Abstract
Purpose
To maximize the discovery of potentially pathogenic variants to better understand the diagnostic utility of genome sequencing (GS) and to assess how the presence of multiple risk events might affect the phenotypic severity in autism spectrum disorders (ASD).
Methods
GS was applied to 180 simplex and multiplex ASD families (578 individuals, 213 patients) with exome sequencing and array comparative genomic hybridization further applied to a subset for validation and cross-platform comparisons.
Results
We found that 40.8% of patients carried variants with evidence of disease risk, including a de novo frameshift variant in NR4A2 and two de novo missense variants in SYNCRIP, while 21.1% carried clinically relevant pathogenic or likely pathogenic variants. Patients with more than one risk variant (9.9%) were more severely affected with respect to cognitive ability compared with patients with a single or no-risk variant. We observed no instance among the 27 multiplex families where a pathogenic or likely pathogenic variant was transmitted to all affected members in the family.
Conclusion
The study demonstrates the diagnostic utility of GS, especially for multiple risk variants that contribute to the phenotypic severity, shows the genetic heterogeneity in multiplex families, and provides evidence for new genes for follow up.
Keywords: autism spectrum disorders, genome sequencing, exome sequencing, multiple-hit events, diagnostic utility
INTRODUCTION
Detailed phenotyping coupled with sequencing of patient cohorts with autism spectrum disorder (ASD) and related neurodevelopmental disorders (NDDs) have allowed the identification of hundreds of risk genes and related variants.1–5 While useful, most of these studies have largely focused on a particular subset of patients or have imposed strict enrollment criteria that have led to phenotypic ascertainment biases. One of the most useful and deeply phenotyped cohorts, the Simons Simplex Collection (SSC),6 for example, was restricted to simplex cases and carried a relatively lower proportion of intellectual disability (ID) cases (<25%). Such biases have likely skewed our understanding of the relative contribution of de novo and private variants as well as the potential diagnostic or predictive utility of genome sequencing (GS) in a clinical setting.
The genetic architecture of ASD has become clearer in the last decades and hundreds of risk genes and related variants have now been identified for both syndromic and idiopathic autism, based on genome-wide microarrays,7 exome sequencing (ES),3,4 and more recently, GS.8,9 Nevertheless, rare genetic variants, including de novo single-nucleotide variants (SNVs) and insertions/deletions (indels), and copy number variants (CNVs) still account for only a limited fraction of simplex cases (10%–30%).3,10 The high heritability of ASD (50%–80%)11 suggests that the monogenic model is likely too simplistic and that other risk variants await discovery. Although hundreds of risk variants have been identified, many of them demonstrate reduced penetrance and/or variable expressivity, including the transmission of potentially pathogenic variants from an unaffected parent to offspring.
One possibility may be that the penetrance of such risk alleles depends upon the genetic background on which these variants occur. Multiple gene-disruptive events, for example, may co-occur in probands and act synergistically or additively to lead to a more severe phenotype as suggested by several recent studies.12–14 Among multiplex families where more than one sibling is affected, differential transmission of such variants in conjunction with additional de novo variants may lead to phenotypic variability, even when Mendelian inheritance seems likely.15 These situations make genetic diagnosis or risk prediction of individuals with ASD and related NDDs particularly challenging.
Comprehensive variant discovery is key to disease association and gene discovery. GS is now regarded as the preferred approach to identify the full spectrum of risk variants and explore the individual-level genetic architecture. In this study, we applied three platforms—GS, ES and array comparative genomic hybridization (aCGH)—in order to study a local cohort of families presenting to the clinic with at least one child with ASD features. Our goal was to maximize the discovery of potentially pathogenic variants to better understand the diagnostic utility of GS compared to a multiplatform approach, identify/validate novel disorder-related variants, and assess how the presence of multiple pathogenic variants might affect the phenotypic severity in individuals with ASD.
MATERIALS AND METHODS
Patients
We selected autism families for genetic investigation where at least one proband had been diagnosed with ASD and had been clinically evaluated at the Seattle Children’s Autism Center over the last five years from the Study of Autism Genetics Exploration (SAGE) collection (Supplementary Methods). SAGE included individuals with ASD and ID as well as individuals with intact cognitive abilities and included children from both multiplex and simplex families. We only selected samples where DNA from both parents was available and probands were diagnosed with ASD by meeting cutoff criteria on the Autism Diagnostic Observation Schedule and/or DSM-5. In total, we investigated 180 families (578 individuals, 213 patients and 5 unaffected siblings), including 149 trios, 23 multiplex quads, 3 simplex quads, 3 multiplex five-member families, 1 simplex five-member family, and 1 seven-member family (Supplementary Figure S1, Supplementary Table S1). Clinical information was extracted from medical record review, standardized psychological evaluation, and/or parent report. For 64 affected individuals from 55 families, quantitative intelligence quotient metrics (full-scale IQ (FSIQ) and/or non-verbal IQ (NVIQ)) were available (Supplementary Table S1). All participants provided informed consent prior to participation in the study (IRB protocol #44219).
GS and analysis
Sequencing and quality control (QC)
All GS samples were analyzed at the New York Genome Center (NYGC) using 1 microgram of DNA, an Illumina PCR-free library protocol, and sequencing on the Illumina HiSeq X Ten platform. Sequence analysis was performed using a Centers for Common Disease Genomics (CCDG)-compliant pipeline as described elsewhere.16 Generated reads were aligned to the genome (GRCh38) using BWA-MEM17 (v0.7.15), duplicate reads were marked using Picard (v2.4.1), and base scores were recalibrated using GATK18 (v3.5). QC analysis included GS metrics estimation (Picard v2.4.1), flagstat estimation (SAMtools v1.3.1), and insert-size estimation (WHAM-Graphening v1.7.0) (Supplementary Figure S2a-d). Genomes were sequenced to a mean coverage of 35.4× (37.3× for the CCDS region). Full QC statistics are available in Supplementary Table S2. Kinship coefficients (ϕ) by KING19 were used to assess family relationships. All family relatedness was estimated as reported (Supplementary Figure S2e, Supplementary Table S2). Mitochondrial haplogroup analysis (Supplementary Figure S2f) indicates that most families are of European descent (consistent with self-reporting).
SNV/indel calls
We used the same pipeline to call SNVs and small indels as described previously.8 In summary, SNVs and indels were called using the GATK HaplotypeCaller (v3.5) on a multiple-samples joint-calling basis and FreeBayes (v1.0.2) on a per-family basis. De novo SNVs and indels were called using a custom pipeline with family-level VCFs for both FreeBayes and GATK. First, a BCFtools (v.1.8) norm was used to left-align and normalize indels. Then, candidate sites were chosen where the father’s genotype was 0/0, the mother’s genotype was 0/0, and the child’s genotype was either 0/1 or 1/1. Finally, we applied allele count, read-depth and allele balance filters: the father alternate allele count = 0, mother alternate allele count = 0, child allele balance > 0.25, father depth > 9, mother depth > 9, child depth > 9, and either child genotype quality > 20 (GATK) or sum of quality of the alternate observations > 20 (FreeBayes). Any sites in low-complexity regions were removed from further analysis.
CNV calls
We use the same pipeline to call CNVs as described previously8 with several changes to the callers applied. In our original pipeline, CNV detection was performed by five SV-calling programs (dCGH,20 Genome STRiP,21 LUMPY,22 WHAMG,23 and VariationHunter). In this study, we excluded VariationHunter and added CNVnator24 and DELLY25 for six total algorithms. Calls generated from those six CNV callers were then merged on a per-sample basis with calls being reported with the breakpoints from one algorithm and supporting algorithms annotated. Breakpoint selection was accomplished by our previously described8 algorithm, which utilizes a combination of relative known breakpoint accuracy (Genome STRiP, LUMPY, WHAMG, DELLY, CNVnator and finally dCGH), read depth, and SVtyper support. In addition, we manually visualized all high-quality, private de novo CNVs using samplot (https://github.com/ryanlayer/samplot) and WSSD read-depth line plot, and only consider the ones that passed our visualization for further analysis.
ES and microarray-based CNV analysis
A subset of families were also subjected to ES and CNV analyses using standard procedures (Supplementary Methods).
RESULTS
GS and variant discovery
We performed GS on all 180 families (578 DNA samples) and applied GATK and FreeBayes to detect SNVs/indels (Supplementary Figure S3). After filtering, 35,384 putative de novo SNVs/indels were detected by both GATK and FreeBayes. We randomly selected 150 putative de novo SNVs for validation distributed from unique (n=50), ancient repetitive (n=50) and recent repetitive (n=50) regions as described previously.8 After excluding variants that cannot be amplified or reliably Sanger sequenced, we estimate a validation rate of 95.5% (42/44) in unique regions, 65.7% (23/35) in ancient repeat regions, and 16.2% (6/37) in recent repeat regions. Correcting for differential validation, we estimate a genome-wide rate of 79 de novo SNVs per child.
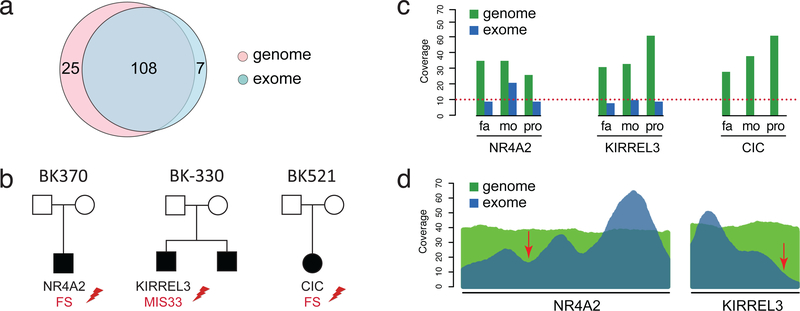
We selected 70 families (90 affected offspring, 230 individuals) for ES (Supplementary Figure S1, Supplementary Methods). To compare the ES and GS results, we analyzed both datasets using the same analysis software and filtering pipeline for de novo variants, while also applying GATK hard filtering to remove high-frequency variants (minor allele frequency > 1% in ExAC). Of the putative de novo coding events detected by either GS or ES, 108 variants were supported by both, 25 by GS only, and 8 by ES only. We attempted to validate all GS-only and ES-only variants. Six GS-only variants could not be validated by Sanger sequencing, one ES-only variant was maternal, and all the others were validated as de novo (Figure 1a, Supplementary Table S3). Of the GS-only de novo events, we discovered a frameshift variant in NR4A2 and a missense variant in KIRREL3 (Combined Annotation Dependent Depletion (CADD) score = 33), both NDD-associated genes missed by ES data due to reduced sequence coverage across these exons (Figure 1b-d). GS also detected eight rare nonsynonymous variants not present in the exome target, including a frameshift variant in CIC, a known autism-associated gene26 (Figure 1b). No variants within NDD-associated genes were identified by only ES.
Figure 1. GS versus ES detection.
(a) Comparison of the number of de novo coding SNVs and indels detected in exome-targeted regions by GS and ES. (b) Families with de novo variants in NDD risk genes missed by ES. (c) Comparison of ES and GS coverage of variants in NR4A2, KIRREL3, and CIC in mother, father, and proband. (d) Mean coverage across all samples sequenced by both GS and ES across NR4A2 and KIRREL3. Arrows point to location of the two variants discovered by GS but missed in ES.
In addition to SNVs/indels, we detected 3,498 private CNVs (specific to a SAGE family) in the offspring of which 623 intersected at least one RefSeq gene (Supplementary Table S4). After visualizing the de novo CNVs, we predicted 41 private CNVs to be de novo, including 19 deletions ranging in size from 302 bp to 6.6 Mbp, and 22 duplications ranging in size from 1 kbp to 9.2 Mbp.
Classification of disorder-related SNVs/indels
We set criteria to define and comprehensively characterize disorder-related SNVs/indels in this study (Supplementary Methods, Supplementary Table S5). We identified and validated 56 de novo disorder-related SNVs/indels (15 likely gene-disruptive (LGD), 40 missense and 1 in-frame) from 52 genes in 49 affected individuals (Table 1, Supplementary Table S6). We identified and validated seven inherited disorder-related SNVs/indels (Table 1, Supplementary Table S7). We estimate that 25.4% of the affected offspring carry disorder-related SNVs/indels in one or more candidate genes. To evaluate how many patients carried clinically relevant variants, we further curated the disorder-related SNVs/indels following the standards and guidelines for the interpretation of sequence variants from the American College of Medical Genetics and Genomics (ACMG).27 In total, we classified 14 as pathogenic and 7 as likely pathogenic variants. Clinically relevant pathogenic or likely pathogenic SNVs/indels account for 8.9% of patients.
Table 1.
Disorder-related SNVs/indels.
| Gene | Funca | NT_AAChangeb | Inheritancec | clinical significanced | sampleID | GeneSetse |
|---|---|---|---|---|---|---|
| ARID1B† | SG | NM_020732:c.C2692T:p.R898* | DN | P | BK-303–03 | SFARI(1)|DD93|BC253|ID526|MG237 |
| ARID1B† | SG | NM_020732:c.C2536T:p.Q846* | DN | P | BK-418–03 | SFARI(1)|DD93|BC253|ID526|MG237 |
| MED13L | SG | NM_015335:c.G4076A:p.W1359* | DN | P | BK-242–03 | SFARI(2)|DD93|BC253|ID_C628 |
| WDFY3 | SG | NM_014991:c.A2932T:p.R978* | DN | P | BK-283–03 | SFARI(2)|BC253|ID_C628 |
| RERE | SG | NM_001042681:c.C2278T:p.Q760* | DN | P | BK-186–03 | SFARI(4) |
| ADNP | FS | NM_001282532:c.2250_2274del:p.V751Mfs*13 | DN | P | BK-246–05 | SFARI(1)|BC253|ID_C628 |
| CIC | FS | NM_001304815:c.884_893del:p.A295Pfs*26 | DN | P | BK-521–03 | SFARI(2) |
| OPHN1 | FS | NM_002547:c.932_933insCA:p.Q311Hfs*7 | DN | P | BK-359–03 | SFARI(3)|ID526 |
| CHD8 | SP | NM_020920:c.2682–2A>G | DN | P | BK-186–03 | SFARI(1)|DD93|BC253|MG237 |
| FOXP1 | SP | NM_001244815:c.1728+1->TGCAGCTTTACAG | DN | P | BK-248–03 | SFARI(2)|DD93|BC253|ID526|MG237 |
| ASXL1 | SG | NM_015338:c.C1045T:p.Q349* | MI | P | BK-483–03 | DD93|ID526 |
| GNB1 | MIS | NM_001282539:c.G229A:p.G77S | DN | P | BK-328–03 | - |
| MEF2C | MIS | NM_001193348:c.C43T:p.R15C | DN | P | BK-192–03 | SFARI(4)|DD93|BC253|ID526|MG237 |
| KMT5B | MIS | NM_016028:c.G791C:p.W264S | DN | P | BK-255–03 | SFARI(1)|DD93|BC253|ID526 |
| NR4A2 | FS | NM_006186:c.601_602insGTCC:p.P201Rfs*82 | DN | LP | BK-370–03 | BC253|ID526|MG237 |
| SMC3 | MIS | NM_005445:c.C2413T:p.R805C | DN | LP | BK-255–03 | SFARI(4)|BC253|MG237|ID_C628 |
| STXBP1 | MIS | NM_001032221:c.C560T:p.P187L | DN | LP | BK-277–03 | SFARI(3)|DD93|BC253|ID526|MG237 |
| GRIA1 | MIS | NM_001258021:c.G2264A:p.G755D | DN | LP | BK-401–03 | SFARI(2)|MG237 |ID_C628 |
| KAT6A | MIS | NM_001305878:c.C1582T:p.P528S | DN | LP | BK-523–03 | SFARI(3)|DD93|BC253 |
| SATB2 | MIS | NM_001172509:c.A1861T:p.I621F | DN | LP | BK-550–03 | SFARI(4)|DD93|BC253|ID526|MG237 |
| POGZ | MIS | NM_015100:c.G3048T:p.E1016D | DN | LP | BK-219–03 | SFARI(1)|DD93|BC253|MG237|ID_C628 |
| DYNC1H1 | MIS | NM_001376:c.A13088C:p.K4363T | DN | LP | BK-283–03 | SFARI(3)|DD93|BC253|ID526 |
| SYNCRIP† | MIS | NM_001159676:c.T629C:p.F210S | DN | PDR | BK-611–01 | BC253|ID_C628 |
| SYNCRIP† | MIS | NM_001159676:c.1573_1574CA_TT:p.Q525L | DN | PDR | BK-252–03 | BC253|ID_C628 |
| JMJD1C | FS | NM_032776:c.667_668insA:p.M223Nfs*3 | DN | PDR | BK-418–03 | SFARI(4) |
| THBS1 | SG | NM_003246:c.C2875T:p.R959* | DN | PDR | BK-396–04 | SFARI|ID_C628 |
| LARP4B | FS | NM_015155:c.801_802del:p.C267* | DN | PDR | BK-205–03 | BC253 |
| MCM3AP | FS | NM_003906:c.276delC:p.F93Lfs*42 | DN | PDR | BK-302–03 | ID_C628 |
| KIRREL3 | MIS | NM_032531:c.G1985A:p.R662H | DN | PDR | BK-330–03 | SFARI(3)|ID526 |
| RPS6KA2 | MIS | NM_001006932:c.G1720A:p.G574R | DN | PDR | BK-222–03 | SFARI(4) |
| TUB | MIS | NM_003320:c.G139A:p.G47S | DN | PDR | BK-141–03 | MG237 |
| DMXL2 | MIS | NM_001174116:c.C6137T:p.A2046V | DN | PDR | BK-175–03 | SFARI(4) |
| TOP1 | MIS | NM_003286:c.A1217T:p.H406L | DN | PDR | BK-254–03 | SFARI(5)|ID_C628 |
| SLC9A3 | MIS | NM_001284351:c.C914T:p.S305L | DN | PDR | BK-523–03 | MG237 |
| KCNK9 | MIS | NM_001282534:c.C907T:p.R303C | DN | PDR | BK-227–03 | ID_C628 |
| SPG11 | MIS | NM_001160227:c.C4955G:p.T1652R | DN | PDR | BK-599–07 | MG237 |
| SLITRK5† | MIS | NM_015567:c.G175T:p.G59C | DN | PDR | BK-354–03 | SFARI|MG237 |
| SLITRK5† | MIS | NM_015567:c.C976T:p.P326S | DN | PDR | BK-372–03 | SFARI|MG237 |
| CLSTN3 | MIS | NM_014718:c.T599C:p.I200T | DN | PDR | BK-187–04 | SFARI(5) |
| SMG9 | MIS | NM_019108:c.A947G:p.H316R | DN | PDR | BK-198–03 | ID_C628 |
| MYT1 | MIS | NM_004535:c.C2138T:p.S713F | DN | PDR | BK-516–03 | ID_C628 |
| ACACB | MIS | NM_001093:c.A1963G:p.S655G | DN | PDR | BK-162–03 | ID_C628 |
| GRM5 | MIS | NM_001143831:c.A523G:p.T175A | DN | PDR | BK-307–03 | SFARI|ID_C628 |
| CSMD1 | MIS | NM_033225:c.A2381C:p.H794P | DN | PDR | BK-146–03 | SFARI |
| SDK1 | MIS | NM_152744:c.G6016A:p.E2006K | DN | PDR | BK-401–03 | SFARI |
| TRANK1† | MIS | NM_014831:c.G2701A:p.V901I | DN | PDR | BK-358–03 | - |
| TRANK1† | MIS | NM_014831:c.C6326A:p.T2109K | DN | PDR | BK-413–03 | - |
| RRP8 | MIS | NM_015324:c.G803A:p.R268H | DN | PDR | BK-590–01 | BC253 |
| UPF2 | MIS | NM_080599:c.G91T:p.V31L | DN | PDR | BK-328–03 | SFARI(5)|ID_C628 |
| PTPRT | MIS | NM_007050:c.G548A:p.R183Q | DN | PDR | BK-261–04 | SFARI|ID_C628 |
| KCNS3 | MIS | NM_001282428:c.G601A:p.A201T | DN | PDR | BK-144–03 | BC253 |
| PAFAH1B3 | MIS | NM_001145939:c.T571C:p.Y191H | DN | PDR | BK-460–03 | ID_C628 |
| NALCN | MIS | NM_052867:c.C682T:p.H228Y | DN | PDR | BK-428–03 | ID_C628 |
| BIRC6 | MIS | NM_016252:c.A3931G:p.I1311V | DN | PDR | BK-280–03 | SFARI|ID_C628 |
| STARD9 | MIS | NM_020759:c.G4802A:p.R1601Q | DN | PDR | BK-473–03 | BC253 |
| FASN | MIS | NM_004104:c.G2719A:p.V907I | DN | PDR | BK-135–03 | ID_C628 |
| DST | NFS | NM_001144769:c.97_98insCCACCATCG:p.V33delinsATIV | DN | PDR | BK-522–03 | SFARI(4)|ID_C628 |
| TNRC6B | SP | NM_001024843:c.46–2A>G | PI | PDR | BK-182–03 | SFARI(2) |
| DLG4 | SP | NM_001365:c.20–1G>C | MI | PDR | BK-201–03 | SFARI|Coe124|ID_C628 |
| LAMB1 | FS | NM_002291:c.144delG:p.K49Sfs*4 | PI | PDR | BK-445–03 | SFARI(3) |
| ATP10A | SG | NM_024490:c.C2397A:p.Y799* | MI | PDR | BK-254–03 | SFARI(3) |
| ELP4 | FS | NM_001288725:c.284delC:p.S95Yfs*64 | PI | PDR | BK-219–03 | SFARI(3) |
| LZTR1 | FS | NM_006767:c.772delT:p.F258Lfs*93 | MI | PDR | BK-384–03 | SFARI(3)|ID_C628 |
SG, stop-gain; FS, frameshift; SP, splicing site; MIS, missense; NFS, nonframeshift.
Canonical isoform presented.
DN, de novo; PI, paternal inheritance; MI, maternal inheritance.
P, pathogenic; LP, likely pathogenic; PDR, potentially disorder-related variants beyond the clinically relevant P and LP variants.
List of NDD gene sets that the genes belong to. SFARI, 970 ASD-associated genes from SFARI gene database; DD93, 93 developmental delay genes identified from DDD study 2017; BC253, 253 significant NDD genes from Coe et al. Nat Genet 2018; MG237, 237 NDD genes with nominal significance for enrichment or clustering of missense de novo variants from Geisheker al. Nat Neurosci 2017; ID526, ID_C628, 526 ID genes and 628 candidate ID genes curated by Gilissen et al. Nature 2014 (also see Supplementary Methods for details and corresponding reference).
Recurrent variant identified.
Multiple occurrences of de novo variants were identified in three NDD genes. We identified and validated two LGD variants in ARID1B, two missense variants in SYNCRIP, and two missense variants in SLITRK5 (Table 1). ARID1B de novo variants have been strongly implicated in ASD and ID. Recurrent SYNCRIP LGD variants were identified in ID1 and the probability of finding two de novo missense variants within this gene in a cohort of this size is significantly low (P = 8.7 × 10−7, Padj = 0.02, one-tailed binomial test; Supplementary Methods). For SLITRK5, after integrating two de novo missense variants from denovo-db v.1.5, a compendium of primarily human de novo NDD variants,28 we identify a potential cluster of them for future investigation (Supplementary Figure S4).
We also identified and validated variants in two other NDD genes, NR4A2 and MYT1, with putative missense clusters. We discovered a de novo frameshift variant in NR4A2, of which a cluster of four missense variants from denovo-db associated with the DNA-binding domain was observed (Supplementary Figure S4). We similarly discovered a de novo missense variant in MYT1 (CADD score = 25), a paralog of the autism-associated MYT1L. Sporadic case reports of MYT1 de novo missense variants were identified in patients with oculo-auriculo-vertebral spectrum (OAVS), which presents with autism-like features.29 Interestingly, a de novo missense variant, which is in close proximity to the one identified in this study, was recently detected in a patient with developmental delay from denovo-db (Supplementary Figure S4).
Classification of disorder-related CNVs
We also set criteria to define the disorder-related CNVs in this study (Supplementary Methods, Supplementary Table S8) and identified 46 disorder-related CNVs and two abnormal karyotypes (48, XXXY and 47, XXY). We attempted to validate these CNVs by two approaches: aCGH validation for relatively large CNVs (>50 kbp) and Sanger sequencing of small deletions that could not be assessed by the aCGH platform (Supplementary Methods). We successfully validated 45/46 disorder-related CNVs or abnormal karyotypes (2/2) accounting for 20.2% of participants (Supplementary Table S9). Once again, we further triaged these 47 CNVs or abnormal karyotypes into those that are clinically relevant following the ACMG standards and guidelines.30 In total, we classified 19 as pathogenic and 11 as likely pathogenic variants accounting for 13.1% of the patients.
Clinically relevant pathogenic CNVs included 13 de novo (10 del, 3 dup) and four inherited (1 del, 3 dup) from 11 genomic regions among 12 affected offspring (Supplementary Table S9). One pathogenic CNV region was recurrent and observed in multiple families: the chromosome 16p11.2 CNV (5 del, 2 dup). The remaining 10 pathogenic CNVs were observed in eight de novo instances: 8p12–11.1 duplication, 5p15.33 deletion, 6p25.3–25.2 deletion, 17p12 deletion, 16p11.2 distal deletion, 15q11–13 duplication, 1q42.11–42.12 deletion, 22q13.32–13.33; one paternal: 17q12 duplication; and one maternal: 1q21.1–21.2 duplication.
Likely pathogenic CNVs included two de novo (1 del, 1 dup) and nine inherited CNVs (6 del, 3 dup) from seven genomic regions among 10 affected offspring from six families (Supplementary Table S9). A chromosome 22 duplication (1.6 Mbp) was identified in the five-member family. This segmental duplication-mediated duplication was transmitted from the affected father (high-functioning autism formerly classified as Asperger’s syndrome) to two affected children and was recently identified in an ASD family.31 A deletion involving almost the entire 3’ untranslated region (UTR) of FOXP2 was detected in the seven-member family and transmitted from the affected father to 4/5 affected siblings. The other likely pathogenic CNVs include large de novo deletions or duplications, or encompass known neurodevelopmental or neuropsychiatric genes. The set includes a 18p11 duplication (4.7 Mbp, de novo), 18p11 deletion (3.2 Mbp, de novo), 2q32.1 duplication (2.8 Mbp, maternal), 13q21.1 deletion (2.9 Mbp, maternal), and TCF4 deletion (4.5 kbp, paternal).
Patients with multiple variants and phenotypic severity
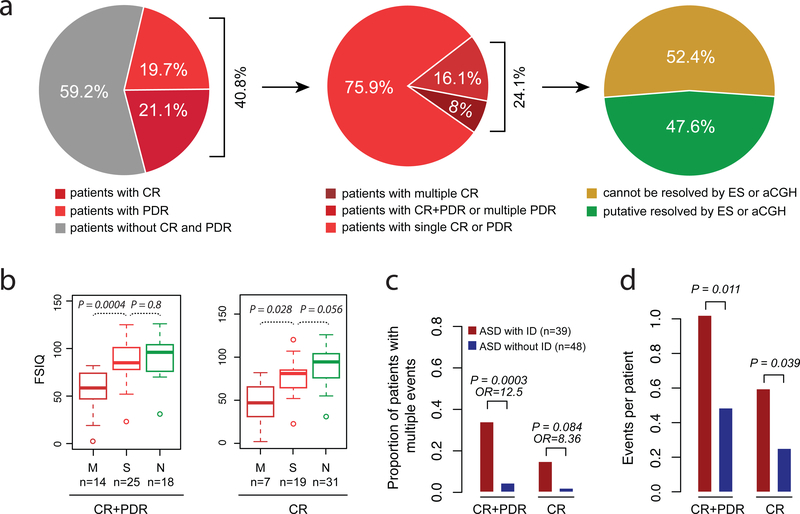
We classified pathogenic or likely pathogenic variants according to ACMG guidelines as stated above. Other disorder-related SNVs or CNVs were classified as potentially disorder-related (PDR) variants (Supplementary Methods). We estimate that 40.8% (87/213) of the affected offspring carry de novo or rare events in pathogenic, likely pathogenic or PDR variants, while 21.1% (45/213) carry one or more event that would be classified as pathogenic or likely pathogenic (Figure 2). One goal of this study is to understand the individual-level genetic architecture of ASD and determine if patients with multiple events are more clinically impaired. Considering only validated events among NDD candidate genes, we identify 21 affected offspring from 21 families with more than one event, accounting for 9.9% (2½13) of the affected offspring (Figure 3). A subset of these (seven affected individuals from seven families) carried multiple events in different genes that would be classified as pathogenic or likely pathogenic. We observed families with all combinations of variant event types (e.g., SNV + SNV, CNV + CNV, SNV + CNV), which are only accessible in a single experiment by GS. Neither ES nor aCGH could detect 52.4% multiple-hit events in this study. Those include the combination of both SNVs and CNVs, and small CNVs (<50 kbp) that could not be detected by the aCGH platform used in this study (Figure 2a).
Figure 2. Diagnostic yield of GS and phenotypic severity in multiple-hit patients.
(a) Diagnostic yield of GS. Pie chart on left compares the proportion of patients with clinically relevant (CR) (pathogenic or likely pathogenic), potentially disorder-related (PDR) (e.g., candidate NDD risk genes), and no risk variant identified. Middle pie chart compares the number of patients with multiple variants versus those with a single event. Right pie chart compares the number of such multiple events that can be resolved by ES or aCGH (green) and those that cannot (yellow). (b) Comparison of full-scale IQ (FSIQ) for patients with multiple events (M), single events (S), and no event (N). Left panel considers both CR and PDR events (CR+PDR); right panel considers CR events only. (c) Burden analysis comparing the proportion of ASD patients with and without ID for all CR+PDR or CR only events. (d) Overall burden analysis for patients with and without ID considering all CR+PDR or CR only events. P values were adjusted for multiple comparisons.
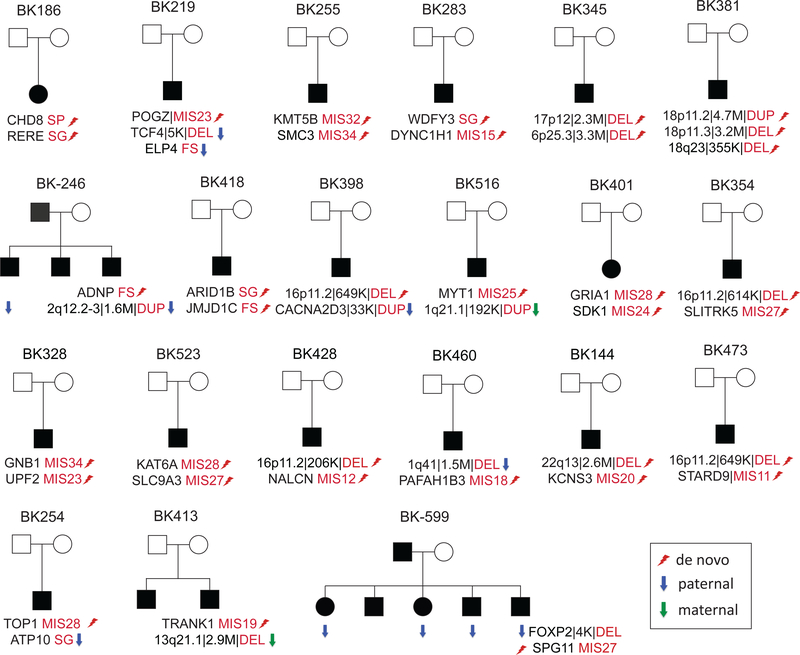
Figure 3. Patients with multiple variants.
Family structures shown for ASD patients with multiple disorder-related variants. The first seven patients from seven families carried multiple pathogenic or likely pathogenic variants. de novo (lightning bolt), paternally (blue arrow) and maternally (green arrow) inherited SNV and CNV (duplication or deletion) events are indicated as well as the severity of the missense variants as determined by CADD score (i.e., MIS27 denotes a missense variant with a CADD score of 27).
To assess the relationship between the number of disorder-related variants and phenotypic severity, we performed two analyses. First, we compared the median distribution of the IQ data across affected offspring with multiple genetic events, single events, and no event. We observed significantly lower FSIQ (Padj = 0.0004, Mann–Whitney U test) in affected offspring with multiple variants in different NDD risk genes when compared to the affected offspring with a single event (Figure 2b). Although there is a weak trend toward lower IQ between affected offspring with a single event and no identified genetic lesion, this difference does not reach significance (Padj = 0.8, Mann–Whitney U test). When we restrict the analysis to variants deemed to be pathogenic or likely pathogenic, the trend still holds (Padj = 0.028) (Figure 2b).
Second, we performed a burden analysis comparing the proportion of individuals with multiple hits with (ASD+ID) and without (ASD-ID) ID. As expected, we observed more individuals with multiple NDD risk gene variants in the ASD+ID group compared to the ASD-ID group (Padj = 0.0003, OR = 12.5, Fisher’s exact test) (Figure 2c). The trend still holds (OR = 8.36) when we restrict to pathogenic or likely pathogenic events, although not yet significant (Padj = 0.084). The same trend was also observed in the overall burden analysis considering all disorder-related events (Padj = 0.011, one-way ANOVA) or clinically relevant events only (Padj = 0.039) (Figure 2d).
Genetic and clinical heterogeneity in multiple affected siblings
In this cohort, there are 27 multiplex families, including a total of 60 affected offspring (Supplementary Figure S1). We identified five clinically pathogenic or likely pathogenic variants in nine affected offspring from five of these families (Supplementary Figure S5). This suggests a reduced diagnostic yield (15%) when compared to simplex and trio families (23%) (P = 0.35, OR = 0.66, Fisher’s exact test). Interestingly, there is no case among these multiplex families where a pathogenic or likely pathogenic variant was transmitted to all affected members in the family, thus implying considerable locus heterogeneity (Supplementary Figure S5) as previously observed.
It is also noteworthy that some of the clinical variability within these families correlates with the number and overall impact of such gene-disruptive variants. In family BK246 (Supplementary Figure S5), for example, the proband with two variants (the de novo frameshift variant in ADNP and the paternally inherited 1.6 Mbp duplication) is the only individual in the family with severe ID (FSIQ=19, NVIQ=20). This contrasts with the sibling with only the inherited duplication who is diagnosed with anxiety and ADHD without ID (FSIQ=94, NVIQ=100). The third sibling with no detected pathogenic variants has the highest IQ (FSIQ=104, NVIQ=107), although the difference is still within the realm of test-retest noise. Similarly in family BK599 (Supplementary Figure S5), the child (BK599.07) with both variants (4 kbp deletion of the 3’ UTR of FOXP2 and a de novo missense variant within SPG11) is more impaired than the other siblings (FSIQ=60, NVIQ=64). Once again, the sibling without any disorder-related event has the highest IQ (NVIQ=129) among all affected siblings.
DISCUSSION
In this study, we set out to determine the phenotypic and genotypic heterogeneity of a minimally ascertained clinical cohort of families with ASD. We demonstrated the diagnostic utility of GS for the discovery of disease-related variants, especially for multiple rare risk variants that contribute to the phenotypic severity of ASD; the genetic heterogeneity within multiplex families with ASD; and the identification of new ASD risk genes for future investigation. Given the narrow clinical definition of pathogenic and likely pathogenic variants, we used available neurodevelopmental gene lists and the literature to define the PDR variants. The expanded definition was necessary to explore the full heterogeneity and correlation with phenotypic severity.
Approximately 10% of the ASD-affected offspring in this study carried multiple risk variants, and multiple hits correlated with increased phenotypic severity. We observed a significant difference in FSIQ and NVIQ scores when comparing affected individuals with multiple hits with those with one or zero hits. The finding is crucial from a clinical perspective as the genetic workup of children with autism and developmental delay often ends if a likely pathogenic SNV or CNV is found by microarray or ES. Since such cases are unlikely to proceed to GS, variants contributing more significantly to the phenotype may remain undiscovered unless such families are subject to full GS. Furthermore, this finding provides support to the oligogenic model of ASD, specifically where multiple rare disruptive variants lead to more severe phenotypes.
We observed considerable genetic heterogeneity within families consistent with earlier observations.15,32 Although such multiplex families are thought to share the same genetic risk event(s), 92% (12/13) of the families failed to segregate phenotype and genotype faithfully when a disorder-related event was discovered (i.e., affected individuals that did not carry the disorder-related variant were present in most families albeit such members tended to be less severely affected). This genetic heterogeneity was not only restricted to de novo variant events (e.g., the de novo LGD within ADNP in family BK246 or de novo 16p11.2 duplication in family BK187) but also observed for transmitted variants (e.g., paternally inherited 16p11.2 duplication in family BK313 and a maternally inherited 13q21.3 deletion in family BK413). These complicated combinations of disorder-related events and phenotypic diversity within families highlight the importance of GS for affected and unaffected members prior to genetic counseling of families.
Variants discovered in this study add substantial evidence confirming ASD candidate genes described in the literature, including but not limited to NR4A2, SYNCRIP, MYT1 and TRANK1. NR4A2 encodes a transcription factor essential for the differentiation of dopaminergic neurons.33 Recently, several de novo deletions covering NR4A2 only or NR4A2 and the adjacent GPD2 were recently reported in patients with ASD, ID and/or language impairment.34 In this study, we identified a de novo frameshift variant and identified a cluster of de novo missense variants within the DNA-binding domain of the predicted protein. Similarly, an excess of de novo truncating variants within SYNCRIP was previously identified in patients with ID.1 In this study, we identified two individuals with ASD with SYNCRIP de novo missense variants. SYNCRIP is a component of mRNA granules bound for the dendrites where it contributes to synaptic plasticity35 and, in Drosophila, is thought to play a role in decommissioning of neural stem cells.36 We also identified two individuals with de novo missense variants in TRANK1. Patients with ID and de novo variants in TRANK1 were previously reported and the locus has been associated with bipolar disorder in different genome-wide association studies;37,38 however, the function of this gene is largely unknown. MYT1 is a paralog of the autism-associated gene MYT1L. Sporadic case reports of MYT1 de novo missense variants were reported in patients with OAVS, which often presents with autism-like features.29
As a diagnostic test, consistent with other recent reports,8,12 GS provides a slight advantage over ES for the detection of protein-encoding risk variants. In this study, GS enabled the discovery of potential de novo ASD-associated variants missed by ES, including a frameshift variant in NR4A2, a frameshift variant in CIC, and a missense variant in KIRREL3. Improvements in capture design and increases in ES coverage have continued to minimize such differences with false negative rates now estimated at less than 2.5%.8 Most GS advantages lie in the greater uniformity of sequence coverage and improved detection of gene-disruptive CNVs. This is especially relevant with respect to the detection of multiple variant events where ES and aCGH could detect and confirm less than half of such cases independently in this study.
The complete genetic architecture of ASD remains to be elucidated. Analysis of the SAGE cohort demonstrated the utility of GS in a clinical setting. The ability to capture most genetic variants enables the discovery of multiple hits that are clinically relevant in determining the severity of presentation of ASD. Our analysis showed that in multiplex families, it is crucial to not assume Mendelian inheritance and suggests that a combination of factors, including genetic background, play a role in phenotypic severity. Moving forward, it will be important to elucidate the full spectrum of genetic variation in clinically relevant cohorts. This will include not only the characterization of unrelated ASD patients with different variants in the same gene but also the comparison of affected siblings with one or more risk alleles within the same family. In addition, GS will provide a platform for assessing the contribution of noncoding regulatory mutation and the interplay between rare and common variants in contributing to the risk of ASD and other NDDs.39,40 Such studies will require much larger sample sizes but will provide an unprecedented opportunity to develop an integrated model for the genetic architecture of autism that will be valuable for future clinical diagnosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sunday Stray, Mary Eng, James Moore, Hannah Kortbawi and Anne Thornton from the laboratory of Mary-Claire King for isolation of DNA from whole blood and Tonia Brown for manuscript editing. We are especially grateful to the families who participated in the SAGE study. This work was supported by the following grants: the Simons Foundation Autism Research Initiative (SFARI 303241) and National Institutes of Health (NIH R01MH101221) to E.E.E. and NIH (R01MH100047) to R.A.B., and NIH (1K99MH117165) to T.N.T.. This work was also supported by the NYGC CCDG (UM1HG008901) and the GSP Coordinating Center (U24HG008956). The CCDG are funded by the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The GSP Coordinating Center contributed to cross-program scientific initiatives and provided logistical and general study coordination. Exome sequencing was provided by the University of Washington Center for Mendelian Genomics (UW-CMG) and was funded by NHGRI and NHLBI grants UM1 HG006493 and U24 HG008956.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. E.E.E. is an investigator of the Howard Hughes Medical Institute.
Footnotes
DISCLOSURE
E.E.E. is on the scientific advisory board (SAB) of DNAnexus, Inc. The other authors declare no competing financial interests.
DATA AVAILABILITY
The SAGE genome sequencing data is available at the database of Genotypes and Phenotypes (dbGaP) under accession: phs001740.v1.p1.
REFERENCES
- 1.Lelieveld SH, Reijnders MR, Pfundt R, et al. Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat Neurosci 2016; 19:1194–1196. [DOI] [PubMed] [Google Scholar]
- 2.Deciphering Developmental Disorders S Prevalence and architecture of de novo mutations in developmental disorders. Nature 2017; 542:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iossifov I, O’Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014; 515:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Rubeis S, He X, Goldberg AP, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014; 515:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coe BP, Stessman HAF, Sulovari A, et al. Neurodevelopmental disease genes implicated by de novo mutation and CNV morbidity. Nat Genet In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron 2010; 68:192–195. [DOI] [PubMed] [Google Scholar]
- 7.Sanders SJ, He X, Willsey AJ, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015; 87:1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner TN, Coe BP, Dickel DE, et al. Genomic Patterns of De Novo Mutation in Simplex Autism. Cell 2017; 171:710–722 e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RK CY, Merico D, Bookman M, et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci 2017; 20:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krumm N, Turner TN, Baker C, et al. Excess of rare, inherited truncating mutations in autism. Nat. Genet 2015; 47:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. JAMA Psychiatry 2011; 68:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner TN, Hormozdiari F, Duyzend MH, et al. Genome Sequencing of Autism-Affected Families Reveals Disruption of Putative Noncoding Regulatory DNA. Am J Hum Genet 2016; 98:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaaf CP, Sabo A, Sakai Y, et al. Oligogenic heterozygosity in individuals with high-functioning autism spectrum disorders. Hum Mol Genet 2011; 20:3366–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girirajan S, Rosenfeld JA, Cooper GM, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet 2010; 42:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen RK, Thiruvahindrapuram B, Merico D, et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat Med 2015; 21:185–191. [DOI] [PubMed] [Google Scholar]
- 16.Regier AA, Farjoun Y, Larson D, et al. Functional equivalence of genome sequencing analysis pipelines enables harmonized variant calling across human genetics projects. Nat Commun 2018; 9:4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010; 26:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010; 20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics 2010; 26:2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudmant PH, Kitzman JO, Antonacci F, et al. Diversity of human copy number variation and multicopy genes. Science 2010; 330:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handsaker RE, Korn JM, Nemesh J, McCarroll SA. Discovery and genotyping of genome structural polymorphism by sequencing on a population scale. Nat Genet 2011; 43:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layer RM, Chiang C, Quinlan AR, Hall IM. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol 2014; 15:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronenberg ZN, Osborne EJ, Cone KR, et al. Wham: Identifying Structural Variants of Biological Consequence. PLoS Comput Biol 2015; 11:e1004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abyzov A, Urban AE, Snyder M, Gerstein M. CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res 2011; 21:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rausch T, Zichner T, Schlattl A, Stutz AM, Benes V, Korbel JO. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 2012; 28:i333–i339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu HC, Tan Q, Rousseaux MW, et al. Disruption of the ATXN1-CIC complex causes a spectrum of neurobehavioral phenotypes in mice and humans. Nat Genet 2017; 49:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner TN, Yi Q, Krumm N, et al. denovo-db: a compendium of human de novo variants. Nucleic Acids Res 2017; 45:D804–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez E, Berenguer M, Tingaud-Sequeira A, et al. Mutations in MYT1, encoding the myelin transcription factor 1, are a rare cause of OAVS. J Med Genet 2016; 53:752–760. [DOI] [PubMed] [Google Scholar]
- 30.Kearney HM, Thorland EC, Brown KK, Quintero-Rivera F, South ST, Working Group of the American College of Medical Genetics Laboratory Quality Assurance C. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med 2011; 13:680–685. [DOI] [PubMed] [Google Scholar]
- 31.Guo H, Peng Y, Hu Z, et al. Genome-wide copy number variation analysis in a Chinese autism spectrum disorder cohort. Sci Rep 2017; 7:44155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leppa VM, Kravitz SN, Martin CL, et al. Rare Inherited and De Novo CNVs Reveal Complex Contributions to ASD Risk in Multiplex Families. Am J Hum Genet 2016; 99:540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph B, Wallen-Mackenzie A, Benoit G, et al. p57(Kip2) cooperates with Nurr1 in developing dopamine cells. Proc Natl Acad Sci U.S.A. 2003; 100:15619–15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy J, Grotto S, Mignot C, et al. NR4A2 haploinsufficiency is associated with intellectual disability and autism spectrum disorder. Clin Genet. 2018; 94:264–268 [DOI] [PubMed] [Google Scholar]
- 35.Bannai H, Fukatsu K, Mizutani A, et al. An RNA-interacting protein, SYNCRIP (heterogeneous nuclear ribonuclear protein Q1/NSAP1) is a component of mRNA granule transported with inositol 1,4,5-trisphosphate receptor type 1 mRNA in neuronal dendrites. J Biol Chem 2004; 279:53427–53434. [DOI] [PubMed] [Google Scholar]
- 36.Yang CP, Samuels TJ, Huang Y, et al. Imp and Syp RNA-binding proteins govern decommissioning of Drosophila neural stem cells. Development. 2017; 144:3454–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen DT, Jiang X, Akula N, et al. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol Psychiatry 2013; 18:195–205. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda M, Takahashi A, Kamatani Y, et al. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol Psychiatry 2017; 23:639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiner DJ, Wigdor EM, Ripke S, et al. Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet 2017; 49:978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mek Neimi, Martin HC, Rice DL, et al. Common genetic variants contribute to risk of rare severe neurodevelopmental disorders. Nature 2018; 562 :268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.