Abstract
Humans throughout the world are exposed regularly to mixtures of environmental toxicants. Four of the most common heavy metal toxicants in the environment are mercury (Hg), cadmium (Cdd), lead (Pb), and arsenic (As). Numerous previous studies assessed the effects and disposition of individual metals in organ systems; however, humans are usually exposed to mixtures of toxicants or metals rather than to a single toxicant. Therefore, the purpose of the current study was to test the hypothesis that exposure to a mixture of toxic heavy metals alters the disposition of single metals in target organs. Wistar rats (Rattus norvegicus) were exposed to Hg, Cd, Pb, or As as a single metal or as a mixture of metals. Rats were injected intravenously for three days, following which kidneys, liver, brain, and blood were harvested. Samples were analyzed for content of Hg, Cd, Pb, and As via inductively coupled plasma mass spectrometry. In general, exposure to a mixture of metals reduced accumulation of single metals in target organs. Interestingly, exposure to mixtures of metals with Pb and/or As increased the concentration of these metals specifically in the liver. The findings from this study indicate that exposure to mixtures of toxic heavy metals may alter significantly the distribution and accumulation of these metals in target organs and tissues.
Keywords: mercury, cadmium, lead, arsenic, mixtures
Introduction
Anthropogenic activities have led to significant contamination of the environment with heavy metals such as mercury (Hg), cadmium (Cd), lead (Pb), and arsenic (As). These metals persist in nature and accumulate in soil, water, and plants. There is a growing awareness of the impact of human exposure to mixtures of toxic heavy metals and other environmental toxicants (Barbosa, 2017). Indeed, Hg, Cd, Pb, and As are recognized by the World Health Organization (WHO) as chemicals of major public health concern (WHO 2018). In addition, As, Pb, Hg, and Cd are listed as numbers one, two, three, and seven, respectively, on the Substance Priority List published by the Agency for Toxic Substances and Disease Registry (ATSDR 2017).
Human exposure to each of these metals occurs in a variety of ways and exposure to each metal may result in significant toxicological consequences. Exposure to Hg may occur through occupational, dietary, and/or environmental routes, which may lead to nephrotoxicity and/or neurotoxicity (Aschner and Syversen 2005; Clarkson and Magos 2006; Branco et al, 2017). In contrast, exposure to Cd occurs primarily through the smoking of tobacco products but may also occur via dietary ingestion of leafy vegetables such as lettuce and spinach (ATSDR 2008; Huang et al. 2017). Cd exerts significant adverse effects on kidneys, liver, lung, and testis (ATSDR 2008). Exposure to As occurs primarily via ingestion of contaminated ground water (ATSDR 2007); however, occupational exposure is also of significant concern (Serrazina et al, 2018). The effects of As exposure are particularly severe in the bladder, kidney, skin, and liver (Tchounwou et al 2003). Human exposure to Pb is often the result of ingestion of contaminated paint, soil, dust, and/or water (Laidlaw et al. 2016; ATSDR 2007). The primary targets of Pb accumulation are the kidney, liver, brain, and bone (Tchounwou et al 2012). Because these metals are found readily throughout the environment, co-exposure of humans to a mixture of these metals is highly likely.
Numerous studies assessed the disposition and adverse effects of a single metal in a mammalian model; however, humans are often exposed to mixtures of metals rather than to a single metal. Based upon data from the US NHANES population, it appears that humans are exposed most frequently to a combination of Hg, Cd, Pb, and/or As (Shim et al. 2017). The toxicological consequences of exposure to a mixture of metals differs significantly from those associated with exposure to an individual metal (Lin et al. 2016; von Stackelberg et al. 2015; Claus Henn et al 2014). In addition, exposure to mixtures of metals was found to lead to adverse health outcomes that are more severe than those associated with exposure to a single metal (Wang et al 2018; von Stackelberg et al. 2015). Several investigators reported that certain mixtures of metals exert synergistic, additive, and/or antagonistic effects on various in vivo models ((Hagopian-Schlekat et al 2001; Montvydiene and Marciulioniene 2004; Lynch et al 2016). Interesting, published reports suggest that co-exposure to As, Pb, and Cd in the presence of one or more additional metals leads to more than an additive effect (von Stackelberg et al. 2015). Similarly, exposure to binary mixtures of metals also leads to toxicological consequences that are different from those following exposure to a single metal (Muthusamy et al 2016). Studies assessing the influence of metal mixtures are critical to develop a more comprehensive understanding of how environmental exposure impacts health outcomes.
In order to understand why the toxicological consequences of exposure to metal mixtures are more severe than those following exposure to individual metals, the changes in corporal disposition of metals within a mixture first need to be characterized. Therefore, the current study was designed to determine how exposure to mixtures of relevant environmental metals alters the disposition and accumulation of individual metals in target organs. Hg, Cd, As, and Pb were selected because of their prevalence in the environment and the frequency to which humans are exposed to these toxic metals. Further, binary combinations of Hg/Cd and As/Pb were utilized because of similarities in the transport mechanisms by which these metals are taken into target cells. The findings from the proposed studies may provide preliminary information regarding how exposure to metal mixtures alters the disposition of individual metals in mammals.
Methods
Animals
Male and female Wistar rats (Rattus norvegicus), weighing 300 g, were obtained from our colony in the Mercer University School of Medicine animal facility. Animals were provided a commercial laboratory diet (Teklad Global Soy Protein Free Extruded Rodent Diet, Harlan Laboratories) and water ad libitum throughout all aspects of the present study. All procedures involving animals were reviewed and approved by the Mercer University Institutional Animal Care and Use Committee. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
Experimental Design
Rats of both genders were divided randomly into 8 groups with 3 rats per group. Dosing regimens were designed based upon published studies using borderline nephrotoxic doses in rat models. Three doses were administered in order to mimic repeated acute exposure to the metals and metal mixtures. Rats were injected intravenously (iv) because this route ensures that the entire dose is delivered to blood and organs. Group 1 was injected iv with 2 ml normal saline • kg−1. Group 2 was injected i.v. for 3 days with 1 µmol (2.7 mg) mercuric chloride (HgCl2) • kg−1 • 2 ml−1 normal saline (Bridges et al 2014; Oliveira et al. 2016). Group 3 was injected i.v. for 3 days with 2.7 mmol (0.5 mg) cadmium chloride (CdCl2) • kg−1 • 2 ml−1 normal saline (Czykier et al 2004; Puri and Saha 2003). Group 4 was injected i.v. for 3 days with 0.8 mmol (0.1 mg) sodium arsenate (Na3AsO4) • kg−1 • 2 ml−1 normal saline (Kobayashi et al 2005; Cui et al. 2004). Group 5 was injected i.v. for 3 days with 0.6 mmol (0.25 mg) lead acetate (Pb(C2H3O2)2) • kg−1 • 2 ml−1 normal saline (Dalley et al. 1989; Stankovic-Keser et al. 1982). Group 6 was injected i.v. for 3 days with a mixture of 1 µmol HgCl2 and 2.7 mmol CdCl2 • kg−1 • 2 ml−1 normal saline. Hg and Cd were administered together because they are each taken up as conjugates of thiol-containing molecules (Bridges and Zalups 2010; Zalups and Ahmad 2003). Group 7 was injected i.v. for 3 days with a mixture of 0.8 mmol Na3AsO4 and 0.6 mmol Pb(C2H3O2)2 • kg−1 • 2 ml−1 normal saline. Pb and As were administered together because they tend to be taken up in their ionic forms (Roggenbeck et al 2016; Bridges and Zalups 2005; Jennette 1981). Group 8 was injected i.v. for 3 days with a mixture of 1 µmol HgCl2, 2.7 mmol CdCl2, 0.8 mmol Na3AsO4, and 0.6 mmol Pb(C2H3O2)2 • kg−1 • 2 ml−1 normal saline.
Intravenous Injections
Injections were carried out according to our previously published protocol (Bridges et al 2008a; 2008b). At the time of injection, each animal was anesthetized with isoflurane and a small incision was made in the skin in the mid-ventral region of the thigh to expose the femoral vein and artery. The respective solution was administered into the vein. The wound was closed using two 9-mm stainless steel wound clips. Animals were subsequently housed individually in metabolic cages. Animals were injected again after 24 and 48 hr with the same dose and solution. Animals were monitored closely for signs of illness and stress; all animals appeared healthy at the end of the time-frame. Seventy-two hr after the first injection (24 hr after the 3rd injection), animals were sacrificed and organs and tissues harvested. It should be noted that euthanasia at a single time point is a limitation of the study in that the kinetics of disposition are unable to be measured.
Collection of Organs
At the time of euthanasia, animals were anesthetized with an intraperitoneal (i.p.) injection of ketamine (70 mg • kg−1) and xylazine (30 mg • kg−1). Two 1-ml samples of blood were obtained from the inferior vena cava and frozen immediately in liquid nitrogen. The total volume of blood was estimated to be 6% of body weight (Lee and Blaufox 1985).
The kidneys, liver, and brain were also removed from each rat. Each sample was placed in a clean microcentrifuge tube, prewashed with trace metal nitric acid. Kidneys were trimmed of fat and fascia, weighed, and cut in half along the mid-traverse plane. The liver was weighed and two 1-g samples were saved for analyses. The brain was also removed, weighed, and saved for analyses. After placing each sample in a tube, the top of the tube was wrapped with lab film, which was subsequently punctured 4 times with a 20-guage needle. Samples were frozen immediately in liquid nitrogen. After collection of all samples was completed, samples were placed in a Labcono FreeZone benchtop freeze dry system (ThermoFisher) for 48 hr. Samples were then pulverized and dry weight of each sample recorded.
Determination of Metal Content
Approximately 0.5 g of lyophilized tissue was digested with 4 ml HNO3, 1 ml H2O2 and 1 ml deionized (DI) water in acid-cleaned Teflon PFA vessels using a 1200 W Ethos microwave digestion system (Milestone Inc.). The acids were high purity (optima grade) from Fisher Scientific and the water was ≥18.2 MΩ from a Milli-Q system (Millipore Corp.). The temperature program consisted of a 20 min ramp to 160°C, a 10 min hold at 160°C, followed by a 5 min ramp to 180°C, and a 15 min hold at 180°C. Digests were diluted to 50 ml with DI water. Then, 1 g diluted solution was transferred to a 15 ml centrifuge tube and made up to 12 g with a 2% (v/v) HNO3 containing 2 ng/g of Rh as the internal standard.
Concentrations of As, Cd, Hg, and Pb were determined using a sector field mass spectrometer (Element-XR; Thermo-Fisher). The sample introduction system consisted of a glass concentric nebulizer outfitted with a glass cyclonic spray chamber. The instrument was tuned prior to analysis for sensitivity and stability, achieving approximately 0.5 million counts per sec and <4% RSD for a 1 ng g−1 solution of 115In in low resolution mode. Instrumental and data acquisition parameters are provided in Table 1. External calibration was used to quantify the elements. Five standards ranging from 0.1 ng g−1 to 20 ng g−1 were prepared in 2% HNO3 using a multi-element standard solution (Spex Certiprep). Linearity (r2 value) for the calibration plots for all isotopes was >0.995. Recoveries for DORM-3 (NRC Canada) reference material were ±15% of certified values.
Table 1.
ICP-MS Instrumental Settings
| Plasma | |
|---|---|
| Cool gas flow | 14 L min−1 |
| Auxiliary gas flow | 1.0 L min−1 |
| Sample gas flow | 0.8 L min−1 |
| RF power | 1260 W |
| Data Acquisition | |
| Isotopes in LR | 111Cd, 202Hg, 208Pb |
| Isotopes in HR | 75As |
| Integration time | 10 ms (LR); 100 ms (HR) |
| Mass window | 20% for LR; 200% for HR |
| Points per peak | 50 |
| Runs/passes | 3/1 |
| Scan type | E-scan |
LR = Low Resolution; HR = High Resolution.
Statistical Analysis
Data were analyzed using the one-way Analysis of Variance (ANOVA) to assess differences among the mean concentrations of each treatment group. When statistically significant F-values were obtained with ANOVA, pairs of means were compared using Tukey’s honestly significant difference (HSD) post hoc multiple comparison test. A p-value of ≤ 0.05 was considered statistically significant, and SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses. Each group of animals contained three rats.
Results
Distribution of Hg in Kidneys, Liver, Blood, and Brain
The concentration of Hg in the kidneys of rats exposed to saline, Hg only, Hg and Cd, or a mixture of Hg, Cd, Pb, and As is presented in Figure 1A. When rats were exposed to a mixture of Hg and Cd, the concentration of Hg in the kidney was reduced significantly. Similarly, when rats were exposed to a mixture of Hg, Cd, Pb, and As, the concentration of Hg in the kidney was also significantly decreased. Interestingly, the concentration of Hg in kidneys of rats exposed to Hg and Cd was not significantly different from that of rats exposed to Hg, Cd, Pb, and As.
Figure 1.
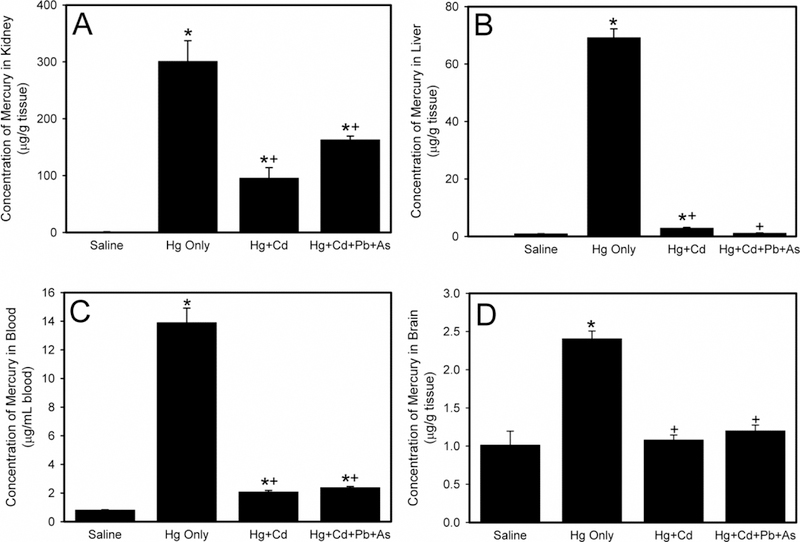
The levels of mercury (Hg) in kidney (A), liver (B), blood (C), and brain (D) following exposure of Wistar rats to saline, Hg only, mercury and cadmium (Hg + Cd), or mercury, cadmium, lead, and arsenic (Hg + Cd + Pb + As). *, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to saline. +, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to Hg only.
Figure 1B shows the concentration of Hg in the liver of rats exposed to saline, Hg only, Hg and Cd, or a mixture of Hg, Cd, Pb, and As. When rats were exposed to Hg and Cd, the amount of Hg in liver was significantly lowered. Interestingly, the amount of Hg in the liver after exposure to Hg, Cd, Pb, and As was significantly reduced compared to rats administered only Hg but was not different from rats treated with to Hg and Cd.
The amount of Hg in blood is depicted in Figure 1C. When rats were exposed to Hg and Cd, the quantity of Hg in blood was significantly decreased compared to blood following exposure to Hg only. When rats were exposed to a mixture of Hg, Cd, Pb, and As, the Hg content in blood was significantly reduced compared to blood following exposure to Hg only. Interestingly, the Hg levels in blood following exposure to the mixture of Hg, Cd, Pb, and As was not significantly different from that following exposure to Hg and Cd.
A similar pattern of Hg distribution was noted in the brain (Figure 1D). Exposure of rats to a mixture of Hg and Cd significantly reduced the amount of Hg in the brain of animals. Similarly, exposure to a mixture of Hg, Cd, Pb, and As led to a significant decrease in the Hg concentration in the brain. The amount of Hg in brain following exposure to Hg and Cd was not significantly different than that following administration of Hg, Cd, Pb, and As mixture. No marked differences in Hg disposition were observed between male and female rats under these conditions.
Distribution of Cd in Kidney, Liver, Blood, and Brain
Figure 2A presents the concentration of Cd in kidneys of rats exposed to saline, Cd only, a mixture of Hg, Cd, Pb, and As, or a mixture of Cd and Hg. When rats were exposed to Cd and Hg, the amount of Cd in the kidney was significantly lower than that in kidneys of rats administered only Cd. Similarly, when rats were treated with a mixture of Cd, Hg, Pb, and As, Cd content in kidneys was significantly less than that in kidneys of rats exposed to Cd only. The amount of Cd in the kidneys of rats exposed to Cd and Hg was not significantly different from that in kidneys of rats administered a mixture of Cd, Hg, Pb, and As.
Figure 2.
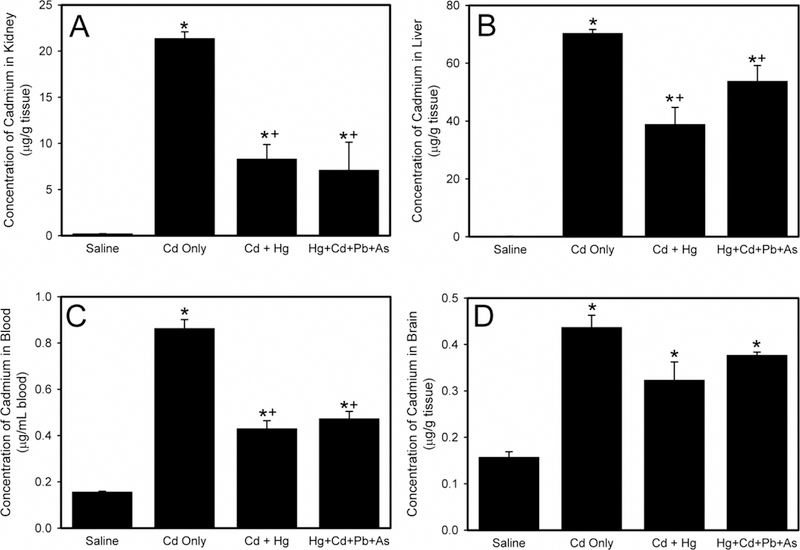
The concentration of cadmium (Cd) in kidney (A), liver (B), blood (C), and brain (D) following exposure of Wistar rats to saline, Cd only, cadmium and mercury (Cd + Hg), or mercury, cadmium, lead, and arsenic (Hg + Cd + Pb + As). *, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to saline. +, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to Cd only.
Figure 2B depicts the hepatic concentration of Cd in rats exposed to saline, Cd only, Cd and Hg, or a mixture of Cd, Hg, Pb, and As. When rats were exposed to a mixture of Cd, Hg, Pb, and As, the Cd concentration in liver was not significantly different than that in liver of rats given only Cd. When rats were treated with Cd and Hg, the amount of Cd in liver was significantly less than that in rats exposed to Cd only.
The Cd levels in blood are shown in Figure 2C. When rats were exposed to Cd and Hg, the quantity of Cd in blood was significantly less than that in blood or rats exposed to Cd only. Similarly, when rats were administered a mixture of Cd, Hg, Pb, and As, the Cd levels in blood were significantly less than that in blood of rats exposed to Cd only. There was no marked difference in Cd content in blood of rats exposed to Cd and Hg and that in blood of rats exposed to a mixture of Cd, Hg, Pb, and As.
The concentration of Cd in brain is shown in Figure 2D. There was no significant difference in the concentration of Cd among the rats exposed to Cd only, Cd and Hg, and the mixture of Cd, Hg, Pb, and As. No marked differences in Cd disposition were observed between male and female rats under these conditions.
Distribution of Pb in Kidney, Liver, Blood, and Brain
The concentration of Pb in the kidney is presented in Figure 3A. When rats were administered Pb and As, the concentration of Pb in kidneys was significantly reduced compared to kidneys after exposure to Pb only. Similarly, when rats were treated with a mixture of Pb, As, Hg, and Cd, the renal Pb levels was significantly lower than that in animals exposed to Pb only. Although it appeared that the amount of Pb in kidneys of rats exposed to Pb and As was greater than that in tissues of rats administered Pb, As, Hg, and Cd, this difference was not significant.
Figure 3.
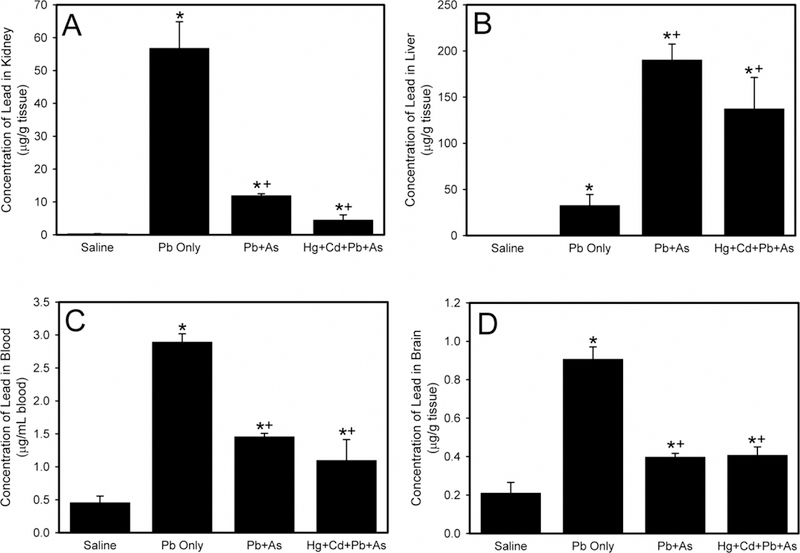
The levels of lead (Pb) in kidney (A), liver (B), blood (C), and brain (D) following exposure of Wistar rats to saline, Pb only, lead and arsenic (Pb + As), or mercury, cadmium, lead, and arsenic (Hg + Cd + Pb + As). *, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to saline. +, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to Pb only.
Data in Figure 3B demonstrate the hepatic concentration of Pb. When rats were exposed to Pb and As, the Pb liver levels was significantly greater than in animals given only Pb. Similarly, when rats were administered a mixture of Pb, As, Hg, and Cd, the accumulation of Pb in the liver was significantly greater compared to Pb only. The hepatic concentration of Pb in rats exposed to Pb, As, Hg, and Cd was not significantly different than levels in rats administered Pb and As.
The concentration of Pb in blood is shown in Figure 3C. When rats were exposed to Pb and As, Pb blood concentration was significantly decreased compared to blood of rats given Pb only. Similarly, when rats were administered Pb, As, Hg, and Cd, the Pb content in blood was significantly lower than in rats given Pb only. There was no significant difference in the concentration of Pb in blood of rats exposed to Pb and As compared to animals rats administered Pb, As, Hg, and Cd.
Data in Figure 3D illustrate concentration of Pb in brain. The brain Pb levels were significantly lower in rats were exposed to Pb and As than in animals administered Pb only. Similarly, the concentrations of Pb in brain of rats treated with a mixture of Pb, As, Hg, and Cd were significantly decreased compared to Pb only. There was no significant difference in Pb content in brain of rats exposed to Pb and As and brains of rats exposed a mixture of Pb, As, Hg, and Cd. No marked differences in Pb disposition were detected between male and female rats under these conditions.
Distribution of As in Kidney, Liver, Blood, and Brain
Figure 4A shows the concentration of As in the kidney. There was no significant difference in renal concentration of As among groups of rats exposed to As only, As and Pb, or a mixture of As, Pb, Cd, and Hg.
Figure 4.
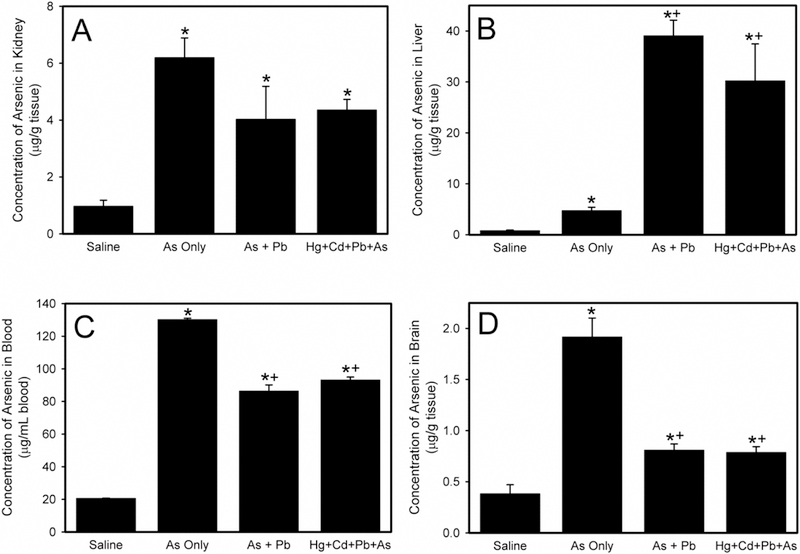
The concentration of arsenic (As) in kidney (A), liver (B), blood (C), and brain (D) following exposure of Wistar rats to saline, As only, As and lead (As + Pb), or mercury, cadmium, lead, and arsenic (Hg + Cd + Pb + As). *, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to saline. +, significantly different (p < 0.05) than the mean of the corresponding group of rats exposed to As only.
The hepatic accumulation of As is presented in Figure 4B. When rats were exposed to As and Pb, the concentration of Pb in liver increased significantly compared to As only. Similarly, when rats were administered As, Pb, Cd, and Hg, the amount of As in liver was significantly greater than that in tissue of animals exposed to As only. The quantity of As in liver of rats exposed to As and Pb was not significantly different from of rats exposed to As, Pb, Cd, and Hg.
Figure 4C shows As blood levels. When rats were exposed to As and Pb, the amount of As in blood was significantly lower compared to blood of rats given As only. Similarly, the concentration of As in blood of rats administered a mixture of As, Pb, Hg, and Cd was significantly diminished compared to As only. The blood As content following exposure to As and Pb was not significantly different from that animals administered As, Pb, Hg, and Cd.
Figure 4D presents the amount of As in brain. In rats exposed to As and Pb, the As levels in brain was significantly decreased compared to As only. Similarly, the amount of As in brain of rats treated with a mixture of As, Pb, Hg, and Cd was significantly lower than in brain of rats exposed to As only. The As concentration in brains of rats exposed to As and Pb was not significantly different from rats given As, Pb, Hg, and Cd. No marked differences in As disposition between male and female rats were observed under these conditions.
Discussion
Understanding how mixtures of toxic metals affect human health is important considering that the environment is contaminated heavily with numerous metals. In addition, humans are exposed to mixtures of these metals on a regular basis. Epidemiological studies showed that exposure to mixtures of metals is more harmful to human health than exposure to a single metal (Wu et al. 2016). Indeed, when organisms are exposed to mixtures of metals, detrimental effects may be observed at concentrations lower than the “no observable effect concentration” (NOEC) (Kortenkamp 2008), suggesting that exposure to a metal within a mixture may be more harmful than exposure to a single metal. While investigators demonstrated that exposure to metal mixtures is harmful (Wu et al. 2016), there is a paucity of data regarding how exposure to a mixture of metals alters the distribution and accumulation of individual metals. Therefore, the current study was designed to determine how exposure to mixtures of toxic heavy metals alters the distribution and accumulation of the individual metals in those mixtures.
The accumulation of Hg in target organs was altered significantly when animals were exposed to a mixture of metals. When rats were exposed to a mixture of Hg and Cd, the amount of Hg in kidneys, liver, blood, and brain was reduced significantly. It is interesting to note that exposure to a mixture of Hg, Cd, Pb, and As yielded dispositional findings that were similar to those following exposure to Hg and Cd. This observation suggests that Pb and As exert minimal influence on the handling and accumulation of Hg in target organs. On the other hand, Cd appears to exert a significant effect on the manner in which Hg is transported by cells of target organs and tissues. Since Hg and Cd typically form complexes with thiol-containing molecules within biological systems, it is likely that the transport mechanisms utilized by these metals are similar to each other and yet, somewhat different from those utilized by Pb and As.
As expected, the majority of Hg accumulation occurred in the kidney. Interestingly, exposure of rats to a mixture of Hg and Cd reduced the renal concentration of Hg by approximately 50%. A possible explanation for this reduction may be related to the expression of export proteins (e.g, multidrug resistance-associated protein (MRP2)) located in the luminal membrane of proximal tubular cells. The expression of MRP2 was found to be upregulated following exposure to xenobiotics and metals, including Hg (Arias et al. 2014; Miller et al. 2007; Vernhet et al. 2001; Aleo et al. 2005) and thus, one would expect co-exposure to Hg and Cd to also enhance the expression of MRP2 in an attempt to transport these metals out of proximal tubular cells for eventual excretion in urine. The multidrug and toxin extrusion proteins (MATE) 1, 2, and 2K may also play roles in this process. Although the MATE carriers have not been shown to mediate the export of mercuric species, these carriers were reported to transport Cd (Yang et al. 2017). The idea that urinary export of Hg and Cd may be enhanced following co-exposure to these metals is supported by the observation that a decrease in the amount of Hg in blood was observed under the same conditions.
In the liver, co-administration of Cd with Hg reduced the accumulation of Hg by approximately 97%. As in renal tissue, reduced accumulation in liver may be due to enhanced expression of export mechanisms, such as MRP2 and MATE, on the canalicular membrane of hepatocytes. It is important to note that the liver appears to be a major site of Cd accumulation (Zalups 1997; Colucci et al 1975; Sabbioni and Marafante 1975), indicating that Cd may easily compete with Hg for uptake via transport mechanisms on the sinusoidal membrane of hepatocytes. Subsequent studies are necessary to characterize fully this phenomenon.
Similarly, when the amount of Cd was measured in organs, data demonstrated that co-administration with Hg significantly altered accumulation of Cd in various organs. As expected, Cd accumulation was greatest in liver. Co-administration of Hg with Cd reduced the accumulation of Cd in liver by approximately 40%. Since this inhibition was not as dramatic as inhibition of Cd on the hepatic uptake of Hg, it was proposed that there are Cd-specific carriers on the sinusoidal membrane of hepatocytes that are not affected by the presence of Hg. These carriers may include the divalent metal transporter (DMT1), zinc-iron-like proteins (ZIP), and/or calcium channels (Zalups and Ahmad 2003). Interestingly, the amount of hepatic Cd following exposure to Cd and Hg was similar to that following administration of Cd, Hg, Pb, and As, suggesting that Pb and As exerted no apparent effect on hepatic handling of Cd. The pattern of accumulation of Cd in kidney, blood, and brain was similar to that of Hg although the quantity of Cd in these tissues was less than that of Hg.
The accumulation of Pb in kidney, blood, and brain was reduced significantly when Pb was co-administered with As, suggesting that As exerted a significant influence on how Pb is handled by cells. In the kidney, the ability of As to decrease accumulation of Pb may be related to the ability of transport proteins on proximal tubular cells to mediate the transport of Pb and As. The multidrug resistance-associated protein 1 (MRP1) is localized on the basolateral membrane of proximal tubular cells (Deeley et al 2006) and Pb and As have been shown to be transportable substrates of this carrier (Carew et al. 2011; Huang et al. 2014). Pb and As may competitively inhibit each other at the site of this carrier, thereby reducing overall uptake of these metals into proximal tubular cells. Indeed, renal accumulation of As was reduced when As was co-administered with Pb. The lack of renal uptake might potentially enhance the hematological burden of Pb and As. The use of a single endpoint in the current study limits the ability to make definitive conclusions regarding the hematologic content of Pb and As. However, the current findings indicate that the hematological burden of Pb was not elevated under the current conditions; therefore, it is conceivable that there is a transient increase in Pb and As in blood followed by a period of rapid uptake at the sinusoidal membrane of hepatocytes. This uptake may occur via MRP1, Ca2+ channels, or another yet unidentified transporter. This idea is supported by the finding that co-administration of Pb and As led to enhanced accumulation of these metals in liver.
It is also important to consider that co-exposure to Pb and As enhances uptake of these metals in the liver. Exposure to As was found to stimulate the opening of L-type voltage gated calcium channels (Pachauri et al. 2013; Rana et al 2018) and these calcium channels have been shown to mediate the transport of Pb into cells (Zhang et al. 2013; Bridges and Zalups 2005). Therefore, exposure of cells to As may lead to more frequent opening of calcium channels, which would facilitate entry of Pb. Therefore, co-exposure of Pb and As may lead to enhanced hepatic accumulation of these metals through this mechanism.
Hg and Cd did not appear to exert a significant effect on renal handling of Pb or As since renal accumulation was not markedly altered further following co-administration of Pb or As with Hg and Cd. As mentioned previously, this finding is likely due to differences in the transport mechanisms responsible for the uptake and export of these metals at the cellular level.
In summary, current data demonstrate that exposure to mixtures of metals altered the disposition of individual metals within the mixture. In some cases, uptake and accumulation was diminished, while in other cases, exposure to a mixture may enhance the accumulation of a particular metal. These findings offer preliminary insight into the mechanisms by which mixtures enhance adverse effects in exposed individuals. More detailed studies are necessary to determine the specific role of individual transport proteins in the toxicity of metal mixtures. Collectively, findings from these studies and others may provide valuable contributions to the body of literature that is used by regulatory agencies to develop guidelines for exposure to toxic heavy metals.
Acknowledgments
Funding Statement
This work was supported by the National Institutes of Health (ES019991) and a grant from the Navicent Health Foundation.
Footnotes
Disclosure Statement
The authors have nothing to disclose.
References
- Aleo MF, Morandini F, Bettoni F, Giuliani R, Rovetta F, Steimberg N, Apostoli P, Parrinello G, and Mazzoleni G. 2005. Endogenous thiols and MRP transporters contribute to Hg2+ efflux in HgCl2-treated tubular MDCK cells. Toxicology 206:137–151. [DOI] [PubMed] [Google Scholar]
- Arias A, Rigalli JP, Villanueva SS, Ruiz ML, Luquita MG, Perdomo VG, Vore M, Catania VA, and Mottino AD. 2014. Regulation of expression and activity of multidrug resistance proteins MRP2 and MDR1 by estrogenic compounds in Caco-2 cells. Role in prevention of xenobiotic-induced cytotoxicity. Toxicology 320:46–55. [DOI] [PubMed] [Google Scholar]
- Aschner M, and Syversen T. 2005. Methylmercury: Recent advances in the understanding of its neurotoxicity. Ther Drug Monit 27: 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. 2018. Substance Priority List U. S. Departmen of Health and Human Services, 9-25-17 2017 [cited 11-6-18 2018]. Available from https://www.atsdr.cdc.gov/spl/. [Google Scholar]
- ATSDR, Agency for Toxic Substance and Disease Registry. 2008. Toxicological Profile for Cadmium. In U.S. Department of Health and Human Services, Public Health Service: Centers for Disease Control. [Google Scholar]
- ATSDR, Agency for Toxic Substances and Disease Registry. 2007. Arsenic: Relevance to Public Health, edited by D. o. H. a. H. Services Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- ATSDR, Agency for Toxic Substances and Disease Registry.. 2007. Public Health Statement: Lead Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Barbosa F Jr. 2017. Toxicology of metals and metalloids: promising issues for future studies in environmental health and toxicology. J Toxicol Environ Health A 80: 137–144. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, and Zalups RK. 2008a. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci 105: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, and Zalups RK. 2008b. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther 324: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, and Zalups RK. 2014. Aging and the disposition and toxicity of mercury in rats. Exp Gerontol 53:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, and Zalups RK. 2005. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 204: 274–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, and Zalups RK. 2010. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B 13: 385–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco V, Caito S, Farina M, da Rocha JT, Aschner M and Carvalho C. 2017. Biomarkers of mercury toxicity: Past, present and future trends. J Toxicol Environ Health B 20: 119–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew MW, Naranmandura H, Shukalek CB, Le XC, and Leslie EM. 2011. Monomethylarsenic diglutathione transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Drug Metab Dispos 39: 2298–2304. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, and Magos L. 2006. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36: 609–662. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Coull BA, and Wright RO. 2014. Chemical mixtures and children’s health. Curr Opin Pediatr 26: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci AV, Winge D, and Krasno J. 1975. Cadmium accumulation in rat liver. Arch Environ Health 30: 153–157. [DOI] [PubMed] [Google Scholar]
- Cui X, Kobayashi Y, Hayakawa T, and Hirano S. 2004. Arsenic speciation in bile and urine following oral and intravenous exposure to inorganic and organic arsenics in rats. Toxicol Sci 82: 478–487. [DOI] [PubMed] [Google Scholar]
- Czykier E, Moniuszko-Jakoniuk J, and Sawicki B. 2004. Effect of acute exposure to cadmium on the expression of calcitonin gene-related peptide (CGRP), calcitonin (CT), somatostatin (SST) and synaptophysin (SYN) in the C cells of the rat thyroid--a preliminary study. Folia Morphol (Warsz) 63: 217–219. [PubMed] [Google Scholar]
- Dalley JW, Gupta PK, Lam FC, and Hung CT. 1989. Interaction of L-ascorbic acid on the disposition of lead in rats. Pharmacol Toxicol 64: 360–364. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, and Cole SP. 2006. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86: 849–899. [DOI] [PubMed] [Google Scholar]
- Hagopian-Schlekat T, Chandler GT, and Shaw TJ. 2001. Acute toxicity of five sediment-associated metals, individually and in a mixture, to the estuarine meiobenthic harpacticoid copepod Amphiascus tenuiremis. Mar Environ Res 51: 247–264. [DOI] [PubMed] [Google Scholar]
- Huang S, Ye J, Yu J, Chen L, Zhou L, Wang H, Li Z, and Wang C. 2014. The accumulation and efflux of lead partly depend on ATP-dependent efflux pump-multidrug resistance protein 1 and glutathione in testis Sertoli cells. Toxicol Lett 226: 277–284. [DOI] [PubMed] [Google Scholar]
- Huang Y, He C, Shen C, Guo J, Mubeen S, Yuan J, and Yang Z. 2017. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct 8: 1373–1401. [DOI] [PubMed] [Google Scholar]
- Jennette KW 1981. The role of metals in carcinogenesis: biochemistry and metabolism. Environ Health Perspect 40: 233–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Cui X, and Hirano S. 2005. Stability of arsenic metabolites, arsenic triglutathione [As(GS)3] and methylarsenic diglutathione [CH3As(GS)2], in rat bile. Toxicology 211:115–123. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A 2008. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int J Androl 31: 233–240. [DOI] [PubMed] [Google Scholar]
- Laidlaw MA, Filippelli GM, Sadler RC, Gonzales CR, Ball AS, and Mielke HW. 2016. Children’s blood lead seasonality in Flint, Michigan (USA), and soil-sourced lead hazard risks. Int J Environ Res Public Health 13: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, and Blaufox MD. 1985. Blood volume in the rat. J Nucl Med 26: 72–76. [PubMed] [Google Scholar]
- Lin X, Gu Y, Zhou Q, Mao G, Zou B, and Zhao J. 2016. Combined toxicity of heavy metal mixtures in liver cells. J Appl Toxicol 36:1163–1172. [DOI] [PubMed] [Google Scholar]
- Lynch NR, Hoang TC, and O’Brien TE. 2016. Acute toxicity of binary-metal mixtures of copper, zinc, and nickel to Pimephales promelas: Evidence of more-than-additive effect. Environ Toxicol Chem 35: 446–457. [DOI] [PubMed] [Google Scholar]
- Miller DS, Shaw JR, Stanton CR, Barnaby R, Karlson KH, Hamilton JW, and Stanton BA. 2007. MRP2 and acquired tolerance to inorganic arsenic in the kidney of killifish (Fundulus heteroclitus). Toxicol Sci 97:103–110. [DOI] [PubMed] [Google Scholar]
- Montvydiene D, and Marciulioniene D. 2004. Assessment of toxic interactions of heavy metals in a multicomponent mixture using Lepidium sativum and Spirodela polyrrhiza. Environ Toxicol 19:351–358. [DOI] [PubMed] [Google Scholar]
- Muthusamy S, Peng C, and Ng JC. 2016. Effects of binary mixtures of benzo[a]pyrene, arsenic, cadmium, and lead on oxidative stress and toxicity in HepG2 cells. Chemosphere 165:41–51. [DOI] [PubMed] [Google Scholar]
- Oliveira CS, Joshee L, Zalups RK, and Bridges CC. 2016. Compensatory renal hypertrophy and the handling of an acute nephrotoxicant in a model of aging. Exp Gerontol 75:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachauri V, Mehta A, Mishra D, and Flora SJ. 2013. Arsenic induced neuronal apoptosis in guinea pigs is Ca2+ dependent and abrogated by chelation therapy: Role of voltage gated calcium channels. Neurotoxicology 35:137–145. [DOI] [PubMed] [Google Scholar]
- Puri VN, and Saha S. 2003. Comparison of acute cardiovascular effects of cadmium and captopril in relation to oxidant and angiotensin converting enzyme activity in rats. Drug Chem Toxicol 26:213–218. [DOI] [PubMed] [Google Scholar]
- Rana MN, Tangpong J, and Rahman MM. 2018. Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: A mini review. Toxicol Rep 5: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenbeck BA, Banerjee M, and Leslie EM. 2016. Cellular arsenic transport pathways in mammals. J Environ Sci (China) 49: 38–58. [DOI] [PubMed] [Google Scholar]
- Sabbioni E, and Marafante E. 1975. Accumulation of cadmium in rat liver cadmium binding protein following single and repeated cadmium administration. Environ Physiol Biochem 5: 465–473. [PubMed] [Google Scholar]
- Serrazina DC, de Andrade VL, Cota M, Mateus ML, Aschner M and dos Santos APM.2018. Biomarkers of exposure and effect in a working population exposed to lead, manganese and arsenic. J Toxicol Environ Health A 81: 983–997. [DOI] [PubMed] [Google Scholar]
- Shim YK, Lewin MD, Ruiz P, Eichner JE, and Mumtaz MM. 2017. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: The United States NHANES, 2007–2012. J Toxicol Environ Health A 80: 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic-Keser M, Stankovic D, Zulic I, and Duricic Z. 1982. [The effect of the single intravenous administration of varying doses of lead acetate on renal function]. Med Arh 36: 3–8. [PubMed] [Google Scholar]
- Tchounwou PB, Patlolla AK, and Centeno JA. 2003. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol Pathol 31:575–588. [DOI] [PubMed] [Google Scholar]
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ 2012. Heavy metals toxicity and the environment. EXS 101:122–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernhet L, Seite MP, Allain N, Guillouzo A, and Fardel O. 2001. Arsenic induces expression of the multidrug resistance-associated protein 2 (MRP2) gene in primary rat and human hepatocytes. J Pharmacol Exp Ther 298: 234–239. [PubMed] [Google Scholar]
- von Stackelberg K, Guzy E, Chu T, and Claus Henn B. 2015. Exposure to mixtures of metals and neurodevelopmental outcomes: A multidisciplinary review using an adverse outcome pathway framework. Risk Anal 35: 971–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, and Park SK. 2018. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int 121: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Ten Chemicals of Major Public Health Concern World Heath Organization; 2018. [cited 07/23/2018. Available from http://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/. [Google Scholar]
- Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, and Yang L. 2016. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int 23: 8244–8259. [DOI] [PubMed] [Google Scholar]
- Yang H, Guo D, Obianom ON, Su T, Polli JE, and Shu Y. 2017. Multidrug and toxin extrusion proteins mediate cellular transport of cadmium. Toxicol Appl Pharmacol 314: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK 1997. Influence of different degrees of reduced renal mass on the renal and hepatic disposition of administered cadmium. J Toxicol Environ Health 51: 245–264. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Ahmad S. 2003. Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol 186:163–188. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cao H, Zhang Y, Ma J, Wang J, Gao Y, Zhang X, Zhang F, and Chu L. 2013. Nephroprotective effect of calcium channel blockers against toxicity of lead exposure in mice. Toxicol Lett 218: 273–280. [DOI] [PubMed] [Google Scholar]


