Abstract
Background.
Excess added sugars, particularly from sugar-sweetened beverages, are a major risk factor for cardiometabolic diseases including cardiovascular disease (CVD) and type 2 diabetes. In 2016, FDA mandated the labeling of added sugar content on all packaged foods and beverages. Yet, potential health impacts and cost-effectiveness of this policy remain unclear.
Methods.
A validated microsimulation model (IMPACT) was used to estimate CVD and type 2 diabetes cases averted, quality-adjusted life-years (QALYs), policy costs, healthcare, informal care and lost productivity {health-related) savings and cost-effectiveness of two policy scenarios: (1) implementation of the FDA added sugar labeling policy (sugar label), and (2) further accounting for corresponding industry reformulation (sugar label+reformulation). The model utilized nationally representative demographic and disease data from CDC Wonder Database, dietary intake from NHANES, policy effects and diet-disease effects from meta-analyses, policy and health-related costs from established sources. Probabilistic sensitivity analysis accounted for model parameter uncertainties and population heterogeneity.
Results.
Between 2018 and 2037, the sugar label would prevent 354,400 CVD (95% UIs: 167,000-673,500) and 599,300 (302,400–957,400) diabetes cases, gain 727,000 (401,300-1,138,000) QALYs, and save $31B (15.7-54.5) in net healthcare costs or $61.9B (33.1-103.3) societal costs (incorporating reduced lost productivity and informal care costs). For the sugar label+reformulation scenario, corresponding gains were 708,800 (369,200-1,252,000) CVD cases, 1.2M (0.7-1.7) diabetes cases, 1.3M (0.8-1.9) QALYs, and $57.6B (31.9-92.4) and $113.2B (67.3-175.2), respectively. Both scenarios were estimated with >80% probability to be cost-saving by 2023.
Conclusions:
Implementing the FDA added sugar labeling policy could generate substantial health gains and cost-savings for the U.S. population.
Keywords: Nutrition, diet, policy, cardiovascular disease, diabetes, prevention, cost-effectiveness
INTRODUCTION
In May 2016, in the first major revision to the Nutrition Facts label since 1993, the U.S. Food and Drug Administration (FDA) announced mandatory labeling of added sugar content as a strategy to reduce intake of added sugars from packaged foods and beverages.1 Overconsumption of added sugars, particularly from sugar-sweetened beverages (SSBs), is a risk factor for cardiometabolic diseases including obesity,2, 3 type 2 diabetes,4, 5 and cardiovascular disease (CVD).4, 6 These conditions pose substantial economic burdens, with total U.S. direct and indirect costs of obesity-related diseases exceeding $1.4 trillion/year and expected to escalate.7 Despite recent declines in added sugar intake in the U.S., largely due to reduced SSB consumption,8 current added sugar intake from SSBs and foods remains high: Americans still consume >300 kcal/day (>15% of total energy (%E)),8 exceeding U.S. guidelines of <10%E.9 The single largest source in the U.S. is SSBs, followed by grain desserts (e.g., cookies, cakes, pastries), fruit drinks, candy, and dairy desserts (e.g., ice cream).10 Considering that 52,000 annual U.S. cardiometabolic deaths are attributed to SSB consumption alone,4 cost-effective approaches to reduce added sugar consumption are a public health priority.
Food labeling supports informed consumer choice, and can effectively change consumer behavior and stimulate industry reformulation,11 for example as supported by recent experience with trans-fat labeling.12 Yet, the health and economic impacts of FDA’s added sugar labeling policy have not been estimated. Even though some companies have already opted to implement the new label on their products,13 FDA recently announced another delay in mandatory implementation of the updated Nutrition Facts label until 2020.14
To elucidate the effects of implementing the added sugar label in the U.S., a validated microsimulation model was used to estimate the potential cardiometabolic impact, costs, and cost-effectiveness of FDA’s added sugar labeling policy based on (a) expected changes in consumer behavior and (b) potential additional impact of corresponding industry reformulation. This investigation was performed as part of the Food-PRICE (Policy Review and Intervention Cost-Effectiveness) Project (www.food-price.org).
METHODS
Study Overview
We extended the previously validated U.S. IMPACT Food Policy model15, 16 to estimate the cost-effectiveness of FDA’s added sugar labeling mandate over a 20-year period (2018-2037), from both healthcare and societal perspectives. The model utilized nationally representative data on population demographics, risk factors, dietary habits and disease to assess cumulative cardiometabolic health outcomes and costs based on current trends. At each stage of the logic pathway, the best available sources (Supplemental Table 1), supplemented with reasoned assumptions (Supplemental Table 2), were used to estimate the potential health and economic consequences of the federal added sugar labeling policy. Data from the National Health and Nutrition Examination Survey (NHANES) were used to generate the simulated population and are publicly available.17 The analytic methods, including elements of the model inputs, structure, and outputs are each described in further detail below and in Supplemental Text 1, which includes Supplemental Figures 1-13. The source code of the model is not publicly available. This modeling investigation was exempt from IRB review as it was based on publicly data and nationally representative, de-identified datasets which included no personally identifiable information.
FDA Added Sugar Labeling Policy Scenarios
We modeled two scenarios including (a) implementation of the FDA added sugar label on all packaged foods and beverages with no change in industry formulations (sugar label), and (b) implementation of the added sugar label plus further accounting for corresponding industry reformulation (sugar label+reformulation). We compared these two scenarios with a counterfactual ‘usual care’ base-case scenario. In all scenarios, we assumed that the recent observed declining trends in added sugar consumption, particularly from SSBs, would continue in the future, providing a conservative estimate of the additional impact of sugar labeling.
Simulated U.S. Population
To generate a U.S. representative synthetic population of adults aged 30-84 years at baseline, the model utilized demographic information, body mass index (BMI) data and added sugar intakes from the two most recent NHANES cycles (2011-2014).17 Demographic information included age, sex, race/ethnicity (race), income and education, and dietary intake included added sugar from SSBs and other foods, estimated based on two 24-hour dietary recalls per person as previously described (further details on dietary intakes are available in Text S1).4, 18 Population size by age, sex, and race and future population projections was derived from the CDC Wonder Database (2014). To first create a static synthetic population, the model drew the traits of the synthetic individuals from conditional distributions that were estimated from multinomial models fitted in the NHANES data. The statistical framework of this method and its extension to epidemiological modeling have been described.19, 20 The model further projected recent trends in BMI and added sugar intake (from SSBs and other foods), as observed from NHANES 2003–2014, to evolve the traits of the model population individuals over time to create a dynamic synthetic population. For these projections we fitted generalized linear models to NHANES data utilizing inverse-probability weighting and design-based standard errors to account for the complex survey design and methods. A detailed description of the method and validation can be found in the Supplement (Text S1).
Policy Effects on Added Sugar Intake
A recent systematic review and meta-analysis of labeling interventions was identified as the best available evidence to estimate the effects of FDA’s labeling policy on added sugar intake.11 Because no interventional studies on added sugar labels were identified, we extended the modest effect of labeling interventions on reducing calorie intake by 6.8% (95% CI: 4.5%-9.0%), with no heterogeneity identified by labeling type (e.g., package labeling, menu labeling, other point-of-purchase labeling) or population characteristics (e.g., age, sex, race and socioeconomic status).11 This proportional effect on calories and its uncertainty were extended to the plausible percentage change in added sugar consumption, as this provided a more conservative effect size than the larger pooled effects of labeling on other additives such as sodium and trans-fat. Consistent with the time period of interventional studies, we assumed the time lag between policy implementation and change in added sugar intake was less than a year, with the intervention effect sustained throughout the 20-year simulated period.
The potential for industry reformulation to reduce added sugars in foods, and subsequently population intake, were based on FDA’s regulatory impact analysis.21 We assumed no reformulation in the first year of labeling implementation, with 7.5% to 9% of sugar-containing products being reformulated to achieve 25% reduction in added sugar content in these products each of years 2 to 5 of the intervention, and no additional reformulation thereafter. In sum, this represents an 8.25% net reduction in added sugar contents of U.S. products over the 20-year intervention period (see Text S1 for details on assumptions and calculations).
For both consumer effects and industry reformulations, we evaluated only the subset of added sugar in NHANES from packaged products that would carry a Nutrition Facts label, i.e. sugars from supermarkets, convenience stores, vending machines. We excluded added sugar consumed from other sources, e.g., in restaurants and as sugar added by consumers.
Effects of Added Sugar Changes on Cardiometabolic Risk
Our detailed methods for reviewing and synthesizing evidence to estimate effect sizes for associations between dietary factors and cardiometabolic endpoints have been reported.4, 22 Considering harms linked to SSB consumption partly due to their liquid form, large dose, and rapid digestion in comparison to solid foods,4, 22 we separately evaluated evidence from meta-analyses of long-term prospective cohorts or randomized clinical trials for associations of added sugars in SSBs vs. other foods with cardiometabolic endpoints, including coronary heart disease (CHD), stroke and diabetes (Figure 1).4 The final model incorporated (a) associations of added sugars from SSBs and from other foods with BMI, (b) subsequent BMI-mediated effects on CHD, stroke and diabetes, and (c) separate BMI-independent associations of added sugars from SSBs (but not from other foods) with CHD and diabetes (Supplemental Table 3). These observed etiologic effects on how changes in added sugars influence BMI (and subsequent disease risk) also inherently account for the average dietary substitutes and complements in the population, and are more conservative than simply translating an observed caloric decrease to a reduction in BMI, as further described in Text S1.
Figure 1. Etiologic pathway through which the U.S. FDA added sugar labeling policy is translated into changes in disease burden.
Considering harms linked to SSB consumption partly due to their liquid form, large dose, and rapid digestion in comparison to solid foods, etiologic effects on cardiometabolic outcomes were evaluated separately for added sugars in SSBs vs. other foods.6
We have published detailed validity analyses comparing the estimated etiologic effects of individual dietary factors on disease risk to findings from prospective studies of diet patterns and randomized control trials of diet patterns.4, 22 These analyses demonstrated that estimated etiologic effects for individual dietary components were very similar to what would be expected based on these other lines of evidence.4, 22 We assumed a median 1-year time lag from change in sugar intake to BMI and a median 1-year time lag from change in BMI to change in disease risk.
The U.S. IMPACT Food Policy Model Structure and Outputs
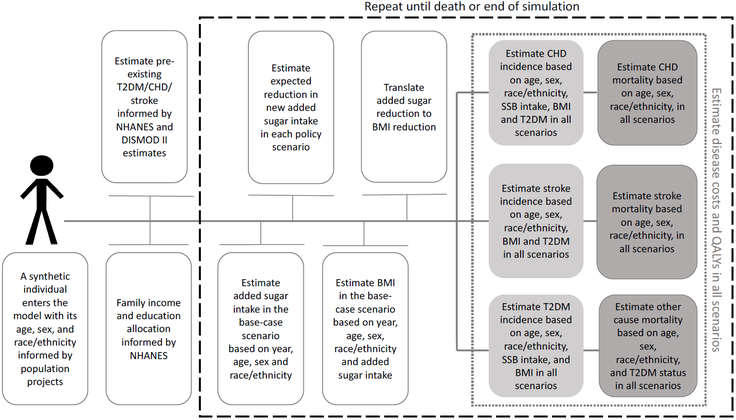
The extended U.S. IMPACT Food Policy model is a stochastic dynamic microsimulation model that simulates the life course of synthetic individuals under different policy scenarios.23 Compared to previous versions of the model,15 it allows for more detailed and flexible simulation of food policies in a competing risk framework, taking into account individual heterogeneity and lag times between exposures and outcomes. The model first simulated the life courses of synthetic individuals aged 30-84 under the base-case scenario and estimated their added sugar intake, BMI, incidence of type 2 diabetes, first episode of CHD and/or stroke, quality-adjusted life-years (QALYs), costs, and death from these diseases or any other cause on an annual basis. Then, it calculated the life courses of the same synthetic individuals under both labeling scenarios and generated annual estimated changes in each health outcome at the individual level (Figure 2).
Figure 2. Simplified IMPACT model structure for estimating the cost-effectiveness of the U.S. FDA added sugar labeling policy.
To generate a U.S. representative synthetic population of adults aged 30-84 years at baseline, the model utilized demographic information, body mass index (BMI) data, and added sugar intakes from the two most recent NHANES cycles (2011-2014). Demographic information included age, sex, race, income and education, and dietary intake included added sugar from SSBs and other foods, estimated based on two 24-hour dietary recalls per person. Population size by age, sex, and race and future population projections was derived from the CDC Wonder Database (2014). The model simulates first the life courses of synthetic individuals under the base-case scenario and estimates their added sugar consumption, body mass index (BMI), incidence of type 2 diabetes (T2DM), the first episode of coronary heart disease (CHD) and/or stroke, quality-adjusted life-years (QALYs), costs, and death from these diseases or any other cause on an annual basis. Then, it calculates the life courses of the same synthetic individuals under both labeling scenarios and generates annual estimated changes in each health outcome at the individual level.
CVD and type 2 diabetes outcomes were modeled as previously reported23 and are fully described in Text S1. Briefly, using a population attributable risk approach, the model estimated the annual risks of the synthetic individuals aged 30-84 years to develop CHD and stroke based on their BMI, type 2 diabetes status, incidence rate forecasts and etiologic effects of added sugar intake (Figure 2). Further, the model calibrated the annual case fatality for CHD, stroke and any other cause to the forecasted mortality rates, in a competing risk framework. Specifically, for ‘any other cause’ mortality we assumed that synthetic individuals with type 2 diabetes have higher mortality rates, to account for diseases other than CHD and stroke that were not explicitly modeled and are causally related to type 2 diabetes.24
Model outputs included the total numbers of relevant outcomes for the simulated population over 20-year open cohort analytic period and estimated cases and deaths prevented or postponed (CHD, stroke (CVD), type 2 diabetes, or other), QALYs, life-years gained (LYG) and disaggregated costs. We calculated the health state utility values (preference weights) using published equations, which used EQ-5D-3L data from the Medical Expenditure Panel Survey (MEPS) 2000-2002.25 Outputs were further stratified by age (30-49, 50-69, 70-84), sex (males, females), and race (non-Hispanic white, non-Hispanic black, Hispanic and other).
Policy Costs
Policy costs included government administration and industry compliance/reformulation costs (Text S1). Government costs to administer, enforce and evaluate the policy were estimated using FDA’s budget reports.26 Industry costs to redesign and reprint the labels to comply with the labeling requirement and (for the sugar label+reformulation scenario) reformulation costs, including uncertainty, were derived from FDA’s regulatory impact analysis.21 FDA estimated industry reformulation costs using a reformulation cost model developed by the Research Triangle Institute (RTI). This reformulation model accounted for variations in product formula complexity, company size, reformulation type, compliance period and other factors, thereby producing a more accurate cost estimate compared to a standard per-product cost approach.
Health-Related Costs
The model evaluated formal healthcare (medical), informal care and productivity costs for CVD (CHD, and stroke) and type 2 diabetes, referred to collectively as health-related costs (Text S1). CVD medical and productivity costs per person per year were derived from an RTI report,27 and CVD informal care (i.e., unpaid caregiving) costs were estimated using published data.28, 29 CVD productivity costs, which included workplace, home and leisure time productivity losses, were divided into morbidity (living with diseases) and mortality costs (premature deaths). Medical and productivity costs for diagnosed and undiagnosed type 2 diabetes, and informal care costs for diagnosed type 2 diabetes, were derived from published sources.30-32 All cost inputs were stratified by age and sex, except informal care costs. CVD costs were additionally stratified by race (Supplemental Table 4).
Statistical Analyses
Cost-effectiveness Analyses
In accordance with recommendations from the U.S. Second Panel on Cost-Effectiveness in Health and Medicine33 we conducted analyses from two perspectives. This included a (1) healthcare perspective incorporating policy costs and medical costs; and (2) societal perspective further incorporating informal care costs and productivity costs. All costs were inflated to 2017 USD using Consumer Price Index, and all costs and QALYs were discounted at 3% annually. Net costs were calculated as policy costs minus health-related costs from cardiometabolic diseases. Incremental cost-effectiveness ratios (ICERs) were calculated as the net change in costs divided by the net change in QALYs. Net monetary benefit (NMB) was calculated by summing net costs (or savings, if negative), and further adding a monetary value of QALYs gained based on a willingness-to-pay threshold per QALY. For this calculation, we assumed a willingness-to-pay threshold of $100,000 per QALY and allowed it to vary in sensitivity analysis, consistent with AHA/ACC and Second U.S. Panel recommendations.33, 34
Sensitivity and Uncertainty Analyses
We used probabilistic sensitivity analysis via second-order Monte Carlo approach that allowed the individual heterogeneity and estimated uncertainty of model parameters to be propagated to the outputs.35 The sources of uncertainty considered included the NHANES sampling design (sampling error of baseline added sugar intake, BMI, and prevalent type 2 diabetes), the sampling error of the relative risks; the time lag between dietary intake change and change in disease risks; the uncertainty of mortality forecasts; the uncertainty around the true incidence of CHD, and stroke; the uncertainty of policy effects; and the uncertainty of costs (Text S1). We summarized the output distributions by reporting the medians and 95% uncertainty intervals (UI). We also plotted the annual probability that a scenario is cost-saving over the simulation period. Discount rate and willingness-to-pay values were included in one-way sensitivity analyses and allowed to vary in steps between 0-9% and $50,000-$150,000 respectively. To identify the threshold % change in added sugar intake that would render the policy cost-effective and cost-saving, we performed additional one-way sensitivity analyses on the overall policy effect size of the sugar label+reformulation scenario, and allowed it to vary in steps between 0.5-6%.
RESULTS
Population Characteristics and Added Sugar Intake
Among packaged products, baseline mean added sugar intake was 60.6 g/day (59.9-61.2) and corresponding median intake was 37.3 g/day (36.7-37.8) (Supplemental Figure 3). Over 20 years without any new intervention, median added sugar intake was projected to decrease by 5.8 g/day (5.3-6.2) (Supplemental Figure 3). Conservatively accounting for those declining underlying trends, the sugar label scenario would reduce median added sugar intake by an additional 2.1 g/day (1.4-2.9), while the sugar label+reformulation scenario by an additional 4.8 g/day (3.9-5.6).
Health Outcomes
Over 20 years, the sugar label scenario was estimated to prevent or postpone 354,400 new CVD cases (167,000-673,500), including 27,830 CVD deaths (9,277-51,950); and 599,300 new type 2 diabetes cases (302,400-957,400) and 16,700 type 2 diabetes-related deaths (3,711-37,110); overall gaining 727,000 discounted QALYs (401,300-1,138,000) (Table 1, Supplemental Figure 4). Adding industry reformulation, health gains would be twice as large, preventing 708,800 CVD cases (369,200-1,252,000), 50,100 CVD deaths (24,030-87,300), 1,184,000 type 2 diabetes cases (666,000-1,703,000), and 31,540 type 2 diabetes-related deaths (9,277-61,230); overall gaining 1.3 million QALYs (0.8-1.9).
Table 1.
Estimated health gains, costs, and cost-effectiveness of the U.S. FDA added sugar labeling policy over 20 years from 2018 -2037.*
| Sugar label (95% UIs) | Label + reformulation (95% UIs) | |
|---|---|---|
| Population in 2037 (million) | 219.41 (216.43 to 221.97) | 219.44 (216.46 to 222.00) |
| Health outcomes | ||
| BMI in 2037 (kg/m2) | 28.58 (28.51 to 28.66) | 28.53 (28.45 to 28.60) |
| CVD cases prevented/postponed | 354,400 (167,000 to 673,500) | 708,800 (369,200 to 1,252,000) |
| CHD cases prevented/postponed | 330,300 (150,300 to 647,600) | 666,100 (324,600 to 119,800) |
| Stoke cases prevented/postponed | 24,120 (5,566 to 57,520) | 46,390 (14,840 to 102,100) |
| CVD deaths prevented/postponed | 27,830 (9,277 to 51,950) | 50,100 (24,030 to 87,300) |
| CHD deaths prevented/postponed | 25,980 (9,277 to 50,100) | 48,240 (22,270 to 85,350) |
| Stroke deaths prevented/postponed | 1,855 (−1,855† to 5,566) | 1,855 (−1,855† to 7,422) |
| T2DM cases prevented/postponed | 599,300 (302,400 to 957,400) | 1,184,000 (666,000 to 1,703,000) |
| T2DM-related deaths prevented/postponed | 16,700 (3,711 to 37,110) | 31,540 (9,277 to 61,230) |
| Life-years gained | 298,700 (124,300 to 539,900) | 532,500 (276,400 to 849,900) |
| QALYs gained | 727,000 (401,300 to 1,138,000) | 1,337,000 (847,900 to 1,905,000) |
| Change in health-related costs‡ ($ billion) | −63.69 (−104.91 to −34.36) | −117.63 (−179.87 to −72.44) |
| CHD medical costs | −11.74 (−24.76 to −4.73) | −22.21 (−44.05 to −9.67) |
| CHD mortality productivity costs | −10.89 (−24.80 to −3.38) | −20.84 (−41.22 to −8.02) |
| CHD morbidity productivity costs | −3.93 (−9.24 to −1.53) | −7.38 (−16.24 to −3.27) |
| CHD informal care costs∥ | −3.10 (−7.19 to −1.30) | −5.81 (−12.64 to −2.58) |
| Stroke medical costs | −0.63 (−1.79 to −0.05) | −1.17 (−3.00 to −0.27) |
| Stroke mortality productivity costs | 0 (−2.38 to 0.40) | −0.49 (−3.27 to 0.51) |
| Stroke morbidity productivity costs | −0.18 (−0.52 to −0.02) | −0.33 (−0.86 to −0.08) |
| Stroke informal care costs∥ | −11.74 (−24.76 to −4.73) | −22.21 (−44.05 to −9.67) |
| T2DM medical costs | −18.14 (−33.00 to −8.28) | −33.05 (−55.37 to −16.61) |
| T2DM productivity costs | −11.71 (−21.02 to −5.40) | −21.44 (−35.66 to −10.71) |
| T2DM informal care costs∥ | −0.36 (−0.74 to −0.12) | −0.62 (−1.17 to −0.26) |
| Change in policy costs‡ ($ billion) | 1.68 (0.67 to 3.79) | 4.32 (2.26 to 7.60) |
| Government administrative costs | 0.02 (0.01 to 0.02) | 0.02 (0.01 to 0.02) |
| Industry compliance costs | 1.66 (0.64 to 3.78) | 1.66 (0.64 to 3.78) |
| Industry reformulation costs | 0 | 2.46 (0.93 to 5.35) |
| Total net cost from healthcare perspective# ($ billion) | −31.01 (−54.53 to −15.74) | −57.62 (−92.42 to −31.89) |
| Total net cost from societal perspective# ($ billion) | −61.92 (−103.26 to −33.07) | −113.25 (−175.21 to −67.33) |
| Net monetary benefit** (valuing QALYs at $100,000) | 134.78 (74.96 to 217.27) | 247.03 (155.96 to 363.94) |
| Incremental cost-effectiveness ratio†† (2017 USD per QALY) | dominant; (dominant to dominant) |
dominant; (dominant to dominant) |
Abbreviations: UIs, uncertainty intervals; BMI, body mass index; CHD, coronary heart disease; T2DM, type 2 diabetes; QALY, quality-adjusted life-years.
Health outcomes and costs were evaluated among U.S. adults aged 30-84 years over a 20-year simulation period (2018-2037). Values are median estimates of each of 2,000 Monte-Carlo distributions (95% UIs). Costs and QALYs were discounted at 3% annually.
Impacts on stroke may be negative in a competing risk framework, with the prevention of other diseases leading to more stroke deaths.
Costs are median from 2,000 Monte-Carlo iterations so may not add up to totals. Negative costs represent savings. Costs are inflated to 2017 USD using the Consumer Price Index. Detailed health-related costs are available in the Appendix (Supplemental Table 4).
Informal care costs refer to unpaid caregiving costs. We conservatively excluded other informal healthcare costs such as transportation costs and patient time costs.
Net costs were calculated as policy costs minus health-related costs from reduced cardiometabolic diseases. Healthcare perspective included policy costs and medical costs; societal perspective further incorporated informal healthcare costs and productivity costs.
Net monetary benefit was calculated by summing net savings and adding a monetary value of QALYs based on a $100,000 willingness-to-pay threshold per QALY.
Incremental cost-effectiveness ratios were calculated as the net change in costs divided by the net change in QALYs. Dominant = cost-saving and more effective than the base-case scenario.
In both scenarios, absolute health benefits were larger in men than in women, reflecting both higher added sugar intakes (mainly from SSBs) and CVD burdens in men (Supplemental Table 5). Estimated CVD and type 2 diabetes benefits were also higher among younger adults (30-49 years), reflecting their higher added sugar intakes (particularly from SSBs), while middle aged adults (50-69 years) gained most net QALYs (Supplemental Table 6). Though absolute health benefits were larger in whites (Supplemental Table 7), health benefits accounting for population size were greater among blacks, consistent with their higher added sugar intakes and higher baseline BMI.
Cost-effectiveness
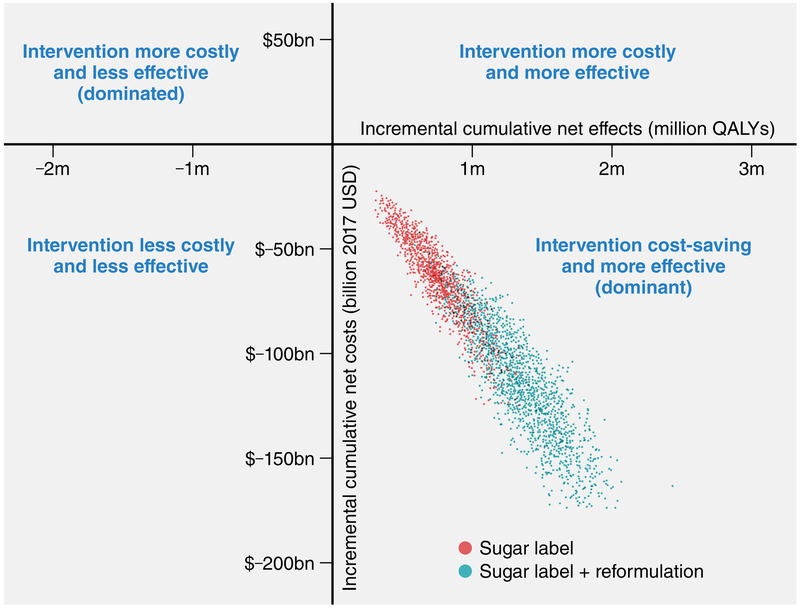
From a healthcare perspective, considering policy costs and medical costs, the sugar label scenario was estimated to save $31 billion (15.7-54.5) in total net costs from 2018-2037 (Table 1, Figure 3). The sugar label+reformulation scenario would generate substantially larger healthcare savings, at $57.6 billion (31.9-92.4). The majority (nearly 60%) of healthcare savings for both scenarios was driven by reduced type 2 diabetes medical costs. From a societal perspective, incorporating informal care and productivity costs, total net savings generated by the policies were about twice as large, $61.9 billion (33.1-103.2) for the sugar label scenario and $113.2 billion (67.3-175.2) for the sugar label+reformulation scenario. Policy costs were estimated at $1.7 billion (0.7-3.8) for the sugar label scenario (99% industry compliance, 1% government costs) and $4.3 billion (2.3-7.6) for the sugar label+reformulation scenario (40% industry compliance, 1% government costs, 59% industry reformulation). Despite substantially higher policy costs when industry reformulation costs were included, scenarios from both healthcare and societal perspectives were dominant (Figure 4), i.e., both cost-saving and gaining health compared to the base-case. Valuing each QALY at $100,000, the sugar label would generate $134.8 billion (75-217.3) in NMB, and the sugar label+reformulation, $247 billion (156-363.9). When assessed in the short- (5-year) and medium-term (10- year), both scenarios were cost-saving (Supplemental Tables 8-9).
Figure 3. Projected policy and health-related costs for the U.S. FDA added sugar labeling policy over the 20-year simulated period (2018 to 2037).
Probabilistic sensitivity analysis via second-order Monte Carlo approach allowed estimated uncertainty of model parameters and individual heterogeneity to be propagated to the outputs. Output distributions are summarized by medians (lines) and 95% uncertainty intervals (shaded areas). Policy costs included government administration and industry compliance/reformulation costs. Health-related costs included medical, informal care and productivity costs related to coronary heart disease, stroke and type 2 diabetes (Supplemental Table 4). The shaded purple area depicts the uncertainty intervals for policy costs; the red area, for informal care costs; the dark green area, for the overlap between medical costs and productivity costs; the light blue area, for productivity costs alone, and the light green area, for medical costs alone. Negative costs represent savings. Costs were inflated to 2017 USD and discounted at 3% annually.
Figure 4. Cost-effectiveness plane for the U.S. FDA added sugar labeling policy by the end of the 20-year simulated period (year 2037).
Each colored dot is the result of each of 2,000 Monte Carlo iterations. Large dots are median combinations of cumulative discounted net costs (2017 USD) and discounted net quality-adjusted life-years (QALYs) for each simulated scenario, and ellipses depict 95% uncertainty intervals. Negative costs represent savings.
Consistent with health gains, larger net savings would accrue in men than in women (Supplemental Table 5), in middle-aged adults (50-69 years) vs. other ages (Supplemental Table 6), and among whites vs. blacks and Hispanics/others (Supplemental Table 7). Accounting for population size, estimated proportional savings were larger among men, middle-aged adults, and blacks.
Probabilistic and Sensitivity Analyses
We estimated a probability of near 100% that the policy would become cost-effective within 5 years (by 2023) for both scenarios, and cost-saving within 7 years (by 2025). Additionally, both scenarios would have more than 80% probability of being cost-effective within 4 years (by 2022), and cost-saving within 5 years (by 2023) (Figure 5). In one-way sensitivity analyses, NMB remained positive when willingness-to-pay was varied down from $100,000 to $50,000 per QALY, and when annual discount rates were varied up from 3% to 9% (Supplemental Tables 10-11). In one-way sensitivity analyses of the policy effect in the sugar label+reformulation scenario, as low as 1% reduction in added sugar intake could be cost-saving over the 20-year simulation period, while a 0.5% reduction could be cost-effective, but not cost-saving over the same period (Supplemental Figures 5-6). Supplemental Table 12 presents model estimates for the base-case scenario.
Figure 5. Estimated probability of cost-effective and cost-saving U.S. FDA added sugar labeling policy over the 20-year simulated period (2018 to 2037).
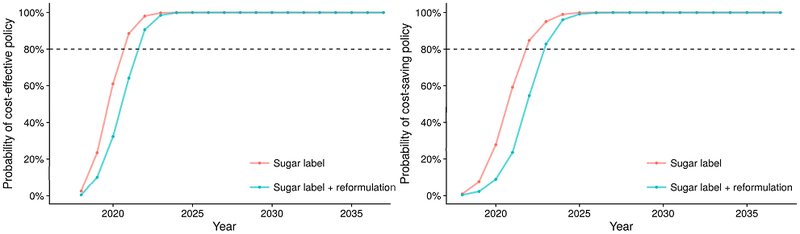
Probabilities for cost-effectiveness and cost-saving of the U.S. FDA added sugar labeling policy as compared with the base-case scenario were estimated via second-order Monte Carlo approach, with willingness-to-pay threshold being valued at $100,000 per quality-adjusted-life year (QALY).
DISCUSSION
Using nationally-representative data, our microsimulation study suggests that implementing the FDA added sugar labeling policy would generate substantial health gains and produce net cost-savings for both the healthcare system and society overall. Over 20 years, the model predicted effects of the added sugar label on consumer behaviors could gain more than 700,000 QALYs, while further accounting for anticipated modest effects on industry reformulation could gain a total of 1.3 million QALYs. Both scenarios were cost-saving, with the label on consumer effects alone generating over $30 billion in healthcare savings and over $60 billion in societal savings. Cost-savings would be twice as large when accounting for potential modest industry reformulation, highlighting industry’s critical role in maximizing the health and economic benefits of the FDA policy.
The economic burdens of cardiometabolic diseases are staggering, with direct and indirect diabetes costs estimated at $245 billion/year30 and corresponding CVD costs at $555 billion/year.36 Suboptimal diet is a leading and preventable cause for cardiometabolic mortality and morbidity, with overconsumption of added sugars being a significant risk factor.4 Our results indicate that implementation of FDA’s added sugar labeling policy to the Nutrition Facts label could substantially reduce U.S. cardiometabolic disease and economic burdens, mainly through reductions in type 2 diabetes incidence and related deaths.
Of all sources of added sugar in the U.S. diet, SSBs are the largest contributor and most consistently linked to cardiometabolic risk.22 Several population-based strategies have been proposed or implemented to target SSBs, including taxation,37 health warning labels,38 and restriction of SSB purchases within food assistance programs such as the Supplemental Nutrition Assistance Program (SNAP).39 Previous studies have modeled the health and economic impacts of these strategies,37, 40, 41 including our own work demonstrating the cost-saving potential of a nationwide tax on SSBs42 and of restricting or disincentivizing SSB purchases within SNAP.39 Despite these ongoing efforts, added sugar intake in the U.S. remains high.8 Declaring added sugar content on the Nutrition Facts label, together with percent Daily Value to help consumers contextualize such information, is a key policy opportunity to target not only SSBs, but added sugar from all packaged products.
Combined approaches to target added sugar appear especially promising. Added sugar labeling can be complemented by government-led reformulation strategies and consumer education, which together can help to level the playing field for industry and to gradually change public taste preference.43 For example, the UK has introduced a front-of-package labeling scheme that includes sugar content43 in combination with nationwide SSB taxes and voluntary industry sugar reformulation targets.44 Nonetheless, targeting added sugar alone will not prevent all obesity and cardiometabolic diseases, and there is a need for complementary policies to improve overall dietary patterns. Such multicomponent policies targeting sugar as well as other dietary factors (e.g., fruit and vegetables, sodium) have been implemented in countries such as France 45 and Chile.46 Our recent study modeling several policy changes within the SNAP program found that combining incentives for purchasing healthier foods (fruits, vegetables, nuts, whole grains and seafood) with disincentives for purchasing unhealthy foods (SSBs, junk food and processed meats) produced the largest health gains and cost-savings.39
Compared to some other types of dietary policies, labeling policies can have the added advantage of stimulating industry reformulation,11 the potential effects of which our investigation quantifies. Prior cost-effectiveness analyses of food labeling strategies have considered a general traffic light front-of-pack label in Australia47 or calorie menu labeling in the U.S.48 Our investigation builds on and extends previous studies by using a microsimulation model to assess the impact of added sugar labeling in the U.S., including potential additional effects of industry reformulation. Our results are consistent with prior labeling studies in finding that these policies are generally “dominant”, due to their relatively low implementation costs and high cost-savings. Even when considering only healthcare savings, such approaches promoting healthy eating were estimated to be far more cost-effective than many federally approved medical interventions, such as drug treatment of hypertension ($20,000/QALY),49 or use of statins for primary CVD prevention ($37,000/QALY).50
To our knowledge this is the first study to assess the health impacts, costs, and cost-effectiveness of implementing FDA’s added sugar labeling policy. Recently the FDA has announced delays to the new label implementation from 2018 to 2020 for large manufacturers, and from 2019 to 2021 for small manufacturers, to provide industry more time for compliance and decrease costs.14 Our findings support more proximal implementation of FDA’s policy, considering the opportunity costs in preventable cardiometabolic events not prevented. Additionally, we demonstrate that the healthcare and societal savings as a result of the added sugar label significantly outweigh policy costs, even when an estimated $2.5 billion of industry reformulation costs were considered.
Our investigation has several strengths. We utilized a validated microsimulation model and national data with probabilistic sensitivity analyses, increasing confidence in the validity of our findings. Potential effects on consumer behavior and industry reformulation were separately evaluated, providing a range of plausible findings. We assumed that recent large observed declines in added sugar intake in the U.S. would continue into the future, moderating the benefits in the policy scenarios and providing more realistic and conservative estimates of potential impact. We separately evaluated added sugar from SSBs and other foods in order to incorporate distinct trends in intake, and to apply etiologic effects for BMI-independent effects of SSBs on cardiometabolic outcomes. Instead of directly translating caloric decrease to BMI reduction, the etiologic effects of changes in added sugar on changes in BMI were based on long-term prospective cohort studies, which were more conservative and inherently incorporate additional health effects of the average dietary substitutes and complements across the population. We accounted for the proportions of added sugar from packaged products that would be affected by the labeling policy, excluding for example added sugars from restaurants. We assumed conservative consumer effects and industry reformulation effects, for example compared to past experience with trans-fat labeling.12 Yet, in one-way sensitivity analyses, we showed that the policy remains cost-saving even if the reduction in added sugar consumption is as low as 1%. Finally, we assessed both short- and long-term health impacts, costs, and cost-effectiveness, providing a range of results across different potential time periods of interest and from distinct relevant perspectives.
Potential limitations should be considered. Modeling approaches cannot prove the health and cost impacts of implementing the FDA added sugar labeling policy. Rather, these estimates provide evidence supporting timely implementation and additional considerations for the monitoring and evaluation of added sugar labeling. As with any medical or public health intervention, our findings should be considered as estimates of the average population effects, and not the effect on any individual person, in whom there may be larger or smaller changes depending on individual variation (e.g., based on age, sex, activity, adiposity, genetics, other risks). Our estimates may be conservative and underestimate the full health and economic impacts, as we included declining added sugar consumption trends and conservative assumptions about industry reformulation. We only evaluated health benefits and cost-savings from cardiometabolic health outcomes; inclusion of increased healthcare costs from competing disease could reduce cost-effectiveness, while other health benefits such as on obesity-related cancers or dental caries would further augment the health gains and cost-savings of added sugar labeling.
In conclusion, our investigation suggests that timely implementation of FDA’s added sugar labeling policy would generate significant health gains and both healthcare and societal cost-savings. Industry reformulation motivated by this policy could provide substantial additional benefits.
Supplementary Material
CLINICAL PERSPECTIVE.
1). What is new?
Using nationally representative data and a validated microsimulation model, we found that implementation of the Food and Drug Administration (FDA) added sugar label would prevent about 354,400 CVD and 599,300 diabetes cases over 20 years, gaining 727,000 QALYs.
Healthcare savings were about $31B and societal savings about $61.9B.
The FDA policy was cost-saving from both a healthcare and societal perspective.
Potential health gains and cost-savings would be twice as large accounting for corresponding industry reformulation, highlighting industry’s critical role in maximizing the health and economic benefits of the FDA policy.
2). What are the clinical implications?
FDA’s added sugar labeling policy could generate substantial health gains and cost-savings for the U.S. population, particularly if the new label stimulates industry reformulation.
Compliance date for updating the Nutrition Facts label, including the added sugar provision, has been continuously delayed. Our findings highlight the need for timely implementation to maximize health and economic gains.
ACKNOWLEDGEMENTS
The authors thank all of the collaborators and advisory groups in the Food Policy Review and Intervention Cost-Effectiveness (Food-PRICE) project (www.food-price.org).
Study concept and design: Huang, Kypridemos, O’Flaherty, Micha.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Huang, Kypridemos, Micha.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Micha.
Study supervision: O’Flaherty, Micha.
Drs Kypridemos and O’Flaherty had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources
This research was supported by the NIH National Heart, Lung, and Blood Institute (R01 HL130735, PI Micha). In addition, Dr. Liu was supported by a postdoctoral fellowship award (17POST33670808) from the American Heart Association. The funding agencies did not contribute to design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Disclosures
All authors report support from NIH grants during the conduct of the study. In addition, Dr. Pearson-Stuttard reports personal fees from ICF consulting for work commissioned by The Directorate-General for Health and Food Safety (DG SANTE). Dr. Micha reports research funding from Unilever and personal fees from the World Bank and Bunge; and Dr. Mozaffarian, personal fees from Astra Zeneca, Acasti Pharma, GOED, DSM, Haas Avocado Board, Nutrition Impact, Pollock Communications, Boston Heart Diagnostics, Bunge, and UpToDate; all outside the submitted work.
REFERENCES
- 1.Food and Drug Administration. Food labeling: revision of the Nutrition and Supplement Facts Labels; final rule. 2016;21 CFR 101. Federal Register 2016;103: 33742–33999. [PubMed] [Google Scholar]
- 2.Te Morenga L, Mallard S and Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492–e7492. [DOI] [PubMed] [Google Scholar]
- 3.Malik VS, Pan A, Willett WC and Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD and Mozaffarian D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA. 2017;317:912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC and Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R and Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA internal medicine. 2014;174:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milken Institute ∣ Weighing down America: the health and economic impact of obesity. 2016. https://www.milkeninstitute.org/publications/view/833. Accessed August 19, 2017.
- 8.Powell ES, Smith-Taillie LP and Popkin BM. Added sugars intake across the distribution of US children and adult consumers: 1977-2012. J Acad Nutr Diet. 2016;116:1543–1550. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services and U.S. Department of Agriculture ∣ Dietary Guidelines for Americans 2015 - 2020. 2015. https://health.gov/dietaryguidelines/2015/. Accessed October 12, 2017.
- 10.Drewnowski A and Rehm CD. Consumption of added sugars among US children and adults by food purchase location and food source. Am J Clin Nutr. 2014;100:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shangguan S, Afshin A, Shulkin M, Ma W, Marsden D, Smith J, Saheb-Kashaf M, Shi P, Micha R, Imamura F and Mozaffarian D. A meta-analysis of food labeling effect on consumer diet behaviors and industry practices. Am J Prev Med. 2019;56(2):300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otite FO, Jacobson MF, Dahmubed A and Mozaffarian D. Trends in trans fatty acids reformulations of US supermarket and brand-name foods from 2007 through 2011. Prev Chronic Dis. 2013;10:E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poinski M The future is now: thousands of products already sport the revamped Nutrition Facts label. 2017. https://www.fooddive.com/news/the-future-is-now-thousands-of-products-already-sport-the-revamped-nutriti/507203/. Accessed November 8, 2017.
- 14.Food and Drug Administration. FDA proposes to extend compliance dates for Nutrition Facts Label final rules. 2017. https://www.fda.gov/food/newsevents/constituentupdates/ucm577264.htm. Accessed October 8, 2017.
- 15.Pearson-Stuttard J, Bandosz P, Rehm CD, Penalvo J, Whitsel L, Gaziano T, Conrad Z, Wilde P, Micha R, Lloyd-Williams F, Capewell S, Mozaffarian D and O’Flaherty M. Reducing US cardiovascular disease burden and disparities through national and targeted dietary policies: A modelling study. PLoS Med. 2017;14:e1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson-Stuttard J, Bandosz P, Rehm CD, Afshin A, Penalvo JL, Whitsel L, Danaei G, Micha R, Gaziano T, Lloyd-Williams F, Capewell S, Mozaffarian D and O’Flaherty M. Comparing effectiveness of mass media campaigns with price reductions targeting fruit and vegetable intake on US cardiovascular disease mortality and race disparities. Am J Clin Nutr. 2017;106:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey [Internet]. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed March 2, 2017.
- 18.Rehm CD, Peñalvo JL, Afshin A and Mozaffarian D. Dietary intake among US adults, 1999-2012. JAMA. 2016;315:2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kypridemos C, Guzman-Castillo M, Hyseni L, Hickey GL, Bandosz P, Buchan I, Capewell S and O’Flaherty M. Estimated reductions in cardiovascular and gastric cancer disease burden through salt policies in England: an IMPACTNCD microsimulation study. BMJ Open. 2017;7:e013791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfons A, Kraft S, Templ M and Filzmoser P. Simulation of close-to-reality population data for household surveys with application to EU-SILC. Stat Methods Appl 2011;20:383–407. [Google Scholar]
- 21.Food and Drug Administration ∣ Regulatory impact analysis for final rules on “Food labeling: revision of the nutrition and supplement facts label”. 2016. https://www.federalregister.gov/documents/2014/03/03/2014-04387/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels. Accessed April 17, 2017.
- 22.Micha R, Shulkin ML, Penalvo JL, Khatibzadeh S, Singh GM, Rao M, Fahimi S, Powles J and Mozaffarian D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE. 2017;12:e0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson-Stuttard J, Kypridemos C, Collins B, Mozaffarian D, Huang Y, Bandosz P, Capewell S, Whitsel L, Wilde P, O’Flaherty M and Micha R. Estimating the health and economic effects of the proposed US Food and Drug Administration voluntary sodium reformulation: Microsimulation cost-effectiveness analysis. PLoS Med. 2018;15:e1002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringhini S, Carmeli C, Jokela M, Avendano M, Muennig P, Guida F, Ricceri F, d’Errico A, Barros H, Bochud M, Chadeau-Hyam M, Clavel-Chapelon F, Costa G, Delpierre C, Fraga S, Goldberg M, Giles GG, Krogh V, Kelly-Irving M, Layte R, Lasserre AM, Marmot MG, Preisig M, Shipley MJ, Vollenweider P, Zins M, Kawachi I, Steptoe A, Mackenbach JP, Vineis P and Kivimaki M. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet. 2017;389:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan PW and Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food and Drug Administration (FDA), Department of Health and Human Services (DHHS)∣ Food and Drug Administration justification of estimates for appropriations committees. Fiscal year 2012 [Internet]. Food and Drug Administration (FDA); 2012. https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/BudgetReports/UCM243370.pdf. Accessed July 10, 2017. [Google Scholar]
- 27.American Heart Association ∣ Cardiovascular disease: a costly burden for America. Projections through 2035. https://healthmetrics.heart.org/wp-content/uploads/2017/10/Cardiovascular-Disease-A-Costly-Burden.pdf. Accessed Nov 4, 2017.
- 28.Joo H, Dunet DO, Fang J and Wang G. Cost of informal caregiving associated with stroke among the elderly in the United States. Neurology. 2014;83:1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leal J, Luengo-Fernández R, Gray A, Petersen S and Rayner M. Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J. 2006;27:1610–1619. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dall TM, Yang W, Halder P, Pang B, Massoudi M, Wintfeld N, Semilla AP, Franz J and Hogan PF. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37:3172–3179. [DOI] [PubMed] [Google Scholar]
- 32.Langa KM, Vijan S, Hayward RA, Chernew ME, Blaum CS, Kabeto MU, Weir DR, Katz SJ, Willis RJ and Fendrick AM. Informal caregiving for diabetes and diabetic complications among elderly americans. J Gerontol B Psychol Sci Soc Sci. 2002;57:S177–186. [DOI] [PubMed] [Google Scholar]
- 33.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK and Prosser LA. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED and Shaw LJ. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–2322. [DOI] [PubMed] [Google Scholar]
- 35.Koerkamp BG, Stijnen T, Weinstein MC and Hunink MM. The combined analysis of uncertainty and patient heterogeneity in medical decision models. Med Decis Making. 2011;31:650–661. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW and Turner MB. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 37.Long MW, Gortmaker SL, Ward ZJ, Resch SC, Moodie ML, Sacks G, Swinburn BA, Carter RC and Claire Wang Y. Cost Effectiveness of a sugar-sweetened beverage excise Tax in the U.S. Am J Prev Med. 2015;49:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pomeranz JL, Mozaffarian D and Micha R. Can the Government Require Health Warnings on Sugar-Sweetened Beverage Advertisements? JAMA. 2018;319:227–228. [DOI] [PubMed] [Google Scholar]
- 39.Mozaffarian D, Liu J, Sy S, Huang Y, Rehm C, Lee Y, Wilde P, Abrahams-Gessel S, de Souza Veiga Jardim T, Gaziano T and Micha R. Cost-effectiveness of financial incentives and disincentives for improving food purchases and health through the US Supplemental Nutrition Assistance Program (SNAP): A microsimulation study. PLoS Med. 2018;15:e1002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BY, Ferguson MC, Hertenstein DL, Adam A, Zenkov E, Wang PI, Wong MS, Gittelsohn J, Mui Y and Brown ST. Simulating the Impact of Sugar-Sweetened Beverage Warning Labels in Three Cities. Am J Prev Med. 2018;54:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu S, Seligman HK, Gardner C and Bhattacharya J. Ending SNAP subsidies for sugar-sweetened beverages could reduce obesity and type 2 diabetes. Health Aff. 2014;33:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilde P, Huang Y, Sy S, Abrahams-Gessel S, Jardim TV, Paarlberg R, Mozaffarian D, Micha R and Gaziano T. Cost-Effectiveness of a US National Sugar-Sweetened Beverage Tax With a Multistakeholder Approach: Who Pays and Who Benefits. Am J Public Health. 2019;109(2):276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyness LA, Butriss JL and Stanner SA. Reducing the population’s sodium intake: the UK Food Standards Agency’s salt reduction programme. Public Health Nutr. 2012;15:254–261. [DOI] [PubMed] [Google Scholar]
- 44.Public Health England ∣ Sugar Reduction, achieving the 20%: A technical report outlining progress to date, guidelines for industry, 2015 baseline levels in key foods and next steps. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/604336/Sugar_reduction_achieving_the_20_.pdf. Accessed December 12, 2017.
- 45.World Cancer Research Fund International ∣ Curbing global sugar consumption. https://www.wcrf.org/sites/default/files/Curbing-Global-Sugar-Consumption.pdf. Accessed Nov 19, 2017.
- 46.Global Food Research Program at University of North Carolina at Chapel Hill. Chile: [Internet]. http://globalfoodresearchprogram.web.unc.edu/multi-country-initiative/countries-where-we-work/chile/. Accessed January 19, 2018. [Google Scholar]
- 47.Sacks G, Veerman JL, Moodie M and Swinburn B. ‘Traffic-light’nutrition labelling and ‘junk-food’tax: a modelled comparison of cost-effectiveness for obesity prevention. Int J Obesity. 2011;35:1001–1009. [DOI] [PubMed] [Google Scholar]
- 48.Gortmaker SL, Wang YC, Long MW, Giles CM, Ward ZJ, Barrett JL, Kenney EL, Sonneville KR, Afzal AS and Resch SC. Three interventions that reduce childhood obesity are projected to save more than they cost to implement. Health Aff. 2015;34:1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park C, Wang G, Durthaler JM and Fang J. Cost-effectiveness Analyses of Antihypertensive Medicines: A Systematic Review. Am J Prev Med. 2017;53:S131–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandya A, Sy S, Cho S, Weinstein MC and Gaziano TA. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA. 2015;314:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.