Abstract
Neonatal hypoxia-ischemia (HI) injury caused by oxygen deprivation is the most common cause of mortality and severe neurologic deficits in neonates. The present work evaluated the preventative effect of photobiomodulation (PBM) preconditioning, and its underlying mechanism of action on brain damage in a HI model in neonatal rats. According to the optimal time response of ATP levels in brain samples removed from normal rats, a PBM preconditioning regimen (808 nm CW laser, 1 cm2 spot, 100 mW/cm2, 12 J/cm2) was delivered to the scalp 6 hours before HI. PBM preconditioning significantly attenuated cognitive impairment, volume shrinkage in the brain, neuron loss, dendritic and synaptic injury after HI. Further mechanistic investigation found that PBM preconditioning could restore HI-induced mitochondrial dynamics and inhibit mitochondrial fragmentation, followed by a robust suppression of cytochrome c release, and prevention of neuronal apoptosis by inhibition of caspase activation. Our work suggests that PBM preconditioning can attenuate HI-induced brain injury by maintaining mitochondrial dynamics and inhibiting the mitochondrial apoptotic pathway.
Keywords: Cognitive impairment, Hypoxia-ischemia, Mitochondria, Neuroprotection, Photobiomodulation preconditioning
Introduction
Hypoxic-ischemic brain injury, also known as neonatal hypoxic-ischemic encephalopathy (HIE), is a type of brain damage that often occurs in human infants, caused by impaired cerebral blood flow and oxygen deprivation affecting the brain within the perinatal period1, 2. It is one of the most serious birth complications with a relatively high incidence rate (~0.4%), high mortality (~20%), and a significant possibility of long-term neurological sequelae (~30%), including severe life-long motor and cognitive deficits3–6. It is known that the brain needs a significant amount of energy to support neuronal survival and function, which is dependent on the continuous supply of oxygen and glucose via cerebral blood flow7. Due to the deprivation of oxygen and glucose occurring within the brain, brain injury in HIE is usually characterized by delayed neuronal cell death in the cortex and the hippocampus8. Neuronal cell death can cause several types of severe neurological dysfunction such as impairments in hearing, learning, behavioral disability, attention deficits, blindness, or even death9–12.
There is compelling evidence that the loss of neurons in HIE can lead to not only the impairment of motor function, but also to impairment in learning and memory during adolescence13, 14. A number of studies demonstrated that learning and memory deficits were closely related to the changes in dendrites and synaptic proteins15–17. Proteins related to synaptic plasticity including microtubule-associated protein-2 (MAP-2) (dendritic marker), synaptophysin (presynaptic marker) and spinophilin (postsynaptic marker) all play a central role in cognitive impairment18, 19. Neonatal brain injured shows marked decreases in the expressions of synaptic proteins20, 21. For this reason, several methods focusing on maintaining the function of dendrites and inhibiting the decreased level of synaptic protein has been proven effective in improving learning and memory deficits16, 22–24.
Mitochondria supply the energy demands of neurons, playing an essential role in powering various cellular activities, handling oxidative reactions, preserving the membrane potential and maintenance of intracellular ionic homeostasis. Many of these functions are mediated (at least in part) by morphological and metabolic changes in the mitochondria10, 25–27. Studies have reported that the rapid depletion of ATP induced by HI insults was able to trigger detrimental pathophysiological responses28–31. These pathophysiological responses and mitochondrial dysfunction will result in mitochondrial permeabilization triggering mitochondrial fission followed by the release of pro-apoptotic proteins including the release of the key pro-apoptotic protein cytochrome c from the mitochondria into the cytosol32, 33. Therefore, mitochondria are an important target to identify new approaches for therapeutic intervention against HIE34–36.
Photobiomodulation (PBM) also known as low-level laser (light) therapy (LLLT) has been well-documented to improve wound healing, alleviate inflammation, reduce oxidative stress and relieve pain37–40. In the past several years, the neuroprotective role of PBM in Alzheimer’s and Parkinson’s diseases, traumatic brain injury, stroke, global cerebral ischemia and major depression has been well demonstrated37, 39, 41–43, and mitochondria have been recognized as one critical mediator of PBM effects (or photoreceptor)44–46. Our previous studies have shown that PBM conferred neuroprotective effects against β-amyloid neurotoxicity by preserving mitochondrial function and suppressing inflammation and oxidative stress42. While many studies, including our own, have revealed the beneficial role of PBM on a range of brain disorders, those studies were primarily concerned with the therapeutic effects of PBM, rather than the potential preventative function of delivering PBM before the occurrence of injury or disease. Therefore, the present work was designed to examine the effect of PBM preconditioning on HIE and its underlying mechanism.
Materials and Methods
Hypoxic-ischemic animal model
As described in our previous work47, 10-day-old neonatal Sprague-Dawley rats of both sexes were used to establish a HI animal model. The establishment of HIE model undergoes a two-step process. The first step is the ligation of the right common carotid artery, which cannot significantly decrease the cerebral blood flow (CBF) due to the existence of circle of Willis. However, the second step in the process with a subsequent exposure to a hypoxic environment is capable to induce a significant CBF decrease and results in ischemic injury. In the present work, the right common carotid artery was ligated permanently following careful dissection. Rat pups were allowed to recover for 1.5 hours in the home cage following the closure of the wound. Next, the animals were enclosed in a hypoxic environment (6% oxygen in nitrogen) at 37 °C for 2 hours. All procedures received ethical approval by the IACUC of Augusta University under protocol #2014–0661.
PBM treatment
Laser irradiation was delivered for two minutes (CW, 808 nm, 100 mW/cm2, 12 J/cm2) onto the scalp. A 1 cm2 round spot was applied via fiber optic cable and multi-mode coupler (SMA connector; Imeter 400um multi model, Dragon Lasers) to the top of the head (collimated round spot centered at 3 mm posterior to the eye and 2 mm anterior to the ear (Fig. 1A) using a diode IR Laser System (808 M100, Dragon Lasers). The device parameters have been reported in detail in our previous study37.
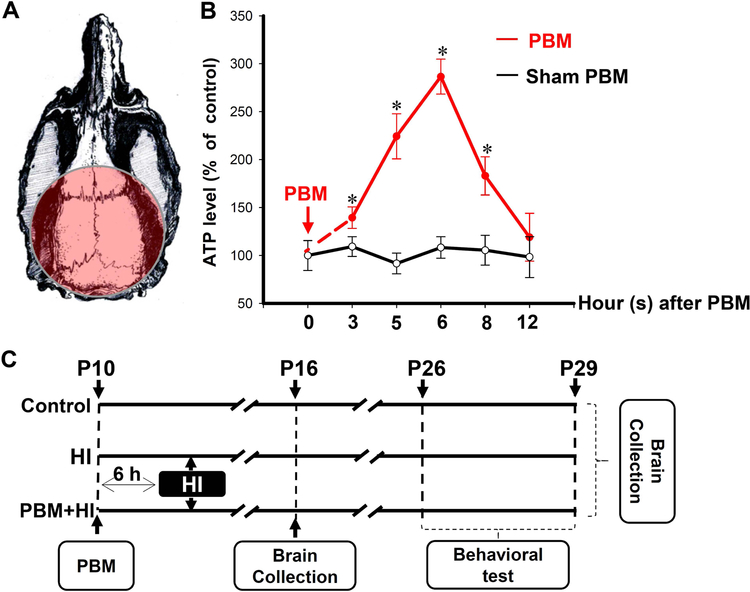
Fig. 1. Schematic diagram of the experimental protocol.
(A) Diagram shows the irradiation position of PBM treatment. (B) ATP contents in total protein samples were collected at the different time point in naive control group and PBM treatment group (n=4–5). (C) Animals were randomly divided into three groups. Group I: healthy animals without HI (control); Group II: animals that received HI insults plus sham PBM preconditioning (HI); Group III: animals that received HI insults with PBM preconditioning (PBM+HI). PBM pretreatment was initiated 6 hours before HI insults, and the behavior tests were performed from P26 to P29. Brain collections were conducted at P16 and P29 respectively. Brains collected at P16 were used for immunofluorescence staining, Western blotting, and biochemical analyses (n=5–6). Brains collected at P29 were used for Creyl violet (n=5–6). All data are presented as mean ± SD. *P < 0.05 versus PBM sham group.
Experimental design
In the present work, two types of experiments were designed to investigate the effects of PBM in the HI animal model. Experiment 1 was performed to determine the most suitable time interval between PBM treatment and initiation of the HI model, by evaluating PBM effects on mitochondrial activity in the normal rat brain. Experiment 2 was designed to test the beneficial preconditioning effect of PBM in the HI animal model and the underlying mechanisms.
Experiment 1: To determine the optimal preconditioning time point of PBM before the HI insult, normal neonatal rats were randomly divided into 11 groups (Groups: PBM-0 sham, PBM-3, PBM-3 sham, PBM-5, PBM-5 sham, PBM-6, PBM-6 sham, PBM-8, PBM-8 sham, PBM-12, PBM-12 sham) according to the interval between PBM pretreatment and brain removal (brain collection procedures were conducted at 0, 3, 5, 6, 8, 12 hours after the 2-minute PBM treatment). ATP levels were measured to reflect the effect of PBM on mitochondrial function at the different time (Fig. 1B).
Experiment 2: After the optimal preconditioning time was confirmed, additional animals were randomly divided into 3 groups: (a) control group (animals without ligation); (b) HI group (HI animal with sham PBM pretreatment); (c) PBM group (HI animal with PBM pretreatment). As shown in Fig. 1C, the HI model was initiated 6 hours after PBM pretreatment on postnatal day 10 (P10). From P26 to P29, Barnes Maze Task and Novel Object Recognition Test were performed. Brains were collected at P16 and P29, respectively.
Brain Collection and Tissue Preparation
Brain collection and tissue preparation were performed as indicated in our previous study37. Briefly, the animal brains were quickly removed half-frozen for protein analysis and half-fixed for tissue section immunohistochemistry under anesthesia following perfusion with ice-cold saline. Brains for tissue section were post-fixed and cryoprotected with 4% paraformaldehyde (PFA) and 30 % sucrose respectively. A Leica Rm2155 microtome was used for cutting frozen sections (25 μm each). Brains for protein analysis were homogenized using a motor-driven Teflon homogenizer in ice-cold homogenization medium. To obtain total protein fractions, the homogenization medium was prepared (50 mM HEPES, 150 mM NaCl, 12-b-glycerophosphate, 3 dithiothreitol (DTT), 2 mM sodium orthovanadate (Na 3VO4), 1 mM EGTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100, and inhibitors of proteases and enzymes (Thermo Scientific, Rockford, IL)) following centrifugation at 15,000 × g for 30 min. The collection of crude mitochondrial fractions was carried out as previously described48. Modified Lowry Protein Assay was performed to determine protein concentrations.
Quantification of total ATP content
The level of ATP was determined by a kit of ® rLuciferase/Luciferin Reagent (FF2021, Promega, Madison, WI) as previously described37. Total protein samples (30 μg) were diluted in 100 μL of reconstituted rL/L reagent buffer consisting of D-luciferin, Tris-acetate buffer (pH 7.75), luciferase, bovine serum albumin, EDTA, magnesium acetate, and dithiothreitol. The light output from sample and “background blank” (rL/L reagent and homogenization buffer) were measured using a standard microplate luminometer (PE Applied Biosystems). The levels of ATP production were expressed as percentage changes versus the control group.
Infarct Volume Assessment
Infarct volumes of different groups were measured as described in detail previously by our laboratory37. Brain sections from different groups were selected and stained with 0.01% (w/v) cresyl violet for 10 min, which was followed by graded ethanol dehydration. AxioVision microscope system (Carl Zeiss, Germany) and Image J (NIH) were applied to acquire and quantify images of brain sections respectively. Shrinking areas was expressed as a percent of the total area of the contralateral hemisphere using the following formula: percent of shrinking area = (area of contralateral hemisphere – area of shrinking hemisphere)/area of contralateral hemisphere × 100%.
Nissl Staining and TUNEL staining
The Nissl substance is abundant in neurons and has been used to identify neurons. In this study, NeuroTrace ™ fluorescent Nissl stain was performed. Briefly, a mixture was prepared using NeuroTrace ™ fluorescent Nissl stain (Thermo Fisher Scientific) and DAPI (Vector Laboratories) (1:1000). Afterwards, brain sections were cover-slipped with this mixture. TUNEL staining was performed as previously described. Selected sections were placed on slides and stained using the Click-iT Plus TUNEL assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Images were captured by LSM510 Meta confocal laser microscope (Carl Zeiss).
Immunofluorescence Staining and Confocal Microscopy
Briefly, after incubation with 10% normal donkey serum and 0.1% Triton X-100 for 1 hour at room temperature, brain sections were incubated with corresponding primary antibodies. In this study, the following primary antibodies were used: MAP2 from Santa Cruz; anti-synaptophysin, spinophilin (Abcam); anti-TOM20 from Proteintech Group; anti-cleaved-3/9 (Cell Signaling). After washes, brain sections were incubated with appropriate secondary antibodies from Thermo Fisher Scientific for 1 hour at room temperature followed by another three washes. Afterwards, brain sections were mounted with mounting medium and cover-slipped with DAPI (Vector Laboratories). Fluorescence images were captured by LSM510 Meta confocal laser microscope (Carl Zeiss), and quantitatively analyzed by Image J (NIH).
Western Blotting Analysis
As previously described48, proteins (30–50 μg) were separated on 4–20 % SDS-polyacrylamide gels and transferred onto PVDF membranes. The PVDF membranes were then blocked with 3% BSA in TBST followed by incubation with anti-Cytochrome c (Proteintech, IL) antibodies at 4 °C for 12 hours. After washing three times, PVDF membranes were incubated with HRP conjugated secondary antibodies (Cell Signaling; Danvers, MA, USA) for 1 hour at room temperature. Membrane-bound proteins were detected and analyzed by CCD digital imaging system and Image J analysis software (Version 1.49; NIH, USA), respectively.
Caspase Activity Assay
Caspase-3 and caspase-9 activities in cytosolic proteins determined as previously described by our laboratory48. Ac-DEVD-AMC and Ac-LEHD-AMC (AnaSpec, Fremont, CA, USA) were used as substrates for detecting caspase-3 and caspase-9, respectively. Fluorescence was measured by a Synergy HT Microplate reader (BioTek Instruments Inc, Winooski, VT, USA). The values in HI and PBM pretreatment groups were expressed as a percentage of the control group.
Novel Object Recognition Test (NOR)
NOR test was applied to measure the effects of PBM pretreatment on recognition memory. Rats have an intrinsic tendency to spend more time on exploring a novel object48. To test the long-term recognition memory, rats were placed in object recognition box with 2 identical objects for 5 min at P28. Twenty-four hours later (P29), the rats were returned to the object recognition box with one familiar object and one novel object. A video camera and ANY-maze video tracking software were used to record and analyze the time animals spent on exploring each object and the time animals approached the zone within 2 cm from the object. The discrimination index (the percentage time spent on exploring the novel object) and the entries index (the percentage times approached the zone of the novel object) were calculated according to data recorded mentioned above.
Objection Location test (OLT)
OLT was applied to measure the effects of PBM pretreatment on spatial cognitive ability49. In the sample trial, two identical objects were fixed in the same location as that in the NOR for 5 min. On the test day (P29), the rats were left to explore one object in the established location and another object in a novel location for 5 min. The behavior of animals was recorded and analyzed in the same way described in NOR test.
Barnes Maze Task (BM)
As previously described48, the Barnes Maze Task was applied to test spatial learning and memory. In the current study, a 100 cm high platform (diameter: 122 cm) with 18 holes around the perimeter was used. The platform was divided into four quadrants with equal area and the area where the target box was located was defined as target quadrant. P26, P27 and P28 were defined as training days and P29 was probe day. During training days, animals were placed on the platform and each rat was allowed 3 min to find the black escape box (20×15×12 cm). The time animals spent on finding the black escape box were recorded and each animal was allowed 1 trial per training day. On the probe day, the black escape box was removed during the 90-second probe test. The time rats spent in the target quadrant were recorded by a video camera and analyzed using ANY-maze video tracking software (Stoelting; Wood Dale, IL, USA).
Statistical Analysis
The tracking data of behavioral tests was analyzed by ANY-maze video tracking software mentioned above. In addition, SigmaStat software (Systat Software; San Jose, CA, USA) were used to perform statistical analysis. One-way analysis of variance (ANOVA) followed by Student-Newman Keul’s was applied to analyze between group differences. The differences were considered significant at the 95% confidence level (P < 0.05). Data were expressed as means ± SD.
Results
Time respon of ATP production after PBM pretreatment in naive neonatal rats at P10
To define the optimal time for PBM preconditioning, brain collection and measurement of ATP content was conducted at 0, 3, 5, 6, 8 and 12 hours after PBM treatment in naïve neonatal rats at P10. As shown in Fig. 1B, the ATP content in the hippocampus was significantly modulated by PBM and displayed a biphasic time response for PBM. Hippocampus collected 6 hours after PBM preconditioning (PBM-6 group) had the highest ATP level compared to ATP in the other animal groups. At 3, 5, 6 and 8 hours after PBM treatment, the ATP levels in the hippocampus were significantly elevated compared to sham treatment at the same time point. ATP levels in sham PBM groups did not present any time response and the ATP content in PBM-6 sham group did not have any difference compared with other groups without PBM pretreatment.
PBM Pretreatment Significantly Alleviates HI-induced Behavioral Deficits
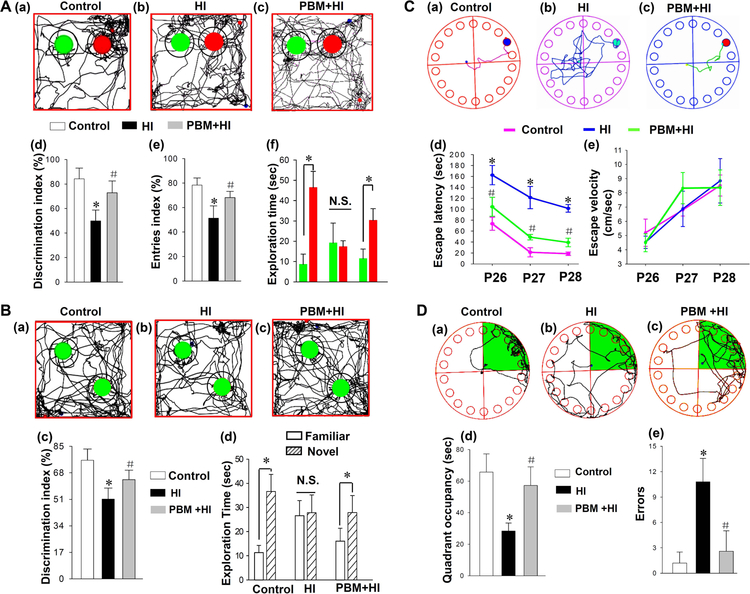
To examine the effect of PBM pretreatment on HI-induced behavioral deficits, NOR test, OLT test and BM test were performed from P26 to P29. As shown in Fig. 2A, the recognition memory of the animals was impaired at P29 after HI insults as indicated by the comparison of the discrimination index, entries index and the time spent on exploring each object. Interestingly, the recognition memory deficit was significantly prevented by PBM pretreatment compared with the sham PBM-treated HI groups. As shown in Fig. 2B, HI induced a decreased preference in investigating the object in a novel location (spatial recognition) compared with the control group, and the impaired spatial recognition memory was attenuated by PBM pretreatment. Furthermore, the BM test was also applied to test hippocampus-dependent spatial learning and memory. During training days, HI animals without PBM pretreatment expended more time finding the black escape box compared to rats of the control group and PBM group (Fig. 2C (a-d)). Notably, as shown in Fig. 2C (d), there were no differences among three groups in escape velocity, suggesting that the difference in finding escape box were not due to variation in motor abilities. During the probe day, animals from the PBM pretreatment group and control groups spent a longer time in the target quadrant compared with animals of HI group (Fig. 2D (a-d). In addition, the exploration errors in HI group were increased, and this increase was attenuated by PBM preconditioning (Fig. 2D (e)), suggesting PBM pretreatment can significantly prevent spatial memory deficit induced by a HI insult.
Fig. 2. Effects of PBM preconditioning against HI-induced behavioral defects.
(A) Novel object recognition tests at P28 and P29 were conducted to test the recognition memory. (a-c) Representative traces of animals were presented for animals’ exploration of the familiar object (green) and a novel object (red). The analysis for discrimination index, entries index (white: Control, black: HI, grey: PBM+HI) and the time spent on exploring each object (green: familiar object, red: novel object) are shown in (d-f). (B) Objection location tests at P28 and P29 were also conducted to test the spatial recognition memory. Representative traces of animals were presented for animals’ exploration of two familiar object (green) in familiar location and new location (a-c). Relative discrimination index and exploration time were analyzed and shown in (c and d). (C, D) The Barnes Maze Task was applied to test spatial learning and memory. (C) Results of test trials in the Barnes maze task were present in (a-e). Representative escaping traces of animals were recorded (a-c). The analysis results of escape latencies and the average velocity were shown in (d and e). (D) Probe trials were conducted at P29. Representative escaping traces of animals from control (a), HI group (b) and PBM group (c) were recorded. The analysis results of target quadrant (green area) and errors occurred in the process of finding target box were shown in (d and e). All data are presented as mean ± SD (n=5–6). *P < 0.05 versus control group; #P<0.05 versus HI group.
PBM Pretreatment Significantly Decreases Brain Shrinkage volume and Protects Neurons after HI Insults
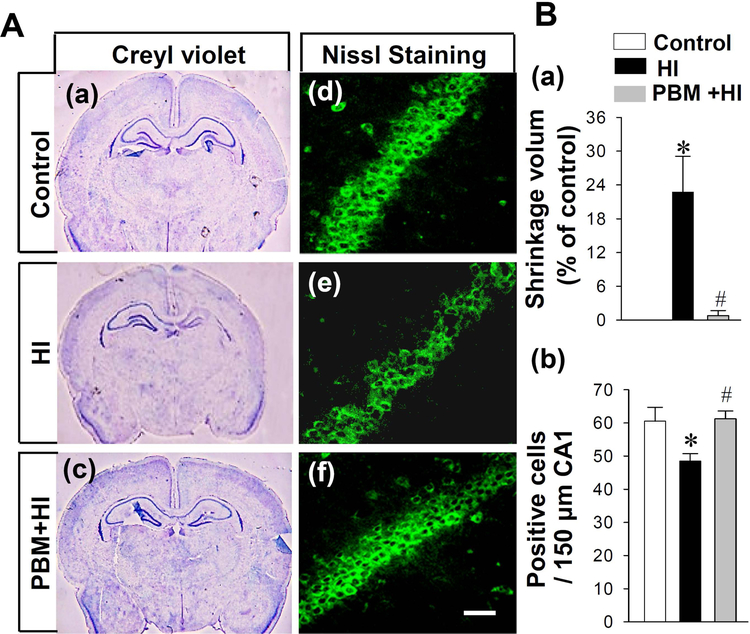
To determine the effects of PBM preconditioning on neonatal HI brain damage at P29, Cresyl violet staining and NeuroTrace™ Fluorescent Nissl Staining were applied for histological evaluation. Cresyl violet staining showed that there was an obvious shrinkage in the brain section of the right hemisphere after HI, compared with sections from the control group, notably, the shrinkage volume was significantly decreased in HI animals with PBM pretreatment (Fig. 3A (a-c) and Fig. 3B (a)).The effect of PBM on the right hippocampus is also shown in Fig. 3A (a-c), PBM pretreatment significantly attenuated HI-induced hippocampal atrophy. In addition, the results of NeuroTrace™ Fluorescent Nissl Staining indicated that PBM pretreatment significantly prevented the decrease of neuronal density in the hippocampal CA1 region after HI insults (Fig. 3A (d-f) and Fig. 3 B (b)).
Fig. 3. Effects of PBM preconditioning on the shrinkage volume and neuronal density in the hippocampal CA1 region.
(A) Representative images of cresyl violet from control (a), HI group (b) and PBM preconditioning group (c). NeuroTraceTM Fluorescent Nissl Staining was performed to mark neurons. Representative immunofluorescence staining of neurons was shown in (d-f). (B) Quantification analysis was performed by analyzing shrinkage volume and counting the survival neurons. (a) Shrinkage volume was quantized by Image-J software (NIH). Shrinkage volume was expressed as a percent of the total area of the contralateral hemisphere. (b) Numbers of surviving neurons was counted and analyzed in (b). All data are expressed as mean ± SD (n = 5–6). *P < 0.05 versus control group, #P < 0.05 versus HI group. Scale bar = 30 μm.
PBM Pretreatment Significantly Inhibits Dendritic and Synaptic Injury after HI Insults
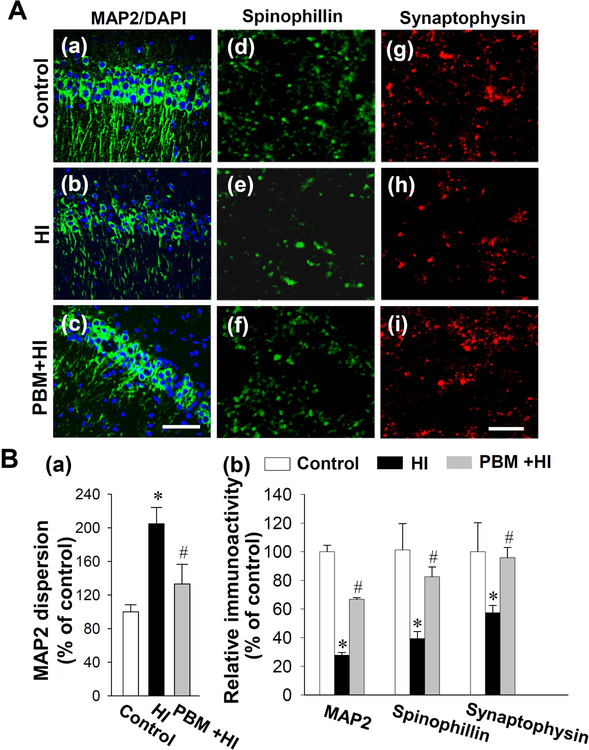
To investigate the effect of PBM preconditioning on dendritic and synaptic damage 6 days after HI insults, brain sections were labeled with MAP2, synaptophysin and spinophilin, respectively37, 48. MAP2 has been widely used as a dendritic marker, which can, at least in part, indicate a change in the morphology of the dendrites18. As shown in Fig. 4A (a-c) and Fig. 4B, confocal microscopy and quantitative analysis demonstrated that the MAP2 fluorescent staining intensity was significantly decreased in HI animals compared with control rats, and the decreased expression of MAP2 was prevented in animals with PBM preconditioning. In addition, it was obviously that HI animals had greater MAP 2 dispersion in the hippocampal CA1 stratum radiatum compared with control animals. In contrast, dendritic morphology was spared in HI animals that received PBM pretreatment. To assess the effects of PBM pretreatment on synaptic changes after HI insults, synaptophysin and spinophilin were stained to determine the effects of PBM on the synaptic injury. As shown in Fig. 4A (d-i) and quantitative analysis in Fig. 4B, the expression of synaptic proteins was decreased in HI animals, and this decreased expression of synaptophysin and spinophilin was prevented by PBM pretreatment.
Fig. 4. Effects of PBM preconditioning on HI-induced neuronal injury in hippocampus on P16.
(A) Typical staining of MAP2 from control animals (a), HI insults with sham PBM pretreatment (b) or PBM preconditioning (c). Scale bar = 30 μm. Confocal microscopy images of spinophilin and synaptophysin were taken from the stratum radiatum layer of the hippocampal CA1 and displayed in (d-f) and (g-i) respectively. Scale bar = 10 μm. (B) The confocal microscopy images of MAP2, spinophilin, and synaptophysin were analyzed by Image J analysis software. The quantitative analyses of MAP2 dispersion in hippocampal CA1 (a) and relative immunoreactivity were analyzed and expressed as percentage changes versus the control group. Values are displayed as mean ± SD (n = 5–6). *P < 0.05 versus control group, #P < 0.05 versus HI.
PBM Pretreatment Significantly Improves HI-induced Changes in Mitochondrial Dynamics in Hippocampal CA1 Neurons
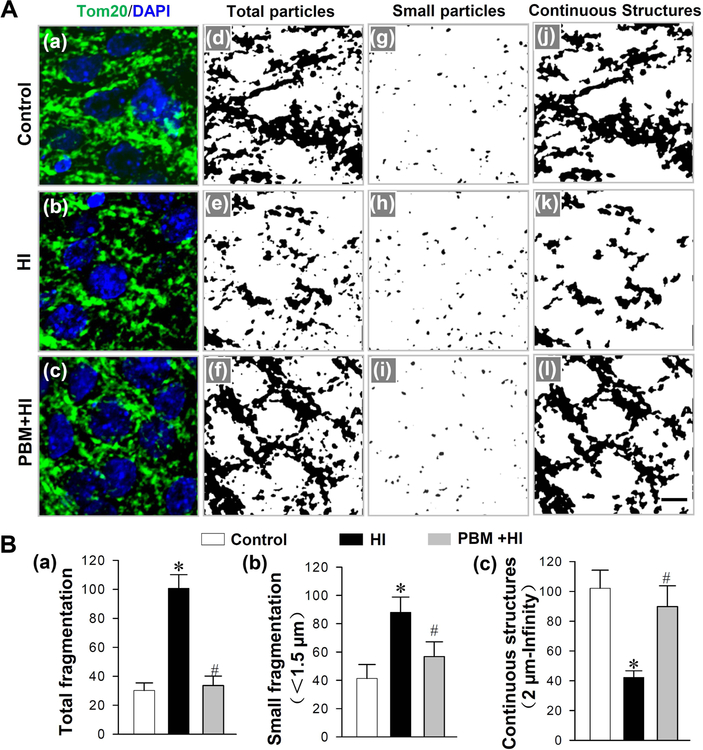
Mitochondrial dynamics play a central role in mitochondrial function and is critical for the maintenance of neuronal function and survival50. We next evaluated the effect of PBM pretreatment on mitochondrial morphology following HI insults. As indicated in Fig. 5A (a-c), the fragmented mitochondria in neurons from HI animals were markedly increased compared with neurons from normal animals, and PBM pretreated animals. To further define and analyze those changes, Image J software was applied to threshold, filter and binarize the images of TOM20 fluorescent staining. As shown in Fig. 5A (d-i) giving the results of quantitative analysis of total mitochondrial particles and small particles in Fig. 5B (a and b), the mitochondrial fragmentation in HI group was significantly elevated compared with the control group, and the increased mitochondrial fragmentation was reduced by PBM. Furthermore, continuous mitochondrial structures were decreased in HI animals, and this was prevented in HI animals with PBM preconditioning (Fig. 5A (j-l) and Fig. 5B (c)).
Fig. 5. Effects of PBM preconditioning on HI-induced changes in mitochondrial fragmentation in hippocampal CA1 region on P16.
(A) Typical confocal microscopy images of Tom 20 (green) and DAPI (blue) were shown in (a-c). The confocal images of Tom 20 were thresholded, filtered (median, 2.0 pixels), and binarized by image J software. As shown in (j-l), mitochondrial segments were separated as total particles (d-f), small particles (g-i, ze: 0–1.5μm), and continuous structures (j-l, size: 2μm- infinity). (B) The analysis of mitochondrial segments was counted and displayed in (a-c). The counts for total mitochondrial segments (a), small mitochondrial segments (b) were calculated as the percentage of the total mitochondrial area. Continuous mitochondrial structures were normalized to the total mitochondrial area (c). Values are presented as mean ± SD (n = 5–6). *P < 0.05 versus control group, #P < 0.05 versus HI group. Scale bar = 5 μm.
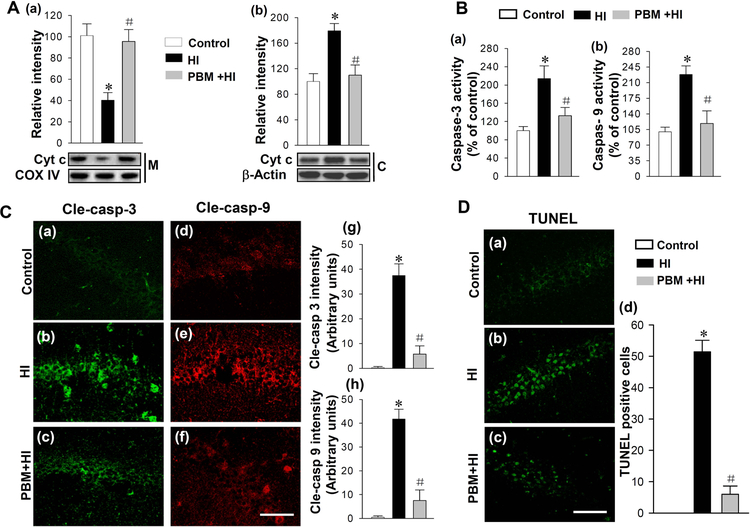
PBM Pretreatment Attenuates HI-induced Cytochrome c Release and the Mitochondrial Apoptotic Pathway
We next investigated the effect of PBM on the cytochrome c content in mitochondria and cytosol after HI insults. Results of western blotting showed that the HI insult resulted in significant decrease in cytochrome c in mitochondria compared with those in controls animals, and the effect was prevented by PBM pretreatment (Fig. 6A (a)). On the contrary, as shown in (Fig. 6A (b)), the levels of cytochrome c in the cyotosol were elevated in HI group compared with the control group, whereas PBM preconditioning significantly suppressed this increase. The changes in cytochrome c in mitochondria and cytosol suggested cytochrome c was released from the mitochondria to the cytosol after the HI insult, and this change of cytochrome c was attenuated by PBM pretreatment. Subsequently, we measured the activity of caspase-9 and caspase-3 in the cytosol. Results showed that both caspase-9 and caspase-3 activities were significantly increased following HI insults, which were effectively inhibited by PBM pretreatment (Fig. 6B). Furthermore, the active form of caspase-3 and caspase-9 were examined by immunofluorescence staining. Fig. 6C shows representative confocal microscopy of staining for cleaved caspase-3 and cleaved caspase-9 taken from the medial hippocampal CA1 region. The active form of caspase-3 and caspase-9 were clearly increased after HI insult compared to control groups, which was attenuated by PBM pretreatment. Finally, TUNEL staining was performed to measure apoptotic neuronal death in the hippocampal CA1. As presented in Fig. 6D (a-d), TUNEL staining revealed a robust increase in the number of TUNEL-positive cells compared to control groups, which was significantly attenuated by PBM pretreatment indicating the inhibitive effect of PBM on HI-induced apoptosis.
Fig. 6. Effects of PBM preconditioning on the content of cytochrome c in mitochondria and cytosol, the activities of caspase-9 and caspase-3, and apoptosis after HI insults.
(A) The content of cytochrome c in mitochondria (M) and cytosol (C) were measured by Western blotting and analyzed by Image J software (a and b). Substrate activity assay was performed to measure caspase-3 and caspase-9 activities using cytosolic proteins from hippocampus (B). (C) The active form of caspase-3 and caspase-9 were stained using anti-cleaved-caspase-3 and anti-cleaved-caspase-9. Representative confocal microscopy images of cleaved-caspase-3 and cleaved-caspase-9 were shown in (a-f). The fluorescent intensity of cle-caspase-3 and cle-caspase-9 were analyzed and shown in (g and h). (D) Apoptotic cells were stained by TUNEL staining as shown in (a-c). The numbers of TUNEL-positive cells were counted and analyzed as shown in (d). Data are presented as mean ± SD (n = 5–6). *P < 0.05 versus control group, #P < 0.05 versus HI group. Scale bar = 40 μm.
Discussion
In the present study, we have demonstrated that PBM preconditioning has the potential to prevent brain injury occurring after a hypoxic-ischemic event. Results suggested that ATP content reached the highest level 6 hours after PBM treatment compared with other PBM pretreatment groups. When PBM pretreatment was performed 6 hours before HI insults, its beneficial effects on preventing neuronal damage and attenuating long-term neurobehavioral deficits induced by HI insults were evident. The underlying mechanism of PBM pretreatment responsible for HI insults can be attributed to its ability to improve mitochondrial function. Morphological changes in mitochondria induced by HI insults can be effectively attenuated by PBM pretreatment. The maintenance of mitochondrial morphology prevented cytochrome c release followed by inhibition of the cytochrome c-mediated mitochondrial apoptotic pathway. Thus, the present study provides evidence for PBM preconditioning against HIE and supports the possible use of PBM treatment for prevention of HI-induced brain injury in the perinatal period in the future.
Currently, the only promising treatment for HIE is therapeutic hypothermia (TH). The underlying mechanism involved the inhibition of ROS production, the maintenance of mitochondrial function and the suppression of mitochondrial apoptotic signaling5, 51–54. While the beneficial effects of TH have been demonstrated in HIE models employing neonatal sheep, pigs and rats, its application for HIE treatment have limited by the requirement for specialized equipment and the narrow time window for effective application55, 56. For TH treatment, TH must be administered within minutes to a few hours, which is difficult to apply to infants who are delivered in rural and remote communities56. Even if the infants were accessible to the equipment for TH, it is still difficult to apply if HIE is only recognized after 6 hours, and serious cardiovascular side effects may occur after TH administration56–59. In a nutshell, possible adverse events and the complicated equipment required together limit the effectiveness and efficiency of TH. Therefore, finding a method to improve the recovery of the fetus after HI insults is important to decrease mortality and morbidity after difficult births.
Since the first publication described ischemic preconditioning, pretreatment regimens have attracted more and more attention45, 60–62. In the field of preconditioning, several regimens including hyperthermia, hypothermia, ischemia, hyperbaric oxygen, and physical exercise, have been applied for improving resistance to ischemic insults60, 63. As a response to preconditioning, pathways mediating resistance to ischemia are activated, and the cells are transformed to a phenotype that is resistant to tissue damage64–67. PBM as a non-invasive approach for modulating many biological processes and signaling pathways, has demonstrated a beneficial effect in improving behavioral performance in several brain disorders39, 42, 68. The non-invasive nature of transcranial PBM relies on its usage of near-IR light at 808 nm which has deep penetrance and minimal tissue media absorption, reaching depths of 2.5–3.0 cm when administered directly to the scalp surface in rabbits69–71. Furthermore, previous work investigating the effect of laser treatment with different wavelengths on brain injury suggested that laser irradiation with wavelength around 808 nm can significantly improve Neurological Severity Score and decrease brain damage compared with laser light at other wavelengths72. Our previous work has found beneficial effects of laser at 808 nm on animal models of stroke, Alzheimer disease and global cerebral ischemia37, 42, 73.
In the past several years, mitochondria have been conclusively demonstrated to be one of the main targets of PBM37, 41, 74, 75. One previous study investigated the time response of muscles to PBM, which suggested the importance of finding the optimal time to apply PBM to tissues as a preconditioning regimen76. In agreement with the results of mitochondrial response to PBM in muscles76, a similar time response of the brain to PBM was also found in the present study. The content of ATP in the hippocampus reached a peak at 6 hours after PBM treatment suggesting mitochondrial function reached its best level at that time. One characteristic effect of HIE is the rapid depletion of ATP in neurons induced by the deprivation of oxygen and glucose in the brain7, 8, 29. Therefore, if PBM can trigger the production of ATP at the highest level at the time of occurrence of HI insults, the damage induced by HI to the brain may be attenuated by PBM pretreatment. In this study, we investigated the effect of PBM treatment performed 6 hours before HI insult to prevent neuronal damage and neurobehavioral deficits.
Dendritic injury, involving the loss of neurons and synaptic proteins is closely related to deficits in learning and memory performance19, 42, 48, 77. It has been shown that MAP2, a neuron-specific cytoskeletal protein, which also functioned as a dendritic marker, played a critical role in the initial formation of neurites78, 79. The evidence demonstrated that decreased expression of MAP2 was correlated with cognitive impairment19, and on the contrary, an improvement of behavioral performance was associated with the prevention of MAP2 reduction42, 48. Furthermore, it has been widely accepted that synaptic proteins play a key role in learning and memory formation80, 81. Synaptophysin and spinophilin are synaptic proteins which are involved in synaptic signal transmission82, 83. Previous studies (including our own) have suggested that the loss of synaptophysin and spinophilin can be observed in neurodegenerative disorders and in brain injury48, 83–85. Since dendritic and synaptic changes are common in patients with poor learning and memory performance, methods that can improve their expression and function are beneficial to learning and memory48. In the present study, PBM pretreatment could prevent HI-induced decreased expressions of MAP2, spinophilin, and synaptophysin, suggesting that PBM preconditioning could improve the resistance of neurons to HI insults and alleviate dendritic and synaptic injury. In line with the effect of PBM pretreatment on limiting dendritic and synaptic damage, the behavioral results were significantly improved. Also, the loss of neurons in the hippocampus and the infarct area in the brain were markedly attenuated by PBM preconditioning, which confirmed the neuroprotective effect of PBM in HI-induced brain injury. Those effects are likely to be related to mitochondria, the main target of PBM.
Mitochondria play a central role in powering numerous cellular activities and modulation of various biological processes86–88. Over the past few decades, mitochondrial dysfunction has been extensively investigated in aged tissues and in brain disorders37, 42, 89. Our previous study demonstrated that the restoration of mitochondrial dynamics and the suppression of mitochondrial dysfunction can significantly attenuate behavioral deficits after stroke and AD37, 48, 90, and several studies have demonstrated that the recovery of mitochondrial function can provide the majority of the energy, needed for neuronal survival and neurogenesis37, 42. The important role of mitochondrial function in Parkinson’s disease, global cerebral ischemia, traumatic brain injury and depression indicate mitochondria are a major target in HI treatment and prevention75, 91–93. In HIE, the rapid depletion of ATP induced by mitochondrial dysfunction can trigger several pathophysiological response including excitotoxic amino acid release, the activation of glutamate receptors (AMPA, AMPA), increased cellular calcium uptake, intracellular Ca2+ accumulation, nitric oxide (NO) release and reactive oxygen species (ROS) production28–31. All these pathophysiological responses to mitochondrial dysfunction are closely related to mitochondrial dynamics, including the balance between mitochondrial fusion and fission. Impaired mitochondrial dynamics can result in mitochondrial fragmentation and facilitate cytochrome c release94. Cytochrome c is a key pro-apoptotic protein which will be released from the mitochondria to the cytosol when mitochondrial fragmentation occurs32, 33, 95–97. The released cytochrome c in the cytosol can trigger the activation of caspase-9 followed by caspase-3 and finally result in neuronal apoptosis98. Our previous work found that PBM posttreatment after HI conferred a robust neuroprotection by the attenuation of mitochondrial dysfunction and inhibition of neuronal apoptosis in the neonatal HI brain99. Consistent with our previous work, the present work demonstrated that PBM pretreatment was also able to contribute to the significant neuroprotection via the mitochondrial regulation.
In conclusion, we demonstrated that PBM preconditioning can maintain mitochondrial dynamics in neurons and inhibited mitochondrial fragmentation, which was in accordance with previous findings that PBM treatment can shift mitochondrial dynamics toward fusion, and maintain a healthy balance of mitochondrial dynamics42, 100. Furthermore, we also found PBM pretreatment can inhibit the release of cytochrome c from mitochondria to cytoplasm followed by the suppression of mitochondria-mediated apoptotic pathway and neuronal apoptosis. The mitochondria-mediated apoptotic pathway is a typical pathway playing a critical role in cell survival with considerable evidence in numerous studies101, 102. Our findings demonstrated that PBM pretreatment could suppress these pathways by the preservation of mitochondria in an HI model. Although the further in-depth investigation of the underlying mechanism of PBM pretreatment on HIE is still needed, the results of our study suggest the possible application to prevent HIE in humans. As indicated in our previous study, several important parameters contribute to the effect of PBM treatment. The effect of different power densities and dosages of PBM pretreatment on HI model are still unclear. Therefore, more work on this aspect is still needed in the future. In addition, further work will need to test whether the application of PBM to human infants that already have suffered a HI insult can produce a therapeutic benefit. It is not beyond the bounds of possibility that non-invasive Near Infrared light could be applied to the fetus during the actual birth process.
Acknowledgements
This study was supported by Research Grant NS086929 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA, and National Natural Science Foundation Grant of China (61575065 to CYL). Michael R Hamblin was supported by US NIH grants R01AI050875 and R21AI121700.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- [1].Xu F, Liu P, Pascual JM, Xiao G, Lu H Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2012, 32, 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kumral A, Uysal N, Tugyan K, Sonmez A, Yilmaz O, Gokmen N, Kiray M, Genc S, Duman N, Koroglu TF, Ozkan H, Genc K Behavioural brain research 2004, 153, 77–86. [DOI] [PubMed] [Google Scholar]
- [3].Juul SE, Ferriero DM Clinics in perinatology 2014, 41, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Colver A, Fairhurst C, Pharoah PO Lancet 2014, 383, 1240–1249. [DOI] [PubMed] [Google Scholar]
- [5].Pfister RH, Soll RF Journal of perinatology : official journal of the California Perinatal Association 2010, 30 Suppl, S82–87. [DOI] [PubMed] [Google Scholar]
- [6].van Handel M, Swaab H, de Vries LS, Jongmans MJ European journal of pediatrics 2007, 166, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mergenthaler P, Lindauer U, Dienel GA, Meisel A Trends in neurosciences 2013, 36, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V Nature neuroscience 2014, 17, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arteaga O, Alvarez A, Revuelta M, Santaolalla F, Urtasun A, Hilario E International journal of molecular sciences 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lu Y, Tucker D, Dong Y, Zhao N, Zhuo X, Zhang Q Journal of neuroscience and rehabilitation 2015, 2, 1–14. [PMC free article] [PubMed] [Google Scholar]
- [11].Low JA The journal of obstetrics and gynaecology research 2004, 30, 276–286. [DOI] [PubMed] [Google Scholar]
- [12].Volpe JJ Mental retardation and developmental disabilities research reviews 2001, 7, 56–64. [DOI] [PubMed] [Google Scholar]
- [13].Xiao J, Huang Y, Li X, Li L, Yang T, Huang L, Yang L, Jiang H, Li H, Li F Neuroscience bulletin 2016, 32, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vannucci SJ, Hagberg H The Journal of experimental biology 2004, 207, 3149–3154. [DOI] [PubMed] [Google Scholar]
- [15].Mi Z, Abrahamson EE, Ryu AY, Fish KN, Sweet RA, Mufson EJ, Ikonomovic MD Neurobiology of aging 2017, 55, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang R, Wang H, Carrera I, Xu S, Lakshmana MK The Journal of biological chemistry 2015, 290, 9299–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS Proceedings of the National Academy of Sciences of the United States of America 2004, 101, 2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eastwood SL, Lyon L, George L, Andrieux A, Job D, Harrison PJ J Psychopharmacol 2007, 21, 635–644. [DOI] [PubMed] [Google Scholar]
- [19].Liu HX, Zhang JJ, Zheng P, Zhang Y Brain research. Molecular brain research 2005, 139, 169–177. [DOI] [PubMed] [Google Scholar]
- [20].Haraldstad K, Rohde G, Stea TH, Lohne-Seiler H, Hetlelid K, Paulsen G, Berntsen S European review of aging and physical activity : official journal of the European Group for Research into Elderly and Physical Activity 2017, 14, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang FX, Sun QJ, Zheng XY, Lin YT, Shang W, Wang AH, Duan RS, Chi ZF Cellular and molecular neurobiology 2014, 34, 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Griva M, Lagoudaki R, Touloumi O, Nousiopoulou E, Karalis F, Georgiou T, Kokaraki G, Simeonidou C, Tata DA, Spandou E Brain research 2017, 1667, 55–67. [DOI] [PubMed] [Google Scholar]
- [23].Beraki S, Diaz-Heijtz R, Tai F, Ogren SO The international journal of neuropsychopharmacology 2009, 12, 243–255. [DOI] [PubMed] [Google Scholar]
- [24].Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS Endocrinology 2001, 142, 1284–1289. [DOI] [PubMed] [Google Scholar]
- [25].Wang C, Youle R Nature 2016, 530, 288–289. [DOI] [PubMed] [Google Scholar]
- [26].Busija DW, Gaspar T, Domoki F, Katakam PV, Bari F Advanced drug delivery reviews 2008, 60, 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chinopoulos C, Adam-Vizi V The FEBS journal 2006, 273, 433–450. [DOI] [PubMed] [Google Scholar]
- [28].Thornton C, Hagberg H Clinica chimica acta; international journal of clinical chemistry 2015, 451, 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hagberg H, Mallard C, Rousset CI, Thornton C The Lancet. Neurology 2014, 13, 217–232. [DOI] [PubMed] [Google Scholar]
- [30].Johnston MV, Fatemi A, Wilson MA, Northington F The Lancet. Neurology 2011, 10, 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Puka-Sundvall M, Gajkowska B, Cholewinski M, Blomgren K, Lazarewicz JW, Hagberg H Brain research. Developmental brain research 2000, 125, 31–41. [DOI] [PubMed] [Google Scholar]
- [32].Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ Progress in neurobiology 2014, 112, 24–49. [DOI] [PubMed] [Google Scholar]
- [33].Jellinger KA, Stadelmann C Journal of neural transmission. Supplementum 2000, 59, 95–114. [DOI] [PubMed] [Google Scholar]
- [34].Leaw B, Nair S, Lim R, Thornton C, Mallard C, Hagberg H Frontiers in cellular neuroscience 2017, 11, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Al Rahim M, Thatipamula S, Hossain MA Neurobiology of disease 2013, 50, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nijboer CH, Heijnen CJ, van der Kooij MA, Zijlstra J, van Velthoven CT, Culmsee C, van Bel F, Hagberg H, Kavelaars A Annals of neurology 2011, 70, 255–264. [DOI] [PubMed] [Google Scholar]
- [37].Yang L, Tucker D, Dong Y, Wu C, Lu Y, Li Y, Zhang J, Liu TC, Zhang Q Experimental neurology 2018, 299, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hamblin MR AIMS biophysics 2017, 4, 337–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hamblin MR BBA clinical 2016, 6, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR Annals of biomedical engineering 2012, 40, 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hamblin MR Journal of neuroscience research 2018, 96, 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME, Zhu L, Liu TC, Cohen RM, Zhang Q Neurobiology of aging 2017, 49, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Naeser MA, Hamblin MR Photomedicine and laser surgery 2015, 33, 443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hamblin MR Photochemistry and photobiology 2018, 94, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Agrawal T, Gupta GK, Rai V, Carroll JD, Hamblin MR Dose-response : a publication of International Hormesis Society 2014, 12, 619–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR PM & R : the journal of injury, function, and rehabilitation 2010, 2, S292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang J, Tucker LD, DongYan Y. Lu, Yang L, Wu C, Li Y, Zhang Q Neurochemistry international 2018, 116, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wu C, Yang L, Tucker D, Dong Y, Zhu L, Duan R, Liu TC, Zhang Q Medicine and science in sports and exercise 2018, 50, 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen W, An D, Xu H, Cheng X, Wang S, Yu W, Yu D, Zhao D, Sun Y, Deng W, Tang Y, Yin S PeerJ 2016, 4, e2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Flippo KH, Strack S Journal of cell science 2017, 130, 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu G, Li ZG, Gao JS European review for medical and pharmacological sciences 2017, 21, 50–53. [PubMed] [Google Scholar]
- [52].Fan J, Cai S, Zhong H, Cao L, Hui K, Xu M, Duan M, Xu J Neuroscience letters 2017, 647, 45–52. [DOI] [PubMed] [Google Scholar]
- [53].Yenari MA, Han HS Nature reviews. Neuroscience 2012, 13, 267–278. [DOI] [PubMed] [Google Scholar]
- [54].Westin B, Miller JA Jr., Nyberg R, Wedenberg E Surgery 1959, 45, 868–879. [PubMed] [Google Scholar]
- [55].Laptook AR, Shankaran S, Tyson JE, Munoz B, Bell EF, Goldberg RN, Parikh NA, Ambalavanan N, Pedroza C, Pappas A, Das A, Chaudhary AS, Ehrenkranz RA, Hensman AM, Van Meurs KP, Chalak LF, Khan AM, Hamrick SEG, Sokol GM, Walsh MC, Poindexter BB, Faix RG, Watterberg KL, Frantz ID 3rd, Guillet R, Devaskar U, Truog WE, Chock VY, Wyckoff MH, McGowan EC, Carlton DP, Harmon HM, Brumbaugh JE, Cotten CM, Sanchez PJ, Hibbs AM, Higgins RD Jama 2017, 318, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ Lancet 2005, 365, 663–670. [DOI] [PubMed] [Google Scholar]
- [57].Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW Resuscitation 2012, 83, 188–196. [DOI] [PubMed] [Google Scholar]
- [58].Nielsen N, Sunde K, Hovdenes J, Riker RR, Rubertsson S, Stammet P, Nilsson F, Friberg H Critical care medicine 2011, 39, 57–64. [DOI] [PubMed] [Google Scholar]
- [59].Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J International journal of cardiology 2011, 151, 333–341. [DOI] [PubMed] [Google Scholar]
- [60].Koch S, Della-Morte D, Dave KR, Sacco RL, Perez-Pinzon MA Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2014, 34, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Das M, Das DK IUBMB life 2008, 60, 199–203. [DOI] [PubMed] [Google Scholar]
- [62].Murry CE, Jennings RB, Reimer KA Circulation 1986, 74, 1124–1136. [DOI] [PubMed] [Google Scholar]
- [63].Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y Progress in neurobiology 2014, 114, 58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Semenza GL Biochimica et biophysica acta 2011, 1813, 1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang X, Cohen MV, Downey JM Cardiovascular drugs and therapy 2010, 24, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dorn GW 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D Proceedings of the National Academy of Sciences of the United States of America 1999, 96, 12798–12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Currie RW Journal of molecular and cellular cardiology 1987, 19, 795–808. [DOI] [PubMed] [Google Scholar]
- [68].Hennessy M, Hamblin MR J Opt 2017, 19, 013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lapchak PA, Han MK, Salgado KF, Streeter J, Zivin JA Stroke 2008, 39, 3073–3078. [DOI] [PubMed] [Google Scholar]
- [70].Lapchak PA, Salgado KF, Chao CH, Zivin JA Neuroscience 2007, 148, 907–914. [DOI] [PubMed] [Google Scholar]
- [71].Lapchak PA, Wei J, Zivin JA Stroke 2004, 35, 1985–1988. [DOI] [PubMed] [Google Scholar]
- [72].Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, Dai T, Dhital S, Sharma SK, Whalen MJ, Hamblin MR Lasers in surgery and medicine 2012, 44, 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang R, Dong Y, Lu Y, Zhang W, Brann DW, Zhang Q Molecular neurobiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR Molecular neurobiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, Zhang G, Yang L, Li J, Du X, Zhang Q, Xu X Molecular neurobiology 2017, 54, 4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ferraresi C, de Sousa MV, Huang YY, Bagnato VS, Parizotto NA, Hamblin MR Lasers in medical science 2015, 30, 1259–1267. [DOI] [PubMed] [Google Scholar]
- [77].Bernstein HG, Dobrowolny H, Keilhoff G, Steiner J Neurochemistry international 2018, 114, 55–57. [DOI] [PubMed] [Google Scholar]
- [78].Cortes-Canteli M, Pignatelli M, Santos A, Perez-Castillo A The Journal of biological chemistry 2002, 277, 5460–5467. [DOI] [PubMed] [Google Scholar]
- [79].Scott EK, Luo L Nature neuroscience 2001, 4, 359–365. [DOI] [PubMed] [Google Scholar]
- [80].Kaang BK, Lee SH, Kim H The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 2009, 15, 430–435. [DOI] [PubMed] [Google Scholar]
- [81].Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ Nature medicine 2008, 14, 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tarsa L, Goda Y Proceedings of the National Academy of Sciences of the United States of America 2002, 99, 1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P Proceedings of the National Academy of Sciences of the United States of America 2000, 97, 9287–9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, Gouras GK The Journal of neuroscience : the official journal of the Society for Neuroscience 2010, 30, 14299–14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, Ma T, Zheng R, Lu B, Nanus DM, Lin MT, Gouras GK The Journal of neuroscience : the official journal of the Society for Neuroscience 2009, 29, 9704–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kiriyama Y, Nochi H Cells 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Burke PJ Trends in cancer 2017, 3, 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhu Z, Wang X Advances in experimental medicine and biology 2017, 1038, 219–230. [DOI] [PubMed] [Google Scholar]
- [89].Theurey P, Pizzo P Genes 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lec FL, Alexopoulos T, Boulu-Reshef B, Fayant MP, Zenasni F, Lubart T, Jacquemet N The Behavioral and brain sciences 2017, 40, e32. [DOI] [PubMed] [Google Scholar]
- [91].Li X, Wang H, Wen G, Li L, Gao Y, Zhuang Z, Zhou M, Mao L, Fan Y Journal of cellular and molecular medicine 2018, 22, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q Mol Neurobiol 2016, 53, 5344–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK The Journal of biological chemistry 2008, 283, 9089–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Otera H, Miyata N, Kuge O, Mihara K The Journal of cell biology 2016, 212, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, Neagu MR, Nilsson M, Eriksson PS, Hagberg H, Luban J, Kroemer G, Blomgren K The Journal of experimental medicine 2007, 204, 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Blomgren K, Hagberg H Free radical biology & medicine 2006, 40, 388–397. [DOI] [PubMed] [Google Scholar]
- [97].Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H The Journal of biological chemistry 2001, 276, 10191–10198. [DOI] [PubMed] [Google Scholar]
- [98].Xiong S, Mu T, Wang G, Jiang Protein X & cell 2014, 5, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tucker LD, Lu Y, Dong Y, Yang L, Li Y, Zhao N, Zhang Q Journal of molecular neuroscience : MN 2018, 65, 514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yin K, Zhu R, Wang S, Zhao RC Stem cells and development 2017, 26, 762–775. [DOI] [PubMed] [Google Scholar]
- [101].Shi M, Zhou L, Zhao L, Shang M, He T, Tang Z, Sun H, Ren P, Lin Z, Chen T, Yu J, Xu J, Yu X, Huang Y PLoS neglected tropical diseases 2017, 11, e0006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A Human molecular genetics 2003, 12, 517–526. [DOI] [PubMed] [Google Scholar]