Abstract
Background:
The role of retinal imaging with optical coherence tomography (OCT) in assessing individuals with radiologically isolated syndrome (RIS) remains largely unexplored.
Objective:
To assess retinal layer thicknesses in RIS and examine their associations with clinical features suggestive of increased risk for conversion to MS.
Methods:
Thirty RIS subjects and 60 age- and sex-matched healthy controls (HC) underwent retinal imaging with spectral-domain OCT, followed by automated segmentation of retinal layers.
Results:
Overall, retinal layer thicknesses did not differ between RIS and HC. However, RIS subjects with spinal cord (SC) lesions had lower ganglion cell+inner plexiform layer (GCIP) thickness compared to HC (−4.41μm; p=0.007) and RIS without SC lesions (−3.53μm; p=0.041). Similarly, RIS subjects with infratentorial (IT) brain lesions had lower GCIP thickness compared to HC (−4.07μm; p<0.001) and RIS without IT lesions (−3.49μm; p=0.029). Multivariate analyses revealed that the presence of SC and IT lesions were independently associated with lower GCIP thickness in RIS (p=0.04 and p=0.03 respectively). Other patient characteristics, including sex, abnormal cerebrospinal fluid and presence of gadolinium-enhancing or juxtacortical lesions, were not associated with retinal layer thicknesses.
Conclusions:
The presence of SC or IT lesions in RIS may be associated with retinal neuro- axonal loss, supporting the presence of more disseminated disease.
Keywords: Multiple Sclerosis, Radiologically Isolated Syndrome, Optical Coherence Tomography, Retina, Spinal Cord, Demyelination
INTRODUCTION
The term radiologically isolated syndrome (RIS) has been used to describe asymptomatic individuals with incidentally identified magnetic resonance imaging (MRI) brain lesions that are suggestive of multiple sclerosis (MS).1 About a third of individuals with RIS develop symptoms of neurological dysfunction within five years of their initial brain MRI, with the majority presenting with a clinical picture consistent with relapsing-remitting MS, while a subset may experience a progressive disease course consistent with primary-progressive MS (PPMS).2,3 Several clinical and paraclinical features have been proposed to confer an increased risk of conversion to MS in RIS subjects, including age <37 years, male sex, infratentorial (IT) lesions, spinal cord (SC) lesions, gadolinium-enhancing lesions, cerebrospinal fluid (CSF)-unique oligoclonal bands and/or elevated CSF IgG index, pregnancy, and abnormal visual evoked potentials (VEP).2,4–7 In the largest studied cohort of RIS subjects to date, male sex, age <37 years, and presence of SC lesions were independently associated with an increased risk of developing a seminal acute or progressive clinical event.2
The anterior visual pathway is a frequent site of clinical and subclinical involvement in MS, and may be rapidly, reliably, and non-invasively assessed by use of optical coherence tomography (OCT).8,9 Reductions in thickness of the inner retinal layers, namely the retinal nerve fiber layer (RNFL) and the ganglion cell + inner plexiform layer (GCIP), have been found not only in eyes with a prior history of optic neuritis (ON), but also in MS eyes without a history of ON, including at the earliest symptomatic stages of the disease (i.e. clinically isolated syndrome).8,10,11 Furthermore, increased thickness of the inner nuclear layer (INL) has been suggested to correlate with clinical and radiological inflammatory disease activity and reductions in INL thickness have been associated with favorable responses to disease-modifying therapies (DMT).12,13
However, there is a paucity of prior studies utilizing OCT to assess for retinal involvement in RIS. In this cross-sectional study, as a primary objective, we sought to compare retinal layer thicknesses between RIS subjects and healthy controls (HC). As a secondary objective, we sought to assess associations between retinal layer thicknesses and proposed high- risk features for conversion from RIS to MS. We hypothesized that, as compared to HC, RIS subjects may have reduced thickness of the inner retinal layers and/or increased thickness of outer retinal layers and that differences in retinal layer thicknesses may be associated with high risk features for conversion to MS.
METHODS
Study subjects
The medical records of individuals presenting to the Johns Hopkins MS Center for evaluation of incidental brain MRI findings, between March 2011 and March 2018, were retrospectively reviewed for inclusion in the study. RIS was defined using the 2009 Okuda diagnostic criteria.1 The clinical diagnosis was confirmed by the treating neurologist, and the MRIs at the time of initial detection of incidental abnormalities were reviewed (see below). Abnormal findings of CSF analysis (if available) were recorded, including pleocytosis (white blood cells ≥5/mm3), elevated protein (≥45 mg/dL), presence of ≥2 CSF-unique oligoclonal bands and/or an elevated IgG index (≥0.8). Age- and sex-matched HC were recruited from amongst Johns Hopkins University staff and spouses of patients evaluated at the Johns Hopkins MS Center. Participants with a history of diabetes mellitus, uncontrolled hypertension,glaucoma, ocular surgery, refractive errors exceeding ±6 diopters, or other neurological or ophthalmological conditions were excluded.
This study was approved by the Johns Hopkins University Institutional Review Board. All participants provided written informed consent.
Magnetic resonance imaging studies
The imaging studies originated from different MRI scanners and magnetic field strengths (1.5 or 3.0 Tesla), since they were acquired at a variety of academic or community imaging centers. All brain examinations included T1-weighted, T2-weighted and Fluid Attenuated Inversion Recovery (FLAIR) sequences and all spinal examinations included T1-weighted, T2-weighted and Short Tau Inversion Recovery (STIR) sequences. These sequences were reviewed, including T1-weighted post-gadolinium (if available), by a single-rater (ESS), in a blinded fashion. Participants were included if the incidentally identified CNS white matter anomalies met the MRI criteria defined in the 2009 Okuda diagnostic criteria: 1) Ovoid, well-circumscribed, and homogeneous foci with or without involvement of the corpus callosum 2) T2 hyperintensities measuring >3mm and fulfilling Barkhof criteria (at least 3 out of 4) for dissemination in space 3) CNS white matter anomalies not consistent with a vascular pattern.1,14
Optical coherence tomography
Retinal imaging was performed with high-definition, spectral-domain Cirrus OCT (Model 5000, Carl Zeiss Meditec, Dublin, CA), using a scanning protocol described in detail elsewhere.9,15 Briefly, peri-papillary and macular scans were obtained, by experienced technicians, with the Optic Disc Cube 200×200 protocol and the Macular Cube 512×128 protocol, respectively. The OCT scans were performed at the time of the initial evaluation of the study subjects at the Johns Hopkins MS center. Scans were reviewed by a rater blinded to clinical status (AF), in order to assess for presence of any retinal pathology and to ensure satisfactory quality. Scans with signal strength below 7/10 or with artifact were excluded, in accordance with OSCAR-IB criteria.16,17
Peri-papillary RNFL (p-RNFL) thickness values were generated by conventional Cirrus HD-OCT software, as described in detail elsewhere.9 Thicknesses of the GCIP, INL and outer nuclear layer (ONL) were automatically derived from macular scans, using a validated segmentation algorithm, as previously described.18,19 Briefly, average thicknesses of the studied retinal layers were calculated within an annulus with an inner radius of 0.5 mm and an outer radius of 2.5mm, centered at the fovea. This retinal layer boundary segmentation was visually inspected and verified for all scans.
Statistical Methods
Statistical analyses were performed with STATA version 13 (StataCorp, College Station, TX). Comparisons of the baseline characteristics between groups were performed with Student’s t-test (age) and Fisher’s exact test (sex, race, CSF, and MRI findings). OCT measures were compared utilizing generalized estimating equations (GEE) with an exchangeable correlation matrix, accounting for within-subject inter-eye correlation, and adjusting for age and sex. Similarly, the relationship of age and retinal layer thicknesses in RIS and HC was assessed utilizing GEE, with age, the group variable (0: HC; 1: RIS) and their interaction included as independent variables. Analyses were based on a priori established research hypotheses and consequently adjustment for multiple comparisons was not performed. A P-value < 0.05 was considered statistically significant.
RESULTS
A total of 30 RIS subjects were identified that were eligible for inclusion in the analyses (Figure 1) and 60 age- and sex-matched HC were recruited to the study. Demographics of the RIS and HC groups are outlined in Table 1.
Figure 1.
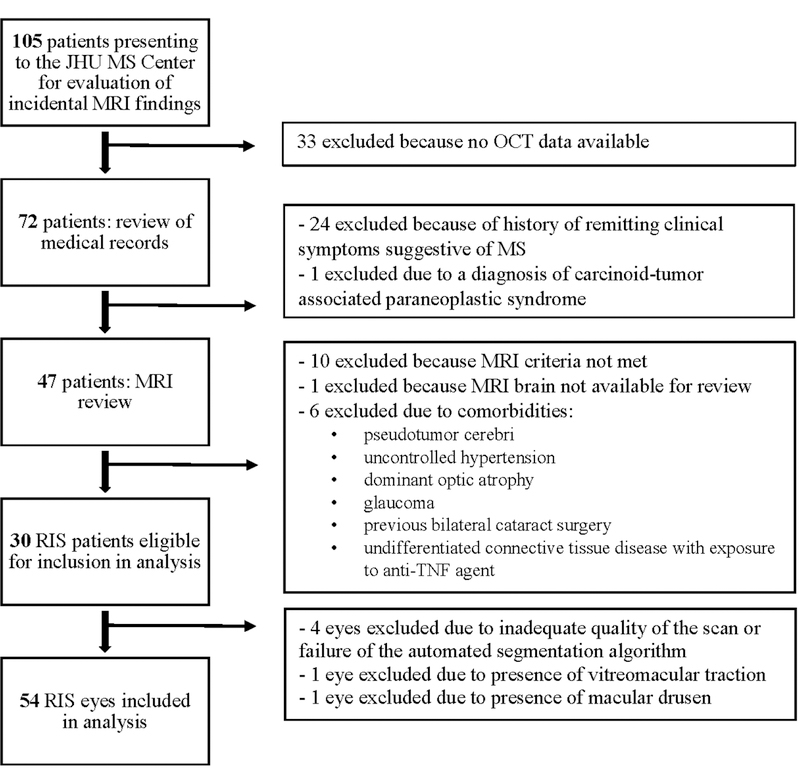
Study Profile
JHU: Johns Hopkins University; MS: multiple sclerosis; MRI: magnetic resonance imaging; OCT: optical coherence tomography; RIS: radiologically isolated syndrome; TNF: tumor necrosis factor
Table 1.
Demographics
| RIS | HC | P-value | |
|---|---|---|---|
| Subjects (eyes) | 30 (54) | 60 (120) | |
| Age, mean (SD) | 42.9 (13.8) | 41.2 (12.4) | 0.56 |
| Female Sex, n (%) | 22 (73%) | 39 (65%) | 0.48 |
| Race, n (%) | |||
| Caucasian-American | 22 (73%) | 44 (73%) | 0.71 |
| African-American | 5 (17%) | 9 (15%) | |
| Other | 3 (10%) | 7 (12%) |
RIS: radiologically isolated syndrome; HC: healthy controls; SD: standard deviation
Reasons for the initial brain MRI were diverse, with headache and head trauma being the most common (Table 2). One subject presented with left upper extremity pain, with history and neurologic exam consistent with a cervical radiculopathy and cervical spine MRI revealing foraminal stenosis at the left C5-C6 level without any cervical SC demyelinating lesions. One subject had a history of transient aphasia lasting for less than five minutes and a negative vascular work-up, while another subject was experiencing brief episodes of vertigo associated with positional changes of the head, with a positive Dix-Hallpike maneuver on exam, and received a diagnosis of benign paroxysmal positional vertigo (BPPV). Two subjects presented with facial pain that was non-specific and attributed to a previous sinus surgery and dental procedure.
Table 2.
Indication for initial brain MRI
| Indication for initial brain MRI | n (%) |
|---|---|
| Headache | 12 (40%) |
| Trauma | 5 (16.7%) |
| Facial pain | 2 (6.7%) |
| Seizure | 2 (6.7%) |
| Episode of transient aphasia | 1 (3.3%) |
| Hyperprolactinemia | 1 (3.3%) |
| Ocular migraine | 1 (3.3%) |
| Family history of intracranial aneurysms | 1 (3.3%) |
| MS family history | 1 (3.3%) |
| Anxiety/panic attacks | 1 (3.3%) |
| Research control subject | 1 (3.3%) |
| Cervical radiculopathy | 1 (3.3%) |
| BPPV with (+) Dix-Hallpike test | 1 (3.3%) |
MRI: magnetic resonance imaging; MS: multiple sclerosis; BPPV: benign positional paroxysmal vertigo
Three RIS subjects were on a DMT at the time of the OCT scan (two were on interferon beta-1a and one was enrolled in a randomized-controlled trial investigating the effects of a DMT in RIS). Imaging and CSF analysis findings are shown in Table 3. SC lesions were identified in 12 RIS subjects, while IT brain lesions were present in 11 subjects and juxtacortical lesions in 26 subjects. Five subjects had both IT and SC lesions. The proportion of subjects with identified SC, IT or juxtacortical lesions did not differ between those who underwent 1.5 vs. 3 Tesla MRI (SC:p=0.71; IT: p=0.82; Juxtacortical: p=0.99). Eight subjects had gadolinium-enhancing lesions identified on brain MRI. CSF analysis was available for 21 RIS subjects, with ≥2 CSF-unique oligoclonal bands found in 16 subjects and any abnormal CSF finding in a total of 18 subjects.
Table 3.
Cerebrospinal fluid and MRI findings in RIS participants
| MRI findings | n (%) |
|---|---|
| Brain Gd-enhancing lesionsa | 8 (30%) |
| Infratentorial lesions | 11 (37%) |
| Juxtacortical lesions | 26 (87%) |
| Spinal cord lesionsb | 12 (40%) |
| CSFc | |
| Pleocytosis (≥5 WBC/mm3) | 3 (14%) |
| ≥2 CSF-specific oligoclonal bands | 16 (76%) |
| Elevated IgG index (≥0.8) | 6 (29%) |
| Elevated protein (≥45 mg/dL) | 4 (19%) |
| Any abnormal CSF finding (CSF pleocytosis and/or elevated IgG index and/or oligoclonal bands and/or elevated protein) | 18 (86%) |
Gadolinium contrast-enhanced brain MRI available for 27 RIS participants
Cervical spine MRI available for all RIS participants and thoracic spine MRI available for 24 participants
CSF analysis available for 21 RIS participants
MRI: magnetic resonance imaging; RIS: radiologically isolated syndrome; Gd: gadolinium; CSF: cerebrospinal fluid; WBC: white blood cells
Overall, retinal layer thicknesses did not differ between RIS and HC (Table 4). However, RIS subjects with SC lesions had lower GCIP thickness compared to HC (−4.41μm; 95% CI: −7.61 to −1.20μm; p=0.007) and RIS subjects without SC lesions (−3.53μm; 95% CI: −6.93 to −0.14μm; p=0.041), in analyses adjusted for age and sex (Table 5, Figure 2A). Furthermore, RIS subjects with IT lesions had lower GCIP thickness compared to HC (−4.07μm; 95% CI: −6.33 to - 1.82μm; p<0.001) and RIS subjects without IT lesions (−3.49μm; 95% CI: −6.62 to −0.35μm; p=0.029) (Table 5, Figure 2B). In a multivariate analysis of RIS participants including age, sex, presence of SC lesions and presence of IT lesions as predictor variables, we found that the presence of SC or IT lesions were independently associated with lower GCIP thickness (SC: - 3.21μm, 95%CI: −6.24 to −0.19μm, p=0.04; IT: −3.21μm; 95% CI: −6.10 to −0.31μm; p=0.03). Other retinal layer thicknesses did not differ between the groups based on presence of SC or IT lesions. RIS subjects with SC lesions were older than those without SC lesions (RIS with SC lesions: 48.9±11.3 years; RIS without SC lesions: 38.8±14.1 years; p=0.047) and HC (HC: 41.2 ±12.4 years; p=0.04), while this was not the case for RIS subjects with IT lesions compared to those without (RIS with IT lesions: 45.1±14.9 years; RIS without IT lesions: 41.6±13.3 years; p=0.50) or HC (p=0.33). Sex ratios did not differ between groups. The RIS with SC lesions group included five African-American patients, while there were none in the RIS group without SC lesions. Race was not adjusted for given the small number of African-American subjects and the collinearity observed between race and presence of SC lesions. Race did not differ between groups based on the presence of IT lesions. The RIS with and without SC or IT lesions groups did not differ in regards to CSF findings and the presence of gadolinium enhancement on brain MRI.
Table 4.
Comparison of retinal layer thicknesses between RIS and HC
| Layer | RIS (n=54 eyes) mean (μm) (SD) | HC (n=120 eyes) mean (μm) (SD) | Coefficient (μm) (95% CI) | P-value |
|---|---|---|---|---|
| p-RNFL | 93.47 (10.53) | 93.12 (10.15) | 0.64 (−3.39 to 4.68) | 0.76 |
| GCIP | 75.31 (6.14) | 76.97 (5.21) | −1.29 (−3.41 to 0.82) | 0.23 |
| INL | 45.17 (2.65) | 45.38 (2.84) | −0.03 (−1.10 to 1.04) | 0.96 |
| ONL | 67.22 (5.35) | 68.23 (5.86) | −0.85 (−3.10 to 1.39) | 0.46 |
RIS: radiologically isolated syndrome; HC: healthy controls; SD: standard deviation; CI: confidence interval; p-RNFL: peri-papillary retinal nerve fiber layer; GCIP: ganglion cell + inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer
Table 5.
Comparison of retinal layer thicknesses between RIS subjects with and without spinal cord or infratentorial lesions and HC
| Layer thicknesses mean (μm) (SD) | RIS with SC lesions vs. RIS without SC lesions | RIS with SC lesions vs. HC | |||||
|---|---|---|---|---|---|---|---|
| Layer | RIS with SC lesions (n=20 eyes) | RIS without SC lesions (n=34 eyes) | HC (n=120 eyes) | Coefficient, (μm) (95% CI) | P-value | Coefficient, (μm) (95% CI) | P-value |
| p-RNFL | 89.62 (11.25) | 95.72 (9.54) | 93.12 (10.15) | −3.70 (−10.28 to 2.87) | 0.27 | −2.07 (−8.22 to 4.09) | 0.51 |
| GCIP | 71.70 (7.01) | 77.44 (4.43) | 76.97 (5.21) | −3.53 (−6.93 to −0.14) | 0.041 | −4.41 (−7.61 to −1.20) | 0.007 |
| Layer thicknesses mean (μm) (SD) | RIS with IT lesions vs. RIS without IT lesions | RIS with IT lesions vs. HC | |||||
| Layer | RIS with IT lesions (n=19 eyes) | RIS without IT lesions (n=35 eyes) | HC (n=120 eyes) | Coefficient, (μm) (95% CI) | P-value | Coefficient, (μm) (95% CI) | P-value |
| p-RNFL | 89.33 (9.01) | 95.89 (10.71) | 93.12 (10.15) | −5.59 (−12.09 to 0.90) | 0.09 | −3.37 (−8.53 to 1.79) | 0.20 |
| GCIP | 72.24 (3.85) | 76.98 (6.54) | 76.97 (5.21) | −3.49 (−6.62 to −0.35) | 0.029 | −4.07 (−6.33 to −1.82) | <0.001 |
RIS: radiologically isolated syndrome; HC: healthy controls; SC: spinal cord; SD: standard deviation; CI: confidence interval; p-RNFL: peri-papillary retinal nerve fiber layer; GCIP: ganglion cell + inner plexiform layer; IT: infratentorial
Figure 2.
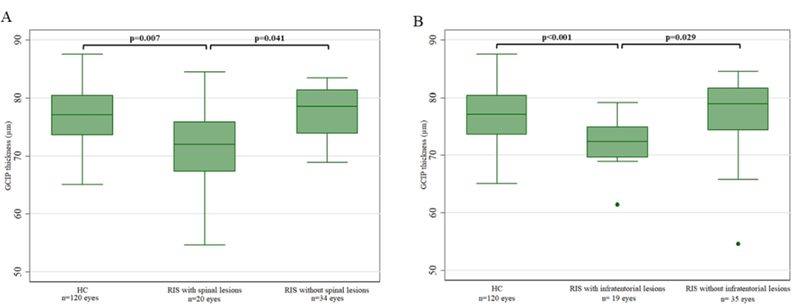
Boxplots of GCIP thickness in HC and RIS by presence of spinal cord lesions (A) and infratentorial lesions (B)
GCIP: ganglion cell + inner plexiform layer; HC: healthy controls; RIS: radiologically isolated syndrome
Furthermore, we examined the differential effect of age on OCT measures in the RIS and HC cohorts. Increasing age was associated with lower GCIP thickness in RIS (−0.21 μm/year, 95% CI: −0.32 to −0.11 μm/year; p<0.001), whereas this was not observed in HC (−0.04 μm/year, 95% CI: −0.12 to 0.04 μm/year; p=0.35). The relationship of age and GCIP thickness in RIS remained significant after accounting for the presence of SC and IT lesions (p=0.001). The slopes of age and GCIP thickness differed significantly between RIS and HC (p=0.01; Figure 3A). In both RIS and HC, increasing age was associated with lower p-RNFL thickness (−0.34 μm/year in RIS, 95% CI: −0.55 to −0.12 μm/year; p=0.002; −0.25 μm/year in HC, 95% CI: −0.42 to −0.09 μm/year; p=0.003) and this relationship did not differ between the two groups (p=0.55) (Figure 3B). Additionally, in RIS, increasing age was associated with lower INL thickness (−0.08 μm/year, 95% CI: −0.13 to −0.02 μm/year; p=0.008), while this was not observed in HC (0.02 μm/year, 95% CI: −0.03 to 0.08 μm/year; p=0.39). The interaction term of age and RIS was also statistically significant, with RIS subjects exhibiting lower INL thickness with increasing age when compared to HC (p=0.012) (Figure 3C). ONL thickness was not related to age in either group.
Figure 3.
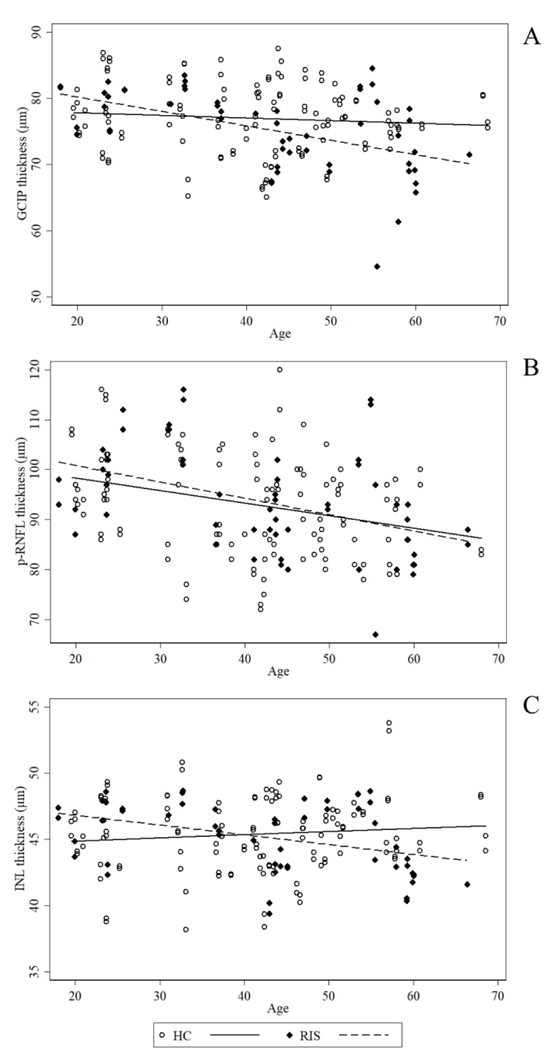
Relationship of retinal layer thicknesses with age in HC and RIS. A: GCIP; B: p-RNFL; C: INL HC: healthy controls; RIS: radiologically isolated syndrome; GCIP: ganglion cell + inner plexiform layer; p-RNFL: peri-papillary retinal nerve fiber layer; INL: inner nuclear layer
Other subgroup analyses based on the presence of CSF abnormalities (presence of ≥2 CSF-unique oligoclonal bands or any CSF abnormality), gadolinium-enhancing lesions, juxtacortical lesions and sex, did not reveal any associations with retinal layer thicknesses.
DISCUSSION
In our study of people with RIS, the presence of SC or IT lesions were independently associated with reduced GCIP thickness. This finding suggests that subclinical retinal neuro- axonal loss may be present at the very earliest, pre-symptomatic stage of MS, especially in individuals who are considered to be at an increased risk for development of clinical demyelinating events.2 These findings are in line with prior work showing correlations between SC structural integrity and inner retinal layer thicknesses in MS, suggesting that there may be associations between ongoing pathologic processes in these two compartments of the central nervous system in MS, or that individuals with SC or IT lesions have more disseminated disease.20
Furthermore, we found that GCIP and INL thicknesses were negatively associated with age in the RIS cohort and that this differed significantly from the HC cohort, in which we did not observe a relationship between age and GCIP or INL thickness. These findings may parallel to some extent what is known about the evolution of retinal layer thicknesses in MS. GCIP thinning in MS has been shown to be more prominent with increasing disease duration, irrespective of ON history.21 Moreover, in addition to ganglion cell loss, INL atrophy was demonstrated pathologically post-mortem in MS retinas, and was most pronounced in subjects with longstanding disease.22 Additionally, electrophysiologic studies employing use of electroretinograms have shown INL dysfunction in MS eyes.23 Also, increased INL thickness has been associated with inflammatory disease activity and INL thickness may have increased variance early in the disease course.13 These findings are consistent with the possibility that in earlier disease stages when inflammatory disease activity is more prominent, INL thickness may be normal or increased, followed by decreasing thickness in the setting of neuronal cell loss and diminished inflammation. It is conceivable that similar, albeit subclinical, pathological mechanisms may be at play in RIS, with age in our analysis serving essentially as a proxy of disease duration.
Interestingly, the mean age of RIS subjects with SC lesions in our study is comparable to the age of PPMS onset in MS populations. Furthermore, it is known that a small percentage of RIS subjects will develop PPMS, and those subjects are older and have a higher prevalence of SC lesions compared to RIS evolving to relapsing-remitting MS.3 The cross-sectional design of our study did not allow us to assess if the RIS subjects in our cohort are in a “pre-progressive” phase, however it is conceivable that our finding of GCIP thinning in this population is reflective of the generalized brain and spinal cord atrophy that has been shown to be present at the time of progressive disease onset.24–26
Another interesting observation is that all African-American RIS subjects in our study had SC lesions. In MS, African-Americans have a more aggressive disease course with an increased incidence of SC involvement, as well as accelerated RNFL and GCIP thinning and worse visual disability, compared to Caucasian-Americans.27–29 Notably however, the number of African-American participants in our study (n=5) is similar to that in the largest longitudinal RIS study cohort reported to date (n=6), highlighting that the effect of race has been understudied in RIS.2 Assessment of large, diverse RIS cohorts will be important to further investigate the prognostic utility of race in this population.
Notably, the visual pathway, despite being the subject of extensive study in MS, has only been assessed in a small number of studies in RIS. Lebrun et al. showed, in a cohort of 70 RIS subjects, that abnormal VEP at presentation may be associated with an increased risk for conversion to MS, however OCT was not performed.5 Interestingly, a significant number (53%) of RIS subjects had abnormal VEP in this study, but these findings contrast with the reports of Knier et al. and Gabelic et al, which found abnormal VEP in 0% and 14.3% of studied RIS subjects respectively.30,31 Only one study (Knier et al.) has reported OCT findings in RIS, in which the authors found lower macular RNFL and GCIP volume in RIS compared to HC, but no difference in p-RNFL thickness, in a sample of 20 RIS subjects (defined utilizing Swanton criteria for dissemination in space).30,32 Additionally, Knier et al. found that INL volume was increased in RIS as compared to subjects with non-specific white matter lesions (but not compared to HC), and that reduced macular RNFL volume or p-RNFL thickness and increased INL volume were associated with measures of radiological disease activity. However, despite our larger sample size and use of more stringent MRI criteria for dissemination in space (Barkhof criteria), we were not able to detect any differences in retinal layer thicknesses in our overall RIS cohort as compared to HC. Notably, in the Knier et al. study, SC MRI data was available only for a small number of participants and the prevalence of SC lesions was low, while the prevalence of IT lesions was not reported. This highlights the importance for larger, independent future studies to further characterize retinal layer thicknesses in RIS and confirm these findings.
Our study has a number of limitations that warrant discussion. Firstly, the cross-sectional study design does not allow us to assess the prognostic value of retinal layer thicknesses in RIS for conversion to MS. This will be important to assess in larger, longitudinal cohorts of RIS. Furthermore, amongst the patients evaluated for inclusion in the study, we were not able to identify a sufficient number of subjects with non-specific white matter abnormalities to include as a comparator group, which is necessary to assess if OCT may add specificity to the diagnostic criteria for RIS. Moreover, our relatively small sample size (although this may be the largest reported OCT study in RIS to date), did not allow us to characterize the contribution of race to our findings. Our observations however clearly highlight this as an important area for further research in RIS. Finally, it is likely that we were insufficiently powered to detect associations of OCT measures with factors such as presence of gadolinium-enhancing lesions, given the low prevalence of this finding in our cohort.
In conclusion, the present work establishes that retinal neuro-axonal loss may be detected in RIS subjects with a high risk of conversion to MS, namely with SC or IT lesions. These findings support the rationale for future, larger, prospective, longitudinal studies to investigate the evolution of OCT measures over time in RIS and the role of baseline retinal layer thicknesses in predicting the risk of clinical demyelinating events. Overall, our study provides further evidence that OCT imaging is a promising tool for clinical monitoring in RIS and an important area for future research.
Acknowledgments
Study Funding:
This study was funded by the NIH (5R01NS082347 to P.C.) and National MS Society (FP-1607-24999 to E.S.; RG-1606-08768 to S.S).
Thomas Shoemaker is funded by a Sylvia Lawry physician fellowship award from the National Multiple Sclerosis Society (NMSS) and has received consulting honorariums from Genentech.
Megan Esch has received postdoctoral fellowship funding from the National Multiple Sclerosis Society (NMSS).
Peter A. Calabresi has received personal honorariums for consulting from Disarm Therapeutics. He is PI on research grants to Johns Hopkins from MedImmune, Annexon, Biogen, and Genzyme.
Shiv Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen-Idec, Genzyme, Genentech Corporation, EMD Serono & Novartis. He is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen Idec, and received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer. He is also the site investigator of a trial sponsored by MedDay Pharmaceuticals.
Ellen M. Mowry has grants from Biogen and Genzyme, is site PI for studies sponsored by Biogen and Sun Pharma, has received free medication for a clinical trial from Teva and receives royalties for editorial duties from UpToDate.
Elias S. Sotirchos is funded by a Sylvia Lawry physician fellowship award from the National Multiple Sclerosis Society (NMSS).
Footnotes
Disclosures:
Angeliki Filippatou has nothing to disclose.
Madiha Qutab has nothing to disclose.
Natalia Gonzalez-Caldito has nothing to disclose.
Jerry Prince is a founder of Sonovex, Inc. and serves on its Board of Directors.
Conflict of interest:
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 2009; 72: 800–805. [DOI] [PubMed] [Google Scholar]
- 2.Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS ONE 2014; 9: e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarci OH, Lebrun C, Siva A, et al. Primary Progressive Multiple Sclerosis Evolving From Radiologically Isolated Syndrome. Annals of Neurology 2016; 79: 288–294. [DOI] [PubMed] [Google Scholar]
- 4.Okuda DT, Mowry EM, Cree BaC, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011; 76: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebrun C, Bensa C, Debouverie M, et al. Association Between Clinical Conversion to Multiple Sclerosis in Radiologically Isolated Syndrome and Magnetic Resonance Imaging, Cerebrospinal Fluid, and Visual Evoked Potential: Follow-up of 70 Patients. Archives of Neurology 2009; 66: 841–846. [DOI] [PubMed] [Google Scholar]
- 6.Lebrun C, Bensa C, Debouverie M, et al. Unexpected multiple sclerosis: follow-up of 30 patients with magnetic resonance imaging and clinical conversion profile. J Neurol Neurosurg Psychiatry 2008; 79: 195–198. [DOI] [PubMed] [Google Scholar]
- 7.Granberg T, Martola J, Kristoffersen-Wiberg M, et al. Radiologically isolated syndrome – incidental magnetic resonance imaging findings suggestive of multiple sclerosis, a systematic review. Multiple Sclerosis Journal 2013; 19: 271–280. [DOI] [PubMed] [Google Scholar]
- 8.Petzold A, Balcer L, Balcer LJ, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. The Lancet Neurology 2017; 16: 797–812. [DOI] [PubMed] [Google Scholar]
- 9.Syc SB, Warner CV, Hiremath GS, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler 2010; 16: 829–839. [DOI] [PubMed] [Google Scholar]
- 10.Oreja-Guevara C, Noval S, Alvarez-Linera J, et al. Clinically isolated syndromes suggestive of multiple sclerosis: an optical coherence tomography study. PLoS ONE 2012; 7: e33907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberwahrenbrock T, Ringelstein M, Jentschke S, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler 2013; 19: 1887–1895. [DOI] [PubMed] [Google Scholar]
- 12.Knier B, Schmidt P, Aly L, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 2016; 139: 2855–2863. [DOI] [PubMed] [Google Scholar]
- 13.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 2012; 11: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkhof F, Filippi M, Miller LH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997; 120: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 15.Saidha S, Syc SB, Ibrahim MA, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain : a journal of neurology 2011; 134: 518–533. [DOI] [PubMed] [Google Scholar]
- 16.Baldewpersad Tewarie PK, Balk LJ, Costello F, et al. The OSCAR-IB Consensus Criteria for Retinal OCT Quality Assessment. PLoS One 2012; 7: e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schippling S, Balk LJ, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler 2015; 21: 163–170. [DOI] [PubMed] [Google Scholar]
- 18.Lang A, Carass A, Hauser M, et al. Retinal layer segmentation of macular OCT images using boundary classification. Biomedical optics express 2013; 4: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhargava P, Lang A, Al-Louzi O, et al. Applying an Open-Source Segmentation Algorithm to Different OCT Devices in Multiple Sclerosis Patients and Healthy Controls: Implications for Clinical Trials. Multiple sclerosis international 2015; 2015: 136295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh J, Sotirchos ES, Saidha S, et al. Relationships between quantitative spinal cord MRI and retinal layers in multiple sclerosis. Neurology 2015; 84: 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013; 80: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green AJ, McQuaid S, Hauser SL, et al. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 2010; 133: 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You Y, Graham EC, Shen T, et al. Progressive inner nuclear layer dysfunction in non-optic neuritis eyes in MS. Neurol Neuroimmunol Neuroinflamm 2018; 5: e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sastre-Garriga J, Ingle GT, Chard DT, et al. Grey and white matter atrophy in early clinical stages of primary progressive multiple sclerosis. Neuroimage 2004; 22: 353–359. [DOI] [PubMed] [Google Scholar]
- 25.Zeydan B, Gu X, Atkinson EJ, et al. Cervical spinal cord atrophy: An early marker of progressive MS onset. Neurol Neuroimmunol Neuroinflamm 2018; 5: e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bieniek M, Altmann DR, Davies GR, et al. Cord atrophy separates early primary progressive and relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2006; 77: 1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cree BAC, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 2004; 63: 2039–2045. [DOI] [PubMed] [Google Scholar]
- 28.Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Multiple Sclerosis 2003; 9: 293. [DOI] [PubMed] [Google Scholar]
- 29.Kimbrough DJ, Sotirchos ES, Wilson JA, et al. Retinal damage and vision loss in African American multiple sclerosis patients. Ann Neurol 2015; 77: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knier B, Berthele A, Buck D, et al. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Multiple Sclerosis Journal 2016; 22: 893–900. [DOI] [PubMed] [Google Scholar]
- 31.Gabelić T, Radmilović M, Posavec V, et al. Differences in oligoclonal bands and visual evoked potentials in patients with radiologically and clinically isolated syndrome. Acta Neurol Belg 2013; 113: 13–17. [DOI] [PubMed] [Google Scholar]
- 32.Swanton JK, Rovira A, Tintore M, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study 2007. [DOI] [PubMed]


