Abstract
Stem cells provide tremendous promise for the development of new therapeutic approaches for musculoskeletal conditions. In addition to their multipotency, certain types of stem cells exhibit immunomodulatory effects that can mitigate inflammation and enhance tissue repair. However, the translation of stem cell therapies to clinical practice has proven difficult due to challenges in intra- and inter-donor variability, engraftment, variability in recipient microenvironment and patient indications, and limited therapeutic biological activity. In this regard, the success of stem cell-based therapies may benefit from cellular engineering approaches to enhance factors such as purification, homing and cell survival, trophic effects, or immunomodulatory signaling. By combining recent advances in gene editing, synthetic biology, and tissue engineering, the potential exists to create new classes of “designer” cells that have prescribed cell-surface molecules and receptors, as well as synthetic gene circuits that provide for autoregulated drug delivery or enhanced tissue repair.
Keywords: CRISPR-Cas9, regenerative medicine, synthetic biology, MSC, iPSC
Stem Cell Research and Therapy in Orthopaedics
The field of orthopaedics has seen tremendous growth in the application of various types of stem cells for musculoskeletal research, as well as in the development of new approaches for translation of cell-based therapies to clinical orthopaedic practice. Stem cell research in orthopaedics has spanned from embryonic stem (ES) cells and induced pluripotent stem cells (iPSCs), to multiple types of adult stem cells, often termed mesenchymal stem cells (MSCs). Indeed, much of the work in adult stem cell research was pioneered by musculoskeletal researchers and initially directed toward the development of novel therapies for orthopaedic conditions such as bone regeneration and cartilage repair.1 Following these initial advances, the field has continued to investigate new methods to enhance regeneration and repair of all the major musculoskeletal tissues, as well as cell-based therapies for diseases such as osteoarthritis, tendonitis and tendon repair, intervertebral disc regeneration, muscle repair, and many other conditions (reviewed in 2).
The prominent emphasis of applied stem cell research on musculoskeletal tissues may be due to the initial identification of a multipotent, non-hematopoietic population of bone marrow cells with the capacity to undergo osteogenic differentiation in culture.3 These cells could be expanded through multiple population doublings and showed the ability to form colony-forming units (CFUs) and self-renewal in culture.4 Under defined in vitro culture conditions, such cells could be induced to express the phenotypic characteristics of multiple musculoskeletal tissues of mesenchymal lineage.5 Thus, they were named “mesenchymal stem cells” to reflect the hypothesis that these cells served in vivo as adult stem cells, responsible for the development and/or regeneration of mesenchymally-derived tissues.1 However, in recent years, the name “MSC” has led to significant controversy and debate.6,7 It can be argued that in vitro, MSCs meet the two fundamental requirements of a “stem cell” (i.e., self-renewal and capability for differentiation).8 However, it remains to be determined if this definition is met broadly in vivo,9 outside of specific situations such as the engraftment and proliferation of immortalized MSCs into irradiated bone marrow.10 Additionally, the early pre-clinical work investigating the contribution of MSCs in vivo has been limited by the techniques available to track and evaluate function of MSCs following implantation. Further confusion has arisen by the identification of similar but distinct populations of multipotent cells, likely of perivascular origin, in tissues such as adipose tissue, muscle, tendon, bone, and synovium, but with mixed and inconsistent terminology being used to describe them. Accumulating evidence indicates that multipotent cells derived from bone marrow, fat, bone, muscle, and other tissues exhibit significantly different properties, identities, and differentiation potential,11,12 but in many cases are referred to as “MSCs”.13,14
Beyond basic science research, the translational applications of stem cells in orthopaedics has focused primarily on cell therapy or tissue engineering, with more recent expansion into the development of microphysiologic systems and in vitro disease modeling. “Cell therapy” implies the introduction of isolated cells without a structural scaffold, usually through injection into the target site (e.g., intra-articularly or intra-discally), or in some cases, intravenously. “Tissue engineering” has generally focused on combining cells, biomaterial scaffolds, and environmental factors (e.g., growth factors, bioreactors) to regenerate tissue replacements for implantation. Despite tremendous progress in these areas, however, there have been few long-term successes in the translation of stem cell therapies to clinical therapies.15,16 Preclinical studies for tissue-engineered repair of cartilage and bone have been quite promising, but most clinical procedures either lack controls or have shown long-term results that are equivocal to standard-of-care.17,18 Similarly, stem cell therapies have shown significant promise in controlled preclinical animal studies,19–21 but have not shown consistent clinical efficacy in prospective and randomized trials.22–24
Despite the lack of clear evidence to support these “stem cell” therapies, numerous clinics continue to offer unproven procedures in the United States, with an unknown number of such clinics outside the United States offering various cell therapies as medical tourism. Not only do many of these procedures not involve actual stem cells, there have been a number of serious adverse effects that have been documented due to unproven stem cell therapies,25 including growth of a mucus-producing nose in the spine,26 tumor formation,27 blindness,28 and infection.29
Indeed, the results of less than half of stem cell clinical trials are published, suggesting that many negative findings in the stem cell field go unreported.30 Furthermore, many of these clinics are misusing the term “stem cell” or “cellular therapy” to treat various musculoskeletal conditions. The United States Food and Drug Administration (FDA) defines a somatic cell therapy as the administration to humans of autologous, allogeneic, or xenogeneic living cells that have been processed ex vivo (FDA Guidance for Industry). This definition is important as many of these clinics claim bone marrow aspirate concentrate (BMAC) as a stem cell therapy, with limited clinical data to support this notion. BMAC is becoming more popular in the clinical community to treat musculoskeletal injuries and diseases due to the limited regulatory barrier for clinical use - bone marrow aspirate is concentrated and therefore, considered to be minimally manipulated but not processed and expanded ex vivo. In fact, 48% of the businesses marketing “stem cells” describe use of autologous stem cells obtained from bone marrow.31
Challenges in Stem Cell Therapies
The promise of stem cell therapies for musculoskeletal conditions remains largely unfulfilled, and despite a wealth of successful preclinical animal studies, proven success in the clinic is still elusive.32,33 At the onset of the field, it was hypothesized that injected stem cells can home directly to sites of injury, exhibit long-term engraftment and survival, and perform the appropriate regenerative and trophic functions for the appropriate amount of time. Unfortunately, most of these hypotheses around stem cell homing and engraftment have turned out to be extremely difficult to show in clinical practice, particularly under the scrutiny of randomized prospective clinical trials.33 More recently, the primary proposed mechanism of action for stem cell therapies is based on the concept that the cells termed “MSCs” may be in fact a type of perivascular cell – the pericyte – that exists with distinct phenotypes in different vascular tissues sites.34 When activated or reintroduced to the body, these cells exhibit trophic and anti-inflammatory effects through paracrine signaling.35 Mounting evidence supports this notion, but similar to past hypotheses, controlled, randomized clinical studies have been limited and have shown somewhat variable response capabilities.
For example, many studies that have shown evidence of therapeutic benefits of stem cell therapies have also shown little, if any, homing and long-term engraftment to sites of injury, with most stem cells dying or being cleared away within days of injection.19,36 Furthermore, without defined exogenous factors to regulate their behavior in situ, the differentiation and growth of stem cells may be uncontrolled post implantation. For example, growing evidence shows that MSCs used in the context of cartilage regeneration can experience hypertrophy and ossification once implanted in vivo.37 Similarly, the first generation of therapies using autologous chondrocyte implantation for focal cartilage defects experienced uncontrolled growth and hyperplasia in approximately 40% of cases, which required additional surgery for removal of the overgrowth.38
An important consideration in this regard is an improved understanding of the role that the microenvironment may play on the therapeutic potential of MSCs. While MSCs exhibit some anti-inflammatory and regenerative effects in controlled pre-clinical environments, in the clinical setting, they are often being introduced into a highly inflammatory microenvironment, such an injured or arthritic joint. In these cases, MSCs may not have the ability to overcome pathophysiologic levels of inflammation or the harsh biomechanical environment of the musculoskeletal system.39 Furthermore, preclinical studies of stem cell therapies rarely consider the underlying disease state and co-morbidities of the recipient. Factors such as obesity, aging, autoimmune disease, or systemic inflammation can greatly influence stem cell responses such as engraftment, viability, and functional response.40
Similarly, intra-donor and inter-donor variability of stem cells still represents a major limitation to clinical translation. Multiple factors can influence intra-donor variability, such as the tissue source14 or the isolation protocol.41 Recent studies suggest that the standard MSC preparation can include multiple distinct populations of cells types, and it remains to be determined which sub-population is responsible for the different properties of these cells.42 These issues are further exaggerated by inter-donor variability, where age, sex, and multiple genetic or epigenetic factors may influence stem cell properties and function. In addition to donor variability, cell therapies in general are limited by current cell culture techniques. In general, current culture systems lack reproducibility and standardization, influence and alter stem cell architecture and function, and limit the ability to scale-up and manufacture.43
Engineering the next generation of stem cells
Clearly, there is tremendous potential for stem cells to form the basis for a variety of regenerative approaches for musculoskeletal conditions. However, in addition to many of the limitations in cell isolation, expansion, and delivery noted above, it is now becoming apparent that stem cells in their “naïve” (i.e., unmodified) form often have limited therapeutic potential and high variability in such responses. In this regard, the ability to “engineer” stem cells to modify their properties and behavior could significantly improve their therapeutic potential. Initial efforts at optimizing stem cell therapeutics involved a number of transgenic or peptide-based approaches.44 For example, transgenic approaches have been very effective in modifying stem cells to overexpress specific growth factors, receptors, or transcription factors to enhance their differentiation into defined lineages, particularly in the context of tissue engineering.45
Similarly, several studies have shown that stem cells can be modified to produce desired genes,46 effectively providing a source for the production of specific cytokines or signaling molecules in the context of autocrine or paracrine based signaling. For example, stem cells that have been transduced to overexpress anti-inflammatory cytokines or inhibitors of fibrosis can enhance wound healing if administered as either cell-based therapies or implantable tissue-engineered constructs.47 For further control of cell-based delivery of biologic drugs, other approaches have incorporated tunable and inducible genetic switches into stem cells, allowing for stem cells or tissue-engineered cartilage with exogenously controllable drug delivery systems.48,49
To address issues of homing and retention, several approaches have shown that stem cells can be targeted to specific tissues in the body by modifying cell surface receptors,44 such as stromal cell-derived factor 1 (SDF-1).50 For example, enzymatic modification of the CD44 surface receptor into an E-selectin binding domain significantly enhanced homing of MSCs to the bone marrow.51 In other studies, anti-cartilage matrix antibodies have been coupled to the surface of MSCs, allowing for the binding of virtually any Fc-bearing protein to the cell and the targeting of cells to tissue-specific proteins in the body (such as type II collagen).52 These approaches provide a number of potential advantages in the application of cell therapy, such as a need for reduced numbers of transplanted cells as well as fewer “off-target” effects,53 but to date, have not been implemented in clinical practice.
Engineering Designer Stem Cells
Using recent advances in genome and epigenetic engineering in combination with synthetic biology, new approaches are being developed for dynamic control of cellular behavior in response to environmental signals. In initial approaches, several studies have used genetic modification of endogenous inflammation-responsive promoters to drive the expression of therapeutic transgenes, allowing for dynamic, self-regulating gene expression driven by synthetic gene circuits. For example, Rachakonda et al. designed a truncated promoter sequence of cyclooxygenase 2 (COX-2) upstream of the IL-4 gene to develop a self-limiting promoter construct that expresses IL-4 in chondrocytes only in the presence of inflammation.54 In other studies, a synthetic gene promoter system was developed based on multiple consensus elements for nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which was used to amplify and drive the expression of anti-cytokine drugs such as IL-1Ra in response to IL-1 (Figure 1A)55.In a similar approach, Lin et al. generated an NF-κB responsive lentiviral system to synthesize IL-4 as a potential regulator of macrophage polarity in response to inflammatory cytokines.56 These strategies have generally involved the use of viral vectors for gene delivery, which has the advantage of broad application to multiple cell types, but could be impaired by epigenetic silencing or pose a risk for insertional mutagenesis.
Figure 1. Synthetic gene circuits used to develop self-regulating biologic drug delivery systems.
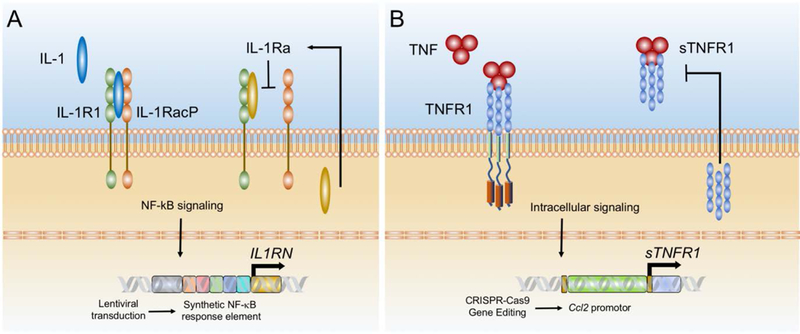
A. Synthetic promoter containing multiple NF-kB recognition motifs upstream of IL1RN (gene for IL-1Ra) to create an inducible promoter that is activated by inflammation. In response to IL-1, the synthetic promoter is activated and produces IL-1 Ra, which then inhibits IL-1 in an autoregulated manner. B. CRISPR-Cas9 targeted gene editing was used to insert a therapeutic transgene (sTNFR1) in the Ccl2 locus. Activation of the endogenous Ccl2 promoter by TNF results in dynamic expression of sTNFR1, which then inhibits TNF, creating a self-regulating system.
Targeted Genome and Epigenome Engineering of Stem Cells
While the concept of cellular engineering is not new,57 the molecular tools available for targeted genome and epigenome engineering have greatly advanced over the past few years, allowing for rapid modifications of cells, with unprecedented precision.58,59 In particular, the emergence of the CRISPR-Cas9 system and its applicability to mammalian cells has revolutionized the ability for gene editing. Because of the highly-targeted nature of this system, the chances for off-target effects or risks of tumorigenicity are considerably reduced.60,61
Nonetheless, in the orthopaedic field, there have been few applications of CRISPR-Cas9 genome engineering in stem cells. Some of the first applications have been in the engineering of stem cells to allow for controlled attenuation of their detrimental responses to inflammatory cytokines [e.g., interleukin-1 (IL-1), tumor necrosis factor (TNF)62,63]. For example, murine iPSCs have been engineered to harbor a functional deletion of the interleukin-1 receptor I (ll1r1).64 These cells were capable of synthesizing a cartilaginous matrix that was protected against IL-1 - mediated inflammation or tissue degradation, as measured by a decreased expression of pro-inflammatory genes and a reduced loss of proteoglycan content. In other studies, dead Cas9 (dCas9)-KRAB was used for epigenome editing at loci encoding cytokine receptors IL1R1 and tumor necrosis factor receptor 1 (TNFR1) to inactivate these receptors.65 This approach showed the ability to inhibit downstream activation of NF-κB and to increase stem cell survival, without changes in the gene sequence. Coupling dCas9 with a transactivation domain such as VP64 has been used for highly targeted gene activation providing alternative approaches for conferring anti-inflammatory properties to stem cells or activation of specific transcription factors for inducing stem cell differentiation.66
Creating Self-Regulating “Smart” Cells
The CRISPR-Cas9 provides a system for targeted gene editing that can allow for epigenetically stable, robust transgene expression for applications in which the use of viral vectors may not be desired. For example, we have used this approach to engineer iPSCs that contain artificial gene circuits that are cytokine-activated and feedback-controlled to regulate the expression of biologic therapies.67 Such “smart” stem cells were used to engineer articular cartilage capable of inducible and transient anti-inflammatory responses to inflammatory cytokines. Specifically, using CRISPR gene editing, targeted gene addition of IL1-Ra or soluble TNF receptor (sTNFRI) cDNA downstream of the Ccl2 promoter was used to produce “smart” iPSCs that initiated a dynamic negative feedback loop upon stimulation with IL-1 or TNF67 (Figure 1B). These iPSCs were engineered to form implantable self-regulating tissue constructs and have shown promising efficacy in early studies in a model of inflammatory arthritis.68
“Designer” stem cells have the potential to overcome challenges with long-term therapeutic delivery of biologic drugs, as well as limitations involved in cell homing and engraftment. The development of self-regulating67 or exogenously controlled48 systems for transgene expression may allow for a new generation of stem cells that can not only be used to engineer tissue replacements, but simultaneously may serve as an inducible and tunable depot for localized delivery of biologic drugs. Furthermore, the field of synthetic biology has made significant advances in the creation of biologic components with precise and controlled responses to stimuli.69 Application of this toolkit of gene switches, classifiers, and synthetic transcription systems to stem cell therapies for orthopaedic problems may allow for more specific control of the delivery of therapeutic transgenes.
Conclusions
Stem cells provide tremendous opportunities for the development of novel therapies for a range of musculoskeletal disorders, but to date, their full potential has not been realized. While stem cells clearly exhibit a range of trophic and anti-inflammatory capabilities, increasing evidence suggests that in many cases, stem cells in their naïve state may not possess sufficient disease-modifying characteristics to justify the added costs and potential risks involved in their use. With the advent of a multitude of new methods for cellular engineering, we propose that a new generation of stem cell therapies will emerge that can provide functional tissue replacements as well as exogenous or even self-regulating capabilities for biologic drug delivery. In addition to such therapeutic applications of stem cells in vivo, genome edited stem cells may also allow for the development of in vitro models70 of disease or reporter systems71 for stem cell purification, or for investigating the consequences of causative or associate genetic elements such as coding/noncoding single nucleotide polymorphisms (SNPs), variable number tandem repeats (VNTRs), etc. identified through genome-wide association studies (GWAS).72
Acknowledgments
This study was supported in part by NIH grants AR50245, AR48852, AG15768, AR48182, AG46927, OD10707, AR057235, AR073752, DK108742, EB108266, the Arthritis Foundation, the Nancy Taylor Foundation for Chronic Diseases, and the Philip and Sima Needleman Fellowship for Regenerative Medicine from the Washington University Center of Regenerative Medicine to LP and AKR.
Footnotes
Conflict of Interest Disclosure
FG is a founder and employee of Cytex Therapeutics Inc.
References
- 1.Caplan AI. 1991. Mesenchymal stem cells. J Orthop Res 9:641–650. [DOI] [PubMed] [Google Scholar]
- 2.Im GI. 2017. Clinical use of stem cells in orthopaedics. Eur Cell Mater 33:183–196. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. 1968. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6:230–247. [PubMed] [Google Scholar]
- 4.Owen M, Friedenstein AJ. 1988. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 136:42–60. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger MF, Mackay AM, Beck SC, et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. [DOI] [PubMed] [Google Scholar]
- 6.Sipp D, Robey PG, Turner L. 2018. Clear up this stem-cell mess. Nature 561:455–457. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI. 2017. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med 6:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimble JM, Guilak F, Nuttall ME, et al. 2008. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus Med Hemother 35:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guimaraes-Camboa N, Cattaneo P, Sun Y, et al. 2017. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 20:345–359 e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin P, Correa D, Kean TJ, et al. 2013. Serial Transplantation and Long-term Engraftment of Intra-arterially Delivered Clonally Derived Mesenchymal Stem Cells to Injured Bone Marrow. Mol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estes BT, Wu AW, Guilak F. 2006. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum 54:1222–1232. [DOI] [PubMed] [Google Scholar]
- 12.Im GI, Shin YW, Lee KB. 2005. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage 13:845–853. [DOI] [PubMed] [Google Scholar]
- 13.Cano E, Gebala V, Gerhardt H. 2017. Pericytes or Mesenchymal Stem Cells: Is That the Question? Cell Stem Cell 20:296–297. [DOI] [PubMed] [Google Scholar]
- 14.Nancarrow-Lei R, Mafi P, Mafi R, et al. 2017. A Systemic Review of Adult Mesenchymal Stem Cell Sources and their Multilineage Differentiation Potential Relevant to Musculoskeletal Tissue Repair and Regeneration. Curr Stem Cell Res Ther 12:601–610. [DOI] [PubMed] [Google Scholar]
- 15.Hurley ET, Yasui Y, Gianakos AL, et al. 2018. Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surg Sports Traumatol Arthrosc 26:3499–3507. [DOI] [PubMed] [Google Scholar]
- 16.lijima H, Isho T, Kuroki H, et al. 2018. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Windt TS, Vonk LA, Slaper-Cortenbach ICM, et al. 2017. Allogeneic MSCs and Recycled Autologous Chondrons Mixed in a One-Stage Cartilage Cell Transplantion: A First-in-Man Trial in 35 Patients. Stem Cells 35:1984–1993. [DOI] [PubMed] [Google Scholar]
- 18.Knutsen G, Drogset JO, Engebretsen L, et al. 2007. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89:2105–2112. [DOI] [PubMed] [Google Scholar]
- 19.Diekman BO, Wu CL, Louer CR, et al. 2013. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transplant 22:1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy JM, Fink DJ, Hunziker EB, et al. 2003. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48:3464–3474. [DOI] [PubMed] [Google Scholar]
- 21.Black LL, Gaynor J, Gahring D, et al. 2007. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther 8:272–284. [PubMed] [Google Scholar]
- 22.Pas HI, Winters M, Haisma HJ, et al. 2017. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med 51:1125–1133. [DOI] [PubMed] [Google Scholar]
- 23.Aghebati-Maleki L, Dolati S, Zandi R, et al. 2018. Prospect of mesenchymal stem cells in therapy of osteoporosis: A review. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- 24.Pas H, Moen MH, Haisma HJ, et al. 2017. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med 51:996–1002. [DOI] [PubMed] [Google Scholar]
- 25.Bauer G, Elsallab M, Abou-El-Enein M. 2018. Concise Review: A Comprehensive Analysis of Reported Adverse Events in Patients Receiving Unproven Stem Cell-Based Interventions. Stem Cells Transl Med 7:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dlouhy BJ, Awe O, Rao RC, et al. 2014. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J Neurosurg Spine 21:618–622. [DOI] [PubMed] [Google Scholar]
- 27.Berkowitz AL, Miller MB, Mir SA, et al. 2016. Glioproliferative Lesion of the Spinal Cord as a Complication of “Stem-Cell Tourism”. N Engl J Med 375:196–198. [DOI] [PubMed] [Google Scholar]
- 28.Kuriyan AE, Albini TA, Townsend JH, et al. 2017. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N Engl J Med 376:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins KM, Spoto S, Rankin DA, et al. 2018. Notes from the Field: Infections After Receipt of Bacterially Contaminated Umbilical Cord Blood–Derived Stem Cell Products for Other Than Hematopoietic or Immunologic Reconstitution. MMWR Morb Mortal Wkly Rep 67:1397–1399. [DOI] [PubMed] [Google Scholar]
- 30.Fung M, Yuan Y, Atkins H, et al. 2017. Responsible Translation of Stem Cell Research: An Assessment of Clinical Trial Registration and Publications. Stem Cell Reports 8:1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner L, Knoepfler P. 2016. Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry. Cell Stem Cell 19:154–157. [DOI] [PubMed] [Google Scholar]
- 32.Anderson JA, Little D, Toth AP, et al. 2014. Stem cell therapies for knee cartilage repair: the current status of preclinical and clinical studies. Am J Sports Med 42:2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks PW, Witten CM, Califf RM. 2017. Clarifying Stem-Cell Therapy’s Benefits and Risks. N Engl J Med 376:1007–1009. [DOI] [PubMed] [Google Scholar]
- 34.Crisan M, Yap S, Casteilla L, et al. 2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313. [DOI] [PubMed] [Google Scholar]
- 35.Caplan AI, Correa D. 2011. The MSC: an injury drugstore. Cell Stem Cell 9:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roddy GW, Oh JY, Lee RH, et al. 2011. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells 29:1572–1579. [DOI] [PubMed] [Google Scholar]
- 37.Hellingman CA, Koevoet W, van Osch GJ. 2012. Can one generate stable hyaline cartilage from adult mesenchymal stem cells? A developmental approach. J Tissue Eng Regen Med 6:e1–e11. [DOI] [PubMed] [Google Scholar]
- 38.Ebert JR, Smith A, Fallon M, et al. 2015. Incidence, degree, and development of graft hypertrophy 24 months after matrix-induced autologous chondrocyte implantation: association with clinical outcomes. Am J Sports Med 43:2208–2215. [DOI] [PubMed] [Google Scholar]
- 39.Wu CL, Diekman BO, Jain D, et al. 2013. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int J Obes (Lond) 37:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costa-Almeida R, Reis RL, Gomes ME. 2019. Metabolic Disease Epidemics: Emerging Challenges in Regenerative Medicine. Trends Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- 41.Paladino FV, Peixoto-Cruz JS, Santacruz-Perez C, et al. 2016. Comparison between isolation protocols highlights intrinsic variability of human umbilical cord mesenchymal cells. Cell Tissue Bank 17:123–136. [DOI] [PubMed] [Google Scholar]
- 42.Huynh NP, Kelly NH, Alonso E, et al. 2019. Single cell RNA sequencing of human MSC chondrogenesis reveals multiple cell subpopulations and intermediate differentiation states as targets for drug discovery. Trans Orthop Res Society:149. [Google Scholar]
- 43.McKee C, Chaudhry GR. 2017. Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces 159:62–77. [DOI] [PubMed] [Google Scholar]
- 44.Wagner J, Kean T, Young R, et al. 2009. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol 20:531–536. [DOI] [PubMed] [Google Scholar]
- 45.Cucchiarini M, Madry H. 2019. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat Rev Rheumatol 15:18–29. [DOI] [PubMed] [Google Scholar]
- 46.Mosca JD, Hendricks JK, Buyaner D, et al. 2000. Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res:S71–90. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Li J, Zhu J, et al. 2007. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther 15:1616–1622. [DOI] [PubMed] [Google Scholar]
- 48.Glass KA, Link JM, Brunger JM, et al. 2014. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 35:5921–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moutos FT, Glass KA, Compton SA, et al. 2016. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc Natl Acad Sci U S A 113:E4513–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau TT, Wang DA. 2011. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther 11:189–197. [DOI] [PubMed] [Google Scholar]
- 51.Sackstein R, Merzaban JS, Cain DW, et al. 2008. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 14:181–187. [DOI] [PubMed] [Google Scholar]
- 52.Dennis JE, Cohen N, Goldberg VM, et al. 2004. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res 22:735–741. [DOI] [PubMed] [Google Scholar]
- 53.Wells CA, Mosbergen R, Korn O, et al. 2013. Stemformatics: visualisation and sharing of stem cell gene expression. Stem Cell Res 10:387–395. [DOI] [PubMed] [Google Scholar]
- 54.Rachakonda PS, Rai MF, Schmidt MF. 2008. Application of inflammation-responsive promoter for an in vitro arthritis model. Arthritis Rheum 58:2088–2097. [DOI] [PubMed] [Google Scholar]
- 55.Ross AK, Pferdehirt LG, Brunger JM, et al. 2018. A Synthetic Transcription System Based on NF-kB Signaling for Cartilage Tissue Engineering using Self-Regulating Delivery of Therapeutic Biologic Drugs. Trans Orthop Res Soc:66. [Google Scholar]
- 56.Lin T, Pajarinen J, Nabeshima A, et al. 2017. Establishment of NF-kappaB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy 19:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nerem RM. 1991. Cellular engineering. Ann Biomed Eng 19:529–545. [DOI] [PubMed] [Google Scholar]
- 58.Doudna JA, Charpentier E. 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096. [DOI] [PubMed] [Google Scholar]
- 59.Gaj T, Gersbach CA, Barbas CF 3rd. 2013. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeder ML, Gersbach CA. 2016. Genome-editing technologies for gene and cell therapy. Molecular Therapy 24:430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ran FA, Hsu PD, Wright J, et al. 2013. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ousema PH, Moutos FT, Estes BT, et al. 2012. The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds. Biomaterials 33:8967–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wehling N, Palmer GD, Pilapil C, et al. 2009. Interleukin-1 beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum 60:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunger JM, Zutshi A, Willard VP, et al. 2017. CRISPR/Cas9 Editing of Murine Induced Pluripotent Stem Cells for Engineering Inflammation-Resistant Tissues. Arthritis Rheumatol 69:1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farhang N, Brunger JM, Stover JD, et al. 2017. CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments. Tissue Eng Part A 23:738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez-Pinera P, Kocak DD, Vockley CM, et al. 2013. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods 10:973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brunger JM, Zutshi A, Willard VP, et al. 2017. Genome Engineering of Stem Cells for Autonomously Regulated, Closed-Loop Delivery of Biologic Drugs. Stem Cell Reports 8:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi YR, Collins KH, Springer LE, et al. 2019. A Genome-engineered Bioartificial Implant for Autonomous Anti-Cytokine Delivery for Rheumatoid Arthritis. Trans Orthop Res Soc:63. [Google Scholar]
- 69.Xie M, Fussenegger M. 2018. Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nature Reviews Molecular Cell Biology:1. [DOI] [PubMed] [Google Scholar]
- 70.Willard VP, Diekman BO, Sanchez-Adams J, et al. 2014. Use of cartilage derived from murine induced pluripotent stem cells for osteoarthritis drug screening. Arthritis Rheumatol 66:3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adkar SS, Wu CL, Willard VP, et al. 2019. Step-wise chondrogenesis of human induced pluripotent stem cells and purification via a reporter allele generated by CRISPR-Cas9 genome editing. Stem Cells 37:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adkar SS, Brunger JM, Willard VP, et al. 2017. Genome Engineering for Personalized Arthritis Therapeutics. Trends Mol Med 23:917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]


