Abstract
Reported cases of uni-leaflet mitral valve (MV) were related to the absence or dysplasia of the posterior mitral leaflet with ample anterior mitral leaflet. We present here a new entity of uni-leaflet MV where the MV appears as a membrane-like structure with a single slit-like orifice at its lateral part with no commissures.
Case report
Continuous Doppler flow revealed a mean pressure gradient of 19 mmHg across the mitral valve indicating severe mitral stenosis. In 3D images from the left atrial view, the MV appeared like a membrane with a single orifice in its lateral part toward the left atrial appendage, the area of this orifice by 3D was 0.52 cm2, there were no commissures or even any residual lines at the site where commissures should be. The diagnosis of congenital severe mitral stenosis due to acommissural MV was confirmed. During surgery, the surgical appearance of the MV confirmed our diagnosis by 3D.
Conclusion
Isolated congenital severe mitral stenosis presenting in adulthood is rare, uni-leaflet MV as a cause is only reported in a few cases. MV replacement is usually indicated due to the abnormal anatomy of MV leaflets and the subvalvular apparatus.
<Learning objectives: How to diagnose uni-leaflet congenital mitral stenosis and how to differentiate it from other causes of congenital mitral stenosis.>
Keywords: Mitral, Valve, Congenital, Uni-leaflet
Introduction
Reported cases of uni-leaflet mitral valve (MV) were related to the absence or dysplasia of the posterior mitral leaflet with ample anterior mitral leaflet. We present here a new entity of uni-leaflet MV where the MV appears as a membrane-like structure with a single slit-like orifice at its lateral part with no commissures. Real-time transesophageal three-dimensional echocardiography (RT3DTEE) provides direct visualization of the MV anomaly which was confirmed during surgery for MV replacement. To the best of our knowledge, this form of uni-leaflet MV has been not reported in adulthood before.
The MV is the inlet valve to the left ventricle (LV). The normal MV is a complex apparatus composed of an annulus and two leaflets that are attached by chordae tendineae to two papillary muscles. The papillary muscles arise from the walls of the LV and secure the chordae tendineae and mitral leaflets, preventing prolapse of the valve during ventricular systole. The proper function of the MV requires an intact MV apparatus and satisfactory LV function [1]. Congenital malformations of the MV are unusual. It represents about 0.4% in patients with congenital heart disease. Among them, congenital stenosis represented in the literature as much as 6% [2]. Congenital mitral stenosis presents with obstruction of little hemodynamic significance involving a structurally normal MV to severe mitral stenosis with extremely abnormal subvalvular apparatus.
Congenital mitral stenosis, a rare entity, takes several forms, Congenital MS presenting in adulthood may be: (1) Parachute MV: all the chordae insert into a single large papillary muscle. (2) Supravalvular mitral ring. (3) Anomalous mitral arcade: The leaflets were found to insert directly into the papillary muscle because of the absence of chordae tendineae [3]. (4) Double-orifice MV: Duplication of the mitral orifice with or without fusion of subvalvular chordal structures [3]. (5) Unicuspid MV: The MV had an ample anterior leaflet and a hypoplastic posterior leaflet. Three-dimensional reconstruction demonstrated a single anterior leaflet with ample movement. The unicuspid MV is the rarest entity between all [3]. (6) MV commissural fusion: Fused one commissure leads to a unicommissural MV [3].
Case report
In this report, we present a case of acommissural MV causing severe MS in an adult female aged 19 years old. She is not diabetic, not hypertensive, has no family history of any cardiac disease, no history of rheumatic arthritis, and no history of long-acting penicillin. She was well until 6 months previously when she started to complain of shortness of breath which was categorized as New York Heart Association (NYHA) class II, which progressed to NYHA class III in the following two months. On general examination she was an averagely built female, her height was 159 cm, weight 55 kg, she looked ill, dyspneic, and tachycardic. On local examination, there was diastolic rumbling murmur grade V/VI with maximum intensity at the apex. There were no systolic murmurs and no additional sounds could be detected. Laboratory investigations were normal including erythrocyte sedimentation rate C-reactive protein, anti-streptolysin O titer, complete blood count, rheumatoid factor, and antinuclear antibodies. Chest X-ray: There was mitralization of left heart border with bilateral pulmonary congestion (Fig. 1). Electrocardiogram showed a biphasic P wave at V1 (Fig. 2). There was mild tricuspid regurgitation with estimated systolic pulmonary artery pressure of 45 mmHg.
Fig. 1.
X-ray chest showed mitralization of left heart border and bilateral pulmonary congestion.
Fig. 2.
12 leads ECG showed biphasic P wave at lead V1.
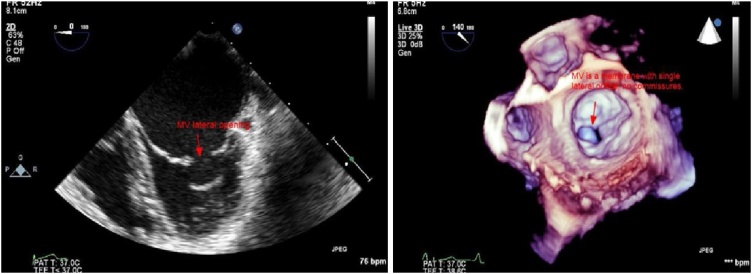
Transthoracic echocardiography (TTE): The parasternal long-axis view showed that the MV appeared to have two leaflets (Fig. 3 left and Video 1), parasternal short-axis view showed the single orifice at the lateral one third of the MV, the medial two thirds were fused (Fig. 3 right and Video 2). Apical 4-chamber view showed flow acceleration through the stenotic lateral orifice of the MV (Video 4). Transesophageal echocardiography (TEE): In mid esophageal 4-chamber view there was what looked like two MV leaflets attached to a thickened chordae, (Fig. 4 left and Video 4) in 2-chamber views, the subvalvular apparatus looked to be amalgamated forming a mass below the posteromedial part of the MV (Fig. 5 left and Video 5). With color, there was an acceleration of flow through a lateral orifice of the MV (Video 5). In transgastric long-axis view of LV, there were two papillary muscles, the posteromedial one which was larger with fused chordae looked like the papillary muscle was directly inserted in the leaflet with the absence of intracordal spaces, the papillary muscle was inserted at the inferolateral wall of the LV near the apex. The anterolateral one was smaller with clearer chordal apparatus and was inserted at the med anterolateral wall of LV. In transgastric short-axis view, there was a clear appearance of both papillary muscles, excluding any possibility of parachute MV (Fig. 5 right and Video 6). Continuous Doppler flow revealed a mean PG of 19 mmHg across the MV indicating severe MS. In 3D images from the left atrial view, the MV appeared like a membrane with a single orifice in its lateral part toward the left atrial appendage, the area of this orifice by 3D was 0.52 cm2, and there were no commissures or even any residual lines at the site where the commissures should present (Fig. 4 right and Video 7). In 3D images from the LV view, the single slit-like orifice is clear with bulky amalgamated subvalvular apparatus (Video 8). The diagnosis of congenital severe mitral stenosis due to acommissural MV was confirmed, the case was discussed in our heart team meeting, and it was decided to go for MV replacement with a bioprosthetic valve. During surgery, the surgical appearance of the MVs confirmed our diagnosis by 3D (Fig. 6).
Fig. 3.
Left: Parasternal long axis view showed the MV seemed to have 2 leaflets. Right: parasternal short axis view showed the single orifice at the lateral one third of the MV, the medial 2 thirds are fused.
Fig. 4.
Left: TEE 2D mid esophageal view, apparent MV with two leaflets. Right: 3D surgical view of the mitral valve, a single oval orifice at the lateral part of the membrane like uni-leaflet mitral valve, there is no commissures seen.
Fig. 5.
Left: TEE 2D showed amalgamated subvalvular apparatus with absent intracordal spaces below the posteromedial part of the mitral valve. Right: TEE transgastric short axis view showed both papillary muscles excluding parachute mitral valve.
Fig. 6.
Left: Surgical view showed a membrane like mitral valve with single orifice. Right: Resected mitral valve, slit like orifice at the lateral part of a membrane like uni-leaflet mitral valve.
Discussion
The congenital abnormalities in MV apparatus including chordae tendineae, papillary muscles, and MV leaflets are rare, it may remain asymptomatic in the lifetime, or may present with mitral stenosis, mitral insufficiency, or both [4]. In the embryological development of MV, there is a prominent horseshoe-shaped myocardial ridge that appears nearly on the 33rd fetal day from the anterior wall of the LV and extends to the posterior wall [5]. This ridge is connected to the endocardial cushion tissue in the atrioventricular region, then the myocardial ridge transforms into two papillary muscles and part of endocardial cushion tissue develops into valve leaflets and chordae tendineae [5]. Abnormal development of the anterior or posterior parts of this ridge from the ventricular wall leads to papillary muscle abnormalities, parachute MV develops if one papillary muscle is absent or rudimentary. Normally after the atrioventricular canal is divided into right and left atrioventricular junctions, the anterior leaflet develops from the inferior and superior cushions while the posterior leaflet develops from the lateral cushion [6]. The described cases of uni-leaflet MV in the literature were related to partial or complete leaflet agenesis or hypoplasia, this leads to the LV wall to take the action of the defected leaflet and this leads to mitral regurgitation. Candan et al. reported one case of a 47-year-old male with uni-leaflet MV, in which there were mitral stenosis and mitral regurgitation [7]. Bezgin et al. described one case of a 45-year-old female with uni-leaflet MV, with mitral regurgitation [8]. Kanagala et al. reported 3 cases of females aged 18, 17, and 46 years with uni-leaflet MV with mitral regurgitation [9]. In the previously reported five cases, we postulate that the defect in the development of the lateral cushion with absent or hypoplastic posterior mitral leaflet and the prominent lesion is mitral regurgitation. In our case, we postulate that the defect is the fusion of the superior, inferior, and lateral cushions forming a single MV leaflet with a single hole forming the MV orifice, and in this case, the lesion was severe mitral stenosis. Zhang et al. reported one case of a 5-year-old boy with congenital uni-cusped MV with severe mitral stenosis, and mild mitral insufficiency, he presented with severe symptoms that required surgery with MV replacement [10]. This case is similar to our case, however to the best of our knowledge our case is the first to be presented at adulthood at 19-years-old. When the patient was discussed in our heart team meeting, the option of balloon mitral valvuloplasty was raised, however as there were no commissures in the MV, even no residual commissural lines seen, this option was excluded as there was nothing to split by the balloon, also the subvalvular apparatus amalgamation excluded any possibility of balloon valvuloplasty. The patient was referred to surgery and underwent successful bioprosthetic MV replacement as she was in the childbearing period.
Conclusion
Isolated congenital severe mitral stenosis presenting in adulthood is rare, uni-leaflet MV as a cause is only reported in a few cases. RT3DTEE is mandatory for the accurate diagnosis of this anomaly. MV replacement is usually indicated due to the abnormal anatomy of MV leaflets and the subvalvular apparatus.
Conflict of interest
All authors indicated no potential conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jccase.2019.01.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Roberts W.C. Morphologic features of the normal and abnormal mitral valve. Am J Cardiol. 1983;51:1005–1008. doi: 10.1016/s0002-9149(83)80181-7. [DOI] [PubMed] [Google Scholar]
- 2.Collins-Nakai R.L., Rosenthal A., Castaneda A.R., Bernhard W.F., Nadas A.S. Congenital mitral stenosis. Circulation. 1977;56:1039–1047. doi: 10.1161/01.cir.56.6.1039. [DOI] [PubMed] [Google Scholar]
- 3.Espinola-Zavaleta N., Vargas-Barrón J., Keirns C., Rivera G., Romero-Cárdenas A., Roldán J. Three-dimensional echocardiography in congenital malformations of the mitral valve. J Am Soc Echocardiogr. 2002;15:468–472. doi: 10.1067/mje.2002.115772. [DOI] [PubMed] [Google Scholar]
- 4.Mohan J.C., Shukla M., Mohan V., Sethi A. Spectrum of congenital mitral valve abnormalities associated with solitary undifferentiated papillary muscle in adults. Indian Heart J. 2016;68:639–645. doi: 10.1016/j.ihj.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oosthoek P.W., Wenink A.C., Wisse L.J., Gittenberger-de Groot A.C. Development of the papillary muscle of the mitral valve: morphologic background of parachute like asymmetric mitral valves and other mitral valve anomalies. J Thoracic Cardiovasc Surg. 1998;116:36–46. doi: 10.1016/S0022-5223(98)70240-5. [DOI] [PubMed] [Google Scholar]
- 6.Sebuela P.-E., Houyel L., Acar P. Congenital malformations of the mitral valve. Arch Cardiovasc Dis. 2011;104:465–479. doi: 10.1016/j.acvd.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Candan O., Guler A., Anug S.M., Gecmen C., Karabay C.Y., Yildiz M. Uni-leaflet mitral valve. Eur J Echocardiogr. 2011;12:640. doi: 10.1093/ejechocard/jer027. [DOI] [PubMed] [Google Scholar]
- 8.Bezgin T., Elveran A., Karagoz A., Canga Y., Yilmaz F. Mitral valve with a single leaflet. Turk Kardiol Dern Ars. 2014;42:80–82. doi: 10.5543/tkda.2014.44380. [DOI] [PubMed] [Google Scholar]
- 9.Kanagala P., Baker S., Green L., Houghton A.R. Functionally uni-leaflet mitral valve in a family: a case series. Eur J Echocardiogr. 2010;11:E27. doi: 10.1093/ejechocard/jeq021. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W., Wang Y., Ma C., Zhang Z., Yang J. Congenital uni-leaflet mitral valve with severe stenosis. A case report with literature review. Echocardiography. 2017;34:468–471. doi: 10.1111/echo.13473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.