatively spliced tissue factor: a new approach to impede the primary growth and spread of pancreatic ductal adenocarcinoma. Oncotarget 2016;7(18):25264–25275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Unruh D, Turner K, Srinivasan R, et al. Alternatively spliced tissue factor contributes to tumor spread and activation of coagulation in pancreatic ductal adenocarcinoma. Int J Cancer 2014;134(1):9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog 2013;18(1–2):43–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2(8):563–572 [DOI] [PubMed] [Google Scholar]
- 82.Shigemori C, Wada H, Matsumoto K, et al. Tissue factor expression and metastatic potential of colorectal cancer. Thromb Haemost 1998;80(6):894–898 [PubMed] [Google Scholar]
- 83.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A 1992;89(24):11832–11836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gil-Bernabe AM, Ferjancic S, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012;119(13):3164–3175 [DOI] [PubMed] [Google Scholar]
- 85.Amirkhosravi A, Meyer T, Chang JY, et al. Tissue factor pathway inhibitor reduces experimental lung metastasis of B16 melanoma. Thromb Haemost 2002;87(6):930–936 [PubMed] [Google Scholar]
- 86.Bromberg ME, Konigsberg WH, Madison JF, Pawashe A, Garen A. Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation. Proc Natl Acad Sci U S A 1995;92(18):8205–8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mueller BM, Ruf W. Requirement for binding of catalytically active factor VIIa in tissue factor-dependent experimental metastasis. J Clin Invest 1998;101(7):1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yokota N, Zarpellon A, Chakrabarty S, et al. Contributions of thrombin targets to tissue factor-dependent metastasis in hyperthrombotic mice. J Thromb Haemost 2014;12(1):71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000;96(10):3302–3309 [PubMed] [Google Scholar]
- 90.Palumbo JS, Potter JM, Kaplan LS, et al. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res 2002;62(23):6966–6972 [PubMed] [Google Scholar]
- 91.Camerer E, Qazi AA, Duong DN, et al. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 2004;104(2):397–401 [DOI] [PubMed] [Google Scholar]
- 92.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005;105(1):178–185 [DOI] [PubMed] [Google Scholar]
- 93.Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res 2004;2(7):395–402 [PubMed] [Google Scholar]
- 94.Villares GJ, Zigler M, Dobroff AS, et al. Protease activated receptor-1 inhibits the Maspin tumor-suppressor gene to determine the melanoma metastatic phenotype. Proc Natl Acad Sci U S A 2011;108(2):626–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang X, Molema G, King S, et al. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997;275(5299):547–550 [DOI] [PubMed] [Google Scholar]
- 96.Ran S, Gao B, Duffy S, et al. Infarction of solid Hodgkin’s tumors in mice by antibody-directed targeting of tissue factor to tumor vasculature. Cancer Res 1998;58(20):4646–4653 [PubMed] [Google Scholar]
- 97.Liu C, Huang H, Donate F, et al. Prostate-specific membrane antigen directed selective thrombotic infarction of tumors. Cancer Res 2002;62(19):5470–5475 [PubMed] [Google Scholar]
- 98.El-Sheikh A, Borgstrom P, Bhattacharjee G, Belting M, Edgington TS. A selective tumor microvasculature thrombogen that targets a novel receptor complex in the tumor angiogenic microenvironment. Cancer Res 2005;65(23):11109–11117 [DOI] [PubMed] [Google Scholar]
- 99.Hu Z, Sun Y, Garen A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc Natl Acad Sci U S A 1999;96(14):8161–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu Z, Garen A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc Natl Acad Sci U S A 2000;97(16):9221–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu Z, Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc Natl Acad Sci U S A 2001;98(21):12180–12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamamoto Y, Hyodo I, Koga Y, et al. Enhanced antitumor effect of anti-tissue factor antibody-conjugated epirubicin-incorporating micelles in xenograft models. Cancer Sci 2015;106(5):627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sugaya A, Hyodo I, Koga Y, et al. Utility of epirubicin-incorporating micelles tagged with anti-tissue factor antibody clone with no anticoagulant effect. Cancer Sci 2016;107(3):335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Breij EC, de Goeij BE, Verploegen S, et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res 2014;74(4):1214–1226 [DOI] [PubMed] [Google Scholar]
- 105.Koga Y, Manabe S, Aihara Y, et al. Antitumor effect of antitissue factor antibody-MMAE conjugate in human pancreatic tumor xenografts. Int J Cancer 2015;137(6):1457–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang X, Li Q, Zhao H, et al. Pathological expression of tissue factor confers promising antitumor response to a novel therapeutic antibody SC1 in triple negative breast cancer and pancreatic adenocarcinoma. Oncotarget 2017;8(35):59086–59102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 2003;102(4):1458–1465 [DOI] [PubMed] [Google Scholar]
- 108.Tsumura R, Manabe S, Takashima H, et al. Influence of the dissociation rate constant on the intra-tumor distribution of antibody-drug conjugate against tissue factor. J Control Release 2018;284:49–56 [DOI] [PubMed] [Google Scholar]


