Abstract
In the last decade, scientific and clinical interest in the pedunculopontine nucleus (PPN) has grown dramatically. This growth is largely a consequence of experimental work demonstrating its connection to the control of gait and of clinical work implicating PPN pathology in levodopainsensitive gait symptoms of Parkinson’s disease (PD). In addition, the development of optogenetic and chemogenetic approaches has made experimental analysis of PPN circuitry and function more tractable. In this brief review, recent findings in the field linking PPN to the basal ganglia and PD are summarized; in addition, an attempt is made to identify key gaps in our understanding and challenges this field faces in moving forward.
The PPN is a functionally heterogenous brainstem region
Historically defined as a population of cholinergic neurons in the rostral brainstem, the pedunculopontine nucleus (PPN) is now known to be not only neurochemically heterogenous but functionally complex. In addition to cholinergic neurons, the PPN is populated by glutamatergic and GABAergic neurons (Clements & Grant, 1990; Clements, Toth, Highfield, & Grant, 1991; Ford, Holmes, Mainville, & Jones, 1995; Lavoie & Parent, 1994b). Cholinergic neurons, which express choline acetyltransferase (ChAT) and NADPH (Clements & Grant, 1990), have medium to large fusiform, triangular, multipolar or round somas (20–40 μm in diameter) with 2 to 6 primary dendrites (Honda & Semba, 1995; Ichinohe, Teng, & Kitai, 2000; Rye, Saper, Lee, & Wainer, 1987). Glutamatergic neurons, which express type 2 vesicular glutamate transporter (VGlut2), have a smaller somata (< 20 μm) with 2 to 4 primary dendrites (Clements & Grant, 1990; Ichinohe et al., 2000; Jia, Yamuy, Sampogna, Morales, & Chase, 2003; Wang & Morales, 2009). GABAergic neurons, which express the 67 KD isoform of glutamate decarboxylase (GAD67), are similar in size to glutamatergic neurons (Clements & Grant, 1990; Ichinohe et al., 2000; Jia et al., 2003; Wang & Morales, 2009).
Despite its original classification as a cholinergic nucleus, cholinergic neurons constitute only 25–30% of the total number of neurons in the PPN. Glutamatergic neurons constitute the largest fraction (40–45%) of PPN neurons, with GABAergic neurons being about as common as cholinergic neurons (Wang and Morales, 2009). There also appears to be a small group of glycinergic neurons in the PPN (Pienaar et al., 2013). The three principal neuronal populations are unevenly distributed in the PPN. In the rostral, par dissipata portion of the PPN, cholinergic neurons are sparse. Whereas, in the caudal, pars compacta region, cholinergic neurons are considerably more abundant. Glutamatergic neurons also increase in density along the rostro-caudal axis, whereas GABAergic neurons have the opposite gradient, being most abundant in the pars dissipata (Wang and Morales, 2009). Although there is some controversy about the co-expression of different neurotransmitters, the prevailing view is that there is essentially no co-expression and these three populations are truly distinct (Clements et al., 1991; Jia et al., 2003; Lavoie & Parent, 1994a; Wang & Morales, 2009).
This neurochemical diversity in the PPN is partially paralleled by physiological diversity. Based upon in vitro electrophysiological studies with intracellular sharp electrodes, Takakusaki and Kitai (Takakusaki & Kitai, 1997) classified PPN neurons into two groups (Type I and Type II). Type I neurons had low threshold spikes (LTS) and sub-threshold voltage oscillations that were sensitive to the Na+ channel blocker tetrodotoxin. They were spontaneously active, spiking in irregular or bursting patterns at up to 18 spikes per second. Type II neurons also were spontaneously active (3–16 Hz), but their spiking was more regular than Type I neurons. These neurons also had a prominent slow ramp to the first spike after a hyperpolarizing step, indicating the presence of a robust population of Kv4 K+ channels; another hallmark of these cells was a prominent spike afterhyperpolarization, which like the Kv4 channels served to regularize spiking. Another distinguishing feature of Type II neurons was the presence of Ca2+ and Ca2+- dependent sub-threshold oscillations. This description also is consistent with work by Scarnati and coworkers in 1987 (Scarnati, Proia, Di Loreto, & Pacitti, 1987).
What is less clear is the relationship between Type I and II neurons and the transmitter phenotype of PPN neurons. A little more than half of Type II neurons were found to have histochemical markers of cholinergic neurons, while all of the Type I neurons lacked these markers (Takakusaki & Kitai, 1997). Using a similar post hoc immunocytochemical approach to identify recorded neurons, Petzold et al. (Petzold, Valencia, Pal, & Mena-Segovia, 2015) found that PPN cholinergic neurons had a stronger spike frequency adaptation and a lower overall firing frequency than their non-cholinergic counterparts – consistent with the conclusions of Takakusaki et al. (Takakusaki, Chiba, Nozu, & Okumura, 2016). More recently, genetic approaches have been used to label cholinergic and GABAergic PPN neurons with fluorescent reporters allowing directed sampling of these populations (Bordas, Kovacs, & Pal, 2015). These studies have largely confirmed previous work suggesting that cholinergic neurons have the Type II phenotype and gone on to suggest that the expression of Kv7 (KCNQ) K+ channels by cholinergic neurons not only contributes to the previously described properties of Type II neurons but also endows them with resonance properties in the 20 Hz range.
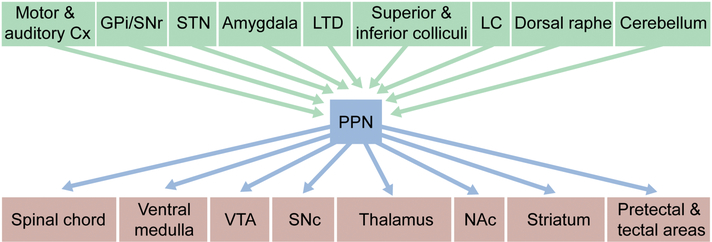
Functional connectivity of the PPN
Both the afferent and efferent connectomes of the PPN are complex. Although monosynaptic rabies virus techniques (Wall, Wickersham, Cetin, De La Parra, & Callaway, 2010; Wickersham, Finke, Conzelmann, & Callaway, 2007) have not been used to generate quantitative, unbiased maps of the regions making synaptic connections with each of the PPN subtypes, conventional anatomical approaches have shown that the basal ganglia and a wide variety of other structures provide a rich innervation of the PPN. In particular, GABAergic neurons in the internal part of the globus pallidus (GPi) and the subtantia nigra pars reticulata (SNr) robustly innervate the PPN (Shink, Sidibe, & Smith, 1997); glutamatergic neurons of the subtalamic nucleus (STN) also innervate the PPN (Granata & Kitai, 1989; Hammond, Rouzaire-Dubois, Feger, Jackson, & Crossman, 1983; Jackson & Crossman, 1983; Kita & Kitai, 1987; Steininger, Rye, & Wainer, 1992). The PPN also receives excitatory innervation from the cerebral cortex, including frontal motor regions and primary auditory cortex (Schofield & Motts, 2009; Semba & Fibiger, 1992; Sesack, Deutch, Roth, & Bunney, 1989). The deep cerebellar nuclei, laterodorsal tegmental nucleus, superior and inferior colliculi, dorsal raphe, and locus coeruleus also innervate the PPN (Hazrati & Parent, 1992; Jones & Yang, 1985; Redgrave, Mitchell, & Dean, 1987; Satoh & Fibiger, 1986; Semba & Fibiger, 1992; Steininger et al., 1992; Steininger, Wainer, Blakely, & Rye, 1997) (Figure 1). Again, what remains is to clearly determine whether there is cellular specificity to the afferent connectome.
Figure 1:
Summary of the afferent and efferent connectivity of PPN.
Abbreviations: Cx: Cortex; GPi: internal Globus pallidus; SNr: Subtantia Nigra pars reticulata; STN: Subthalamic nucleus; LTD: Laterodorsal tegmental nucleus; LC: Locus coeruleus; VTA: Ventral tegmental area; SNc: Subtantia Nigra pars compacta; NAc: Nucleus accumbens.
The efferent connectome of the PPN also is diffuse. The longest ranging PPN projections arise from the cholinergic neurons, which as a population, send both ascending projections to the thalamus, basal ganglia, and limbic structures and descending projections to other brainstem nuclei (Dautan, Hacioglu Bay, Bolam, Gerdjikov, & Mena-Segovia, 2016; Dautan et al., 2014; Lavoie & Parent, 1994c; Mena-Segovia, Sims, Magill, & Bolam, 2008; Semba & Fibiger, 1992; Takakusaki, Shiroyama, Yamamoto, & Kitai, 1996)(Figure 1). In contrast, the GABAergic and glutamatergic neurons appear to largely target neighboring areas within the midbrain and brainstem (Bevan & Bolam, 1995; Ford et al., 1995; Mena-Segovia et al., 2008; Ros, Magill, Moss, Bolam, & Mena-Segovia, 2010)(Figure 1). These projections are somewhat topographically organized – neurons within the caudal PPN project primarily to limbic structures, including the dopaminergic neurons within the ventral tegmental area (VTA), while neurons in the rostral portions of the PPN primarily send projections to motor structures such as the dorsal striatum and substantia nigra pars compacta (SNc) (Dautan et al., 2014; Inglis & Winn, 1995; Jackson & Crossman, 1983; Lavoie & Parent, 1994c; Martinez-Gonzalez, Bolam, & Mena-Segovia, 2011; Oakman, Faris, Kerr, Cozzari, & Hartman, 1995; Winn, 2006).
One of the most studied targets of the PPN is the SNc. The PPN is well established as one of the primarily sources of excitatory synapses on SNc dopaminergic neurons. Electrical stimulation in the PPN in ex vivo slices produces excitatory post-synaptic potentials in SNc neurons (Futami, Takakusaki, & Kitai, 1995), while in vivo studies have shown that PPN activation produces an increase in spike discharge rate in SNc neurons (Di Loreto, Florio, & Scarnati, 1992; Scarnati, Proia, Campana, & Pacitti, 1986; Scarnati et al., 1987). As expected from these results, PPN stimulation increases dopamine release in the nucleus accumbens (Floresco, West, Ash, Moore, & Grace, 2003). Putting this result into a functional context, inhibition of activity in the PPN blunts the response of SNc dopaminergic neurons to salient behavioral cues, arguing that this key feature of SNc dopaminergic neurons is controlled by the PPN (Pan & Hyland, 2005).
What is more controversial is the type of PPN neuron responsible for excitation of SNc dopaminergic neurons. Recent studies examining the PPN cholinergic projection to SNc dopaminergic neurons have produced conflicting results (Estakhr, Abazari, Frisby, McIntosh, & Nashmi, 2017; Xiao et al., 2016). Despite clear behavioral effects associated with cholinergic activation, Xiao et al. (2016) found that a large fraction of SNc dopaminergic neurons failed to respond to optogenetic activation PPN cholinergic neurons when antagonists for glutamatergic synaptic transmission were present, suggesting that the effects of cholinergic stimulation on SNc activity were largely, if not entirely, mediated by indirect, network based signaling. In contrast, there is little doubt that glutamatergic PPN neurons provide a robust excitatory innervation of SNc dopaminergic neurons. Early in vivo studies found that the excitatory effects of PPN stimulation on SNc discharge rate were dramatically attenuated by antagonists of ionotropic glutamate receptors (Di Loreto et al., 1992; Scarnati et al., 1986; Scarnati et al., 1987). More recently, it has been shown that selective, optogenetic activation of PPN glutamatergic neurons excites ventral tegmental area (VTA) dopaminergic neurons and that mice will lever press for optical stimulation (Yoo et al., 2017). In agreement with these findings, recent work from our group found that PPN glutamatergic neurons made monosynaptic synapses on the proximal dendrites of SNc dopaminergic neurons near the spike-generating axon initial segment, allowing PPN synapses to effectively drive single spikes and bursts of spikes (Galtieri, Estep, Wokosin, Traynelis, & Surmeier, 2017).
PPN and the symptoms of PD
One of the most compelling motivations to study PPN cholinergic neurons was the recognition that nearly half of them are gone in late stage PD patients (Hirsch, Graybiel, Duyckaerts, & Javoy-Agid, 1987; Shinotoh et al., 1999; Zweig, Jankel, Hedreen, Mayeux, & Price, 1989). Given that the PPN appears to be part of the reticular activating system and the mesencephalic locomotor region, it was natural to hypothesize that the sleep and gait symptoms of PD – neither of which are levodopa-responsive – were caused by PPN degeneration (Muslimovic et al., 2008; Sethi, 2008; Zweig et al., 1987). This clinical motivation has been buttressed by work showing a correlation between PD patient falls and the reduction in acetycholine release in the thalamus, which was presumably due to the loss of PPN cholinergic neurons (Bohnen et al., 2009; Bohnen et al., 2012). In support this conclusion, functional MRI work has shown that in healthy human subjects PPN activity increases during imagined walking (Karachi et al., 2010).
A number of animal studies have supported the connection between PPN cholinergic neurons and gait control. Karachi et al. (2010) reported that selectively lesioning PPN cholinergic neurons with urotensin II-conjugated diphtheria toxin induced gait and postural deficits in monkeys. They also found that MPTP treatment induced a significant loss of PPN cholinergic neurons, similar to that observed in PD patients. Selectively increasing the excitability of PPN cholinergic neurons with a chemogenetic strategy reportedly ameliorated gait and postural disturbances in a rat model of PD (Pienaar et al., 2015).
However, the connection between PPN cholinergic neurons and gait is far from resolved. Pioneering work done by Garcia-Rill and colleagues (1991;(Garcia-Rill & Skinner, 1987a, 1987b, 1988; Kinjo et al., 1990; Skinner RD, 1990), show that the input from the PPN, in relation to the activation of locomotion, to the ventral medulla and the spinal chord is mainly non-cholinergic. Based on their work, Pahapill and Lozano (Pahapill & Lozano, 2000) speculated that the PPN glutamatergic neurons, which they suggest provide the main PPN output to the spinal chord and the ventral medulla, are critical for the initiation of programed movements and gait, whereas PPN cholinergic neurons relay sensory information about movement back to the basal ganglia and thalamus, helping to maintain gait. This hypothesis has been supported by two compelling, high profile studies using optogenetic approaches, both of which show that PPN glutamatergic neurons – not cholinergic neurons –control gait and locomotion (Caggiano et al., 2018; Roseberry et al., 2016).
A closely related question is the nature of the pathophysiology that might underlie a gait disturbance. Clearly, frank loss of PPN cholinergic could result in dysregulation of target neuronal populations, one of which is likely to be PPN glutamatergic neurons. But, electrophysiological studies in animal models of PD have not yielded a clear picture of what this pathophysiology might be. Some studies have found an increase in the firing rate of unidentified PPN neurons (Aravamuthan et al., 2008; Breit, Bouali-Benazzouz, Benabid, & Benazzouz, 2001; Geng et al., 2016; Zhang et al., 2008), others have reported decreased spiking (Florio et al., 2007; Gomez-Gallego, Fernandez-Villalba, Fernandez-Barreiro, & Herrero, 2007) while others found no change (Mena-Segovia et al., 2005; (Heise & Mitrofanis, 2006). Frankly, these studies, which have all relied upon the use of toxins to create a model of PD, are difficult to interpret for a variety of reasons. One is that the PPN is very close to the SNc, where toxin models induce inflammation, creating a potential artifact. Another concern is that none of the animal PD models faithfully reproduce the pattern of pathology in human PD patients, particularly that seen outside of the SNc in the brainstem (Surmeier, Obeso, & Halliday, 2017a). As PPN receives a convergent input from many of these structures, it could very well be the case that PPN pathophysiology will never be recapitulated in a PD model that only targets the SNc.
In PD patients, there is very little relevant data. It has been reported that there is a decrease in alpha oscillations in the field potentials (Tattersall et al., 2014; Thevathasan et al., 2012) or a synchronization between alpha and beta oscillations (Lau et al., 2015) during the imagined gait. But how this translates to pathological activity patterns in identified cell types is completely unclear. Given the differences in the functional roles played by these different groups of PPN neuron, this is a critical question.
Another key question that remains unanswered is the relationship between gait disturbances and falls. Several studies have implicated basal forebrain cholinergic systems, rather than those of the PPN, in fall frequency in PD patients (Kucinski & Sarter, 2015; Sarter, Albin, Kucinski, & Lustig, 2014).
In spite of all this uncertainty, the clinical importance of gait and balance disturbances in PD patients have motivated an attempt to use deep brain stimulation (DBS) of the PPN region as a palliative treatment. Unfortunately, these studies have yielded conflicting results (Ferraye et al., 2010; Stefani et al., 2007; Thevathasan et al., 2012; Thevathasan et al., 2018). In a recent review, Thevathasan et al. (2018) conclude that despite this inconsistency, PPN DBS has the potential to improve gait and reduce falls in PD patients. They also conclude that the bilateral PPN DBS has better outcomes than the unilateral DBS. Of course, given the complexity of the PPN and neighboring structures linked to gait, this is not surprising. In agreement with an earlier review (Stefani et al., 2013), recent work by Takakusaki et al. (2016) suggests that one issue is the location of the DBS electrode. They argue that the caudal portions of the PPN and the neighboring cuneiform nucleus, which is closely linked to the mesencephalic locomotor region, are the most effective targets for gait disturbances in PD patients; in contrast, stimulation of the more rostral portions of the PPN produced an inhibition of brain stem motor regions and concomitant muscle atonia. In relation to this, Gut and Winn (Gut & Winn, 2015), in a mice model of PD, described that an anterior PPN DBS worsened gait, but posterior PPN DBS mildly improved it, wich emphasize the importance of the DBS location inside the PPN.
The effects of PPN DBS appear not to be limited to movement. Sleep and cognition may also be affected by PPN DBS in PD patients (Alessandro et al., 2010; Ceravolo et al., 2011; Costa et al., 2010; Peppe et al., 2012; Romigi et al., 2008). In particular, bilateral, low frequency PPN-DBS has been reported to improve sleep quality and architecture (Alessandro et al., 2010; Peppe et al., 2012; Romigi et al., 2008). Verbal fluency, long-term memory, and executive functions might also be improved by PPN DBS (Ceravolo et al., 2011). Studies reporting alleviation of non-motor symptoms in PD patients have consistently targeted the caudal PPN, where cholinergic neurons are more abundant.
Frankly, given the complexity of the PPN region, it seems unlikely that the indiscriminate electrical stimulation afforded by DBS is ever likely to yield reliable clinical outcomes, even its role in PD symptoms is resolved. Rather, optogenetic or chemogenetic targeting of genetically defined PPN (or neighboring) neurons is much more likely to be clinically useful. To make this happen, cellspecific promoters that would allow targeted gene delivery [e.g., (Roseberry et al., 2016)] need to be developed for human use.
PPN and PD pathogenesis
Another set of questions for which there are no clear answers has to do with PD pathogenesis, rather than symptomology. There are two specific questions that merit discussion in this regard. First, why do PPN neurons die in PD patients? Second, does PPN pathology contribute to the loss of SNc dopaminergic neurons and the cardinal motor symptoms of PD?
Although the initial description of PPN pathology in PD patients focuse on cholinergic neurons (Hirsch et al., 1987; Shinotoh et al., 1999; Zweig et al., 1989), more recent work has suggested that neuronal loss is not limited to this population but includes GABAergic and glycinergic neurons as well (Pienaar et al., 2013). In rodent toxin and proteostasis inhibition models of PD, neuronal loss in the PPN also includes non-cholinergic neurons (Elson, Yates, & Pienaar, 2016; Pienaar & van de Berg, 2013). It is not clear whether loss extends to glutamatergic neurons implicated in gait control.
What remains to be determined is why any of these neurons die. One possibility is that mitochondrial dysfunction is a culprit, as in the SNc (Surmeier, Obeso, & Halliday, 2017b). Pienaar et al. (Pienaar, Vernon, & Winn, 2016) suggest that alterations in nitric oxide (NO) signaling triggers mitochondria-mediated cell arrest and apoptosis of cholinergic neurons. Another possibility is that the loss of PPN cholinergic neurons is induced by pathophysiology elsewhere in the brain. Bensaid et al. (Bensaid, Michel, Clark, Hirsch, & Francois, 2016) suggest that there is a reciprocity between SNc dopaminergic neurons and PPN cholinergic neurons, such that when one dies, the other does as well. Precisely why this would be the case is unclear and it must be noted that this hypothesis is based upon acute lesion studies. Although inflammation seems not to be a factor in the results of Bensaid et al. (2016), there is a clear need to show that slow, progressive, non-toxin-induced loss of SNc dopaminergic neurons has the same effect as acute, toxin-induced loss.
Another possibility is that the loss of PPN cholinergic neurons is the consequence of alphasynuclein pathology. Given the large axonal field and abundance of presynaptic release sites of PPN cholinergic neurons, it is likely that they express high levels of alpha-synuclein, which functions in the vesicular release of neurotransmitter. As calcium promotes the aggregation of alpha synuclein (Lautenschlager et al., 2018; Llinas, Sugimori, & Silver, 1992; Nielsen, Vorum, Lindersson, & Jensen, 2001; Schneggenburger & Neher, 2000), the combination of elevated expression of alpha-synuclein and high intracellular calcium, which presumably exists in Type II cholinergic neurons, could promote alpha synuclein pathology and ultimately neuronal death. Another possibility that has received a great deal of attention is that PPN cholinergic neurons are vulnerable to propagated alpha synuclein pathology (Braak, Ghebremedhin, Rub, Bratzke, & Del Tredici, 2004; Dijkstra et al., 2014). The robust synaptic connectivity of PPN cholinergic neurons with brainstem nuclei that manifest Lewy pathology is consistent with this idea (Surmeier et al., 2017a). That said, this model has difficulty explaining the selectivity of the PPN loss, as PPN glutamatergic neurons, which appear to be resistant to PD pathology, also are richly connected with the same brainstem regions. Clearly, more study is needed on this point.
A related question is whether PPN pathology contributes to the loss of SNc dopaminergic neurons. One of the earliest theories about PD pathogenesis was that it was driven by glutamatergic N-methyl-d-aspartate (NMDA) receptor-mediated, excitotoxicity (Beal, 1998; German, Manaye, Sonsalla, & Brooks, 1992; Meredith, Totterdell, Beales, & Meshul, 2009; Obeso et al., 2008). NMDA receptor antagonists, are neuroprotective in toxin models of PD (Armentero, Fancellu, Nappi, Bramanti, & Blandini, 2006; Calabresi et al., 2013). Lesioning the STN, a source of glutamatergic innervation of to SNc dopaminergic neurons, also is neuroprotective in toxin models (Piallat, Benazzouz, & Benabid, 1996; Wallace et al., 2007). Given the potent glutamatergic innervation of SNc dopaminergic neurons by the PPN (Galtieri et al., 2017), it is possible that elevated activity in these neurons could drive excitotoxicity. However, this could all be an artifact of toxin models that produce local inflammation and activation of astrocytes and microglia, leading to impaired glutamate homeostasis. Indeed, in ex vivo brain slices from toxin models, extracellular glutamate is elevated in the SNc even though it has been cut off from the glutamatergic neurons innervating it – clearly implicating deficits in astrocytic regulation of extracellular glutamate (Meredith et al., 2009).
Conclusions
The PPN is a complex region from a variety of standpoints. Although it is clearly implicated in movement control, its specific role in gait and posture is unclear, as is its role in arousal, sleep and cognition. Even with refinements in electrode placement, attempts to target this region using DBS are likely to yield inconsistent results because of the complexity of the region. Alternative strategies using optogenetic or chemogenetic approaches are more likely to produce consistent clinical outcomes. It also remains to be resolved why PPN neurons are lost in PD and how this loss affects pathology in other regions, including the SNc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, . . . Peppe A (2010). Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci, 289(1–2), 44–48. doi: 10.1016/j.jns.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Aravamuthan BR, Bergstrom DA, French RA, Taylor JJ, Parr-Brownlie LC, & Walters JR (2008). Altered neuronal activity relationships between the pedunculopontine nucleus and motor cortex in a rodent model of Parkinson’s disease. Exp Neurol, 213(2), 268–280. doi: 10.1016/j.expneurol.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentero MT, Fancellu R, Nappi G, Bramanti P, & Blandini F (2006). Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol Dis, 22(1), 1–9. doi: 10.1016/j.nbd.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Beal MF (1998). Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann Neurol, 44(3 Suppl 1), S110–114. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Michel PP, Clark SD, Hirsch EC, & Francois C (2016). Role of pedunculopontine cholinergic neurons in the vulnerability of nigral dopaminergic neurons in Parkinson’s disease. Exp Neurol, 275 Pt 1, 209–219. doi: 10.1016/j.expneurol.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Bevan MD, & Bolam JP (1995). Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J Neurosci, 15(11), 7105–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, & Albin RL (2009). History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology, 73(20), 1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, . . . Frey KA (2012). Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J Cereb Blood Flow Metab, 32(8), 1609–1617. doi: 10.1038/jcbfm.2012.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordas C, Kovacs A, & Pal B (2015). The M-current contributes to high threshold membrane potential oscillations in a cell type-specific way in the pedunculopontine nucleus of mice. Front Cell Neurosci, 9, 121. doi: 10.3389/fncel.2015.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, & Del Tredici K (2004). Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res, 318(1), 121–134. doi: 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Breit S, Bouali-Benazzouz R, Benabid AL, & Benazzouz A (2001). Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat. Eur J Neurosci, 14(11), 1833–1842. [DOI] [PubMed] [Google Scholar]
- Caggiano V, Leiras R, Goni-Erro H, Masini D, Bellardita C, Bouvier J, . . . Kiehn O (2018). Midbrain circuits that set locomotor speed and gait selection. Nature, 553(7689), 455–460. doi: 10.1038/nature25448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Di Filippo M, Gallina A, Wang Y, Stankowski JN, Picconi B, . . . Dawson TM (2013). New synaptic and molecular targets for neuroprotection in Parkinson’s disease. Mov Disord, 28(1), 51–60. doi: 10.1002/mds.25096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceravolo R, Brusa L, Galati S, Volterrani D, Peppe A, Siciliano G, . . . Stefani A (2011). Low frequency stimulation of the nucleus tegmenti pedunculopontini increases cortical metabolism in parkinsonian patients. Eur J Neurol, 18(6), 842–849. doi: 10.1111/j.1468-1331.2010.03254.x [DOI] [PubMed] [Google Scholar]
- Clements JR, & Grant S (1990). Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci Lett, 120(1), 70–73. [DOI] [PubMed] [Google Scholar]
- Clements JR, Toth DD, Highfield DA, & Grant SJ (1991). Glutamate-like immunoreactivity is present within cholinergic neurons of the laterodorsal tegmental and pedunculopontine nuclei. Adv Exp Med Biol, 295, 127–142. [DOI] [PubMed] [Google Scholar]
- Costa A, Carlesimo GA, Caltagirone C, Mazzone P, Pierantozzi M, Stefani A, & Peppe A (2010). Effects of deep brain stimulation of the peduncolopontine area on working memory tasks in patients with Parkinson’s disease. Parkinsonism Relat Disord, 16(1), 64–67. doi: 10.1016/j.parkreldis.2009.05.009 [DOI] [PubMed] [Google Scholar]
- Dautan D, Hacioglu Bay H, Bolam JP, Gerdjikov TV, & Mena-Segovia J (2016). Extrinsic Sources of Cholinergic Innervation of the Striatal Complex: A Whole-Brain Mapping Analysis. Front Neuroanat, 10, 1. doi: 10.3389/fnana.2016.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, & Mena-Segovia J (2014). A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci, 34(13), 4509–4518. doi: 10.1523/JNEUROSCI.5071-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Loreto S, Florio T, & Scarnati E (1992). Evidence that non-NMDA receptors are involved in the excitatory pathway from the pedunculopontine region to nigrostriatal dopaminergic neurons. Exp Brain Res, 89(1), 79–86. [DOI] [PubMed] [Google Scholar]
- Dijkstra AA, Voorn P, Berendse HW, Groenewegen HJ, Rozemuller AJ, & van de Berg WD (2014). Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov Disord, 29(10), 1244–1251. doi: 10.1002/mds.25952 [DOI] [PubMed] [Google Scholar]
- Elson JL, Yates A, & Pienaar IS (2016). Pedunculopontine cell loss and protein aggregation direct microglia activation in parkinsonian rats. Brain Struct Funct, 221(4), 2319–2341. doi: 10.1007/s00429-015-1045-4 [DOI] [PubMed] [Google Scholar]
- Estakhr J, Abazari D, Frisby K, McIntosh JM, & Nashmi R (2017). Differential Control of Dopaminergic Excitability and Locomotion by Cholinergic Inputs in Mouse Substantia Nigra. Curr Biol, 27(13), 1900–1914 e1904. doi: 10.1016/j.cub.2017.05.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, . . . Pollak P (2010). Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain, 133(Pt 1), 205–214. doi: 10.1093/brain/awp229 [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, & Grace AA (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci, 6(9), 968–973. doi: 10.1038/nn1103 [DOI] [PubMed] [Google Scholar]
- Florio T, Scarnati E, Confalone G, Minchella D, Galati S, Stanzione P, . . . Mazzone P (2007). High-frequency stimulation of the subthalamic nucleus modulates the activity of pedunculopontine neurons through direct activation of excitatory fibres as well as through indirect activation of inhibitory pallidal fibres in the rat. Eur J Neurosci, 25(4), 1174–1186. doi: 10.1111/j.1460-9568.2007.05360.x [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, & Jones BE (1995). GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol, 363(2), 177–196. doi: 10.1002/cne.903630203 [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, & Kitai ST (1995). Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res, 21(4), 331–342. [DOI] [PubMed] [Google Scholar]
- Galtieri DJ, Estep CM, Wokosin DL, Traynelis S, & Surmeier DJ (2017). Pedunculopontine glutamatergic neurons control spike patterning in substantia nigra dopaminergic neurons. Elife, 6. doi: 10.7554/eLife.30352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, & Skinner RD (1987a). The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res, 411(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, & Skinner RD (1987b). The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res, 411(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, & Skinner RD (1988). Modulation of rhythmic function in the posterior midbrain. Neuroscience, 27(2), 639–654. [DOI] [PubMed] [Google Scholar]
- Geng X, Xie J, Wang X, Wang X, Zhang X, Hou Y, . . . Wang M (2016). Altered neuronal activity in the pedunculopontine nucleus: An electrophysiological study in a rat model of Parkinson’s disease. Behav Brain Res, 305, 57–64. doi: 10.1016/j.bbr.2016.02.026 [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, Sonsalla PK, & Brooks BA (1992). Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci, 648, 42–62. [DOI] [PubMed] [Google Scholar]
- Gomez-Gallego M, Fernandez-Villalba E, Fernandez-Barreiro A, & Herrero MT (2007). Changes in the neuronal activity in the pedunculopontine nucleus in chronic MPTP-treated primates: an in situ hybridization study of cytochrome oxidase subunit I, choline acetyl transferase and substance P mRNA expression. J Neural Transm (Vienna), 114(3), 319–326. doi: 10.1007/s00702-006-0547-x [DOI] [PubMed] [Google Scholar]
- Granata AR, & Kitai ST (1989). Intracellular analysis of excitatory subthalamic inputs to the pedunculopontine neurons. Brain Res, 488(1–2), 57–72. [DOI] [PubMed] [Google Scholar]
- Gut NK, & Winn P (2015). Deep brain stimulation of different pedunculopontine targets in a novel rodent model of parkinsonism. J Neurosci, 35(12), 4792–4803. doi: 10.1523/jneurosci.3646-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Rouzaire-Dubois B, Feger J, Jackson A, & Crossman AR (1983). Anatomical and electrophysiological studies on the reciprocal projections between the subthalamic nucleus and nucleus tegmenti pedunculopontinus in the rat. Neuroscience, 9(1), 41–52. [DOI] [PubMed] [Google Scholar]
- Hazrati LN, & Parent A (1992). Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Res, 585(1–2), 267–271. [DOI] [PubMed] [Google Scholar]
- Heise CE, & Mitrofanis J (2006). Fos immunoreactivity in some locomotor neural centres of 6OHDA-lesioned rats. Anat Embryol (Berl), 211(6), 659–671. doi: 10.1007/s00429-006-0130-0 [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Graybiel AM, Duyckaerts C, & Javoy-Agid F (1987). Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci U S A, 84(16), 5976–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, & Semba K (1995). An ultrastructural study of cholinergic and non-cholinergic neurons in the laterodorsal and pedunculopontine tegmental nuclei in the rat. Neuroscience, 68(3), 837–853. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Teng B, & Kitai ST (2000). Morphological study of the tegmental pedunculopontine nucleus, substantia nigra and subthalamic nucleus, and their interconnections in rat organotypic culture. Anat Embryol (Berl), 201(6), 435–453. [DOI] [PubMed] [Google Scholar]
- Inglis WL, & Winn P (1995). The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol, 47(1), 1–29. Jackson A & Crossman AR (1983). Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience, 10(3), 725–765. [DOI] [PubMed] [Google Scholar]
- Jia HG, Yamuy J, Sampogna S, Morales FR, & Chase MH (2003). Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res, 992(2), 205–219. [DOI] [PubMed] [Google Scholar]
- Jones BE, & Yang TZ (1985). The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol, 242(1), 56–92. doi: 10.1002/cne.902420105 [DOI] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, . . . Francois C (2010). Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest, 120(8), 2745–2754. doi: 10.1172/JCI42642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, & Garcia-Rill E (1990). Medioventral medulla-induced locomotion. Brain Res Bull, 24(3), 509–516. [DOI] [PubMed] [Google Scholar]
- Kita H, & Kitai ST (1987). Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol, 260(3), 435–452. doi: 10.1002/cne.902600309 [DOI] [PubMed] [Google Scholar]
- Kucinski A, & Sarter M (2015). Modeling Parkinson’s disease falls associated with brainstem cholinergic systems decline. Behav Neurosci, 129(2), 96–104. doi: 10.1037/bne0000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Welter ML, Belaid H, Fernandez Vidal S, Bardinet E, Grabli D, & Karachi C (2015). The integrative role of the pedunculopontine nucleus in human gait. Brain, 138(Pt 5), 1284–1296. doi: 10.1093/brain/awv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager J, Stephens AD, Fusco G, Strohl F, Curry N, Zacharopoulou M, . . . Schierle GSK (2018). C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction. Nat Commun, 9(1), 712. doi: 10.1038/s41467-018-03111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, & Parent A (1994a). Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol, 344(2), 232–241. doi: 10.1002/cne.903440205 [DOI] [PubMed] [Google Scholar]
- Lavoie B, & Parent A (1994b). Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol, 344(2), 190–209. doi: 10.1002/cne.903440203 [DOI] [PubMed] [Google Scholar]
- Lavoie B, & Parent A (1994c). Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol, 344(2), 210–231. doi: 10.1002/cne.903440204 [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, & Silver RB (1992). Microdomains of high calcium concentration in a presynaptic terminal. Science, 256(5057), 677–679. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, & Mena-Segovia J (2011). Topographical organization of the pedunculopontine nucleus. Front Neuroanat, 5, 22. doi: 10.3389/fnana.2011.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Sims HM, Magill PJ, & Bolam JP (2008). Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol, 586(12), 2947–2960. doi: 10.1113/jphysiol.2008.153874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Beales M, & Meshul CK (2009). Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp Neurol, 219(1), 334–340. doi: 10.1016/j.expneurol.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ, & Group CS (2008). Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology, 70(23), 2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80 [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Vorum H, Lindersson E, & Jensen PH (2001). Ca2+ binding to alpha-synuclein regulates ligand binding and oligomerization. J Biol Chem, 276(25), 22680–22684. doi: 10.1074/jbc.M101181200 [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, & Hartman BK (1995). Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci, 15(9), 5859–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, . . . Olanow CW (2008). The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol, 64 Suppl 2, S30–46. doi: 10.1002/ana.21481 [DOI] [PubMed] [Google Scholar]
- Pahapill PA, & Lozano AM (2000). The pedunculopontine nucleus and Parkinson’s disease. Brain, 123 ( Pt 9), 1767–1783. [DOI] [PubMed] [Google Scholar]
- Pan WX, & Hyland BI (2005). Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci, 25(19), 4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppe A, Pierantozzi M, Baiamonte V, Moschella V, Caltagirone C, Stanzione P, & Stefani A (2012). Deep brain stimulation of pedunculopontine tegmental nucleus: role in sleep modulation in advanced Parkinson disease patients: one-year follow-up. Sleep, 35(12), 1637–1642. doi: 10.5665/sleep.2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A, Valencia M, Pal B, & Mena-Segovia J (2015). Decoding brain state transitions in the pedunculopontine nucleus: cooperative phasic and tonic mechanisms. Front Neural Circuits, 9, 68. doi: 10.3389/fncir.2015.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piallat B, Benazzouz A, & Benabid AL (1996). Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci, 8(7), 1408–1414. [DOI] [PubMed] [Google Scholar]
- Pienaar IS, Elson JL, Racca C, Nelson G, Turnbull DM, & Morris CM (2013). Mitochondrial abnormality associates with type-specific neuronal loss and cell morphology changes in the pedunculopontine nucleus in Parkinson disease. Am J Pathol, 183(6), 1826–1840. doi: 10.1016/j.ajpath.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar IS, Harrison IF, Elson JL, Bury A, Woll P, Simon AK, & Dexter DT (2015). An animal model mimicking pedunculopontine nucleus cholinergic degeneration in Parkinson’s disease. Brain Struct Funct, 220(1), 479–500. doi: 10.1007/s00429-013-0669-5 [DOI] [PubMed] [Google Scholar]
- Pienaar IS, & van de Berg W (2013). A non-cholinergic neuronal loss in the pedunculopontine nucleus of toxin-evoked parkinsonian rats. Exp Neurol, 248, 213–223. doi: 10.1016/j.expneurol.2013.06.008 [DOI] [PubMed] [Google Scholar]
- Pienaar IS, Vernon A, & Winn P (2016). The Cellular Diversity of the Pedunculopontine Nucleus: Relevance to Behavior in Health and Aspects of Parkinson’s Disease. Neuroscientist. doi: 10.1177/1073858416682471 [DOI] [PubMed] [Google Scholar]
- Redgrave P, Mitchell IJ, & Dean P (1987). Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp Brain Res, 68(1), 147–167. [DOI] [PubMed] [Google Scholar]
- Romigi A, Placidi F, Peppe A, Pierantozzi M, Izzi F, Brusa L, . . . Stefani A (2008). Pedunculopontine nucleus stimulation influences REM sleep in Parkinson’s disease. Eur J Neurol, 15(7), e64–65. doi: 10.1111/j.1468-1331.2008.02167.x [DOI] [PubMed] [Google Scholar]
- Ros H, Magill PJ, Moss J, Bolam JP, & Mena-Segovia J (2010). Distinct types of non-cholinergic pedunculopontine neurons are differentially modulated during global brain states. Neuroscience, 170(1), 78–91. doi: 10.1016/j.neuroscience.2010.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, & Kreitzer AC (2016). Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell, 164(3), 526–537. doi: 10.1016/j.cell.2015.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, & Wainer BH (1987). Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol, 259(4), 483–528. doi: 10.1002/cne.902590403 [DOI] [PubMed] [Google Scholar]
- Sarter M, Albin RL, Kucinski A, & Lustig C (2014). Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol, 257, 120–129. doi: 10.1016/j.expneurol.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, & Fibiger HC (1986). Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol, 253(3), 277–302. doi: 10.1002/cne.902530302 [DOI] [PubMed] [Google Scholar]
- Scarnati E, Proia A, Campana E, & Pacitti C (1986). A microiontophoretic study on the nature of the putative synaptic neurotransmitter involved in the pedunculopontine-substantia nigra pars compacta excitatory pathway of the rat. Exp Brain Res, 62(3), 470–478. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Proia A, Di Loreto S, & Pacitti C (1987). The reciprocal electrophysiological influence between the nucleus tegmenti pedunculopontinus and the substantia nigra in normal and decorticated rats. Brain Res, 423(1–2), 116–124. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, & Neher E (2000). Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature, 406(6798), 889–893. doi: 10.1038/35022702 [DOI] [PubMed] [Google Scholar]
- Schofield BR, & Motts SD (2009). Projections from auditory cortex to cholinergic cells in the midbrain tegmentum of guinea pigs. Brain Res Bull, 80(3), 163–170. doi: 10.1016/j.brainresbull.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, & Fibiger HC (1992). Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol, 323(3), 387–410. doi: 10.1002/cne.903230307 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, & Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol, 290(2), 213–242. doi: 10.1002/cne.902900205 [DOI] [PubMed] [Google Scholar]
- Sethi K (2008). Levodopa unresponsive symptoms in Parkinson disease. Mov Disord, 23 Suppl 3, S521–533. doi: 10.1002/mds.22049 [DOI] [PubMed] [Google Scholar]
- Shink E, Sidibe M, & Smith Y (1997). Efferent connections of the internal globus pallidus in the squirrel monkey: II. Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol, 382(3), 348–363. [PubMed] [Google Scholar]
- Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka S, Iyo M, . . . Irie T (1999). Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson’s disease and progressive supranuclear palsy. Ann Neurol, 46(1), 62–69. [PubMed] [Google Scholar]
- Skinner RD KN, Ishikawa Y, Biedermann JA, Garcia-Rill E (1990). Locomotor projections from the pedunculopontine nucleus to the medioventral medulla. Neuroreport, 1(3–4), 207–210. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, . . . Mazzone P (2007). Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain, 130(Pt 6), 1596–1607. doi: 10.1093/brain/awl346 [DOI] [PubMed] [Google Scholar]
- Stefani A, Peppe A, Galati S, Bassi MS, D’Angelo V, & Pierantozzi M (2013). The serendipity case of the pedunculopontine nucleus low-frequency brain stimulation: chasing a gait response, finding sleep, and cognition improvement. Front Neurol, 4, 68. doi: 10.3389/fneur.2013.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, & Wainer BH (1992). Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol, 321(4), 515–543. doi: 10.1002/cne.903210403 [DOI] [PubMed] [Google Scholar]
- Steininger TL, Wainer BH, Blakely RD, & Rye DB (1997). Serotonergic dorsal raphe nucleus projections to the cholinergic and noncholinergic neurons of the pedunculopontine tegmental region: a light and electron microscopic anterograde tracing and immunohistochemical study. J Comp Neurol, 382(3), 302–322. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, & Halliday GM (2017a). Parkinson’s Disease Is Not Simply a Prion Disorder. J Neurosci, 37(41), 9799–9807. doi: 10.1523/jneurosci.1787-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, & Halliday GM (2017b). Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci, 18(2), 101–113. doi: 10.1038/nrn.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Chiba R, Nozu T, & Okumura T (2016). Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J Neural Transm (Vienna), 123(7), 695–729. doi: 10.1007/s00702-015-1475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, & Kitai ST (1997). Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience, 78(3), 771–794. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shiroyama T, Yamamoto T, & Kitai ST (1996). Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J Comp Neurol, 371(3), 345–361. doi:10.1002/(SICI)1096–9861(19960729)371:3<345::AID-CNE1>3.0.CO;2–2 [DOI] [PubMed] [Google Scholar]
- Tattersall TL, Stratton PG, Coyne TJ, Cook R, Silberstein P, Silburn PA, . . . Sah P (2014). Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat Neurosci, 17(3), 449–454. doi: 10.1038/nn.3642 [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, Brittain JS, . . . Brown P (2012). A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain, 135(Pt 5), 1446–1454. doi: 10.1093/brain/aws039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Debu B, Aziz T, Bloem BR, Blahak C, Butson C, . . . Functional N (2018). Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: A clinical review. Mov Disord, 33(1), 10–20. doi: 10.1002/mds.27098 [DOI] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, & Callaway EM (2010). Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc Natl Acad Sci U S A, 107(50), 21848–21853. doi: 10.1073/pnas.1011756107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, & Benabid AL (2007). Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain, 130(Pt 8), 2129–2145. doi: 10.1093/brain/awm137 [DOI] [PubMed] [Google Scholar]
- Wang HL, & Morales M (2009). Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci, 29(2), 340–358. doi: 10.1111/j.1460-9568.2008.06576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, & Callaway EM (2007). Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods, 4(1), 47–49. doi: 10.1038/nmeth999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn P (2006). How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci, 248(1–2), 234–250. doi: 10.1016/j.jns.2006.05.036 [DOI] [PubMed] [Google Scholar]
- Xiao C, Cho JR, Zhou C, Treweek JB, Chan K, McKinney SL, . . . Gradinaru V (2016). Cholinergic Mesopontine Signals Govern Locomotion and Reward through Dissociable Midbrain Pathways. Neuron, 90(2), 333–347. doi: 10.1016/j.neuron.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Wu J, Punta C, Ramajayam N, Shen X, . . . Hnasko TS (2017). Activation of Pedunculopontine Glutamate Neurons Is Reinforcing. J Neurosci, 37(1), 38–46. doi: 10.1523/JNEUROSCI.3082-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Liu J, Wang Y, Wang S, Wu ZH, Yan W, . . . Ali U (2008). The firing activity of presumed cholinergic and non-cholinergic neurons of the pedunculopontine nucleus in 6-hydroxydopamine-lesioned rats: an in vivo electrophysiological study. Brain Res, 1243, 152–160. doi: 10.1016/j.brainres.2008.09.028 [DOI] [PubMed] [Google Scholar]
- Zweig RM, Jankel WR, Hedreen JC, Mayeux R, & Price DL (1989). The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol, 26(1), 41–46. doi: 10.1002/ana.410260106 [DOI] [PubMed] [Google Scholar]
- Zweig RM, Whitehouse PJ, Casanova MF, Walker LC, Jankel WR, & Price DL (1987). Loss of pedunculopontine neurons in progressive supranuclear palsy. Ann Neurol, 22(1), 18–25. doi: 10.1002/ana.410220107 [DOI] [PubMed] [Google Scholar]