Abstract
Chronic obstructive pulmonary disease (COPD) is the leading cause of respiratory mortality worldwide. Genetic risk loci provide novel insights into disease pathogenesis. We performed a genome-wide association study in 35,735 cases and 222,076 controls from the UK Biobank and additional studies from the International COPD Genetics Consortium. We identified 82 loci with P-value < 5 × 10−8; 47 were previously described in association with either COPD or population-based lung function. Of the remaining 35 novel loci, 13 were associated with lung function in 79,055 individuals from the SpiroMeta consortium. Using gene expression and regulation data, we identified enrichment for loci in lung tissue, smooth muscle and several lung cell types. We found 14 COPD loci shared with either asthma or pulmonary fibrosis. COPD genetic risk loci clustered into groups of quantitative imaging features and comorbidity associations. Our analyses provide further support to the genetic susceptibility and heterogeneity of COPD.
Editorial summary
Genome-wide analysis of chronic obstructive pulmonary disease identifies 82 loci, 35 of which are new. Integration of gene expression and genomic annotation data shows enrichment of signals in lung tissue, smooth muscle and several lung cell types.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease of enormous and growing global burden1, ranked third as a global cause of death by the World Health Organization in 20162. Environmental risk factors, predominately cigarette smoking, account for a large fraction of disease risk, but there is considerable variability in COPD susceptibility among individuals with similar smoking exposure. Studies in families and in populations demonstrate that genetic factors account for a substantial fraction of disease susceptibility. Similar to other adult-onset complex diseases, common variants likely account for the majority of population genetic susceptibility3,4. Our previous efforts identified 22 genome-wide significant loci5. Expanding the number of loci can lead to novel insights into disease pathogenesis, not only through discovery of novel biology at individual loci6,7, but also across loci via identification functional links and specific cell types and phenotypes5.
We performed a genome-wide association study combining previously described studies from the International COPD Genetics Consortium (ICGC)5 with additional subjects from the UK Biobank8, a population-based study of several hundred thousand subjects with lung function and cigarette smoking assessment. We determined, through bioinformatic and computational analysis, the likely set of variants, genes, cell types, and biologic pathways implicated by these associations. Finally, we assessed our genetic findings for relevance to COPD-specific, respiratory, and other phenotypes.
Results
Genome-wide association study of COPD
We included a total of 257,811 individuals from 25 studies in the analysis, including studies from International COPD Genetics Consortium and UK Biobank (Figure 1). We defined COPD based on pre-bronchodilator spirometry according to modified Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for moderate to very severe airflow limitation9, as done previously5. This definition resulted in 35,735 cases and 222,076 controls (Supplementary Table 1). We tested association of COPD and 6,224,355 variants in a meta-analysis of 25 studies using a fixed-effects model. We found no evidence of confounding by population substructure using linkage disequilibrium score regression10 (LDSC) intercept (1.0377, s.e. 0.0094).
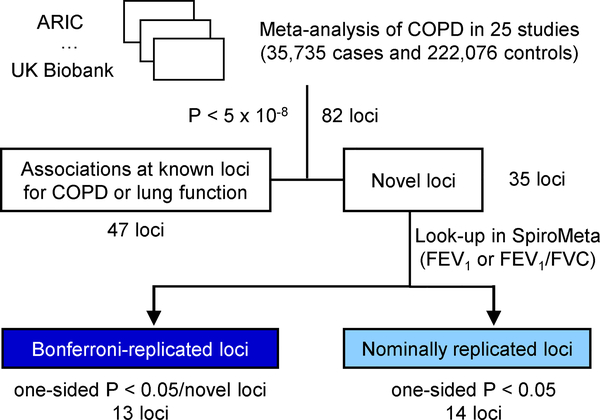
Figure 1. Study design.
COPD, chronic obstructive pulmonary disease; FEV1, force expiratory volume in one second; FVC, forced vital capacity. ARIC, Atherosclerosis Risk in Communities.
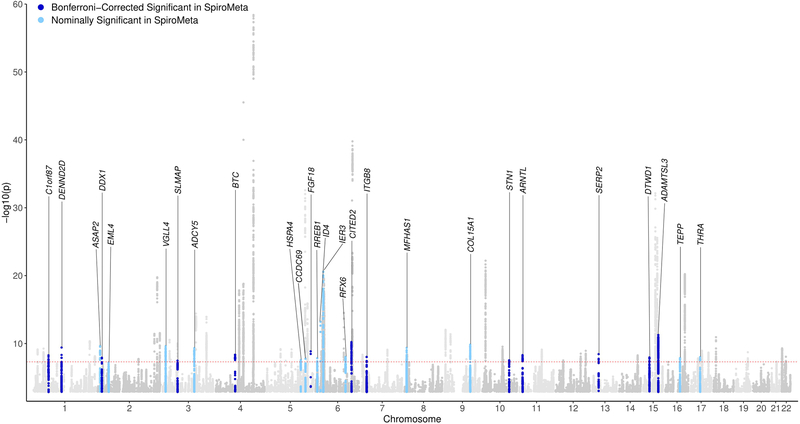
We identified 82 loci (defined using 2-Mb windows) at genome-wide significance (P < 5 × 10−8) (Figure 1 and 2; Supplementary Figures 1 and 2). Forty-seven of 82 loci were previously described as genome-wide significant in COPD5,11 or lung function12–20 (Supplementary Table 2), leaving 35 novel loci (Table 1) at the time of analysis. We then sought to replicate these loci. Given the strong genetic correlation between population-based lung function and COPD, we tested the lead variant at each locus for association with forced expiratory volume in 1 s (FEV1)or FEV1/forced vital capacity (FVC) in 79,055 individuals from SpiroMeta21 (Supplementary Table 3). We identified 13 loci - C1orf87, DENND2D, DDX1, SLMAP, BTC, FGF18, CITED2, ITGB8, STN1, ARNTL, SERP2, DTWD1, and ADAMTSL3 – that replicated using a Bonferroni correction for a one-sided P < 0.05/35; Table 1). Although not meeting the strict Bonferroni threshold, additional 14 novel loci were nominally significant in SpiroMeta (consistent direction of effect and one-sided P < 0.05): ASAP2, EML4, VGLL4, ADCY5, HSPA4, CCDC69, RREB1, ID4, IER3, RFX6, MFHAS1, COL15A1, TEPP, and THRA (Table 1), and all 82 loci showed consistent direction of effect with either FEV1 or FEV1/FVC ratio in SpiroMeta (Table 1 and Supplementary Table 2). We note that 9 of our 35 novel loci were recently described in a contemporaneous analysis of lung function in UK Biobank21. None of the novel loci appeared to be explained by cigarette smoking, and variant effect sizes in ever- and never-smokers and including and excluding self-reported asthmatics were similar (Supplementary Note). In addition, we found no significant differences in variant effects by sex (Supplementary Note). Including all 82 genome-wide significant variants, we explain up to 7.0% of the phenotypic variance in liability scale, using a 10% prevalence of COPD, acknowledging that these effects are likely overestimated in the discovery sample. This represents up to a 48% increase in COPD phenotypic variance explained by genetic loci compared to the 4.7% explained by 22 loci reported in a recent GWAS of COPD5.
Figure 2. Manhattan plot.
P-values are two-sided based on Wald statistics (35,735 cases and 222,076 controls) without multiple comparison adjustment. Loci are labeled with the closest gene to the lead variant.
Table 1.
Meta-analysis results showing 35 loci novel for COPD and lung function
| rsID | HGVS name | Closest gene | Locus | Risk/alt. allele | Risk allele frequency | UK Biobank | ICGC Cohorts | Overall meta-analysis | SpiroMeta FEV1 | SpiroMeta FEV1/FVC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | Beta | P value | Beta | P value | ||||||
| rs72673419 | NC_000001.10:g.60913143C>T | C1orf87 | 1p32.1 | T/C | 0.05 | 1.13 | 1.07–1.18 | 2.0E-06 | 1.19 | 1.08–1.31 | 2.9E-04 | 1.14 | 1.09–1.19 | 4.0E-09 | −0.02 | 1.3E-01 | −0.05 | 3.0E-04 |
| rs629619 | NC_000001.10:g.111738108C>T | DENND2D | 1p13.3 | T/C | 0.20 | 1.08 | 1.05–1.11 | 7.5E-08 | 1.10 | 1.04–1.16 | 8.2E-04 | 1.08 | 1.06–1.11 | 2.9E-10 | −0.01 | 4.6E-01 | −0.02 | 9.3E-04 |
| rs10929386 | NC_000002.11:g.15906179C>T | DDX1 | 2p24.3 | C/T | 0.49 | 1.06 | 1.03–1.08 | 1.2E-06 | 1.07 | 1.03–1.12 | 1.8E-03 | 1.06 | 1.04–1.08 | 9.1E-09 | −0.02 | 3.1E-06 | −0.02 | 7.1E-06 |
| rs62259026 | NC_000003.11:g.57746515C>T | SLMAP | 3p14.3 | C/T | 0.75 | 1.07 | 1.04–1.09 | 1.8E-06 | 1.07 | 1.02–1.12 | 3.8E-03 | 1.07 | 1.04–1.09 | 2.4E-08 | −0.03 | 3.3E-05 | −0.01 | 6.4E-02 |
| rs4585380 | NC_000004.11:g.75673363G>A | BTC | 4q13.3 | G/A | 0.74 | 1.07 | 1.04–1.10 | 1.2E-07 | 1.06 | 1.02–1.11 | 8.1E-03 | 1.07 | 1.05–1.09 | 3.4E-09 | −0.02 | 3.7E-04 | −0.02 | 1.3E-03 |
| rs12519165 | NC_000005.9:g.170901586A>T | FGF18 | 5q35.1 | A/T | 0.38 | 1.08 | 1.05–1.10 | 2.7E-10 | 1.02 | 0.97–1.08 | 4.0E-01 | 1.07 | 1.05–1.09 | 1.1E-09 | −0.02 | 6.5E-03 | −0.03 | 2.4E-07 |
| rs646695 | NC_000006.11:g.140280398T>C | CITED2 | 6q24.1 | C/T | 0.24 | 1.07 | 1.05–1.10 | 3.0E-08 | 1.09 | 1.04–1.14 | 3.4E-04 | 1.08 | 1.05–1.10 | 4.6E-11 | −0.02 | 5.6E-04 | 0.00 | 7.5E-01 |
| rs2040732 | NC_000007.13:g.20418134C>T | ITGB8 | 7p21.1 | C/T | 0.58 | 1.07 | 1.05–1.09 | 5.2E-09 | 1.03 | 0.99–1.07 | 1.7E-01 | 1.06 | 1.04–1.08 | 6.9E-09 | −0.02 | 1.8E-03 | −0.01 | 1.0E-02 |
| rs1570221 | NC_000010.10:g.105656874G>A | STN1 | 10q24.33 | A/G | 0.35 | 1.06 | 1.03–1.08 | 3.5E-06 | 1.07 | 1.03–1.12 | 1.4E-03 | 1.06 | 1.04–1.08 | 2.2E-08 | −0.02 | 9.5E-04 | −0.01 | 3.9E-02 |
| rs4757118 | NC_000011.9:g.13171236C>T | ARNTL | 11p15.2 | T/C | 0.54 | 1.07 | 1.04–1.09 | 1.1E-08 | 1.04 | 1.00–1.08 | 7.3E-02 | 1.06 | 1.04–1.08 | 3.8E-09 | −0.01 | 2.8E-01 | −0.02 | 2.2E-03 |
| rs9525927 | NC_000013.10:g.44842503G>A | SERP2 | 13q14.11 | G/A | 0.19 | 1.07 | 1.04–1.10 | 2.9E-06 | 1.10 | 1.05–1.16 | 1.4E-04 | 1.08 | 1.05–1.10 | 2.8E-09 | −0.02 | 2.2E-02 | −0.02 | 2.2E-03 |
| rs72731149 | NC_000015.9:g.49984710G>C | DTWD1 | 15q21.2 | G/C | 0.91 | 1.12 | 1.07–1.17 | 8.9E-07 | 1.12 | 1.04–1.20 | 2.6E-03 | 1.12 | 1.08–1.16 | 8.3E-09 | −0.05 | 7.9E-07 | −0.03 | 9.0E-04 |
| rs10152300 | NC_000015.9:g.84392907G>A | ADAMTSL3 | 15q25.2 | G/A | 0.23 | 1.09 | 1.06–1.11 | 2.4E-10 | 1.08 | 1.02–1.13 | 4.7E-03 | 1.08 | 1.06–1.11 | 4.2E-12 | −0.01 | 6.4E-02 | −0.02 | 1.5E-03 |
| rs955277 | NC_000002.11:g.9290357C>T | ASAP2 | 2p25.1 | T/C | 0.61 | 1.08 | 1.05–1.10 | 2.7E-10 | 1.04 | 0.99–1.08 | 8.6E-02 | 1.07 | 1.05–1.09 | 1.9E-10 | 0.00 | 9.6E-01 | −0.01 | 3.3E-02 |
| rs12466981 | NC_000002.11:g.42433247C>T | EML4 | 2p21 | C/T | 0.73 | 1.06 | 1.03–1.08 | 8.1E-06 | 1.08 | 1.03–1.13 | 1.2E-03 | 1.06 | 1.04–1.09 | 4.9E-08 | −0.01 | 4.4E-02 | −0.01 | 6.4E-02 |
| rs2442776 | NC_000003.11:g.11640601G>A | VGLL4 | 3p25.3 | G/A | 0.15 | 1.09 | 1.06–1.13 | 5.7E-08 | 1.10 | 1.04–1.16 | 8.9E-04 | 1.09 | 1.06–1.12 | 2.0E-10 | −0.01 | 6.4E-02 | −0.01 | 1.3E-01 |
| rs4093840 | NC_000003.11:g.123077042T>A | ADCY5 | 3q21.1 | A/T | 0.47 | 1.07 | 1.05–1.09 | 1.6E-09 | 1.04 | 1.00–1.09 | 4.8E-02 | 1.06 | 1.04–1.08 | 3.9E-10 | −0.01 | 8.5E-02 | −0.00 | 3.8E-01 |
| rs62375246 | NC_000005.9:g.132439010T>A | HSPA4 | 5q31.1 | A/T | 0.26 | 1.08 | 1.05–1.10 | 9.6E-09 | 1.03 | 0.98–1.08 | 2.7E-01 | 1.06 | 1.04–1.09 | 2.2E-08 | −0.02 | 1.3E-02 | −0.01 | 2.2E-01 |
| rs979453 | NC_000005.9:g.150595073A>G | CCDC69 | 5q33.1 | G/A | 0.34 | 1.07 | 1.05–1.10 | 1.6E-09 | 1.02 | 0.97–1.06 | 5.1E-01 | 1.06 | 1.04–1.08 | 1.4E-08 | −0.01 | 7.2E-02 | −0.00 | 6.4E-01 |
| rs1334576 | NC_000006.11:g.7211818G>A | RREB1 | 6p24.3 | A/G | 0.42 | 1.07 | 1.05–1.09 | 4.2E-09 | 1.02 | 0.99–1.06 | 2.2E-01 | 1.06 | 1.04–1.08 | 1.2E-08 | 0.00 | 6.9E-01 | −0.01 | 2.9E-02 |
| rs9350191 | NC_000006.11:g.19842661C>T | ID4 | 6p22.3 | T/C | 0.85 | 1.11 | 1.08–1.15 | 6.2E-12 | 1.14 | 1.05–1.23 | 1.9E-03 | 1.12 | 1.09–1.15 | 5.1E-14 | −0.02 | 4.9E-02 | −0.01 | 1.5E-01 |
| rs2284174 | NC_000006.11:g.30713580T>C | IER3 | 6p21.33 | C/T | 0.22 | 1.12 | 1.10–1.15 | 3.4E-19 | 1.12 | 1.05–1.21 | 1.5E-03 | 1.12 | 1.10–1.15 | 2.1E-21 | −0.01 | 5.2E-02 | −0.02 | 3.0E-02 |
| rs674621 | NC_000006.11:g.117257018T>C | RFX6 | 6q22.1 | C/T | 0.32 | 1.06 | 1.03–1.08 | 1.3E-06 | 1.07 | 1.03–1.12 | 1.4E-03 | 1.06 | 1.04–1.08 | 7.6E-09 | −0.01 | 3.6E-02 | −0.01 | 6.9E-02 |
| rs9329170 | NC_000008.10:g.8697658C>G | MFHAS1 | 8p23.1 | C/G | 0.86 | 1.10 | 1.06–1.14 | 2.0E-08 | 1.09 | 1.03–1.15 | 4.9E-03 | 1.10 | 1.07–1.13 | 3.6E-10 | −0.02 | 4.6E-02 | −0.01 | 6.5E-02 |
| rs10760580 | NC_000009.11:g.101661650G>A | COL15A1 | 9q22.33 | G/A | 0.71 | 1.08 | 1.06–1.11 | 1.6E-10 | 1.04 | 0.99–1.09 | 1.1E-01 | 1.07 | 1.05–1.10 | 1.2E-10 | −0.02 | 1.5E-02 | −0.02 | 1.4E-02 |
| rs8044657 | NC_000016.9:g.58022625G>A | TEPP | 16q21 | G/A | 0.90 | 1.12 | 1.07–1.16 | 1.7E-07 | 1.09 | 1.01–1.17 | 1.8E-02 | 1.11 | 1.07–1.15 | 1.1E-08 | −0.02 | 9.9E-02 | −0.02 | 2.8E-02 |
| rs62065216 | NC_000017.10:g.38218773G>A | THRA | 17q21.1 | A/G | 0.42 | 1.06 | 1.04–1.09 | 1.2E-07 | 1.06 | 1.01–1.10 | 2.2E-02 | 1.06 | 1.04–1.08 | 8.1E-09 | −0.01 | 7.8E-02 | −0.00 | 8.0E-01 |
| rs4660861 | NC_000001.10:g.45946636G>T | TESK2 | 1p34.1 | G/T | 0.57 | 1.05 | 1.03–1.08 | 7.2E-06 | 1.07 | 1.03–1.12 | 1.2E-03 | 1.06 | 1.04–1.08 | 4.4E-08 | −0.01 | 3.0E-01 | −0.00 | 4.1E-01 |
| rs7650602 | NC_000003.11:g.141147414T>C | ZBTB38 | 3q23 | C/T | 0.45 | 1.07 | 1.04–1.09 | 6.7E-09 | 1.01 | 0.97–1.06 | 5.1E-01 | 1.06 | 1.04–1.08 | 4.9E-08 | 0.00 | 8.4E-01 | −0.01 | 3.2E-01 |
| rs34651 | NC_000005.9:g.72144005C>T | TNPO1 | 5q13.2 | C/T | 0.08 | 1.10 | 1.06–1.15 | 7.5E-07 | 1.12 | 1.02–1.22 | 1.2E-02 | 1.11 | 1.07–1.15 | 3.0E-08 | −0.00 | 7.6E-01 | 0.00 | 7.5E-01 |
| rs798565 | NC_000007.13:g.2752152G>A | AMZ1 | 7p22.3 | G/A | 0.71 | 1.09 | 1.06–1.11 | 2.8E-11 | 1.00 | 0.95–1.05 | 8.8E-01 | 1.07 | 1.04–1.09 | 3.9E-09 | −0.01 | 1.2E-01 | −0.00 | 6.1E-01 |
| rs7866939 | NC_000009.11:g.85126163T>C | RASEF | 9q21.32 | C/T | 0.33 | 1.06 | 1.03–1.08 | 1.3E-06 | 1.07 | 1.02–1.12 | 3.8E-03 | 1.06 | 1.04–1.08 | 1.7E-08 | −0.01 | 2.5E-01 | −0.01 | 1.1E-01 |
| rs7958945 | NC_000012.11:g.115947901A>G | MED13L | 12q24.21 | G/A | 0.36 | 1.06 | 1.04–1.09 | 1.0E-07 | 1.07 | 1.02–1.11 | 2.8E-03 | 1.06 | 1.04–1.09 | 1.0E-09 | −0.01 | 1.5E-01 | −0.00 | 6.2E-01 |
| rs72626215 | NC_000019.9:g.46294136G>A | DMWD | 19q13.32 | G/A | 0.73 | 1.06 | 1.04–1.09 | 1.7E-06 | 1.11 | 1.05–1.17 | 7.3E-05 | 1.07 | 1.05–1.10 | 1.7E-09 | 0.00 | 4.8E-01 | −0.01 | 1.2E-01 |
| rs73158393 | NC_000022.10:g.33335386C>G | SYN3 | 22q12.3 | C/G | 0.74 | 1.06 | 1.04–1.09 | 1.1E-06 | 1.09 | 1.03–1.14 | 1.4E-03 | 1.07 | 1.05–1.09 | 7.7E-09 | −0.00 | 7.7E-01 | −0.01 | 3.3E-01 |
CI, confidence interval; OR, odds ratio; ICGC, International COPD Genetics Consortium; HGVS, Human Genome Variation Society. This table shows association statistics in UK Biobank (21,081 cases and 179,711 controls), ICGC cohorts (14,654 cases and 42,365 controls), the overall meta-analysis (35,735 cases and 222,076 controls), and SpiroMeta studies (FEV1 and FEV1/FVC; n=79,055). P values are two-sided based on Wald statistics (COPD) and t statistics (FEV1 and FEV1/FVC) without multiple comparison adjustment. White = Significant in SpiroMeta using Bonferroni correction for novel loci (one-sided P < 0.05/35); Grey = Nominally significant in SpiroMeta (one-sided P < 0.05), Dark grey = not significant (directionally consistent only)
Identification of secondary association signals
We used approximate conditional and joint analysis22 to find secondary signals at each of the 82 genome-wide significant loci. We found 82 secondary signals at 50 loci, resulting in a total of 164 independent associations in 82 loci (Supplementary Table 4). Of 50 loci containing secondary associations, 33 were at loci previously described for COPD or lung function, and six at Bonferroni-replicated novel loci. Of 82 secondary associations, 20 reached genome-wide significance (P < 5 × 10−8) (Supplementary Table 4). Of 61 novel (not previously described in COPD or lung function) independent associations, 21 reached a region-wise Bonferroni-corrected threshold (one-sided P < 0.05/novel independent association(s) in each locus) in unconditioned associations from SpiroMeta (Methods and Supplementary Table 4).
Tissue and specific cell types
In determining the tissue in which COPD genetic variants function to increase COPD risk, lung is the obvious tissue to consider. However, COPD is a systemic disease23,24 and within the lung the cell-types collectively contributing to disease pathogenesis are largely unknown. Furthermore, available databases include cell types relevant to lung (e.g. smooth muscle) but from other organs (e.g. the gastrointestinal tract). To identify putative causal tissues and cell types, we assessed the heritability enrichment in integrated genome annotations at the single tissue level25 and tissue-specific epigenomic marks26. Lung tissue showed the most significant enrichment (enrichment = 9.25, P = 1.36 × 10−9), as previously described, though significant enrichment was also seen in heart (enrichment = 6.85, P = 3.83 × 10−8) and the gastrointestinal (GI) tract (enrichment = 5.53, P = 6.45 × 10−11). In an analysis of enriched epigenomic marks, the most significant enrichment was in fetal lung and GI smooth muscle DNase hypersensitivity sites (DHS) (P = 6.75 × 10−8) and H3K4me1 (P = 7.31 × 10−7) (Supplementary Table 5). To identify the source of association within lung tissue, we tested for heritability enrichment using single-cell chromatin accessibility27 (ATAC-Seq) and gene expression (RNA-Seq) from human28,29 and murine30 lung (Supplementary Table 5). Using LD score regression in murine ATAC-Seq data, we found enrichment of chromatin accessibility in several cell types, including endothelial cells (most significant), type 1, and type 2 alveolar cells (the latter among the highest fold-enrichment [Supplementary Table 5a]). Results using LD score regression31 or SNPsea32 on single-cell RNA-Seq varied, with nominal P-values for genes expressed in type 2 alveolar cells, basal-like cells, club cells, fibroblasts and smooth muscle cells (Supplementary Tables 5b,c).
Fine-mapping of associated loci
To identify the most likely causal variants at each locus, we performed fine mapping using Bayesian credible sets33. Including 160 potential primary and secondary association signals (excluding four variants in the major histocompatibility complex [MHC] region), 61 independent signals had a 99% credible set with fewer than 50 variants; 34 signals had credible sets with fewer than 20 variants (Supplementary Figure 3). Eighteen loci had a single variant with a posterior probability of driving association (PPA) greater than 60% including the NPNT (4q24) locus, where the association could be fine-mapped to a single intronic variant, rs34712979 (NC_000004.11:g.106819053G>A, see Supplementary Note and Supplementary Table 6). Most sets included variants that overlapped genic enhancers of lung-related cell types (e.g., fetal lung fibroblasts, fetal lung, and adult lung fibroblasts) and were predicted to alter transcription binding motifs (Supplementary Table 6). Of 61 credible sets with fewer than 50 variants, eight sets contained at least one deleterious variant. These deleterious variants included 1) missense variants affecting TNS1, RIN3, ADGRG6, ADAM19, ATP13A2, BTC, and CRLF3; and 2) a splice donor variant affecting a lincRNA - AP003059.2.
Candidate target genes
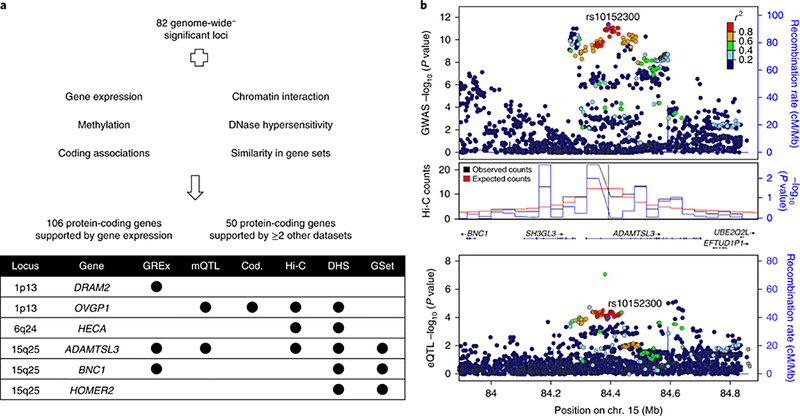
In most cases, the closest gene to a lead SNP will not be the gene most likely to be the causal or effector gene of disease-associated variants34–36. Thus, to identify the potential effector (‘target’) genes underlying these genetic associations, we integrated additional molecular information including gene expression, gene regulation (open chromatin and methylation data), chromatin interaction, co-regulation of gene expression with gene sets, and coding variant data (Methods and Figure 3).
Figure 3. Identification of target genes.
(a) Overview of datasets used to identify target genes at genome-wide significant loci (b) Regional association plots at ADAMTSL3 locus showing GWAS (top), chromatin interaction in lung tissue (middle) and expression quantitative trait loci (bottom). GWAS P-values are two-sided based on Wald statistics (35,735 cases and 222,076 controls). Expression quantitative trait loci (eQTL) P-values are two-sided based on t statistics (1,038 samples). P-values for Hi-C data were calculated using binomial distribution from spline-fitted and outlier-filtered distribution of contacts. All P-values were not adjusted for multiple comparison. GREx, gene-based association using gene expression; mQTL, colocalization with methylation quantitative trait loci; Cod., significant single variant or gene-based association tests for deleterious coding variants from exome data; Hi-C, significant chromatin interaction identified in human lung or the IMR90 cell line; DHS, overlap with DNase hypersensitivity sites; GSet, prioritized genes from DEPICT.
At 82 loci, 472 genes within +/− 1 Mb of top associated variants were implicated by analysis of least one dataset; 106 genes were implicated by lung gene expression37,38, and an additional 50 genes by >= 2 other datasets (methylation39, chromatin interaction40, open chromatin regions41, similarity in gene sets42 or deleterious coding variants43 [Figure 3]), for a total of 156 genes meeting more stringent criteria. Excluding loci in the MHC region, the median number of potentially implicated genes per locus was four, with a maximum of 17 genes (7q22.1 and 17q21.1). The median distance of implicated genes to top associated variants was 346 Kb. Among 82 loci, 60 (73%) included the nearest gene. We identified 20 genes with supportive evidence from exome sequencing data. Two genes (ADAM19 and ADAMTSL3) were implicated by five datasets (Figure 3) and another two (EML4 and RIN3) were implicated by four datasets. A summary of all genes implicated using these approaches is included in Supplementary Table 7.
Associated pathways
To gain further functional insight of associated genetic loci, we performed gene-set enrichment analysis using DEPICT42. Among 165 enriched gene sets at false discovery rate (FDR) < 5%, 44% of them were related to the developmental process term, with nominal P for lung development of 1.02 × 10−6; significant sub-terms included lung alveolus development (P = 0.0003) and lung morphogenesis (P = 0.0005). We also found enrichment of extracellular matrix-related pathways including laminin binding, integrin binding, mesenchyme development, cell-matrix adhesion, and actin filament bundles. Additional pathways of note included histone deacetylase binding, the Wnt receptor signaling pathway, SMAD binding, the MAPK cascade, and the transmembrane receptor protein serine/threonine kinase signaling pathway. Full enrichment analysis results including the top genes for each DEPICT gene set are shown in Supplementary Table 8.
Identification of drug targets
GWAS is also useful for identifying drug targets either at the individual gene18,44,45 or genome-wide level46,47. Of 482 candidate target genes, 60 genes could be targeted by at least one approved or in-development drug48, totaling 428 drugs with 144 different modes of action (Supplementary Table 9). Druggable targets at novel loci for COPD and lung function included ABHD6, CDKL2, GSTO2, KCNC4, PDHB, SLK, and TRPM7. We also identified drugs for repurposing in COPD using transcriptome-wide associations and drug-induced gene expression signatures49 (Supplementary Note).
Phenotypic effects of COPD-associated variants
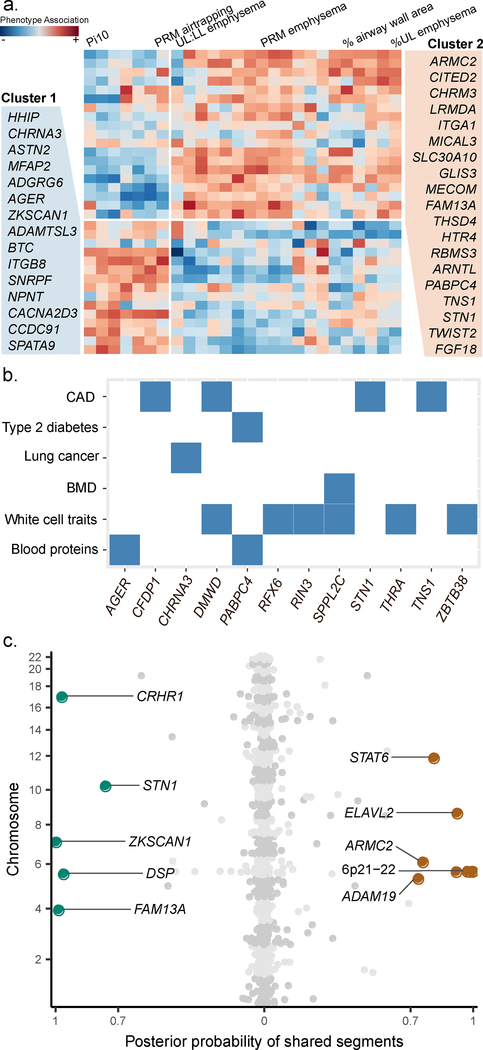
To characterize the phenotypic effects of 82 genome-wide significant loci, we performed a phenome-wide association analysis within the deeply phenotyped COPDGene study (Methods). We assessed for common patterns of phenotype associations for the 82 loci by using hierarchical clustering across scaled Z scores of phenotype-variant associations. We identified two clusters of variants differentially associated with two sets of phenotypes (Supplementary Figure 4). As these two variant-phenotype clusters appeared to be driven by computed tomography (CT) imaging features, we repeated variant clustering limited to quantitative computed tomography imaging features. We again found two clusters of variants, differentiated by association with quantitative emphysema, emphysema distribution, gas trapping, and airway phenotypes (Figure 4a). Additionally, we evaluated the association of the 82 genome-wide significant variants in a prior GWAS of emphysema and airway quantitative computed tomography features50 (Supplementary Table 10).
Figure 4. Effects on COPD-related and other phenotypes.
(a) Heatmap of scaled computed tomography (CT) quantitative imaging associations with the 34 genome-wide significant variants (known and replicated novel associations) with at least nominal (P < 0.05) association with any CT imaging feature in COPDGene non-Hispanic white participants. Cluster 1 variants are more associated with airway imaging features and Cluster 2 variants are more associated with emphysema imaging features. Variants are referred to by the closest gene. (b) Overlap of genome-wide significant loci of COPD and select traits from GWAS Catalog (c) Genome-wide overlapping results between COPD with pulmonary fibrosis (left) and asthma (right). PRM emphysema, emphysema quantified by parametric response mapping; UL, upper lobe of the lung; LL, lower lobe of the lung; Pi10, airway wall thickness calculated from regressing the square root of the airway wall area with the airway internal perimeter. CAD, coronary artery disease; BMD, bone mineral density.
We also examined all genome-wide significant loci in the NHGRI-EBI GWAS Catalog51 (Supplementary Figure 5, Supplementary Table 11) and looked for trait-associated variants in linkage disequilibrium (r2 > 0.2) with our lead COPD-associated variants. Many variants were associated with anthropometric measures including height and body mass index (BMI), measurements on blood cells (red and white cells), and cancers. COPD is well known to have many common comorbidities, such as coronary artery disease (CAD), type 2 diabetes mellitus (T2D), osteoporosis, and lung cancer. Of these diseases and 13 additional traits, we confirmed previously reported overall genetic correlation (using linkage disequilibrium score regression52) of COPD with lung function, asthma, and height, and found evidence of modest correlation between COPD and lung cancer (Supplementary Note). However, at individual loci, and using more stringent linkage disequilibrium (r2 > 0.6), we found evidence of shared risk factors for these comorbid diseases and COPD including a genome-wide significant variant near PABPC4 associated with T2D, four variants with CAD (near CFDP1, DMWD, STN1, and TNS1), and a variant near SPPL2C with bone density (Figure 4b).
Overlapping loci with asthma and pulmonary fibrosis
Based on our previous identification of genetic overlap of COPD with asthma, and COPD with pulmonary fibrosis, we examined loci for specific overlap with these two diseases. In asthma, we noted an r2 > 0.2 with one of our variants and previously reported variants at ID2, ZBTB38, C5orf56, MICA, AGER, HLA-DQB1, ITGB8, CLEC16A, and THRA. In pulmonary fibrosis, in addition to our previously described overlap at FAM13A, DSP, and 17q21, we noted overlapping associations at ZKSCAN1 and STN1 (Supplementary Table 12). To more closely examine overlap, we applied a Bayesian method (gwas-pw53) of COPD associations from our current GWAS with previous GWASs of asthma (limited to those of European ancestry) and pulmonary fibrosis54,55. To mitigate the results of including asthma among our COPD cases, we performed analysis for overlap with asthma removing self-reported asthmatics from UK Biobank for this analysis (Methods). We identified 14 shared genome segments (posterior probability > 70%), 9 with asthma and 5 with pulmonary fibrosis (Figure 4c, Supplementary Table 13). In addition to the three segments shared with pulmonary fibrosis identified in the previous study5 (FAM13A, DSP, and the 17q21 locus – here nearest CRHR1), we identified two new segments including loci near ZKSCAN1 and STN1 (formerly known as OBFC1). Shared variants between COPD and pulmonary fibrosis all had an opposite effect (i.e., increasing risk for COPD but protective for pulmonary fibrosis). In asthma, we identified five shared segments in the 6p21–22 regions, as well as ADAM19, ARMC2, ELAVL2, and STAT6. With the exception of STAT6, overlapping variants showed the same direction of effect.
Discussion
Genetic factors play an important role in COPD susceptibility. We examined genetic risk of COPD in a genome-wide association study of 35,735 cases and 222,076 controls. We identified 82 genome-wide significant loci for COPD, of which 47 were previously identified in genome-wide association studies of COPD or population-based lung function. Of 35 loci not previously described at the time of analysis, 13 replicated in an independent study of population-based lung function. We used several data sources to attempt to assign causal genes at each locus, identifying 156 genes at 82 loci that were supported by either gene expression or a combination of at least 2 other data sources. Our results identify specific genes, cell types, and biologic pathways for targeted study and also suggest a genetic basis for the clinical heterogeneity seen in COPD.
Our study supports the role of early life events in the risk of COPD. Gene set enrichment analysis identified developmental pathways both specific to the lung (e.g., lung morphogenesis and lung alveolar development) and related to the lung (e.g., the canonical Wnt receptor56,57, the MAPK/ERK, and the nerve growth factor receptor signaling pathways). We also confirmed enrichment of heritability in epigenomic marks of fetal lung. Our findings are consistent with epidemiologic studies demonstrating that a substantial portion of the risk of COPD may develop in early life: genetic variants may set initial lung function58 and patterns of growth58–60. While further work will be needed to confirm the causal variants and genes affected by our variants, testing the role of these genes in lung development-relevant murine or ex-vivo models – for example, determining whether the perturbation of these genes changes proliferation and differentiation of lung epithelial progenitors in induced pluripotent cell-derived lung alveolar type 2 cells44 – could provide experimental evidence of the role of these genes in early life susceptibility. Ultimately, the goal of this work would be to identify targets for or subsets of high risk individuals early in the disease course, or molecular candidates that may affect lung repair and regeneration61.
Apart from genes related to lung development, our analyses highlighted several genes and pathways already of interest in COPD therapy (e.g. CHRM3 / acetylcholine receptor inhibitors, the MAPK pathway) – supporting the role of genetic analyses in finding therapeutic targets18,62 – and newer genes that could inform future functional studies. We identified interleukin 17 receptor D (IL17RD), as a potential effector gene at the 3p14 locus. Numerous studies have examined the role of IL-17A in COPD63, and IL17RD can differentially regulate pathways employed by IL-17A64. Chitinase acidic (CHIA) at 1p13.3, which encodes a protein that degrades chitin65, exhibits lung-specific expression66,67. CHIA variants have been associated with FEV168, asthma69–72, and acid mammalian chitinase activity71,73. We identified several potential effector genes related to extracellular matrix, cell adhesion, cell-cell interactions, and elastin-associated microfibrils74–76, some of which have been previously identified in studies of lung function15. These include integrin family members that mediate cell-matrix communication (e.g., ITGA1, ITGA2, ITGA877–79), an integrin ligand encoding gene (NPNT80), and genes encoding matrix proteins (e.g., MFAP2 and ADAMTSL3). ADAMTSL3 plays a role in cell-matrix interactions related to the assembly of fibrillin and microfibril biogenesis81–83 and of our candidate effector genes was supported by the greatest number of bioinformatic analyses. Recombinant forms of other ADAMTS-like proteins demonstrate experimental evidence of promoting and enhancing fibrillin and microfibril deposition and assembly84,85. ADAMTSL3 may play a role in preventing emphysematous destruction of lung tissue by ADAMTS in COPD.
In addition to identifying the effector gene, knowing the effector cell type is critical for functional studies. We identified an overall enrichment of epigenomic marks in lung tissue and smooth muscle (also identified in studies of lung function16). This latter association was found in gastrointestinal tissue cell types; respiratory smooth muscle is absent in the analyzed datasets. We also performed analyses of single-cell data in an attempt to identify the specific lung cell types in which our top variants are potentially functioning. We found evidence for enrichment of several cell types, including but not limited to endothelial cells, alveolar type 2 cells, and basal-like cells. Each of these cell types has been postulated to have a role in the development of COPD86–88, and our data are consistent with the likely heterogeneity of lung cell types contributing to COPD susceptibility. The lung comprises at least 40 different resident cell types89, most of which are not distinctly represented in these datasets. Thus, while our findings support the investigation of specific cell types for further functional studies, they also highlight the need for profiling of lung-relevant cell types and loci-specific analyses.
Characterization of functional variant effects could lead to better disease subtyping and more targeted therapy for COPD. Cluster analysis on hundreds of COPD-associated features in the more extensively phenotyped COPDGene cohort showed heterogeneous effects of genetic variants on COPD-related phenotypes, including computed tomography (CT) measurements of airway abnormalities and emphysema – well-described sources of heterogeneity in COPD90–92. Analyzing hundreds of diseases/traits in GWAS Catalog, we identified overlapping associations with various diseases/traits in multiple organ systems, comorbidities such as coronary artery disease, bone mineral density, and type 2 diabetes mellitus (T2D). The COPD-associated PABPC4 locus was associated with T2D93 and C-reactive protein (CRP) level94. Although a causal gene in this locus and its contribution to COPD is unknown, its association with T2D may suggest a shared disease pathway and drug targets. Together, the identification of variable COPD risk loci associations with sub-phenotypes and other diseases95,96 may have potential for more nuanced approaches to therapy for COPD. Overall, our phenotype, gene, and pathway analyses illustrate the utility of both searching for enrichment of genetic signals overall, and performing a more detailed identification of the effects of individual variants or groups of variants.
We performed additional specific analysis in two diseases that overlap with COPD, asthma and pulmonary fibrosis. While a genome-wide genetic correlation of COPD and asthma has been previously described5, our analysis is the first to identify specific shared genetic segments between asthma and COPD. While the effects at most of these shared segments were concordant in direction, one of the segments of particular interest was near STAT6, which had opposite directions of effect in the two diseases. STAT6 plays a role in T helper (Th) type 2-dependent inflammation, and is activated by interleukin-4 and interleukin-13 (IL-4 and IL-13)97. IL-13, in turn, has been found to be increased in asthmatic airways98 but decreased in severe emphysema99. In pulmonary fibrosis, variants at all overlapping loci have an opposite direction of effect compared to COPD5. These effects raise the possibility that specific therapies for one disease could increase the risk of the other disease, which may be worth evaluating in treatment trials. The reasons why genetic effects are divergent between COPD and fibrosis are unclear, but these identified opposite effects could point to molecular switches that influence why some smokers develop emphysema while others develop pulmonary fibrosis. While pulmonary fibrosis is an uncommon disease and specifically excluded in several of our COPD case-control cohorts, interstitial lung abnormalities are increasingly being recognized as a potential precursor to fibrosis, and an inverse relationship between these abnormalities and emphysema has been previously identified100. Mechanistically, some have hypothesized that the divergent derangement of Wnt and Notch signaling pathways101 and mesenchymal cell fate102 may be responsible for the distinct development of these two diseases. We also describe an overlapping region at the STN1 (previously known as OBFC1) locus. STN1 plays a role in telomere maintenance103; shortened telomeres have been observed in both COPD and idiopathic pulmonary fibrosis (IPF)104,105, and rare genetic variants in the telomerase pathway have been implicated in both pulmonary fibrosis and emphysema – albeit with concordant effects on either disease106.
While our study a large genome-wide association study of COPD, individuals meeting our criteria for COPD in the UK Biobank may be different from other studies, especially for smoking history. We used the same definition of COPD as in our prior analysis5, which included non-smokers. Our use of pre-bronchodilator spirometry to define COPD (allowing us to maximize sample size) as well as population-based lung function for replication could bias our findings against variants that are only associated with more severe forms of COPD. We did not exclude other causes of airway obstruction such as asthma, noting that asthma frequently overlaps with, and is misdiagnosed in COPD107. We performed several additional analyses to determine whether our results were driven by, or markedly different, by smoking status, asthma, or use of pre- instead of post-bronchodilator spirometry to define COPD. The results of these additional analyses did not indicate a substantial impact of these factors on our overall findings, and together with prior analyses5,16, suggest that bias due to these factors is likely small. However, our study was not designed to identify differences between subgroups, and we cannot rule out a role for studying more severe disease or disease subtypes. We note that the alpha-1 antitrypsin locus (SERPINA1) was identified as genome-wide significant in smaller studies of emphysema and in smokers with severe COPD108. In the current study, the association of the PiZ allele (NC_000014.8:g.94844947C>T, rs28929470) had P = 2.2 × 10−5 using moderate-to-severe cases (FEV1 < 80% predicted), and a smaller P-value (1.4 × 10−6) in severe cases (FEV1 < 50% predicted) despite a smaller sample size, a phenomenon we have previously described11. Thus, despite the strong overlap of COPD with quantitative spirometry, new loci may be identified through studies of sufficiently large subsets of COPD patients and with more specific and homogeneous COPD phenotypes. Given suggestive evidence for replication using a related (but not identical) phenotype for additional novel loci beyond the 13 meeting a Bonferroni-corrected threshold for significance, we chose to include all loci significant in discovery in subsequent analyses, recognizing that we likely included some false positive associations. Our study focused on relatively common variants, predominantly in individuals of European ancestry; more detailed studies of rare variants, the human leukocyte antigen (HLA) regions, and other ethnicities are warranted, but broader multi-ethnic analyses are limited by the number of cases in currently available cohorts. Although COPD sex differences have been reported109, we did not identify significant sex-specific differences in effect sizes of the 82 top variants. Future studies including more subjects and methodological advances may be needed to elucidate this effect.
The global burden of COPD is increasing. Our work finds a substantial number of new loci for COPD and uses multiple lines of supportive evidence to identify potential genes and pathways for both existing and novel loci. Further investigation of the genetic overlap of COPD with other respiratory diseases and the phenotypic effects of top loci finds new shared loci for asthma and idiopathic pulmonary fibrosis and suggests heterogeneity across COPD-associated loci. Together, these insights provide multiple new avenues for investigation of the underlying biology and the potential therapeutics in this deadly disease.
Methods
Study populations
The UK Biobank is a population-based cohort consisting of 502,682 individuals8. To determine lung function, we used measures of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) derived from the spirometry blow volume-time series data, subjected to additional quality control based on ATS/ERS criteria110 (Supplementary Note). As in our previous study5, we defined COPD using pre-bronchodilator spirometry according to modified Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for moderate to very severe airflow limitation9: FEV1 less than 80% of predicted value (using reference equations from Hankinson et al.111), and the ratio between FEV1 and FVC less than 0.7. Consistent with our previous analyses and enrollment criteria for COPD case-control datasets112, we did not exclude individuals based on self-reported asthma. Genotyping was performed using Axiom UK BiLEVE array and Axiom Biobank array (Affymetrix, Santa Clara, California, USA) and imputed to the Haplotype Reference Consortium (HRC) version 1.1 panel113.
We invited participants in the prior International COPD Genetics Consortium (ICGC) COPD genome-wide association study to provide case-control association results (with the exception of the 1958 British Birth Cohort, to avoid overlapping samples with the replication sample). ICGC cohorts performed case-control association analysis based on pre-bronchodilator measurements of FEV1 and FEV1/FVC, and cases were identified using modified GOLD criteria, as above. Studies were imputed to 1000 Genomes reference panels. Detailed cohort descriptions and cohort-specific methods have been previously published5 (Supplementary Note). All studies comply to all relevant ethical regulations. Ethical/regulatory boards approved the study protocol for each study (Supplementary Note). We obtained informed consent from all participating individuals.
Based on the strong genetic overlap of lung function and COPD5, we performed lookups of select significant variants for FEV1 and FEV1/FVC in the SpiroMeta consortium meta-analysis21. Briefly, SpiroMeta comprised a total of 79,055 individuals from 22 studies imputed to either the 1000 Genomes Project Phase 1 reference panel (13 studies) or the HRC (9 studies). Each study performed linear regression adjusting for age, age2, sex, and height, using rank-based inverse normal transforms, adjusting for population substructure using principal components or linear mixed models, and performing separate analyses for ever- and never- smokers or using a covariate for smoking (for studies of related subjects). Genomic control was applied to individual studies, and results were combined using a fixed-effects meta-analysis21.
Genome-wide association analysis
In UK Biobank, we performed logistic regression of COPD, adjusting for age, sex, genotyping array, smoking pack-years, ever smoking status, and principal components of genetic ancestry. Association analysis was done using PLINK 2.0 alpha114 (downloaded on December 11, 2017) with Firth-fallback settings, using Firth regression when quasi-complete separation or regular-logistic-regression convergence failure occurred. We performed a fixed-effects meta-analysis of all ICGC cohorts and UK Biobank using METAL (version 2010–08-01)115. We assessed population substructure and cryptic relatedness by linkage disequilibrium (LD) score regression intercept10. We defined a genetic locus using a 2-Mb window (+/−1 Mb) around a lead variant, with conditional analyses as described below.
To maximize our power to identify existing and discover new loci, we examined all loci at the genome-wide significance value of P < 5 × 10−8. We first characterized loci as being previously described (evidence of prior association with lung function12–20,116,117 or COPD5,11,118) or novel. We defined previously reported signals if they were in the same LD block in Europeans119 and in at least moderate LD (r2 >= 0.2). For novel loci we attempted replication through association of each lead variant with either FEV1 or FEV1/FVC ratio in SpiroMeta, using one-sided P-values with Bonferroni correction for the number of novel loci examined. Novel loci failing to meet a Bonferroni-corrected P-value were assessed for nominal significance (one-sided P < 0.05) or directional consistence with FEV1 and FEV1/FVC ratio in SpiroMeta.
Cigarette smoking is the major environmental risk factor for COPD and genetic loci associated with cigarette smoking have been reported5,120. While we adjusted for cigarette smoking in our analysis, we further examined these effects by additionally testing for association of each locus with cigarette smoking and by looking at two separate analyses of ever- and never- smokers in UK Biobank. We tested for sex-specific genetic effects of genome-wide significant variants via a stratified analysis and interaction testing, using a 5% Bonferroni-corrected threshold to determine significance (Supplementary Note).
Identification of independent associations at genome-wide significant loci
We identified specific independent associations at genome-wide significant loci using GCTA-COJO22. This method utilizes an approximate conditional and joint analysis approach requiring summary statistics and representative LD information. As the UK Biobank provided the predominant sample, we used 10,000 randomly drawn unrelated individuals from this discovery dataset as a LD reference sample. We scaled genome-wide significance to a 2-Mb region, resulting in a locus-wide significant threshold of 8 × 10−5, or 2 × 10−6 for variants in the major histocompatibility complex (MHC) region (chr6:28477797–33448354 in hg19). We created regional association plots via LocusZoom using 1000 Genomes EUR reference data121 (Nov2014 release).
Identification and prioritization of tissues and cell types, candidate variants, genes, and pathways
Identification of enriched tissues and specific cell types
We used LD Score Regression (LDSC) to estimate the enrichment of functional annotations26 and specifically expressed gene regions31 on disease heritability. We utilized LDSC baseline models (e.g., conserved region, promoter flanking region), tissue-specific annotations from the Roadmap Epigenomics Program31, integrated tissue annotations from GenoSkyline25, and cell type-specific chromatin accessibility data27 (ATAC-Seq). We used four single-cell gene expression (RNA-Seq) datasets to identify specific cell types (Supplementary Note), including 1) lung epithelial cells from normal and pulmonary fibrosis human lung28 (Gene Expression Omnibus [GEO] accession GSE86618), 2) human induced pluripotent stem cells (iPSCs)-derived putative alveolar type 2 cells29 (GSE96642), 3) mouse lungs at embryonic day 18.5 (E18.5) and 4) postnatal day 1 (P1) by Whitsett et al. (unpublished, available at LungMAP30). We also used SNPsea32 to identify enriched cell types in genome-wide significant loci (Supplementary Note). We reported only estimates of coefficients and P-values for the Roadmap annotations and gene expression datasets, as theses analyses used –h2-cts, which does not report fold enrichment.
Fine-mapping of independent association signals at genome-wide significant loci
We used Bayesian fine-mapping at each locus to identify the credible set: the set of variants with a 99% probability of containing a causal variant. Briefly, for each genome-wide significant loci we calculated approximate Bayes factors33 of association. We then selected variants in each locus, so that their cumulative posterior probability was equal or greater than 0.99 using an unscaled variance. At loci with multiple independent associations, we used statistics from approximate conditional analysis with GCTA software on each index variant adjusting for other independent variants in the loci. Otherwise, we used unconditioned statistics from our meta-analysis. Details on characterization of variant effects are summarized in the Supplementary Note.
Identification of target genes
We used several computational approaches with corresponding available datasets to identify target genes in genome-wide significant loci. We used two methods that utilized gene expression data: 1) S-PrediXcan and 2) DEPICT. We used S-PrediXcan37 to identify genes with genetically regulated expression associated with COPD. We used data from the Lung-eQTL consortium38 (1,038 lung tissue samples) as an expression quantitative trait loci (eQTL) and gene expression reference database. S-PrediXcan tests for association between a trait and imputed gene expression using summary statistics. Here, we performed S-PrediXcan using models for protein-coding genes +/− 1 Mb from top-associated variants at genome-wide significant loci. We used DEPICT (Data-driven Expression Prioritized Integration for Complex Traits)42 to prioritize genes from ‘reconstituted’ gene sets.
We also used additional information on gene regulation, including epigenetic data: 1) regulatory fine mapping, 2) methylation quantitative trait loci (mQTL), and 3) chromosome conformation capture. We used regulatory fine mapping (regfm41) to overlap 99% credible interval (CI) variants at each GWAS locus with open chromatin regions based on DNAse hypersensitivity sites (DHS). DHS cluster accessibility state was then associated with gene expression levels (for 13,771 genes) from 22 tissues in the Roadmap Epigenomics Project41. Using both the 99% CI and DHS overlap, as well as the DHS state and transcript level association, regfm calculates a posterior probability of association of each gene +/− 1 Mb of the lead SNP at each GWAS locus. We also searched for overlapping mQTL data from lung tissue, as recently described39. To determine whether these signals co-localized (rather than being related due to linkage disequilibrium), we performed colocalization analysis between our GWAS and mQTL in genome-wide significant loci using eCAVIAR122 (eQTL and GWAS CAusal Variants Identification in Associated Regions, Supplementary Note). We also sought information from publicly available chromosome conformation capture data40. We queried association statistics of chromatin contact (i.e., long range chromatin interactions) between top associated variants and gene promoters nearby in a lung (fetal lung fibroblast cell line (IMR90) and human lung tissue40) using HUGIn123 (Hi-C Unifying Genomic Interrogator). We retained only the strongest associations (i.e., smallest P-value) for each cell line/primary cell in the analysis.
Finally, we searched for signals from deleterious variants by querying consequences of variants within 99% credible sets containing fewer than 50 variants (Supplementary Note). We also searched for rare coding variants, based on exome sequencing results in the COPDGene, Boston Early-Onset COPD (BEOCOPD), and International COPD Genetics Network (ICGN) studies, as previously described43. In brief, we performed exome sequencing on 485 severe COPD cases and 504 smoking resistant controls from the COPDGene study and 1,554 subjects ascertained through 631 probands with severe COPD from the BEOCOPD and the ICGN study. Details on statistical tests for single-variant and gene-based analyses are summarized in the Supplementary Note.
For each dataset described above, we used Bonferroni-corrected P-values, or a fixed posterior probability threshold to determine target genes at each locus. We reported protein-coding genes +/−1 Mb from a top associated variant. We restricted our search to genes from the GRCh37 server in biomaRt124 with updated HUGO Gene Nomenclature Committee (HGNC) names (downloaded from HGNC database of human gene names on June 7, 2018). For each locus, we used a 5% Bonferroni-corrected threshold (i.e., P < 0.05 divided by number of genes at that locus) to determine significance for 4 data types: gene expression data, chromatin conformation capture data, co-regulation of gene expression, and exome sequencing results. For two remaining datasets, we used a fixed posterior probability (of gene association with a GWAS locus) threshold of 0.1 for regfm and eCAVIAR. We considered genes that were implicated by gene expression or >= 2 combination of other datasets (e.g., methylation and chromatin conformation capture data) as target genes.
Identification of pathways
To identify enriched pathways in COPD-associated loci, we performed gene-set enrichment analysis using the “reconstituted” genes sets from DEPICT, as described above42. We defined significant gene sets using false discovery rate (FDR) < 5%.
Effects on COPD-related and other phenotypes
COPD is a complex and heterogeneous disorder, comprised of different biologic processes and specific phenotypic effects. In addition, many loci discovered by GWAS have pleiotropic effects. To identify these effects, we performed analyses of a) identification of overlapping genetic loci between related disorders (asthma and pulmonary fibrosis) b) genetic association studies of our genome-wide significant findings using COPD-related phenotypes, including a cluster analysis to identify groups of variants that may be acting via similar mechanisms; c) look up of top variants in prior COPD-related quantitative computed tomography (CT) imaging feature GWAS, d) look up of associations with other diseases/traits using GWAS Catalog, and e) estimate the genetic correlation between COPD and other diseases/traits.
To identify overlapping loci between COPD and other respiratory disorders, we used gwas-pw53 to perform pairwise analysis of GWAS. This method searches for shared genomic segments119 using adaptive significance threshold, allowing detection of sub genome-wide significant loci. We identified shared segments or variants using posterior probability of colocalization greater than 0.753. We obtained GWAS summary statistics from previous studies of pulmonary fibrosis55 and asthma in Europeans54. For the overlap analysis of COPD with asthma, we examined the influence of the inclusion of individuals with self-reported asthma on both the overlap of discrete GWAS loci (using gwas-pw) and genome-wide genetic correlation (using LD score regression) by performing these analyses in the meta-analysis of ICGC studies and the UK Biobank (with individuals with asthma removed from cases in the latter). To assess heterogeneous effects of COPD susceptibility loci on COPD-related features (phenotypes), we evaluated associations of our genome-wide significant SNPs with 121 detailed phenotypes (e.g., lung function, computed tomography-derived metrics, biomarkers, and comorbidities) available in 6,760 COPDGene non-Hispanic whites. We calculated Z-scores for each SNP-phenotype combination relative to the COPD risk allele to create a SNP by phenotype Z-score matrix. We tested each COPD-related phenotype with at least one nominally significant association with one of our genome-wide significant COPD SNPs, leaving us with 107 phenotypes. We then oriented all Z-scores to be positive (based on sign of median Z score) in association with each phenotype to avoid clustering based on direction of association. To avoid clustering phenotypes only by strength of association with SNPs, we scaled Z-scores within each phenotype by subtracting mean Z-scores and dividing by the standard deviation of Z-scores within each phenotype. We then scaled Z-scores across SNPs to circumvent clustering of SNPs according only to relative strength of association with phenotypes. We then performed hierarchical clustering of the scaled Z-scores of associations between SNPs and phenotypes to identify clusters of SNPs and phenotypes for all 107 phenotypes as well as in the subset of 26 quantitative imaging phenotypes. We performed the clustering of variants both in the set of all genome-wide significant variants in discovery as well as in the subset of known variants plus novel variants meeting a strict Bonferroni threshold in SpiroMeta replication (Supplementary Note). We further examined top variant associations with COPD-related traits through a look-up of top variants in a prior GWAS of 12,031 subjects with quantitative emphysema and airway CT features50. To examine overlap of our COPD results with other traits, we downloaded genome-wide significant associations from the GWAS Catalog51 (P < 5 × 10−8; downloaded on April 10, 2018). Between a pair of COPD- and trait- associated variants within the same LD block in Europeans119, we computed the LD using the European ancestry panel125 and considered the overlap if variants were in at least in moderate LD (r2 >= 0.2). We estimated genetic correlation between COPD and other diseases/traits using a web engine for LDSC, LD Hub52. We assessed the results using a 5% Bonferroni-corrected significance level.
Identification of drug targets
We queried our target genes using the Drug Repurposing Hub48. This resource contains comprehensive annotations of launched drugs, drugs in phases 1–3 of clinical development, previously approved and preclinical or tool compounds, curated using publicly available sources (e.g., ChEMBL and Drugbank) and proprietary sources. We performed drug-gene expression similarity analysis49 (the Query) using a ranked gene set from a gene-based association test37 (Supplementary Note).
Reporting Summary
We provide further information on research design in the Life Sciences Reporting Summary linked to this article.
Data availability statement
The genome-wide association summary statistics are available at the database of Genotypes and Phenotypes (dbGaP) under accession phs000179.v5.p2 and via the UK Biobank. Derived phenotypic data for COPD case control status is also available in the UK Biobank.
Supplementary Material
Acknowledgements
This work was supported by the Prince Mahidol Award Youth Program Scholarship (P.S.); NHLBI R01HL084323, R01HL113264, R01HL089856, and P01HL105339 (E.K.S.); K08HL136928 (B.D.H.), the Parker B. Francis Research Opportunity Award (B.D.H.); R01HL113264, R01HL137927, P01HL105339 and P01HL132825 (M.H.C.). This research has been conducted using the UK Biobank Resource under application number 20915 (M.H.C.) and 648 (M.D.T.). Please refer to the Supplementary Note for full acknowledgements. The funding body has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing interests statement
M.H.C., E.K.S., L.V.W., M.D.T., D.A.L. and I.P.H. have received grant funding from GlaxoSmithKline (GSK). E.K.S. has received honoraria from Novartis for Continuing Medical Education Seminars and travel support from GSK. I.P.H. has received grant support from BI. R.T-S. is employee and shareholder of GSK. J.V. has received personal fees from GSK, Chiesi Pharmaceuticals, BI, Novartis, and AstraZeneca. D.L.D. has received grants from the National Institutes of Health for research of COPD and personal fees from Novartis. D.A.L has received honoraria from GSK and chaired the Respiratory Therapy Area Board 2012–15. Outside the submitted work, L.L. reports expert consultation for Boehringer Ingelheim GmbH and Novartis and unrestricted grants from AstraZeneca and Chiesi.
Footnotes
URLs
HGNC database of human gene names, https://www.genenames.org/; The Drug Repurposing Hub, https://clue.io/repurposing; The Query, https://clue.io/query; Human Genome Region MHC by Genome Reference Consortium, https://www.ncbi.nlm.nih.gov/grc/human/regions/MHC?asm=GRCh37.p13; EMBL-EBI GWAS Catalog, https://www.ebi.ac.uk/gwas/; LungMAP, https://www.lungmap.net/.
Full author list
The SpiroMeta Consortium:
Nick Shrine5, Anna L Guyatt5, Chiara Batini5, Jing Hua Zhao53, Matthias Wielscher54, Understanding Society Scientific Group55, Stefan Weiss56, Katherine A Kentistou57,58, James P Cook59, Jennie Hui60,61,62,63, Stefan Karrasch64,65,66, Medea Imboden67,68, Sarah E Harris69,70, Jonathan Marten71, Stefan Enroth72, Shona M Kerr71, Ida Surakka73,74, Veronique Vitart71, Terho Lehtimäki75, Ralf Ewert76, Christian Gieger77, Georg Homuth56, Peter K Joshi57, Claudia Langenberg78, Lars Lind79, Jian’an Luan78, Anubha Mahajan80, Alison Murray81, David J Porteous69,70, Rajesh Rawal77,82, Blair H Smith83, Paul RHJ Timmers57, Olli T Raitakari84,85, Mika Kähönen86, Ozren Polasek87,57, Ulf Gyllensten72, Igor Rudan57, Ian J Deary69,88, Nicole M Probst-Hensch67,68, Holger Schulz 64,66, Alan L James60,89,90, James F Wilson57,71, Beate Stubbe76, Eleftheria Zeggini91,92, Marjo-Riitta Jarvelin93,94,95,54,96, Nick Wareham78, Caroline Hayward71, Andrew P Morris59,80, David P Strachan41, Ian P Hall28,29, Martin D Tobin5,49, Louise V Wain5,49
53 Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK
54 Department of Epidemiology and Biostatistics, MRC–PHE Centre for Environment & Health, School of Public Health, Imperial College London, London, UK
55 A list of contributors can be found in the Supplementary Note
56 Interfaculty Institute for Genetics and Functional Genomics, Department of Functional Genomics, University Medicine Greifswald, Greifswald, Germany
57 Centre for Global Health Research, Usher Institute for Population Health Sciences and Informatics, University of Edinburgh, Edinburgh, UK
58 Centre for Cardiovascular Sciences, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
59 Department of Biostatistics, University of Liverpool, Liverpool, UK
60 Busselton Population Medical Research Institute, Sir Charles Gairdner Hospital, Nedlands WA, Australia
61 School of Population Health, The University of Western Australia, Crawley WA, Australia
62 PathWest Laboratory Medicine of WA, Sir Charles Gairdner Hospital, Crawley WA, Australia
63 School of Pathology and Laboratory Medicine, The University of Western Australia, Crawley WA, Australia
64 Institute of Epidemiology, Helmholtz Zentrum Muenchen – German Research Center for Environmental Health, Neuherberg, Germany
65 Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine, Ludwig-Maximilians-Universität, Munich, Germany
66 Comprehensive Pneumology Center Munich (CPC-M), Member of the German Center for Lung Research (DZL), Munich, Germany
67 Swiss Tropical and Public Health Institute, Basel, Switzerland
68 University of Basel, Switzerland
69 Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, UK
70 Centre for Genomic and Experimental Medicine, Institute of Genetics & Molecular Medicine, University of Edinburgh, Western General Hospital, Edinburgh, UK
71 Medical Research Council Human Genetics Unit, Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK
72 Department of Immunology, Genetics and Pathology, Uppsala Universitet, Science for Life Laboratory, Uppsala, Sweden
73 Institute for Molecular Medicine Finland (FIMM), University of Helsinki, Helsinki, Finland
74 The National Institute for Health and Welfare (THL), Helsinki, Finland
75 Department of Clinical Chemistry, Fimlab Laboratories, and Finnish Cardiovascular Research Center - Tampere, Faculty of Medicine and Life Sciences, University of Tampere, Tampere, Finland
76 Department of Internal Medicine B - Cardiology, Intensive Care, Pulmonary Medicine and Infectious Diseases, University Medicine Greifswald, Greifswald, Germany
77 Research Unit of Molecular Epidemiology, Institute of Epidemiology, Helmholtz Zentrum Muenchen – German Research Center for Environmental Health, Neuherberg, Germany
78 MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine, Cambridge, UK
79 Department of Medical Sciences, Cardiovascular Epidemiology, Uppsala University, Uppsala, Sweden
80 Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, UK
81 The Institute of Medical Sciences, Aberdeen Biomedical Imaging Centre, University of Aberdeen, Aberdeen, UK
82 Institute of Epidemiology and Social Medicine, University of Münster, Münster, Germany
83 Division of Population Health and Genomics, Ninewells Hospital and Medical School, University of Dundee, Dundee, UK
84 Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland
85 Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland
86 Department of Clinical Physiology, Tampere University Hospital, and Finnish Cardiovascular Research Center - Tampere, Faculty of Medicine and Life Sciences, University of Tampere, Tampere, Finland
87 University of Split School of Medicine, Split, Croatia
88 Department of Psychology, University of Edinburgh, Edinburgh, UK
89 Department of Pulmonary Physiology and Sleep Medicine, Sir Charles Gairdner Hospital, Nedlands WA, Australia
90 School of Medicine and Pharmacology, The University of Western Australia, Crawley, Australia
91 Wellcome Sanger Institute, Hinxton, UK
92 Institute of Translational Genomics, Helmholtz Zentrum Muenchen – German Research Center for Environmental Health, Neuherberg, Germany
93 Center for Life Course Health Research, Faculty of Medicine, University of Oulu, Oulun yliopisto, Finland
94 Biocenter Oulu, University of Oulu, Finland
95 Unit of Primary Health Care, Oulu University Hospital, OYS, Oulu, Finland
96 Department of Life Sciences, College of Health and Life Sciences, Brunel University London, Uxbridge, UK
The International COPD Genetics Consortium:
Alvar Agusti97, Wayne Anderson98, Nawar Bakerly99,100, Per Bakke14, Robert Bals101, Kathleen C. Barnes37, R Graham Barr15, Terri H. Beaty16, Eugene R. Bleecker12, H. Marike Boezen22,23, Yohan Bossé4,45, Russell Bowler21, Christopher Brightling102,49, Marleen de Bruijne103,104, Peter J. Castaldi1, Bartolome Celli24, Michael H. Cho1,24, Harvey O. Coxson105, James D. Crapo21, Ron Crystal106, Pim de Jong107, Asger Dirksen108, Jennifer Dy109, Marilyn Foreman110, Judith Garcia-Aymerich111,112,113, Pierre Gevenois114, Soumitra Ghosh42, Hester Gietema115, Amund Gulsvik14, Ian P. Hall28,29, Nadia Hansel116, Craig P. Hersh1,24, Brian D. Hobbs1,24, Eric Hoffman117, Noor Kalsheker118, Hans-Ulrich Kauczor119, Woo Jin Kim31, Deog Kyeom Kim9, Tarja Laitinen120, Diether Lambrechts121,122, Sang-Do Lee123, Augusto A. Litonjua124, David A. Lomas32, Stephanie J. London10, Daan W. Loth18, Sharon M. Lutz125, David Lynch126, William MacNee127, Merry-Lynn McDonald128, Deborah A. Meyers12, John D. Newell117, Borge G. Nordestgaard129,130, George T. O’Connor33,34, Ma’en Obeidat13, Yeon-Mok Oh123, Peter D. Paré13,131, Massimo Pistolesi132, Dirkje S. Postma23, Milo Puhan133, Elizabeth Regan134, Stephen S. Rich46, Joon Beom Seo135, Andrea Short136, Edwin K. Silverman1,24, David Sparrow40, Berend Stoel137, David P. Strachan41, Nicola Sverzellati138, Ruth Tal-Singer42, Gerben ter Riet139, Yohannes Tesfaigzi17, Martin D. Tobin5,49, Edwin J.R. Van Beek140, Bram van Ginneken141, Jørgen Vestbo43, Claus F. Vogelmeier142, Louise V. Wain5,49, Adam Wanner143, George Washko144, Els Wauters145, Emiel FM Wouters146, Robert P. Young147, Loems Zeigler-Heitbrock148
97 Respiratory Institue, Hospital Clinic, University of Barcelona, IDIBAPS, CIBERES, Spain
98 University of North Carolina at Chapel Hill, Medicine, Chapel Hill, NC, USA
99 Salford Royal NHS Foundation Trust, Salford , UK
100 Manchester Academic Health Sciences Centre, The University of Manchester, Manchester, UK
101 Department of Internal Medicine V - Pulmonology, Allergology and Critical Care Medicine, Saarland University, Homburg, Germany
102 Department of Respiratory Medicine, Allergy and Thoracic Surgery, Glenfield Hospital, University of Leicester, Leicester, UK
103 Biomedical Imaging Group Rotterdam, Departments of Radiology and Medical Informatics, Erasmus MC, Rotterdam, the Netherlands
104 Department of Computer Science, University of Copenhagen, Copenhagen, Denmark
105 Department of Radiology, University of British Columbia, Canada
106 Department of Genetic Medicine and Division of Pulmonary and Critical Care Medicine, Department of Medicine, Weill Cornell Medical College, New York, NY, USA
107 Department of Respiratory Medicine, University Medical Center Utrecht, Utrecht, the Netherlands
108 Department of Respiratory Medicine, Herlev and Gentofte Hospital, Copenhagen, Denmark
109 Department of Electrical and Computer Engineering, Northeastern University, Boston, MA, USA
110 Morehouse School of Medicine, Atlanta, GA, USA
111 ISGlobal, Barcelona, España
112 Universitat Pompeu Fabra (UPF), Barcelona, España
113 CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, España
114 Department of Radiology, Hôpital Erasme, Université libre de Bruxelles, Belgium
115 Department of Radiology, Maastricht University Medical Center, Maastricht, the Netherlands
116 Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
117 Department of Radiology, University of Iowa, Iowa City, IA, USA
118 School of Life Sciences, The University of Nottingham, Nottingham, UK
119 Department of Diagnostic and Interventional Radiology, University of Heidelberg, Translational Lung Research Center Heidelberg, Heidelberg, Germany
120 Department of Pulmonary Diseases and Clinical Allergology, Turku University Hospital, University of Turku, Turku, Finland
121 Vesalius Research Center (VRC), VIB, Leuven, Belgium
122 Laboratory for Translational Genetics, Department of Oncology, KU Leuven, Leuven, Belgium
123 Department of Pulmonary and Critical Care Medicine and Clinical Research Center for Chronic Obstructive Airway Diseases, Asan Medical Center, University of Ulsan College of Medicine, Songpa-gu, Seoul, Republic of Korea
124 Division of Pediatric Pulmonary Medicine, Department of Pediatrics, Golisano Children’s Hospital at Strong, University of Rochester Medical Center, Rochester, NY, USA
125 Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, MA, USA
126 Department of Radiology, National Jewish Health, Denver, CO, USA
127 Department of Respiratory Medicine, University of Edinburgh, Edinburgh, UK
128 Division of Pulmonary, Allergy and Critical Care Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA
129 Department of Clinical Biochemistry and the Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Herlev, Denmark
130 Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
131 University of British Columbia, Department of Medicine, Institute for Heart and Lung Health, St. Paul’s Hospital, Vancouver, BC, Canada
132 Department of Clinical and Experimental Medicine, University Hospital Careggi, Largo Brambilla 1, 50134 Florence, Italy
133 Epidemiology, Biostatistics and Prevention Institute (EBPI), University of Zurich, Zurich, Switzerland
134 Department of Medicine, Division of Rheumatology, National Jewish Health, Denver, CO, USA
135 Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Songpa-gu, Seoul, Republic of Korea
136 School of Social Sciences, University of Manchester, Manchester, UK
137 Leiden University Medical Center Leiden, the Netherlands
138 Radiology, Department of Medicine and Surgery, University of Parma, Italy
139 Department of General Practice, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
140 Edinburgh Imaging, University of Edinburgh, Edinburgh, UK
141 Department of Radiology and Nuclear Medicine, Radboud University Medical Center, Nijmegen, the Netherlands
142 University of Marburg, Pulmonary Diseases, Marburg, Germany
143 Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, University of Miami, Miller School of Medicine, Miami, Florida, USA
144 Department of Radiology, Brigham and Women’s Hospital, Boston, MA, USA
145 Laboratory of Pneumology, Department of Chronic Diseases, Metabolism and Ageing, KU Leuven, Belgium
146 Respiratory Medicine, Maastricht University Medical Center, the Netherlands
147 School of Biological Sciences and Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand
148 EvA Study Center, Helmholtz Zentrum Muenchen, Gauting, Germany
References
- 1.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. Respir. Med 5, 691–706 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. (2018). [Google Scholar]
- 3.Fuchsberger C et al. The genetic architecture of type 2 diabetes. Nature 536, 41–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou JJ et al. Heritability of chronic obstructive pulmonary disease and related phenotypes in smokers. Am. J. Respir. Crit. Care Med 188, 941–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobbs BD et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet 49, 426–432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Z et al. A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of beta-Catenin. Am J Respir Crit Care Med 194, 185–197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lao T et al. Hhip haploinsufficiency sensitizes mice to age-related emphysema. Proc. Natl. Acad. Sci. U. S. A 113, E4681–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudlow C et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogelmeier CF et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med 195, 557–582 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Bulik-Sullivan BK et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet 47, 291–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho MH et al. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir Med 2, 214–225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilk JB et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet 5, e1000429 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repapi E et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet 42, 36–44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock DB et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42, 45–52 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soler Artigas M et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 43, 1082–1090 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wain LV et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med 3, 769–781 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soler Artigas M et al. Sixteen new lung function signals identified through 1000 Genomes Project reference panel imputation. Nat Commun 6, 8658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wain LV et al. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat. Genet 49, 416–425 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyss AB et al. Multiethnic meta-analysis identifies ancestry-specific and cross-ancestry loci for pulmonary function. Nat. Commun 9, 2976 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson VE et al. Meta-analysis of exome array data identifies six novel genetic loci for lung function. Wellcome open Res. 3, 4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrine N et al. New genetic signals for lung function highlight pathways and pleiotropy, and chronic obstructive pulmonary disease associations across multiple ancestries. bioRxiv (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet 44, 369–75, S1–3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agusti A & Soriano JB COPD as a systemic disease. COPD 5, 133–8 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Barnes PJ & Celli BR Systemic manifestations and comorbidities of COPD. Eur. Respir. J 33, 1165–85 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Lu Q et al. Systematic tissue-specific functional annotation of the human genome highlights immune-related DNA elements for late-onset Alzheimer’s disease. PLoS Genet. 13, e1006933 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finucane HK et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet 47, 1228–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cusanovich DA et al. A Single-Cell Atlas of In Vivo Mammalian Chromatin Accessibility. Cell 174, 1309–1324.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI insight 1, e90558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob A et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 21, 472–488.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ardini-Poleske ME et al. LungMAP: The Molecular Atlas of Lung Development Program. Am. J. Physiol. Lung Cell. Mol. Physiol 313, L733–L740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finucane HK et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet 50, 621–629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slowikowski K, Hu X & Raychaudhuri S SNPsea: an algorithm to identify cell types, tissues and pathways affected by risk loci. Bioinformatics 30, 2496–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakefield J A Bayesian measure of the probability of false discovery in genetic epidemiology studies. Am. J. Hum. Genet 81, 208–27 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visscher PM et al. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet 101, 5–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X et al. Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum. Mol. Genet 21, 1325–35 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claussnitzer M, Hui C-C & Kellis M FTO Obesity Variant and Adipocyte Browning in Humans. N. Engl. J. Med 374, 192–3 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Barbeira AN et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat. Commun 9, 1825 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamontagne M et al. Leveraging lung tissue transcriptome to uncover candidate causal genes in COPD genetic associations. Hum. Mol. Genet 27, 1819–1829 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow JD et al. Human Lung DNA Methylation Quantitative Trait Loci Colocalize with COPD Genome-wide Association Loci. Am. J. Respir. Crit. Care Med (2018). doi: 10.1164/rccm.201707-1434OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt AD et al. A Compendium of Chromatin Contact Maps Reveals Spatially Active Regions in the Human Genome. Cell Rep. 17, 2042–2059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shooshtari P, Huang H & Cotsapas C Integrative Genetic and Epigenetic Analysis Uncovers Regulatory Mechanisms of Autoimmune Disease. Am. J. Hum. Genet 101, 75–86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pers TH et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun 6, 5890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao D et al. Whole exome sequencing analysis in severe chronic obstructive pulmonary disease. Hum. Mol. Genet (2018). doi: 10.1093/hmg/ddy269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanseau P et al. Use of genome-wide association studies for drug repositioning. Nat. Biotechnol 30, 317–20 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Lencz T & Malhotra AK Targeting the schizophrenia genome: a fast track strategy from GWAS to clinic. Mol. Psychiatry 20, 820–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamb J et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–35 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Sirota M et al. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Transl. Med 3, 96ra77 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corsello SM et al. The Drug Repurposing Hub: a next-generation drug library and information resource. Nat. Med 23, 405–408 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subramanian A et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 171, 1437–1452.e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho MH et al. A Genome-Wide Association Study of Emphysema and Airway Quantitative Imaging Phenotypes. Am. J. Respir. Crit. Care Med 192, 559–69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacArthur J et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 45, D896–D901 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng J et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pickrell JK et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet 48, 709–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demenais F et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat. Genet 50, 42–53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fingerlin TE et al. Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia. BMC Genet. 17, 74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skronska-Wasek W et al. Reduced Frizzled Receptor 4 Expression Prevents WNT/β-Catenin-driven Alveolar Lung Repair in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med 196, 172–185 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Sakornsakolpat P et al. Integrative genomics identifies new genes associated with severe COPD and emphysema. Respir. Res. 19, 46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bui DS et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet. Respir. Med (2018). doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 59.McGeachie MJ et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N. Engl. J. Med 374, 1842–1852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross JC et al. Longitudinal Modeling of Lung Function Trajectories in Smokers with and without COPD. Am. J. Respir. Crit. Care Med (2018). doi: 10.1164/rccm.201707-1405OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boucherat O, Morissette MC, Provencher S, Bonnet S & Maltais F Bridging Lung Development with Chronic Obstructive Pulmonary Disease. Relevance of Developmental Pathways in Chronic Obstructive Pulmonary Disease Pathogenesis. Am. J. Respir. Crit. Care Med 193, 362–75 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Nelson MR et al. The support of human genetic evidence for approved drug indications. Nat. Genet 47, 856–60 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Miossec P & Kolls JK Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov 11, 763–76 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Mellett M et al. Orphan receptor IL-17RD tunes IL-17A signalling and is required for neutrophilia. Nat. Commun 3, 1119 (2012). [DOI] [PubMed] [Google Scholar]
- 65.O’Leary NA et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fagerberg L et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 13, 397–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saito A, Ozaki K, Fujiwara T, Nakamura Y & Tanigami A Isolation and mapping of a human lung-specific gene, TSA1902, encoding a novel chitinase family member. Gene 239, 325–31 (1999). [DOI] [PubMed] [Google Scholar]
- 68.Aminuddin F et al. Genetic association between human chitinases and lung function in COPD. Hum. Genet 131, 1105–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birben E et al. The effects of an insertion in the 5’UTR of the AMCase on gene expression and pulmonary functions. Respir. Med 105, 1160–9 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Chatterjee R, Batra J, Das S, Sharma SK & Ghosh B Genetic association of acidic mammalian chitinase with atopic asthma and serum total IgE levels. J. Allergy Clin. Immunol 122, 202–8, 208.e1–7 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Ober C & Chupp GL The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr. Opin. Allergy Clin. Immunol 9, 401–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinzmann A et al. Joint influences of Acidic-Mammalian-Chitinase with Interleukin-4 and Toll-like receptor-10 with Interleukin-13 in the genetics of asthma. Pediatr. Allergy Immunol 21, e679–86 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Okawa K et al. Loss and Gain of Human Acidic Mammalian Chitinase Activity by Nonsynonymous SNPs. Mol. Biol. Evol 33, 3183–3193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang J et al. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J. Cell Biol 159, 431–40 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibson MA, Hughes JL, Fanning JC & Cleary EG The major antigen of elastin-associated microfibrils is a 31-kDa glycoprotein. J. Biol. Chem 261, 11429–36 (1986). [PubMed] [Google Scholar]
- 76.Massaro GD et al. Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol. Genomics 4, 51–7 (2000). [DOI] [PubMed] [Google Scholar]
- 77.Markovics JA et al. Interleukin-1beta induces increased transcriptional activation of the transforming growth factor-beta-activating integrin subunit beta8 through altering chromatin architecture. J. Biol. Chem 286, 36864–74 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitamura H et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Invest 121, 2863–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Araya J et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J. Clin. Invest 117, 3551–62 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeltz C & Gullberg D The integrin-collagen connection - a glue for tissue repair? J. Cell Sci 129, 1284 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Hall NG, Klenotic P, Anand-Apte B & Apte SS ADAMTSL-3/punctin-2, a novel glycoprotein in extracellular matrix related to the ADAMTS family of metalloproteases. Matrix Biol. 22, 501–10 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Apte SS A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J. Biol. Chem 284, 31493–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kutz WE et al. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem 286, 17156–67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabriel LAR et al. ADAMTSL4, a secreted glycoprotein widely distributed in the eye, binds fibrillin-1 microfibrils and accelerates microfibril biogenesis. Invest. Ophthalmol. Vis. Sci 53, 461–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsutsui K et al. ADAMTSL-6 is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J. Biol. Chem 285, 4870–82 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghosh M et al. Exhaustion of Airway Basal Progenitor Cells in Early and Established Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med 197, 885–896 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crystal RG Airway basal cells. The ‘smoking gun’ of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 190, 1355–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giordano RJ et al. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J. Biol. Chem 283, 29447–60 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franks TJ et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc. Am. Thorac. Soc 5, 763–6 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Boschetto P et al. Predominant emphysema phenotype in chronic obstructive pulmonary. Eur. Respir. J 21, 450–4 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Castaldi PJ et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax 69, 415–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cerveri I et al. The rapid FEV(1) decline in chronic obstructive pulmonary disease is associated with predominant emphysema: a longitudinal study. COPD 10, 55–61 (2013). [DOI] [PubMed] [Google Scholar]
- 93.Bonàs-Guarch S et al. Re-analysis of public genetic data reveals a rare X-chromosomal variant associated with type 2 diabetes. Nat. Commun 9, 321 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dehghan A et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 123, 731–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hersh CP et al. Non-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitus. BMC Pulm. Med 14, 164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Higami Y et al. Increased Epicardial Adipose Tissue Is Associated with the Airway Dominant Phenotype of Chronic Obstructive Pulmonary Disease. PLoS One 11, e0148794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chung KF & Barnes PJ Cytokines in asthma. Thorax 54, 825–57 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kroegel C, Julius P, Matthys H, Virchow JC & Luttmann W Endobronchial secretion of interleukin-13 following local allergen challenge in atopic asthma: relationship to interleukin-4 and eosinophil counts. Eur. Respir. J 9, 899–904 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Boutten A et al. Decreased expression of interleukin 13 in human lung emphysema. Thorax 59, 850–4 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Washko GR et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N. Engl. J. Med 364, 897–906 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chilosi M, Poletti V & Rossi A The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir. Res 13, 3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kulkarni T, O’Reilly P, Antony VB, Gaggar A & Thannickal VJ Matrix Remodeling in Pulmonary Fibrosis and Emphysema. Am. J. Respir. Cell Mol. Biol 54, 751–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wan M, Qin J, Songyang Z & Liu D OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J. Biol. Chem 284, 26725–31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albrecht E et al. Telomere length in circulating leukocytes is associated with lung function and disease. Eur. Respir. J 43, 983–92 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Armanios M Telomerase and idiopathic pulmonary fibrosis. Mutat. Res 730, 52–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stanley SE et al. Telomerase mutations in smokers with severe emphysema. J. Clin. Invest 125, 563–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tinkelman DG, Price DB, Nordyke RJ & Halbert RJ Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J. Asthma 43, 75–80 [DOI] [PubMed] [Google Scholar]
- 108.Foreman MG et al. Alpha-1 Antitrypsin PiMZ Genotype Is Associated with Chronic Obstructive Pulmonary Disease in Two Racial Groups. Ann. Am. Thorac. Soc 14, 1280–1287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han MK et al. Gender and chronic obstructive pulmonary disease: why it matters. Am. J. Respir. Crit. Care Med 176, 1179–84 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller MR et al. Standardisation of spirometry. Eur. Respir. J 26, 319–38 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Hankinson JL, Odencrantz JR & Fedan KB Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med 159, 179–87 (1999). [DOI] [PubMed] [Google Scholar]
- 112.Regan EA et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 7, 32–43 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCarthy S et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet 48, 1279–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang CC et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Willer CJ, Li Y & Abecasis GR METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lutz SM et al. A genome-wide association study identifies risk loci for spirometric measures among smokers of European and African ancestry. BMC Genet. 16, 138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loth DW et al. Genome-wide association analysis identifies six new loci associated with forced vital capacity. Nat Genet 46, 669–677 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hobbs BD et al. Exome Array Analysis Identifies a Common Variant in IL27 Associated with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 194, 48–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berisa T & Pickrell JK Approximately independent linkage disequilibrium blocks in human populations. Bioinformatics 32, 283–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Consortium, T. and G. & Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet 42, 441–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pruim RJ et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hormozdiari F et al. Colocalization of GWAS and eQTL Signals Detects Target Genes. Am. J. Hum. Genet 99, 1245–1260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martin JS et al. HUGIn: Hi-C Unifying Genomic Interrogator. Bioinformatics 33, 3793–3795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Durinck S, Spellman PT, Birney E & Huber W Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc 4, 1184–91 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Machiela MJ & Chanock SJ LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome-wide association summary statistics are available at the database of Genotypes and Phenotypes (dbGaP) under accession phs000179.v5.p2 and via the UK Biobank. Derived phenotypic data for COPD case control status is also available in the UK Biobank.