Abstract
BACKGROUND:
This study aims to describe patterns of truncal versus peripheral fat deposition measured by truncal-to-leg fat ratio (TLR) in adolescents, and examine associations of TLR with cardiometabolic (CMD) risk factors.
METHODS:
Data were from 3,810 adolescents (12–19 yrs old) in the National Health and Examination Survey (NHANES) 2003–2006. Body fat was assessed by dual-energy X-ray absorptiometry, and CMD risk factors were determined by blood samples and physical examination. Linear and logistic regression adjusted for BMI z-score and other covariates were used to examine associations of TLR with CMD risk factors as continuous and dichotomized outcomes, respectively.
RESULTS:
Adolescents who were Mexican American, lower income, and with obesity had the highest mean TLR (all p<0.05). In linear regression, increasing TLR was associated positively with homeostasis model assessment of insulin resistance (HOMA-IR), triglycerides, total cholesterol, systolic blood pressure (BP), c-reactive protein, and alanine aminotransferase (ALT), and negatively with high-density lipoprotein (HDL) cholesterol in both sexes (p<0.05). TLR was also associated with diastolic BP in boys and low-density lipoprotein cholesterol in girls (p<0.05). A similar pattern of findings resulted from logistic regression. When further stratified by race/ethnicity, TLR was positively associated with high triglycerides, total cholesterol, and ALT for white and/or Mexican American (p<0.05), but not black adolescents, while associations with HOMA-IR and HDL were significant for all race/ethnicities.
CONCLUSIONS:
In this cohort of adolescents, TLR was associated with several risk factors independent of BMI z-score, though some findings were sex or race/ethnicity-specific. Body fat distribution may be an important determinant of future CMD.
Keywords: Body composition, obesity, cardiometabolic disease, pediatric, trunk fat, leg fat
INTRODUCTION
The increasing incidence of cardiometabolic disease (CMD) risk factors among children and adolescents presents an important public health challenge in the United States and worldwide.1 In particular, children with obesity based on age- and sex-adjusted body mass index (BMI) are at increased risk of metabolic syndrome compared to normal weight counter parts.2,3 However, the relationship between obesity and CMD risk is complex, as some children with obesity can be classified as “metabolically healthy,” and vice versa.4 This suggests that factors beyond obesity influence CMD risk.
Body fat distribution is one determinant of metabolic dysfunction that cannot be measured by BMI alone.5 Similar to adult studies, pediatric studies have also found strong associations of abdominal fat, which includes both visceral and subcutaneous adipose tissue, with CMD risk factors independent of total adiposity measured as total body fat or BMI.6 Although the exact contribution of individual abdominal adipose compartments to metabolic risks is controversial, the general hypothesis is that excess abdominal fat contributes to increased systemic free fatty acid flux, as well as a pro-inflammatory state, which promotes insulin resistance and other metabolic syndrome components.7
In contrast, increased peripheral subcutaneous adipose tissue, especially in the leg or gluteo-femoral regions, has been proposed as a protective fat depot against metabolic dysfunction.8 Though most evidence has been found in adults, a few studies have also been completed in children and adolescents.9–11 Staiano et al. found that percent leg fat (calculated as leg fat/total body fat) was associated with decreased risk of low high density lipoprotein-cholesterol (HDL-C), elevated blood triglycerides, and insulin resistance assessed by the homeostatic model of insulin resistance assessment (HOMA-IR) among U.S. white and African American children and adolescents ages 5–18 yrs old.10 These associations were independent of total body fat, age, sex, race, maturation stage, and physical activity. Similarly, Samouda et al. found significant negative correlations between leg fat mass and HOMA-IR, blood triglycerides, systolic blood pressure, and C-reactive protein (CRP), and positive correlations with HDL-C, among Caucasian European children and adolescents ages 7–17 yrs old with adjustment for similar covariates as above.11 One proposed mechanism for the potential “protective” properties of leg fat is that an increased capacity for peripheral subcutaneous fat storage may prevent the spillover of fatty acids and triglycerides into abdominal fat depots during positive energy balance. However, the exact biological pathways are still being investigated.
These findings suggest that children with a higher ratio of abdominal-to-peripheral fat deposition may be at greater risk for the development of CMD. A few studies have examined this question in children using an android-to-gynoid fat ratio,12–15 which found this marker to be significantly associated with certain measures of CMD risk. However, these studies were limited by relatively small sample sizes (ranging 66 to 193 participants), which limits the potential for adequately powered stratified analyses by sex and race/ethnicity, and their generalizability by including only white and/or African American children or only children with overweight or obesity. Additionally, none of these pediatric studies used a ratio of truncal to leg fat, which has been associated with CMD-related outcomes adults.16
Dual-energy X-ray absorptiometry (DXA) is a precise and accurate clinical technology for directly measuring body fat and lean tissue mass, and bone mineral density. From 1999–2006, the National Health and Nutrition Examination Survey (NHANES), a nationally-representative survey of U.S. citizens released in two-year cycles, performed whole-body DXA scans to assess body composition, in addition to other clinical and dietary assessments. Using these data, the objective of this study was to describe patterns of truncal-to-leg fat ratio (TLR) assessed by DXA among U.S. adolescents, and to examine the associations of TLR with cardiometabolic disease risk factors, independent of BMI, among adolescents overall and stratified by sex and race/ethnicity. We also compared these associations to other individual body composition variables, including waist circumference, as well trunk fat mass and leg fat mass as individual variables.
METHODS
NHANES is a continuous surveillance survey conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS) to assess the health and nutritional status of the U.S. population. It uses a stratified, multi-stage probability sampling design to obtain nationally-representative estimates, and includes a household interview followed by additional assessments at mobile examination centers (MEC). Data for this study were obtained from NHANES cycles 2003–2004 and 2005–2006, as these were the two most recent cycles during which DXA body composition assessment was performed. The initial eligible sample was 4,455 adolescents ages 12 to 19 yrs old. We chose this age group due to the availability of more complete laboratory assessments that were not performed in children younger than 12 yrs old. Among this sample, 131 individuals were excluded for not having valid multiply-imputed DXA data, and 514 individuals were excluded for having missing or invalid data for the covariates of interest, described next, including income status, total energy intake, physical activity level, or BMI z-score, resulting in an analytical sample of 3,810 adolescents. For analyses requiring fasting biomarkers, we used a sub-sample of 1,625 adolescents who were examined in the morning after an overnight fast.
Assessment of Anthropometrics and Body Composition
Height, weight, and waist circumference were measured using standardized protocols.17 Age- and sex- adjusted BMI percentiles and z-scores were calculated using the 2000 Centers for Disease Control and Prevention (CDC) growth charts.18 Children and adolescents were categorized into the following weight status categories: underweight = < 5th percentile; normal weight = 5th – 84th percentile; overweight = 85th to 94th percentile; obese = ≥ 95th percentile.19 Total and regional body composition measurements were collected by DXA for participants ages 8 yrs and older using a Hologic QDR-4500A fan-beam densitometer (Hologic, Inc., Bedford, MA, USA), and scans were analyzed using Hologic Discovery software v12.1.20 Participants were excluded from the DXA scan if they were pregnant, had amputations other than toes or fingers, weighed more than 300 lbs (136 kg), or were taller than 6’5” (195 cm). In each scan, soft tissue measures for fat mass and lean mass were obtained for the head, arms, legs, and trunk regions. Based on evidence that the densitometer’s algorithm overestimated lean mass and underestimated fat mass, the DXA values for lean mass were decreased by 5% and an equivalent weight was added to the fat mass.20,21 It was also found that the percentage of valid data from DXA decreased with age and BMI, due to the increasing prevalence of implants and higher rates of obesity, and due to the exclusion of individuals greater than 300 lbs, respectively. To address this nonrandom nature of missing data for the DXA scans, multiple imputation was performed by NCHS using a sequential regression multivariate imputation method to generate five imputed datasets. Thus, the DXA data used in this analysis included individuals who completed the scan and individuals who were excluded for reasons other than pregnancy, but whose data was imputed. We calculated truncal-to-leg fat ratio (TLR) using the DXA data as: (trunk fat mass (grams) divided by the sum of left and right leg fat mass (grams)), multiplied by 100.
Assessment of Cardiometabolic Risk Factors
Cardiometabolic risk factors were determined from blood samples and the physical examination. For all participants, serum CRP was quantified using latex-enhanced nephelometry, and serum HDL-C (mg/dL) and total-cholesterol (TC, mg/dL) were analyzed by direct immunoassay and enzymatically, respectively. Blood pressure (BP) was measured up to three times by a trained examiner after participants rested quietly for 5 minutes. In our sample, 73% of subjects had 3 measurements, 13% had 2 measurements, and 12% had 1 measurement for BP. Mean systolic BP and diastolic BP were calculated as the average of all available blood pressure measurements. The liver enzyme alanine aminotransferase (ALT), a marker of non-alcoholic fatty liver disease (NAFLD), was analyzed using an enzymatic rate method. Hemoglobin A1C, a measure of 3-month glucose control, was measured using high-performance liquid chromatography (HPLC). A sub-sample of participants also underwent fasting blood draws during the morning visit. For these participants, fasting plasma glucose (mg/dL) was measured by a hexokinase method, and fasting plasma insulin (uU/mL) was measured by a two-site enzyme immunoassay. HOMA-IR was calculated using the following formula: [(fasting insulin (mU/L) x fasting glucose (mg/dL)) / 405].22 Fasting plasma triglycerides (mg/dL) were measured enzymatically using a series of coupled reactions and fasting low-density lipoprotein cholesterol (LDL-C, mg/dL) was calculated using the Friedewald equation.23
Assessment of Other Covariates
Age, sex, and race/ethnicity were collected by self-report during the household interview. Household income status, measured by poverty-income ratio (PIR), was also collected during the household interview and included as a covariate in this analysis as prior studies have found lower socioeconomic status to be associated with greater risk of abdominal obesity and future CMD in children and adolescents.24 Total energy intake in kilocalories per day was estimated using day one 24-hour dietary recall data collected during the in-person interview using the USDA’s Automated Multiple Pass Method.25 Physical activity level (PAL) was estimated based on NHANES’ self-reported physical activity questionnaire. Specifically, the questionnaire asked for the frequency and duration that they performed a list of activities in the past 30 days. Each activity was assigned a metabolic equivalent (MET) value based on the intensity. For each participants, we estimated PAL by calculating the MET minutes per week of moderate and vigorous physical activity (MVPA), where “moderate” activities were defined as those that cause light sweating or a slight to moderate increasing breathing or heart rate, and “vigorous” activities were defined as those that cause heavy sweating or large increases in breathing or heart rate. Lastly, maturation based on tanner staging was not assessed during these cycles, but we were able to dichotomize females based on whether they had started their menstrual period or not using data from the reproductive health questionnaire, which asked “how old were you when you had your first menstrual cycle?”.
Statistical Analysis
SAS v9.4 and the SAS-callable SUDAAN (SAS Institute Inc., Cary, NC, USA) were used for all statistical analyses. We adjusted for NHANES’ complex survey design using sampling strata, primary sampling unit, and individual sampling weights, which were either the MEC exam weights or fasting sub-sample weights as appropriate. We also accounted for the multiply-imputed DXA data by carrying out the analysis five times, once on each of the imputed data sets, and combining estimates using appropriate pooling methods in SUDAAN. Descriptive statistics of the sample were summarized as counts and weighted frequencies for categorical variables and means and standard errors for continuous variables. Mean TLR was calculated for each subgroup level and compared to a reference group using t-tests. Linear regression was performed to examine overall associations of TLR with CMD risk factors. This analysis was performed with the sample overall and stratified by sex due to sex-specific changes in body composition and metabolism in childhood and adolescence.26 All variables were assessed for normality prior to analysis using descriptive plots, including histograms and normal probability plots, and the following variables were log-transformed due to skewness: TLR, CRP, triglycerides, fasting glucose, fasting insulin, HOMA-IR, and ALT. Models were adjusted for sex (for the overall model), race/ethnicity, age (years), PIR, total energy intake (kcal/day), physical activity level, presence of menarche (for the girls only model), and BMI z-score.
We then performed logistic regression, adjusted for the same covariates, to examine associations of log-transformed TLR with the following dichotomized outcomes: high triglycerides (≥ 130 mg/dL), low HDL-C (< 40 mg/dL), high LDL-C (≥ 130 mg/dL), high TC (≥ 130 mg/dL), high ALT (>25 U/L for males; > 22 U/L for females), and high blood pressure (systolic or diastolic BP ≥ 95th percentile for age, sex, and height if 12–17 yrs old; systolic BP ≥130 mmHg and/or diastolic BP ≥80 mmHg if 18–19 yrs old; or self-report of taking prescription for hypertension).27 High HOMA-IR (≥ 3) was defined based on prior research, though it should be noted that no standard cut-off exists in children.28 As a comparison, this analysis was repeated first using waist circumference (WC), a readily available anthropometric measurement of abdominal fat, and second using individual variables of trunk fat mass (kg) and leg fat mass (kg) in place of TLR. For the trunk fat and leg fat mass models, we also controlled for leg and trunk fat mass, respectively, to assess the independent associations of each fat mass with the CMD risk factors. Log-transformed TLR, WC, and trunk and leg fat masses were standardized prior to analysis; thus, results are presented for each standard deviation increase in the predictor variable. We also performed the logistic regression models stratified according to non-Hispanic white, Mexican American, and non-Hispanic black adolescents to assess racial/ethnic differences. We did not control for multiple testing in the analysis due to the exploratory nature of this analysis, but presented all results from linear and logistic regression as beta coefficients and odds ratios, respectively, with 95% confidence intervals.
RESULTS
Descriptive statistics of the analytical sample are presented in Table 1. TLR was significantly increased in the lowest income compared to the highest income group, and in participants with overweight or obesity compared to normal weight (all p<0.05). Additionally, compared to non-Hispanic white race/ethnicity, Mexican American had significantly higher mean TLR, while non-Hispanic black had significantly lower mean TLR (both p<0.05).
Table 1:
Sociodemographic, health, and body composition characteristics of the analytical sample of adolescents 12–19 yrs (n=3,810), NHANES 2003–2006
| Variable | Count (%) | TLR mean ± SE | p-value* |
|---|---|---|---|
| Full sample | 3,810 (100%) | 97.6 ± 0.7 | - |
| Sex | |||
| Boys | 1985 (52.0%) | 98.1 ± 1.0 | Ref |
| Girls | 1825 (48.0%) | 97.1 ± 0.9 | 0.44 |
| Race/Ethnicity | |||
| Non-Hispanic White | 1042 (64.5%) | 97.7 ± 1.0 | Ref |
| Mexican American | 1161 (10.7%) | 106.2 ± 1.3 | <0.001 |
| Non-Hispanic Black | 1334 (14.7%) | 90.0 ± 0.8 | <0.001 |
| Other | 273 (10.1%) | 99.2 ± 2.4 | 0.56 |
| PIR | |||
| < 130% | 1577 (28.6%) | 101.1 ± 1.3 | <0.001 |
| 130–300% | 1174 (29.6%) | 99.6 ± 1.3 | 0.002 |
| > 300% | 1059 (41.8%) | 93.8 ± 1.0 | Ref |
| Weight Status | |||
| Underweight | 86 (2.9%) | 88.0 ± 3.4 | 0.95 |
| Normal Weight | 2249 (60.8%) | 88.2 ± 0.7 | Ref |
| Overweight | 649 (17.3%) | 106.1 ± 1.7 | <0.001 |
| Obese | 826 (19.0%) | 121.5 ± 1.2 | <0.001 |
| Menarche (girls only) | |||
| Pre-Menarche | 125 (8.3%) | 84.7 ± 2.3 | <0.001 |
| Post-Menarche | 1700 (91.7%) | 98.2 ± 1.0 | Ref |
P-values were calculated using independent t-tests comparing each variable sub-level to the reference category, indicated by “Ref”. Abbreviations: TLR, truncal-to-leg fat ratio; PIR, poverty income ratio
The results from linear regression of TLR with CMD risk factors as continuous outcomes are summarized in Table 2. In the sample overall, higher TLR was associated with more deleterious levels of all CMD risk factors, except for fasting plasma glucose and hemoglobin A1C in both sexes, LDL-C in boys only, and diastolic BP in girls only. These findings were significant after adjustment for sex (for the overall model), BMI z-score, race/ethnicity, PIR, total energy intake, physical activity, and menarche (for the girls only model).
Table 2:
Results from linear regression of TLR with continuous CMD risk factors among adolescents (12–19 yrs) stratified by sex and age, NHANES 2003–2006
| Full Sample | Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CMD Risk Factor | n | β* | 95% CI | n | β | 95% CI | n | β | 95% CI |
| HOMA-IR (log ratio) | 1590 | 0.6 | (0.4, 0.8) | 849 | 0.9 | (0.5, 1.2) | 741 | 0.5 | (0.3, 0.7) |
| Fasting glucose (log mg/dL) | 1624 | −0.01 | (−0.1, 0.1) | 860 | −0.03 | (−0.2, 0.1) | 764 | 0.02 | (−0.03, 0.1) |
| Fasting insulin (log mg/dL) | 1590 | 0.6 | (0.4, 0.8) | 849 | 0.9 | (0.5, 1.3) | 741 | 0.5 | (0.3, 0.7) |
| Hemoglobin A1C (%) | 3542 | 0.01 | (−0.1, 0.1) | 1844 | −0.1 | (−0.3, 0.1) | 1698 | 0.1 | (−0.01, 0.2) |
| Triglycerides (log mg/dL) | 1593 | 0.6 | (0.4, 0.8) | 849 | 0.8 | (0.5, 1.1) | 744 | 0.5 | (0.3, 0.7) |
| LDL cholesterol (mg/dL) | 1588 | 17.9 | (5.3, 30.4) | 847 | 17.9 | (−3.1, 38.8) | 741 | 22.4 | (8.6, 36.3) |
| HDL cholesterol (mg/dL) | 3502 | −12.1 | (−15.1, −9.1) | 1831 | −12.9 | (−17.0, −8.9) | 1671 | −9.3 | (−13.8, −4.9) |
| Total cholesterol (mg/dL) | 3502 | 14.4 | (7.0, 21.7) | 1831 | 19.2 | (7.7, 30.6) | 1671 | 18.0 | (6.0, 30.0) |
| Systolic BP (mm Hg) | 3727 | 4.6 | (1.7, 7.4) | 1933 | 4.5 | (0.5, 8.6) | 1794 | 3.1 | (0.4, 6.6) |
| Diastolic BP (mm Hg) | 3712 | 5.0 | (2.0, 7.9) | 1920 | 6.5 | (2.6, 10.4) | 1792 | 3.1 | (−0.6, 6.9) |
| CRP (log mg/dL) | 3522 | 1.2 | (0.8, 1.6) | 1837 | 1.1 | (0.7, 1.6) | 1685 | 1.5 | (1.0, 2.0) |
| ALT (log U/L) | 3479 | 0.2 | (0.1, 0.3) | 1820 | 0.4 | (0.3, 0.5) | 1659 | 0.1 | (0.02, 0.3) |
βs and 95% confidence intervals (CIs) were from linear regression of TLR with each CMD risk factor adjusted for sex (for the overall model), race/ethnicity, age, household income status, total energy intake, physical activity level, BMI z-score, and menarche (for the girls only model). TLR was log-transformed prior to analysis. Bolding indicates statistically significant 95% CIs. Abbreviations: TLR, truncal-to-leg fat ratio; CMD, cardiometabolic disease; BP, blood pressure; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, Homeostatic model of assessment of insulin resistance; ALT, alanine aminotransferase.
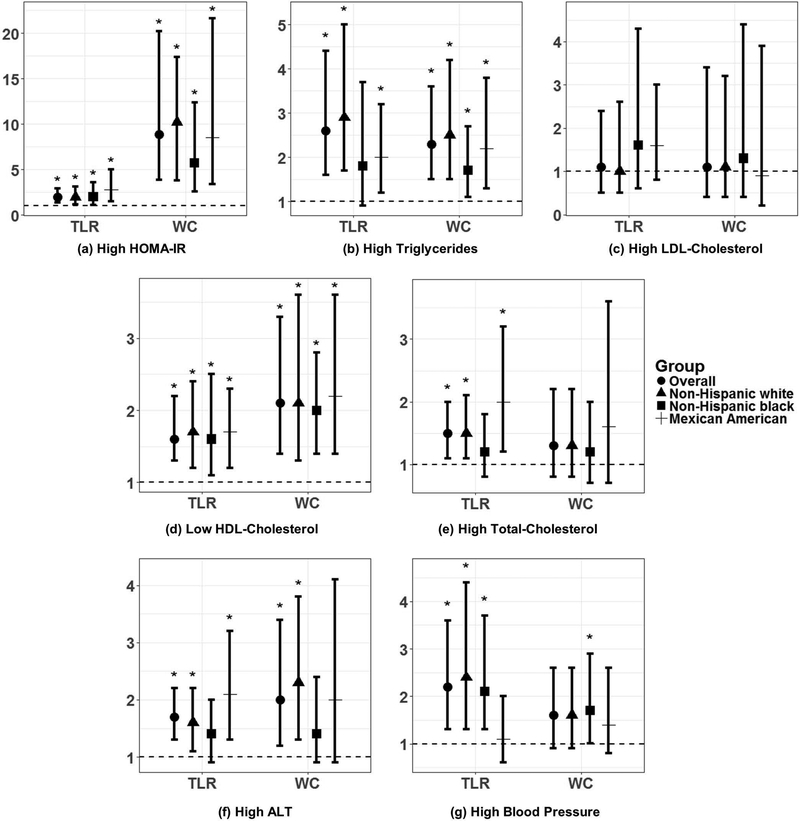
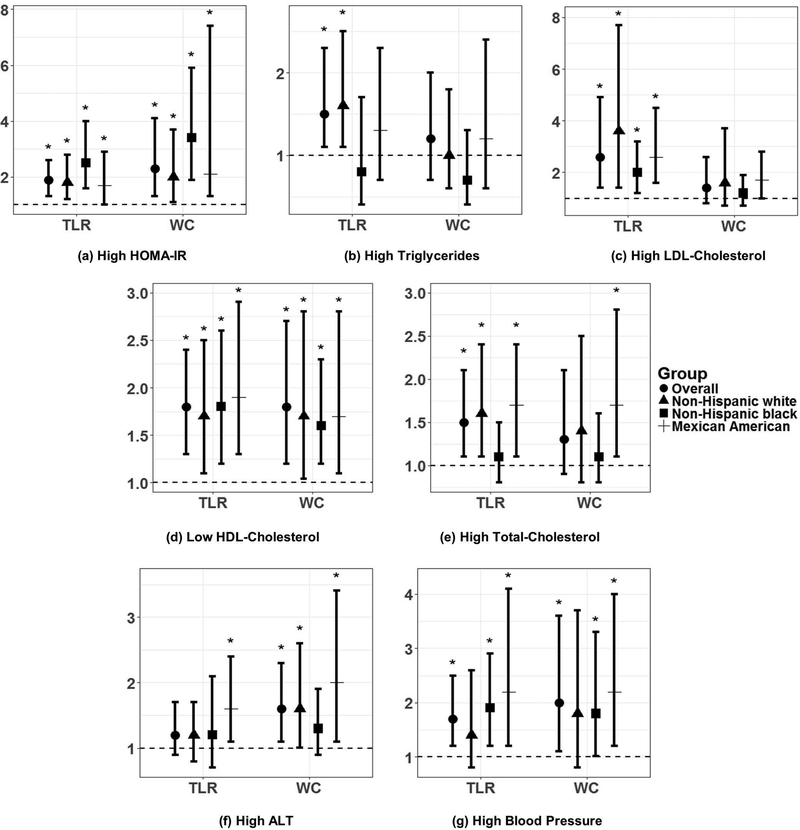
In sex-stratified logistic regression of TLR with odds of dichotomized CMD risk factors, each standard deviation increase in log-transformed TLR was associated with greater odds of high HOMA-IR [OR (95% CI) in boys (B): 2.0(1.4, 2.9) and girls (G): 1.9 (3.6, 2.6)], high triglycerides [B: 2.6 (1.6, 4.4), G: 1.5 (1.1, 2.3)], low HDL-C [B: 1.6 (1.3, 2.2), G: 1.8 (1.3, 2.4)], high TC [B: 1.5 (1.1, 2.0), G: 1.5 (1.1, 2.1)], and high BP [B: 2.5 (1.3, 3.6), G: 1.7 (1.2, 2.5)], after adjustment for the same covariates as in linear regression (Figures 1 and 2). Additionally, increasing TLR was associated with odds of high ALT in boys [1.7 (1.3, 2.2)], and high LDL-C in girls [2.6 (1.4, 4.9)]. In analyses stratified by race/ethnicity, the association of TLR with several CMD risk factors was significant for non-Hispanic white and/or Mexican American adolescents, but not non-Hispanic black adolescents, including high triglycerides (boys only), high TC (boys and girls), and high ALT (boys and girls) (Figures 1 & 2). Odds ratios and 95% confidence intervals for each model are presented in Online Supplementary Table S1.
Figure 1:
Results from logistic regression of TLR and WC with dichotomized CMD risk factors (a-g) among adolescent boys (12–19 yrs), NHANES 2003–2006. Y-axis is on the log-odds scale. Points and errors bars represent ORs and 95% confidence intervals (CIs). Asterisks (*) indicate significant 95% CIs. All models were adjusted for race/ethnicity, age, household income status, total energy intake, physical activity level, and BMI z-score. TLR and WC were standardized to z-scores prior to analysis; thus, each odds ratio refers to each 1 standard deviation increase in TLR or WC. “Other” race/ethnic group was not included in this analysis due to small sample size and insignificant findings. Abbreviations: CMD, cardiometabolic disease; TLR, truncal-to-leg fat ratio; WC, waist circumference; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, Homeostatic Model of Assessment Insulin Resistance; ALT, alanine aminotransferase.
Figure 2:
Results from logistic regression of TLR and WC with dichotomized CMD risk factors (a-g) among adolescent girls (12–19 yrs), NHANES 2003–2006. Y-axis is on the log-odds scale. Points and errors bars represent ORs and 95% confidence intervals (CIs). Asterisks (*) indicate significant 95% CIs. All models were adjusted for race/ethnicity, age, household income status, total energy intake, physical activity level, menarche status, and BMI z-score. TLR and WC were standardized to z-scores prior to analysis; thus, each odds ratio refers to each 1 standard deviation increase in TLR or WC. “Other” race/ethnic group was not included in this analysis due to small sample size and insignificant findings. Abbreviations: CMD, cardiometabolic disease; TLR, truncal-to-leg fat ratio; WC, waist circumference; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, Homeostatic Model of Assessment Insulin Resistance; ALT, alanine aminotransferase.
When comparing TLR and WC in sex-stratified logistic regression, several of the associations of TLR with CMD risk were also significant for WC (Figures 1 & 2). In boys, the exception was for high TC and high BP, which were significantly associated with TLR, but not with WC. In girls, the exception was for high triglycerides, high LDL-C, and high TC, which were all significantly associated with TLR, but not with WC (Figures 1 and 2, Online Supplementary Table S1). The results of logistic regression of trunk fat mass and leg fat mass with CMD risk factors are also summarized in Online Supplementary Table S2. Briefly, for both boys and girls overall, each standard deviation increase in trunk fat mass was associated with greater odds of high HOMA-IR [B:8.8 (3.2, 23.8), G: 3.4 (1.2, 9.1)], low HDL-C [B: 2.3 (1.3, 3.9), G: 2.5 (1.3, 4.8)], high TC [B: 2.4 (1.4, 4.2), G: 2.5 (1.4, 4.3)], high ALT [B: 3.0 (1.5, 5.8), G: 1.9 (1.1, 3.3)], and high BP [B: 5.1 (2.7, 9.5), G: 3.4 (1.7, 7.1)], while leg fat was associated with lower odds of high triglycerides [B: 0.3 (0.1, 0.7), G: 0.6 (0.3, 0.98)], high TC [B: 0.5 (0.3, 0.9), G: 0.5 (0.3, 0.8)], and high BP [B: 0.3 (0.1, 0.5), G: 0.3 (0.2, 0.6)].
DISCUSSION
In this study we found that TLR differs according to race/ethnicity, income status, and weight status among children and adolescents. More specifically, individuals who were Mexican American had the highest mean TLR, while non-Hispanic black adolescents had the lowest mean TLR, aligning with prior studies that have shown that, despite similar total body fat, black individuals tend to have less abdominal fat, while Hispanic individuals tend to have more abdominal fat compared to white counterparts.29,30 We also found that adolescents from lower-income households had the highest mean TLR. This may be attributed to several potential factors, including poorer living conditions, lifestyle habits, higher stress levels and reduced access to healthcare, which may converge to predispose individuals to a greater propensity for abdominal fat deposition.31 Finally, among girls, those who were post-menarchal had a higher mean TLR compared to those who were pre-menarchal likely due to the significant growth and accrual of fat mass that occurs in females during pubertal maturation.
We next found several associations of TLR with CMD risk factors among adolescents in linear and logistic regression, independent of BMI z-score and other potential confounding variables, supporting the potential importance of body fat distribution patterns in future disease risk. In linear regression, we found TLR to be positively associated with nearly all CMD risk factors assessed, including HOMA-IR, triglycerides, TC, ALT, CRP and systolic BP, and negatively associated with HDL-cholesterol, both in the sample overall and in individual models for boys and girls. Similarly, when analyzed in logistic regression models stratified by sex, increasing TLR again was significantly associated with odds of high HOMA-IR, high triglycerides, low HDL-C, high TC, and high BP in both boys and girls, independent of BMI z-score. Conversely, there was no association of TLR with fasting plasma glucose or hemoglobin A1C in adolescents, and there were sex-specific discrepancies in the associations of TLR with LDL-C, such that in both linear and logistic regression, TLR was only associated with LDL-C among girls.
These findings have similarities with prior studies of body fat distribution and CMD risk factors in children and adolescents. In an early study by Daniels et al., DXA-measured android-to-gynoid (A/G) ratio was associated with triglycerides and HDL-cholesterol, but not LDL-C; glucose and insulin metabolism were not reported.12 In a later study by Aucouturier et al, which included measures of glucose and insulin metabolism, there was no association of android/gynoid ratio tertile with fasting plasma glucose, but associations with HOMA-IR and fasting plasma insulin were significant.13 Our study found a similar pattern in terms of glycemic outcomes in boys and girls, and lipid outcomes in boys, which, taken together, suggests that these CMD risk factors may be less strongly linked to body composition phenotype in adolescence.
We also found sex-specific associations of TLR with blood pressure. Specifically, TLR was associated with systolic BP among both sexes, but with diastolic BP only among boys in linear regression. Prior studies have reported an increase in BP levels during puberty.32,33 Because we could not adjust for pubertal stage in all participants, only girls, it is possible that the associations of TLR with BP in boys are confounded by maturation stage. It has also been reported that boys are more likely than girls to develop high BP during adolescence,34 and that among adolescents associations of intra-abdominal fat with increased BP are stronger in boys than girls.35,36 Similar to these findings, our results also showed a stronger association of TLR with high BP in boys than girls in logistic regression. This pattern of findings may be due to sex differences in patterns of growth during adolescence, with girls gaining more subcutaneous fat mass and less fat-free mass compared to boys, while both fat and fat-free mass were associated with BP in prior studies.36 Differences in sex hormones may also play a role, with boys having increased production of testosterone, which may in turn be linked to BP through increased sympathetic activity.35 Nonetheless, the results from logistic regression support an overall association of increased TLR with odds of high BP in both sexes.
When further stratified by race/ethnicity, logistic regression revealed several racial/ethnic differences in the associations of TLR with CMD risk factors. In particular, for blood lipid outcomes (i.e., high triglycerides and/or high TC) and ALT in both sexes, the associations with TLR were significant in Mexican American, but not black adolescents. This may be explained by differences in the proportion of abdominal visceral versus subcutaneous fat among adolescents by race/ethnicity. Prior studies have shown that Hispanic and Caucasian adolescents tend to have a higher proportion of visceral fat than African American adolescents, despite similar levels of total body fat, which could contribute to a more atherogenic profile.30,37 In contrast, the association of TLR with HOMA-IR was significant for all race/ethnicities. Both visceral and abdominal subcutaneous fat have been correlated with measures of insulin resistance in pediatric studies,38–41 suggesting that the type of abdominal fat may be less important in relation to HOMA-IR, though more research is needed to explore this.
In comparison to the other anthropometric and fat mass variables, several of the associations we found for TLR with CMD risk factors were also significant when WC was substituted for TLR. The key differences were for the associations of WC with high TC for boys, and WC with high triglycerides, LDL-C, and TC for girls, which were significant for TLR, but not for WC overall. Thus, WC may also be adequate in predicting certain CMD risk factors independent of BMI z-score; but, for the above risk factors, i.e., triglycerides, LDL-C, and TC, TLR may be a more strongly associated risk factor. Our analyses of trunk and leg fat mass as individual independent variables may also help to explain the added benefit of TLR. In both boys and girls, we found that trunk fat mass was associated with high HOMA-IR, TC, ALT, and BP, and low-HDL-C in logistic regression after adjustment for BMI z-score, leg fat mass, and other covariates. In boys, trunk fat mass was also associated with high triglycerides, and in girls it was associated with high LDL-C. On the other hand, similar to prior studies,10,11 leg fat mass was associated with lower odds of several outcomes, including high triglycerides, TC and BP in both sexes, and high LDL-C in girls only. Thus, it is not surprising that TC, for example, was associated with TLR, but not WC, possibly because the synergistic combination of high trunk fat in the setting of low leg fat was a stronger predictor than WC alone.
Unlike other studies in children, this analysis was the first of its kind to examine truncal-to-leg fat ratio in a large, multi-ethnic cohort of adolescents, which allowed for stratification by sex and race/ethnicity. Another strength of this study was the use of DXA for total and regional body composition assessment, which is considered a “gold standard” technology and avoids the limitations of anthropometric measures that are more subject to measurement error. We were also able to obtain a comprehensive assessment of each participant in terms of their CMD risk profile and other potential confounding variables with a combination of laboratory, physical examination, and questionnaire assessments performed at NHANES.
Limitations of this analysis include its cross-sectional nature, which inhibits our ability to assess causality, and reliance on self-reported questionnaire data for the assessment of several covariates, which may be subject to recall and social desirability biases.42,43 Because we did not control for multiple testing, it is possible that some findings are a result of chance. Additionally, only a sub-sample of adolescents had fasting laboratory data, which limited our sample size and power in these analyses, especially when we stratified by both sex and race/ethnicity. Another limitation was using fasting measures of glucose and insulin to measure insulin resistance, as opposed to the hyerpinsulinemic-euglycemic clamp; however, studies have found that fasting-based indices are suitable for population-based studies.44,45 Lastly, with DXA we could not differentiate abdominal subcutaneous versus visceral fat, nor could we measure fat content in specific organs such as the liver. Thus, we could not assess the contribution of these individual fat depots to the associations of TLR with metabolic outcomes, and this remains an important topic of future research.
Collectively, these findings confirm prior research suggesting that body fat distribution is an independent correlate of certain measures of metabolic dysfunction in adolescents, and show that TLR may be able to predict which children are at greater risk of cardiometabolic dysfunction, regardless of their overall weight status. This has important implications for healthcare professionals who are screening pediatric patients for CMD risk. For future implementation, we found that TLR was more consistently associated with CMD risk than WC, in particular for non-Hispanic white and Mexican American adolescents. On the other hand, given the associations we found of WC with certain outcomes, especially HOMA-IR and low HDL-C, WC may be an adequate and affordable alternative for assessing risk of these risk factors, while additional research examines cost-effective and accurate surrogates for TLR. Finally, studies aiming to elucidate the genetic and environmental factors that contribute to this fat deposition pattern are necessary for future development of personalized medicine and nutrition strategies.
Supplementary Material
Acknowledgments
FUNDING STATEMENT
The doctoral training of C.E.C. was supported by an institutional training grant from the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) no. T32-DK007734. J.A.A. is supported by NIDDK grant no. K01-DK102851. M.B.V. is supported by National Institute of Child Health and Human Development (NICHD) grant no. R21-HD089056.
Footnotes
CONFLICT OF INTEREST STATEMENT
No conflict of interest declared.
REFERENCES
- 1.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among us children and adolescents, 1999–2012. JAMA Pediatrics. 2015;169(3):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JM, Kaylor MB, Johannsson M, Bay C, Churilla JR. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001–2010 National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2014;12(10):527–532. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–2374. [DOI] [PubMed] [Google Scholar]
- 4.Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GDC. Predictors of Metabolically Healthy Obesity in Children. Diabetes Care. 2014;37(5):1462–1468. [DOI] [PubMed] [Google Scholar]
- 5.Wajchenberg BL. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocrine Reviews. 2000;21(6):697–738. [DOI] [PubMed] [Google Scholar]
- 6.Samara A, Ventura EE, Alfadda AA, Goran MI. Use of MRI and CT for fat imaging in children and youth: what have we learned about obesity, fat distribution and metabolic disease risk? Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(8):723–732. [DOI] [PubMed] [Google Scholar]
- 7.Caprio S, Perry R, Kursawe R. Adolescent Obesity and Insulin Resistance: Roles of Ectopic Fat Accumulation and Adipose Inflammation. Gastroenterology. 2017;152(7):1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond). 2010;34(6):949–959. [DOI] [PubMed] [Google Scholar]
- 9.Teixeira PJ, Sardinha LB, Going SB, Lohman TG. Total and regional fat and serum cardiovascular disease risk factors in lean and obese children and adolescents. Obes Res. 2001;9(8):432–442. [DOI] [PubMed] [Google Scholar]
- 10.Staiano AE, Gupta AK, Katzmarzyk PT. Cardiometabolic Risk Factors and Fat Distribution in Children and Adolescents. J Pediatr. 2014;164(3):560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samouda H, Beaufort CD, Stranges S, et al. Cardiometabolic risk: leg fat is protective during childhood. Pediatr Diabetes. 2016;17(4):300–308. [DOI] [PubMed] [Google Scholar]
- 12.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999;99(4):541–545. [DOI] [PubMed] [Google Scholar]
- 13.Aucouturier J, Meyer M, Thivel D, Taillardat M, Duche P. Effect of android to gynoid fat ratio on insulin resistance in obese youth. Arch Pediatr Adolesc Med. 2009;163(9):826–831. [DOI] [PubMed] [Google Scholar]
- 14.Samsell L, Regier M, Walton C, Cottrell L. Importance of Android/Gynoid Fat Ratio in Predicting Metabolic and Cardiovascular Disease Risk in Normal Weight as well as Overweight and Obese Children. J Obes. 2014;2014:846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnberg K, Larnkjaer A, Michaelsen KF, Molgaard C. Central adiposity and protein intake are associated with arterial stiffness in overweight children. J Nutr. 2012;142(5):878–885. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JP, Kanaya AM, Fan B, Shepherd JA. Ratio of Trunk to Leg Volume as a New Body Shape Metric for Diabetes and Mortality. PLoS ONE. 2013;8(7):e68716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics. The National Health and Nutrition Examination Survey (NHANES): Anthropometry and Physical Activity Monitor Procedures Manual. https://wwwn.cdc.gov/nchs/data/nhanes/2005-2006/manuals/BM.pdf. Accessed May 2018.
- 18.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth charts for the United States: methods and development. Vital Health Stat 11 2002;11. [PubMed] [Google Scholar]
- 19.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 Suppl 4:S164–192. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center for Health Statistics. The National Health and Nutrition Examination Survey (NHANES): Technical Documentation for the 1999–2004 Dual Energy X-Ray Absorptiometry (DXA) Multiple Imputation Data Files. 2008; https://wwwn.cdc.gov/Nchs/Data/Nhanes/Dxa/dxa_techdoc.pdf.
- 21.Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81(5):1018–1025. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18. [PubMed] [Google Scholar]
- 24.Puolakka E, Pahkala K, Laitinen TT, et al. Childhood Socioeconomic Status in Predicting Metabolic Syndrome and Glucose Abnormalities in Adulthood: The Cardiovascular Risk in Young Finns Study. Diabetes Care. 2016;39(12):2311–2317. [DOI] [PubMed] [Google Scholar]
- 25.Raper N, Perloff B, Ingwersen L, Steinfeldt L, Anand J. An overview of USDA’s Dietary Intake Data System. J Food Compost Anal. 2004;17(3–4):545–555. [Google Scholar]
- 26.Moran A, Jacobs DR Jr., Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117(18):2361–2368. [DOI] [PubMed] [Google Scholar]
- 27.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128(Suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tresaco B, Bueno G, Pineda I, Moreno LA, Garagorri JM, Bueno M. Homeostatic model assessment (HOMA) index cut-off values to identify the metabolic syndrome in children. J Physiol Biochem. 2005;61(2):381–388. [DOI] [PubMed] [Google Scholar]
- 29.Casazza K, Dulin-Keita A, Gower BA, Fernandez JR. Intrabdominal fat is related to metabolic risk factors in Hispanic Americans, African Americans and in girls. Acta Paediatr. 2009;98(12):1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE. 2007;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamayo T, Christian H, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health. 2010;10:525–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA. 2004;291(17):2107–2113. [DOI] [PubMed] [Google Scholar]
- 33.Vartiainen E, Tuomilehto J, Nissinen A. Blood pressure in puberty. Acta Paediatr Scand. 1986;75(4):626–631. [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta K, O’Loughlin J, Chen S, et al. Emergence of sex differences in prevalence of high systolic blood pressure: analysis of a longitudinal adolescent cohort. Circulation. 2006;114(24):2663–2670. [DOI] [PubMed] [Google Scholar]
- 35.Syme C, Abrahamowicz M, Leonard GT, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: Sex differences and underlying mechanisms. Archives of Pediatrics and Adolescent Medicine. 2008;162(5):453–461. [DOI] [PubMed] [Google Scholar]
- 36.Syme C, Abrahamowicz M, Leonard GT, et al. Sex differences in blood pressure and its relationship to body composition and metabolism in adolescence. Arch Pediatr Adolesc Med. 2009;163(9):818–825. [DOI] [PubMed] [Google Scholar]
- 37.Staiano AE, Katzmarzyk PT. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. Int J Obes (Lond). 2012;36(10):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caprio S Insulin resistance in childhood obesity. J Pediatr Endocrinol Metab. 2002;15(SUPPL. 1):487–492. [PubMed] [Google Scholar]
- 39.Maffeis C, Manfredi R, Trombetta M, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93(6):2122–2128. [DOI] [PubMed] [Google Scholar]
- 40.Ali O, Cerjak D, Kent JW, James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes. 2014;9(3):e58–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He F, Rodriguez-Colon S, Fernandez-Mendoza J, et al. Abdominal Obesity and Metabolic Syndrome Burden in Adolescents-Penn State Children Cohort Study. J Clin Densitom. 2015;18(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams SA, Matthews CE, Ebbeling CB, et al. The Effect of Social Desirability and Social Approval on Self-Reports of Physical Activity. Am J Epidemiol. 2005;161(4):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Börnhorst C, Huybrechts I, Ahrens W, et al. Prevalence and determinants of misreporting among European children in proxy-reported 24 h dietary recalls. Br J Nutr. 2013;109(7):1257–1265. [DOI] [PubMed] [Google Scholar]
- 44.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: A validation study. Diabetes Care. 2004;27(2):314–319. [DOI] [PubMed] [Google Scholar]
- 45.Henderson M, Rabasa-Lhoret R, Bastard JP, et al. Measuring insulin sensitivity in youth: How do the different indices compare with the gold-standard method? Diabetes Metab. 2011;37(1):72–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.