Abstract
Primary extrarenal Wilms tumor of the gynecologic tract is extremely rare with scattered case reports occurring in the ovary, uterine corpus and cervix. Only nine cases of primary ovarian Wilms tumor have been reported to date. Here, we provide an extensive literature review and describe two patients with ovarian Wilms tumor: a 36-year-old female (patient 1) and a 16-year-old female (patient 2), both presenting with abdominal pain and suspected ovarian torsion. They were each found to have unilateral ovarian masses measuring greater than 15 cm in size which were removed by unilateral salpingo-oophorectomy. Microscopically, the tumors exhibited the typical triphasic histology of Wilms tumor. In addition, the tumor from patient 1 contained elements of mature cystic teratoma, while an extensive rhabdomyosarcomatous component was identified in patient 2. Both tumors were diffusely and strongly positive for WT1 with variable staining for other biomarkers. The cases were diagnostically challenging and referred to our center for an expert opinion. Teratoid Wilms tumor in patient 1 is the second reported case of ovarian Wilms tumor arising in association with teratoma. Recognition of primary ovarian Wilms tumor requires a high index of suspicion and exclusion of other entities based on tumor morphology and immunohistochemical studies.
Keywords: Wilms tumor, ovary, differential diagnosis, immunohistochemistry, teratoma, rhabdomyosarcoma
Wilms tumor, also known as nephroblastoma, is a malignant neoplasm derived from nephrogenic blastema cells that frequently shows multiphasic differentiation patterns recapitulating the appearance of embryonic kidney. Wilms tumor is very uncommon in adults, but it accounts for greater than 80% of renal tumors in children with a peak incidence at 2–3 years of age (1). Approximately 10% of cases are associated with genetic conditions such as WAGR syndrome (Wilms tumor, Aniridia, Genitourinary malformations, mental Retardation), Denys-Drash syndrome (Wilms tumor, mesangial sclerosis, 46, XY disorder of sex development), Beckwith-Wiedemann syndrome (Wilms tumor, hemihypertrophy, macroglossia, omphalocele, visceromegaly) and familial nephroblastoma (1, 2). Microscopically, Wilms tumors typically exhibit a characteristic triphasic pattern consisting of undifferentiated blastemal, epithelial and mesenchymal (stromal) components. However, some tumors may be mono- or biphasic. The blastemal component is composed of closely packed small round blue cells with scant cytoplasm and overlapping primitive nuclei with evenly distributed coarse chromatin, small nucleoli, brisk mitotic activity and apoptotic debris. The epithelial component is composed of variably sized rosette-like or well-formed tubules, ill-defined glomeruloid structures and papillary structures lined by primitive columnar or cuboidal cells with elongated nuclei. The mesenchymal component typically contains spindle cells, skeletal muscle, smooth muscle, rarely hyaline cartilage, fat, bone, ganglion cells or neuroglia. The background stroma can be myxomatous or edematous (3). The presence of markedly enlarged (3x) tumor cell nuclei with hyperchromasia and multipolar mitotic figures constitutes anaplasia. The presence of diffuse anaplasia is clinically significant as it correlates with chemoresistance, p53 mutations and thus poor prognosis (4).
Extrarenal Wilms tumors are rare and have been described in various locations including perirenal, lumbar, sacrococcygeal and pelvic areas (5). Few case reports have described primary Wilms tumors of the gynecologic tract, mainly occurring in the uterine corpus and cervix (6–12). Nine cases of primary ovarian Wilms tumor have been reported to date (13–21), only one of which was associated with teratoma (13). Here, we present two additional patients with primary Wilms tumor of the ovary.
CASE REPORTS
Patient 1
A 36-year-old female presented to the emergency room with abdominal pain secondary to ovarian torsion. She subsequently underwent a right salpingo-oophorectomy. Macroscopically, the ovary measured 16.1 × 10.5 × 7.5 cm and weighed 717 grams. The external surface of the ovary was gray-tan to purple-pink and smooth with focal disruption measuring 7.0 × 4.7 cm. The cut surface was multiloculated with multiple granular, tan-pink and firm to gelatinous nodules ranging from 2.4 cm to 9.5 cm in size. The cyst wall thickness ranged from 0.1 cm to 0.9 cm and contained serous fluid. The fallopian tube was unremarkable. A diagnosis of malignant struma ovarii arising in a mature cystic teratoma was rendered, and the case was referred to our institution for a second opinion.
Microscopically, the tumor was composed of primitive small round blue cells with varying architectural growth patterns: epithelioid tubules growing in nests and cords with interspersed stromal elements and a diffuse spindle cell component with minimal cytoplasm. In addition, adjacent to this primitive component was a mature cystic teratoma consisting of skin and cutaneous adnexal structures, bronchial-type epithelium, cartilage and gastric-type mucosa (Figure 1). The ovarian surface was not involved, and the fallopian tube was unremarkable.
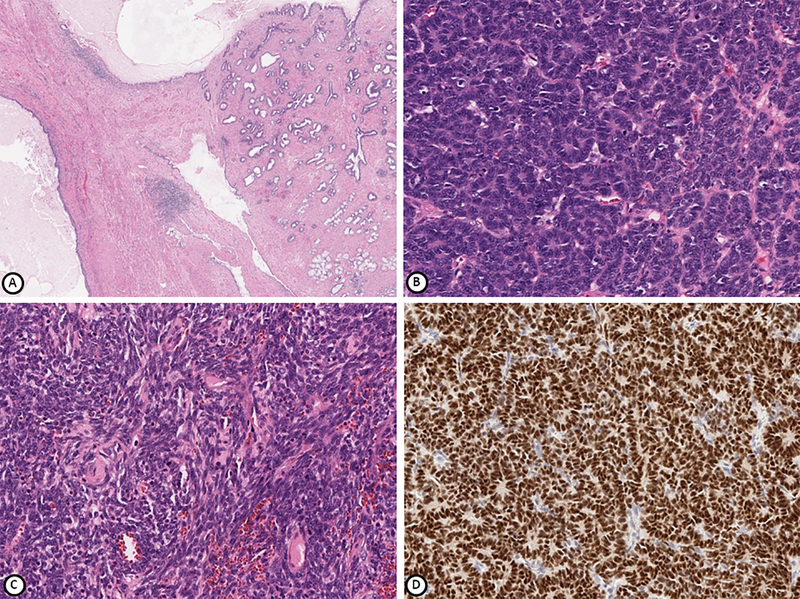
Figure 1.
Tumor from patient 1 comprised of mature teratomatous elements (A), blastemal and epithelial components (B) and spindle cell mesenchymal component (C). WT1 is positive in epithelial and blastemal cells (D). Hematoxylin-eosin stain (A-C), immunohistochemical stain (D); magnification x100 (A, B), x200 (C, D).
Immunohistochemical studies showed that the blastemal and epithelial components were diffusely and strongly positive for WT1 (Figure 1), PAX8, TTF-1, CAM5.2, CD56 and vimentin. Other variably positive markers included estrogen and progesterone receptors (ER, PR), CD99, FLI-1, EMA, pan-cytokeratin AE1/AE3, cytokeratin 7 (CK7), Ber-EP4 and CD10. The tumor was negative for cytokeratin 20 (CK20), chromogranin, synaptophysin, thyroglobulin, S100, placental alkaline phosphatase (PLAP), inhibin, calretinin, calcitonin, desmin, smooth muscle actin (SMA), MART1, HMB-45, OCT-4, AFP, GATA-3, Napsin-A, monoclonal carcinoembryonic antigen (mCEA), glial fibrillary acidic protein (GFAP) and neurofilament, with an approximately 30–50% Ki-67 proliferative index. The tumor was reported as “extrarenal Wilms tumor of the ovary arising in a mature cystic teratoma.” The patient was counseled to see a gynecologic oncologist; unfortunately, she never returned and was lost to follow-up.
Patient 2
A 16-year-old female presented with fever and worsening abdominal pain over two months. Computed tomography with contrast showed an 18.5 × 8.5 × 16.5 cm hemorrhagic left ovarian mass concerning for ovarian torsion. She subsequently underwent a left salpingo-oophorectomy during which a ruptured, hemorrhagic left ovarian cyst was found. Macroscopically, the specimen was received fragmented and consisted of blood clots and red-yellow gelatinous tissue pieces measuring 14 × 12 × 7 cm that weighed 500 grams in aggregate. The fallopian tube was unremarkable. Intraoperative frozen section was reported as “ovarian cyst with hemorrhage and fibrin consistent with torsion; negative for malignancy.” Based on the microscopic examination of permanent sections, a diagnosis of low grade immature teratoma was rendered, and the case was referred to our institution for a second opinion.
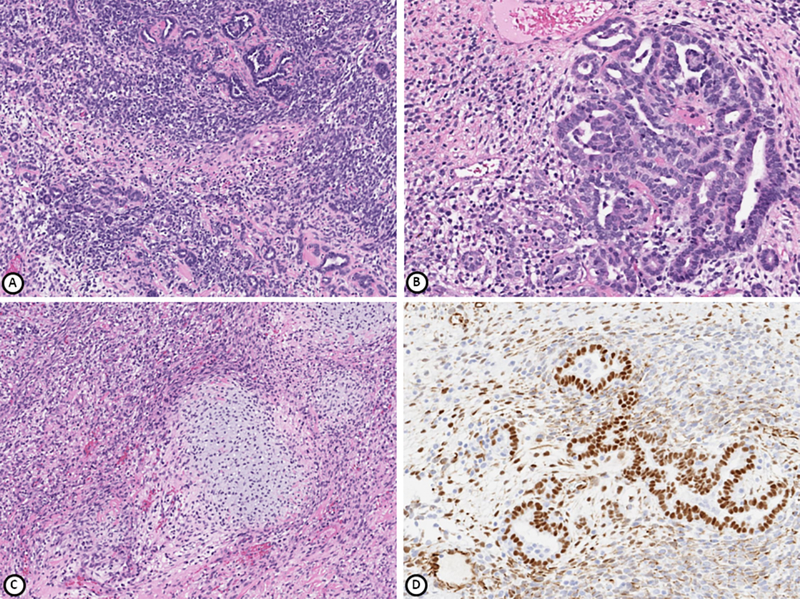
Microscopically, the tumor consisted of undifferentiated mesenchymal elements, blastemal and tubular structures, consistent with Wilms tumor. The mesenchymal elements included skeletal muscle and fetal-type cartilage (Figure 2). The blastemal and epithelial components morphologically resembled a poorly differentiated retiform Sertoli-Leydig cell tumor which was considered in the differential diagnosis. Immunohistochemical studies showed that WT1 was positive in the blastemal and epithelial components (Figure 2), with variable staining for GATA-3 in the spindled/mesenchymal component and no immunoreactivity for inhibin. Some of the tubules expressed steroidogenic factor 1 (SF-1) and FOXL2, markers of sex cord stromal differentiation, further raising the possibility of Sertoli-Leydig cell tumor. However, given the absence of Leydig cells and negative inhibin staining, this diagnosis was considered less likely. No neuroepithelial elements were identified on H&E or immunohistochemical stains. GFAP, synaptophysin and NeuN were negative, while neurofilament was focally positive. No teratomatous elements were identified. Given the amount of skeletal muscle and undifferentiated mesenchymal elements in the tumor, the diagnostic considerations were Wilms tumor with a predominant heterologous component in the form of skeletal muscle and cartilage or an embryonal rhabdomyosarcoma in association with Wilms tumor. The latter was favored. Within just two weeks of the diagnosis, the patient already had recurred with a large lobulated mass in the right lower abdomen and pelvis, inseparable from the right ovary. Biopsy of this mass showed mostly immature cartilage and immature mesenchymal components composed of small spindled blue cells, similar to the original tumor. The patient then received the Children’s Oncology Group Study #ARST0531 Regimen B for intermediate-risk rhabdomyosarcoma, consisting of vincristine, dactinomycin and cyclophosphamide (VAC), along with Granulocyte-colony stimulating factor (G-CSF) for local control. Eighteen weeks into her chemotherapy, the patient underwent resection of residual tumor, which included peritoneal implants, a right pelvic mass, the right ovary and fallopian tube, omentum and appendix, all of which contained residual tumor. The morphology of the residual tumor consisted predominantly of cartilage and nests of undifferentiated mesenchymal components in a background of extensive treatment related changes such as macrophages, fibrosis, inflammation and necrosis. Immunohistochemistry showed positive MyoD1 staining but negative myogenin and desmin. After surgery, she resumed chemotherapy (vincristine and cyclophosphamide) with concurrent radiation therapy. After completing 3 months of radiation and another 3 months of chemotherapy, a positron emission tomography (PET) scan showed no evidence of fluorodeoxyglucose (FDG) avid malignancy.
Figure 2.
Tumor from patient 2 comprised of blastemal and epithelial components (A, B), mesenchymal component containing rhabdomyosarcoma and hyaline cartilage (C). WT1 is positive in epithelial and blastemal cells (D). Hematoxylin-eosin stain (A-C), immunohistochemical stain (D); magnification x100 (A, C), x200 (B, D).
DISCUSSION
Primary Wilms tumor of the gynecologic tract is extremely rare with scattered case reports (6–12). Review of the nine published cases of primary ovarian Wilms tumor demonstrates that the mean patient age was 21 years (range 1–56) (13–21). The most frequent clinical symptom at presentation was abdominal pain (six patients). The median tumor size was 11.3 cm (range 2.5–13), and all tumors were unilateral (Table 1). Our two cases fall within the reported age range (16- and 36-years-old). Both patients presented with abdominal pain and unilateral ovarian masses. The tumors were larger (16.1 cm and 18 cm) than the previously reported size range.
Table 1.
Summary of the clinicopathologic features for 11 cases of ovarian Wilms tumor, including the two current cases.
| Case No. | Reference | Age | Clinical presentation | Treatment | Tumor size, greatest dimension (cm) | Laterality | Histology | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Nicod, 1965 (16) | 35 | Menorrhagia | Left oophorectomy, radium therapy | 10 | left, unilateral | Wilms tumor | No recurrence at 24 months |
| 2 | Sahin and Benda (20) | 56 | Bilateral calf pain due to deep vein thrombosis, pelvic mass | Total abdominal hysterectomy, bilateral salpingo-oophorectomy, appendectomy, pelvic radiotherapy, chemotherapy | 12 | left, unilateral | Wilms tumor | No recurrence at 108 months |
| 3 | O’Dowd and Ismail (17) | 20 | Amenorrhea, elevated androgen | Right salpingo-oophorectomy | 2.5 | Right, unilateral | Wilms tumor associated with granulosa cell tumor | No recurrence, became pregnant, follow-up interval unknown |
| 4 | Isaac et al, 2000 (14) | 21 | Abdominal pain, menorrhagia | Right oophorectomy, wedge resection of left ovary, chemotherapy | 19 | Right, unilateral | Wilms tumor | No recurrence at 6 months |
| 5 | Pereira et al, 2000 (19) | 3.5 | Abdominal pain and distension | Right oophorectomy, left ovarian biopsy, chemotherapy | 13 | Right, unilateral | Wilms tumor | No recurrence at 78 months |
| 6 | Oner et al, 2002 (18) | 3.5 | Abdominal pain, vomiting | Left salpingo-oophorectomy, appendectomy, partial omentectomy, peritoneal biopsies, retroperitoneal lymphadenectomy, chemotherapy | 13 | left, unilateral | Wilms tumor | No recurrence at 7 months |
| 7 | Liang et al, 2008 (15) | 22 | Abdominal pain and distension | Right oophorectomy | 9 | Right, unilateral | Wilms tumor | Unknown |
| 8 | Marwah et al, 2012 (21) | 1 | Abdominal pain, vomiting | Right salpingo-oophorectomy, chemotherapy | 10 | Right, unilateral | Wilms tumor | No recurrence at 3 months |
| 9 | Alexander et al, 2017 (13) | 26 | Abdominal pain | Right salpingo-oophorectomy | 13 | Right, unilateral | Teratoid Wilms tumor | No recurrence at 11 months |
| 10 | Current patient 1 | 36 | Abdominal pain, ovarian torsion | Right salpingo-oophorectomy | 16.1 | Right, unilateral | Teratoid | Unknown |
| 11 | Current patient 2 | 16 | Abdominal pain, ovarian torsion, fever | Left salpingo-oophorectomy | 18 | left, unilateral | Wilms tumor | No recurrence at 6 months |
Diagnosis of extrarenal Wilms tumors requires exclusion of intrarenal tumor and supernumerary kidney. All nine reported cases of ovarian Wilms tumor as well as our patients had no evidence of primary renal Wilms tumor. Extrarenal Wilms tumors can be classified as pure (composed solely of Wilms tumor) and teratoid (composed of a combination of Wilms tumor and teratoma) (22), defined as a triphasic tumor containing greater than 50% heterologous elements (23). Pure extrarenal Wilms tumors have been suggested to arise from persistent mesonephric duct remnants in the wall of the uterine cervix and vagina, ovary and inguinal regions, or from cells with persistent embryonic potential (Connheims’ cell rest theory) (5). In contrast, the most likely origin for the teratoid variant is thought to be misplaced totipotent primitive nephrogenic blastemal elements (5). Teratoid renal Wilms tumors are rare with approximately 33 reported cases to date. These tumors usually present as advanced bilateral disease and have a high mortality rate (24, 25). Only seven cases of teratoid extrarenal Wilms tumor have been reported in various locations (11, 13, 26–29), including the uterine cervix (26), uterine corpus (11) and ovary (13). Patient 1 in this report represents the second published case of an ovarian Wilms tumor associated with teratoma.
Histopathologic diagnosis of renal Wilms tumor is straightforward in most cases based on the identification of the typical triphasic pattern. Mono- or biphasic variants of Wilms tumor, especially in extrarenal locations, can be diagnostically challenging. Metastatic Wilms tumor of the kidney should always be considered in patients aged <10 years. These patients usually have a prior history of renal Wilms tumor or concomitant renal mass. The differential diagnosis for ovarian Wilms tumors includes pure embryonal rhabdomyosarcoma, immature teratoma, carcinosarcoma, moderately to poorly differentiated Sertoli-Leydig cell tumor and peripheral/central neuroectodermal tumor (3). Pure embryonal rhabdomyosarcoma usually contains subepithelial and periglandular cambium layers, and lacks glomerular or tubular differentiation. Immature teratoma contains immature neuroepithelium and tissues from three embryologic layers. Carcinosarcoma is a biphasic neoplasm comprised of high grade carcinoma and sarcoma with marked nuclear pleomorphism in both components. Moderately to poorly differentiated Sertoli-Leydig cell tumors are composed of varying amounts of immature tubules, solid areas and Leydig cells, with or without heterologous elements that are positive for inhibin, epithelial membrane antigen, WT1 and SF-1 (30). Peripheral/central neuroectodermal tumors may contain rosettes but lack epithelial differentiation and exhibit immunoreactivity for GFAP (if central) or FLI-1 with chromosomal translocation t(11;22) (if Ewing/peripheral). When present, blastemal components typically exhibit nuclear molding and early tubular differentiation with nuclei arranged around tubular lumina. Presence of true tubular lumina typically favors Wilms tumor (3, 31). In addition, any of these tumors may contain heterologous elements. However, the presence of characteristic morphologic features and/or immunohistochemical studies should lead to an accurate diagnosis.
Immunohistochemical studies are often required to resolve the above differential diagnoses. Wilms tumor is usually positive for WT1, CD56, CD99 and Neuron-Specific Enolase (NSE), and negative for ER, PR, CD10 and GFAP. Desmin and myoglobin show positive staining in rhabdomyoblasts, and desmin may label blastemal cells. Blastemal and epithelial elements are usually positive for WT1, while they are negative for muscle markers such as SMA, myogenin and Myo-D1. Vimentin and cytokeratin are negative to focally positive in blastemal cells, and cytokeratin is usually positive in the epithelial component. CK7 may be positive in more differentiated epithelial cells. PAX8 and PAX2 are usually positive (3, 31). TTF-1 expression has also been described in 16.6% of Wilms tumors (32). Both tumors in our patients were positive for WT1 and negative for GFAP and synaptophysin. In addition, the tumor from patient 1 was positive for PAX8, TTF-1, CAM5.2, CD56, vimentin, with variable staining for epithelial markers and no immunoreactivity for germ cell, smooth muscle and sex cord stromal markers, chromogranin, thyroglobulin and GATA-3. The tumor from patient 2 showed focal staining for GATA-3 in the mesenchymal component, focal SF-1 and FOXL2 in the tubules and no immunoreactivity for inhibin. Tumor morphology, absence of immunoreactivity for inhibin (patients 1 and 2), strong WT1 positivity, as well as the lack of Leydig cells (patient 2) were essential for diagnosis of Wilms tumor in both cases.
Wilms tumor of the kidney is usually treated with primary resection followed by adjuvant therapy (Children’s Oncology Group) or preoperative therapy followed by surgical resection and adjuvant therapy (International Society of Pediatric Oncology). Overall survival of pediatric patients is greater than 90% (33–35). Most significant unfavorable factors include high stage at presentation and the presence of diffuse anaplasia (“unfavorable” histology) (4, 34). There are no accepted staging or treatment guidelines for extrarenal Wilms tumor, including the teratoid variant. The National Wilms Tumor Study (NWTS) recommends considering all extrarenal Wilms tumors Stage II or higher (36, 37) and thus all cases requiring chemotherapy irrespective of other clinicopathologic features. However, most extrarenal Wilms tumors have a “favorable” histology (lack of diffuse anaplasia) and long-term disease-free survival (33). Only four of nine reported cases of ovarian Wilms tumor were treated with adjuvant chemotherapy. The only reported case of teratoid Wilms tumor was treated with surgery but no chemotherapy. No recurrences have been reported in any of the nine patients (median follow up 33.9 months, range 3–108).
Patient 2 is of particular interest because of the presence of extensive rhabdomyoblasts associated with an undifferentiated mesenchymal component which compelled the diagnosis of rhabdomyosarcoma arising in a background of Wilms tumor. The presence of cartilage does not preclude the diagnosis since chondroid differentiation is a well known association in gynecologic rhabdomyosarcomas (38). There are no well established criteria for distinguishing rhabdomyosarcoma from extensive skeletal muscle differentiation in Wilms tumor, and therefore, it is possible that this case may have represented the latter. However, the presence of the undifferentiated spindle cell component with MyoD1 positivity, the aggressive behavior of the tumor and the good response to rhabdomyosarcoma specific chemotherapy suggests that this was indeed heterologous sarcoma in the form of rhabdomyosarcoma arising in the setting of extrarenal Wilms tumor.
In conclusion, primary ovarian Wilms tumor is very rare and can be diagnostically challenging. It can occur in isolation or in association with teratomatous elements. Recognition of primary ovarian Wilms tumor requires a high index of suspicion and exclusion of other entities. Careful histologic examination will allow for accurate diagnosis based on tumor morphology and often extensive immunohistochemical studies. Both cases described in this report were diagnostically challenging, referred to our center for an expert opinion. Teratoid Wilms tumor in patient 1 is the second reported case of ovarian Wilms tumor arising in association with teratoma.
ACKNOWLEDGMENTS
We would like to thank Dr. Asya Ali and Dr. Xiaoling Guo for referring the cases and for providing clinical information for this manuscript.
FUNDING
This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
References:
- 1.Szychot E, Apps J, Pritchard-Jones K. Wilms’ tumor: biology, diagnosis and treatment. Transl Pediatr. 2014;3(1):12–24. Epub 2014/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43(9):705–15. Epub 2006/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popov SD, Sebire NJ, Vujanic GM. Wilms’ Tumour - Histology and Differential Diagnosis. In: van den Heuvel-Eibrink MM, editor. Wilms Tumor; Brisbane (AU)2016. [PubMed] [Google Scholar]
- 4.Faria P, Beckwith JB, Mishra K, et al. Focal versus diffuse anaplasia in Wilms tumor--new definitions with prognostic significance: a report from the National Wilms Tumor Study Group. Am J Surg Pathol. 1996;20(8):909–20. Epub 1996/08/01. [DOI] [PubMed] [Google Scholar]
- 5.Andrews PE, Kelalis PP, Haase GM. Extrarenal Wilms’ tumor: results of the National Wilms’ Tumor Study. J Pediatr Surg. 1992;27(9):1181–4. Epub 1992/09/01. [DOI] [PubMed] [Google Scholar]
- 6.Bittencourt AL, Britto JF, Fonseca LE Jr. Wilms’ tumor of the uterus: the first report of the literature. Cancer. 1981;47(10):2496–9. Epub 1981/05/15. [DOI] [PubMed] [Google Scholar]
- 7.Benatar B, Wright C, Freinkel AL, Cooper K. Primary extrarenal Wilms’ tumor of the uterus presenting as a cervical polyp. Int J Gynecol Pathol. 1998;17(3):277–80. Epub 1998/07/10. [DOI] [PubMed] [Google Scholar]
- 8.Babin EA, Davis JR, Hatch KD, Hallum AV 3rd. Wilms’ tumor of the cervix: a case report and review of the literature. Gynecol Oncol. 2000;76(1):107–11. Epub 2000/01/06. [DOI] [PubMed] [Google Scholar]
- 9.Muc RS, Grayson W, Grobbelaar JJ. Adult extrarenal Wilms tumor occurring in the uterus. Arch Pathol Lab Med. 2001;125(8):1081–3. Epub 2001/07/28. [DOI] [PubMed] [Google Scholar]
- 10.McAlpine J, Azodi M, O’Malley D, et al. Extrarenal Wilms’ tumor of the uterine corpus. Gynecol Oncol. 2005;96(3):892–6. Epub 2005/02/22. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Galvis OF, Stolnicu S, Munoz E, Aneiros-Fernandez J, Alaggio R, Nogales FF. Adult extrarenal Wilms tumor of the uterus with teratoid features. Hum Pathol. 2009;40(3):418–24. Epub 2008/09/16. [DOI] [PubMed] [Google Scholar]
- 12.Cao MM, Huang CP, Wang YF, Ma DM. Extrarenal Wilms’ Tumor of the Female Genital System: A Case Report and Literature Review. Chin Med Sci J. 2017;32(4):274–8. Epub 2018/01/06. [DOI] [PubMed] [Google Scholar]
- 13.Alexander VM, Meisel J, O’Brien S, Khanna N. Wilms’ tumor of the ovary. Gynecol Oncol Rep. 2017;19:18–21. Epub 2016/12/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaac MA, Vijayalakshmi S, Madhu CS, Bosincu L, Nogales FF. Pure cystic nephroblastoma of the ovary with a review of extrarenal Wilms’ tumors. Hum Pathol. 2000;31(6):761–4. Epub 2000/06/29. [DOI] [PubMed] [Google Scholar]
- 15.Liang L, Zhou XH, Deng YJ, Zhang HH, Ding YQ. [Adult extrarenal Wilms’ tumor occurring in ovary: report of a case]. Zhonghua Bing Li Xue Za Zhi. 2008;37(4):284–5. Epub 2008/10/11. [PubMed] [Google Scholar]
- 16.Nicod JL. [Wilms tumors in the ovary]. Bull Assoc Fr Etud Cancer. 1965;52(2):173–8. Epub 1965/04/01. Tumeur de Wilms dans l’ovaire. [PubMed] [Google Scholar]
- 17.O’Dowd J, Ismail SM. Juvenile granulosa cell tumour of the ovary containing a nodule of Wilms’ tumour. Histopathology. 1990;17(5):468–70. Epub 1990/11/01. [DOI] [PubMed] [Google Scholar]
- 18.Oner UU, Tokar B, Acikalin MF, Ilhan H, Tel N. Wilms’ tumor of the ovary: A case report. J Pediatr Surg. 2002;37(1):127–9. Epub 2002/01/10. [DOI] [PubMed] [Google Scholar]
- 19.Pereira F, Carrascal E, Canas C, Florez L. Extrarenal Wilms tumor of the left ovary: a case report. J Pediatr Hematol Oncol. 2000;22(1):88–9. Epub 2000/03/01. [DOI] [PubMed] [Google Scholar]
- 20.Sahin A, Benda JA. Primary ovarian Wilms’ tumor. Cancer. 1988;61(7):1460–3. Epub 1988/04/01. [DOI] [PubMed] [Google Scholar]
- 21.Marwah N, Rattan KN, Rana P, Goyal V, R. S. Extrarenal Wilms’ Tumor of the Ovary: A Case Report and Short Review of the Literature. J Gynecol Surg. 2012;28(4):306–8. [Google Scholar]
- 22.Variend S, Spicer RD, Mackinnon AE. Teratoid Wilms’ tumor. Cancer. 1984;53(9):1936–42. Epub 1984/05/01. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes ET, Parham DM, Ribeiro RC, Douglass EC, Kumar AP, Wilimas J. Teratoid Wilms’ tumor: the St Jude experience. J Pediatr Surg. 1988;23(12):1131–4. Epub 1988/12/01. [DOI] [PubMed] [Google Scholar]
- 24.Myers JB, Dall’Era J, Odom LF, McGavran L, Lovell MA, Furness P 3rd. Teratoid Wilms’ tumor, an important variant of nephroblastoma. J Pediatr Urol. 2007;3(4):282–6. Epub 2008/10/25. [DOI] [PubMed] [Google Scholar]
- 25.Ghamdi DA, Bakshi N, Akhtar M. Teratoid Wilms Tumor: Report of Three Cases and Review of the Literature. Turk Patoloji Derg. 2017. Epub 2017/03/09. Teratoid Wilms Tumor: Report of Three Cases and Review of the Literature. [DOI] [PubMed] [Google Scholar]
- 26.Song JS, Kim IK, Kim YM, Khang SK, Kim KR, Lee Y. Extrarenal teratoid Wilms’ tumor: two cases in unusual locations, one associated with elevated serum AFP. Pathol Int. 2010;60(1):35–41. Epub 2010/01/09. [DOI] [PubMed] [Google Scholar]
- 27.Pawel BR, de Chadarevian JP, Smergel EM, Weintraub WH. Teratoid Wilms tumor arising as a botryoid growth within a supernumerary ectopic ureteropelvic structure. Arch Pathol Lab Med. 1998;122(10):925–8. Epub 1998/10/24. [PubMed] [Google Scholar]
- 28.Parikh B, Trivedi P, Shukla K. A unilateral teratoid Wilms’ tumor with raised serum alpha-fetoprotein level. Indian J Pathol Microbiol. 2007;50(2):317–9. Epub 2007/09/22. [PubMed] [Google Scholar]
- 29.Chowhan AK, Reddy MK, Javvadi V, Kannan T. Extrarenal teratoid Wilms’ tumour. Singapore Med J. 2011;52(6):e134–7. Epub 2011/07/07. [PubMed] [Google Scholar]
- 30.Zhao C, Barner R, Vinh TN, McManus K, Dabbs D, Vang R. SF-1 is a diagnostically useful immunohistochemical marker and comparable to other sex cord-stromal tumor markers for the differential diagnosis of ovarian sertoli cell tumor. Int J Gynecol Pathol. 2008;27(4):507–14. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- 31.Vujanic GM, Sandstedt B. The pathology of Wilms’ tumour (nephroblastoma): the International Society of Paediatric Oncology approach. J Clin Pathol. 2010;63(2):102–9. Epub 2009/08/19. [DOI] [PubMed] [Google Scholar]
- 32.Bisceglia M, Ragazzi M, Galliani CA, Lastilla G, Rosai J. TTF-1 expression in nephroblastoma. Am J Surg Pathol. 2009;33(3):454–61. Epub 2008/11/18. [DOI] [PubMed] [Google Scholar]
- 33.Lopes RI, Lorenzo A. Recent advances in the management of Wilms’ tumor. F1000Res. 2017;6:670 Epub 2017/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dome JS, Graf N, Geller JI, et al. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J Clin Oncol. 2015;33(27):2999–3007. Epub 2015/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kieran K, Ehrlich PF. Current surgical standards of care in Wilms tumor. Urol Oncol. 2016;34(1):13–23. Epub 2015/07/01. [DOI] [PubMed] [Google Scholar]
- 36.Shojaeian R, Hiradfar M, Sharifabad PS, Zabolinejad N. Extrarenal Wilms’ Tumor: Challenges in Diagnosis, Embryology, Treatment and Prognosis. In: van den Heuvel-Eibrink MM, editor. Wilms Tumor; Brisbane (AU)2016. [PubMed] [Google Scholar]
- 37.Madanat F, Osborne B, Cangir A, Sutow WW. Extrarenal Wilms tumor. J Pediatr. 1978;93(3):439–43. Epub 1978/09/01. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson SE, Gerald W, Barakat RR, Chi DS, Soslow RA. Clinicopathologic features of rhabdomyosarcoma of gynecologic origin in adults. Am J Surg Pathol. 2007. March;31(3):382–9 [DOI] [PubMed] [Google Scholar]