Abstract
Purpose
Wrist fractures are common, contribute significantly to morbidity in women with postmenopausal osteoporosis, and occur predominantly at the ultradistal radius, a site rich in trabecular bone. This exploratory analysis of the phase 3 ACTIVE study evaluated effects of abaloparatide versus placebo and teriparatide on forearm bone mineral density (BMD) and risk of wrist fracture.
Methods
Forearm BMD was measured by dual energy X-ray absorptiometry in a subset of 982 women from ACTIVE, evenly distributed across the three treatment groups. Wrist fractures were ascertained in the total cohort (N = 2463).
Results
After 18 months, ultradistal radius BMD changes from baseline were 2.25 percentage points greater for abaloparatide compared with placebo (95% confidence interval (CI) 1.38, 3.12, p < 0.001) and 1.54 percentage points greater for abaloparatide compared with teriparatide (95% CI 0.64, 2.45, p < 0.001). At 18 months, 1/3 radius BMD losses (versus baseline) were similar for abaloparatide compared with placebo (−0.42; 95% CI −1.03, 0.20; p = 0.19) but losses with teriparatide exceeded those of placebo (−1.66%; 95% CI −2.27, −1.06; p < 0.001). The decline with abaloparatide was less than that seen with teriparatide (group difference 1.22%; 95% CI 0.57, 1.87; p < 0.001). The radius BMD findings, at both ultradistal and 1/3 sites, are consistent with the numerically lower incidence of wrist fractures observed in women treated with abaloparatide compared with teriparatide (HR = 0.43; 95% CI 0.18, 1.03; p = 0.052) and placebo (HR = 0.49, 95% CI 0.20, 1.19, p = 0.11).
Conclusions
Compared with teriparatide, abaloparatide increased BMD at the ultradistal radius (primarily trabecular bone) and decreased BMD to a lesser extent at the 1/3 radius (primarily cortical bone), likely contributing to the numerically lower wrist fracture incidence observed with abaloparatide.
Electronic supplementary material
The online version of this article (10.1007/s00198-019-04890-2) contains supplementary material, which is available to authorized users.
Keywords: Abaloparatide, Bone mineral density, Osteoporosis, Teriparatide, Wrist fracture
Introduction
Osteoporotic fractures place a large burden on patients and a major economic toll on society [1]. Fractures of the distal radius are the most common upper extremity fracture in older adults, comprising approximately 22% of all fractures in women aged 50 and above, with an annual incidence of 8–10 per 1000 person-years, similar to the rate of hip fractures (7 per 1000 person-years) [2, 3]. At age 50 years, a white woman’s lifetime risk of a wrist fracture is 16% [4]. In the Study of Osteoporotic Fractures, elderly women with wrist fractures were almost 50% more likely to have a clinically important functional decline than those without fractures [3]. Mortality in elderly patients with distal radius fractures is significantly higher than a matched cohort of the general population without fracture [5].
Wrist fractures are also associated with an increased subsequent risk of vertebral and hip fractures [6–8]. An analysis of the National Osteoporosis Risk Assessment (NORA) study of 158,940 postmenopausal women showed a threefold risk of subsequent wrist fracture, and a twofold risk of any osteoporotic fracture, in women with prior wrist fracture, after adjusting for multiple covariates [6]. An analysis of the Women’s Health Initiative Observational Study and Clinical Trials showed that, after a mean duration of follow-up of 11.8 years, 15.5% of women who experienced wrist fracture subsequently experienced a non-wrist fracture, with a hazard ratio (HR) of 1.40 (95% confidence interval (CI) 1.33–1.48) compared with women without prior wrist fracture, after adjusting for age, race, and BMI [7].
Most wrist fractures involve the ultradistal radius, a zone with a high proportion of trabecular bone [9]. The 1/3 radius site is predominantly denser cortical bone (Fig. 1) [10]. Although the 1/3 radius is the forearm site most commonly considered with dual-energy x-ray absorptiometry (DXA) measurements [11], use of areal bone mineral density (BMD) at the ultradistal radius has recently been shown to improve fracture risk estimation compared with BMD at the femoral neck alone [12].
Fig. 1.
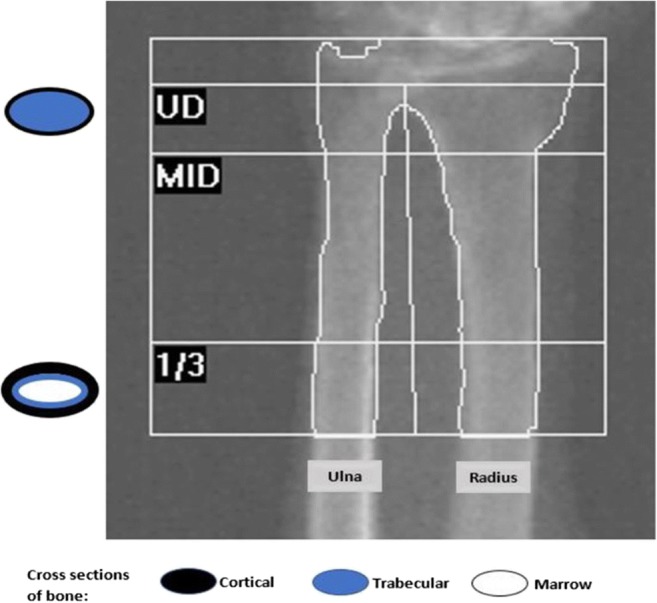
Composition of the distal radius and regions of interest in dual X-ray absorptiometry measurements
Abaloparatide selectively binds to the RG versus R0 conformation of the parathyroid hormone type 1 receptor (PTHR1), resulting in transient receptor signaling consistent with a net anabolic effect [13, 14]. Preclinical studies demonstrated increases in BMD, restoration of bone microarchitecture, and increased bone strength [15–17]. In a 24-week phase II clinical trial in postmenopausal women with osteoporosis, abaloparatide demonstrated dose-dependent increases in BMD at the lumbar spine, femoral neck, and total hip compared with placebo [14], and improvements in skeletal microarchitecture of the lumbar spine, assessed indirectly by trabecular bone score [18]. In the 18-month phase 3 Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE), postmenopausal women with osteoporosis were randomized to receive double-blind abaloparatide or placebo or open-label teriparatide. In ACTIVE, abaloparatide decreased the risk of vertebral and nonvertebral fractures compared with placebo and decreased the risk of major osteoporotic fractures compared with teriparatide [19].
The objectives of this exploratory analysis of ACTIVE were to determine the effect of abaloparatide on BMD at an anatomical site with a high proportion of trabecular bone relevant to wrist fractures (the ultradistal radius), as well as at a site with a higher proportion of cortical bone (the 1/3 radius), and to assess the effect on wrist fracture incidence.
Methods
Study subjects
The multicenter, multinational, randomized controlled ACTIVE study (clinicaltrials.gov identifier: NCT01343004) enrolled 2463 postmenopausal women, ages 49 to 86 years, with osteoporosis as defined by prior radiographic vertebral fracture or recent (within 5 years of enrollment) nonvertebral fracture with a BMD T-score ≤ −2.5 at the lumbar spine or femoral neck if age ≤ 65 years or ≤ −2.0 if age > 65 years. For those aged > 65 years, no prior fracture was required if the lumbar spine or femoral neck BMD T-score was ≤ −3.0. Other inclusion/exclusion criteria have been previously described [19].
Study design
The protocol was approved by the respective institutional review boards. After informed written consent was obtained, women were screened and those eligible were randomized 1:1:1 to receive double-blinded daily subcutaneous injections of abaloparatide 80 μg or matching placebo, or open-label daily injections of teriparatide 20 μg for 18 months [19]. All women received supplements of 500 to 1000 mg/day calcium and 400 to 800 IU vitamin D based on regional standard of care. The study was conducted in accordance with the ethical principles contained in the declaration of Helsinki and in compliance with good clinical practice guidelines and all applicable local regulations and ethical requirements.
Endpoints
The primary endpoint of ACTIVE was the incidence of new vertebral fractures from baseline to 18 months in women treated with abaloparatide compared with placebo [19]. Between-group comparisons of changes in BMD from baseline at the ultradistal and 1/3 radius, and the incidence of wrist fractures, were prespecified exploratory efficacy endpoints for abaloparatide versus placebo and abaloparatide versus teriparatide. All comparisons between teriparatide and placebo, and BMD changes from baseline within treatment groups, were post hoc exploratory analyses. Changes in BMD from baseline were assessed at the ultradistal and 1/3 radius at 6, 12, and 18 months in a subset of women who had at least one baseline and post-baseline measurement. BMD was measured by Lunar (GE Healthcare; Madison, WI, USA) or Hologic (Hologic, Inc.; Marlborough, MA, USA) dual energy X-ray absorptiometry (DXA) and centrally analyzed (Bioclinica-Synarc, Newark, CA, USA) using the percentage change from baseline in BMD, adjusted for machine differences. Clinical wrist fractures were ascertained from the total ACTIVE intent-to-treat population of 2463 randomized women, initially self-reported and then verified from source documents. All wrist fractures that caused a patient to seek medical care, regardless of the level of trauma, were included.
Study oversight was performed and safety was assessed as previously described [19], and all assessors of BMD and fracture were blinded to treatment group assignments.
Statistical analyses
Percent changes in BMD from baseline were evaluated at each study visit, with missing data imputed based on last observation carried forward. Comparisons within treatment groups were performed using t test. Comparisons among treatment groups were performed using analysis of covariance (ANCOVA) models. DXA scanner (Lunar or Hologic) and baseline BMD were included as covariates in ANCOVA models. Kaplan-Meier curves were used to display cumulative wrist fracture incidence. Time to first wrist fracture was compared using the log-rank test; hazard ratios (HR) were calculated using the Cox proportional hazards model. Similar analyses were conducted for analyses of wrist fracture by prior wrist fracture status. p values were not adjusted for multiple comparisons and were considered significant for p < 0.05.
Results
Subject disposition and demographics
The subject disposition and demographic characteristics in ACTIVE have been previously described [19]. BMD measurements at the ultradistal radius and the 1/3 radius were performed in a subset of 982 women representing 14 study sites randomized to receive abaloparatide (n = 321), placebo (n = 334), or teriparatide (n = 327). Baseline characteristics of this subset (Table 1) were similar to the full cohort with mean age 68.4 years, mean femoral neck T-score − 2.2, 24.6% having prevalent vertebral fracture and 32.7% reporting prior nonvertebral fracture within the past 5 years. Approximately 22% of the subset reported any prior history of wrist fracture.
Table 1.
Demographics and baseline characteristics of the ACTIVE overall population and women with wrist BMD measurements
| Characteristic | Placebo | Abaloparatide | Teriparatide | |||
|---|---|---|---|---|---|---|
| Overall, n = 821 | Wrist, n = 334 | Overall, n = 824 | Wrist, n = 321 | Overall, n = 818 | Wrist N = 327 | |
| Age, years, mean (SD) | 68.7 (6.5) | 68.1 (6.6) | 68.9 (6.5) | 68.7 (6.2) | 68.8 (6.6) | 68.2 (6.2) |
| BMI, kg/m2, mean (SD) | 25.1 (3.6) | 24.4 (3.5) | 25.0 (3.5) | 24.5 (3.6) | 25.2 (3.6) | 24.6 (3.7) |
| Race, n (%) | ||||||
| White | 655 (79.8) | 234 (70.1) | 663 (80.5) | 221 (68.8) | 645 (78.9) | 221 (67.6) |
| Asian | 131 (16.0) | 98 (29.3) | 128 (15.5) | 99 (30.8) | 137 (16.7) | 104 (31.8) |
| Black or African-American | 23 (2.8) | 2 (0.6) | 26 (3.2) | 0 | 24 (2.9) | 1 (0.3) |
| Other | 12 (1.5) | 0 | 7 (0.8) | 1 (0.3) | 12 (1.5) | 1 (0.3) |
| Hispanic or Latino, n (%) | 199 (24.2) | 12 (3.6) | 199 (24.2) | 11 (3.4) | 194 (23.7) | 11 (3.4) |
| BMD T-score, mean (SD) | ||||||
| Total hip | −1.9 (0.8) | −2.0 (0.8) | −1.9 (0.7) | −1.9 (0.7) | −1.9 (0.8) | −1.9 (0.7) |
| Femoral neck | −2.2 (0.7) | −2.2 (0.7) | −2.2 (0.6) | −2.2 (0.6) | −2.1 (0.7) | −2.2 (0.7) |
| Lumbar spine | −2.9 (0.8) | −2.9 (0.9) | −2.9 (0.9) | −2.8 (0.8) | −2.9 (0.9) | −2.9 (0.9) |
| Ultradistal radius | – | −3.3 (1.3) | – | −3.3 (1.3) | – | −3.3 (1.3) |
| 1/3 radius | – | −2.8 (1.2) | – | −2.8 (1.1) | – | −2.8 (1.1) |
| Prevalent vertebral fracture at baseline, n (%) | 188 (22.9) | 82 (24.6) | 177 (21.5) | 71 (22.1) | 220 (26.9) | 88 (26.9) |
| Prior nonvertebral fracture within last 5 years, n (%) | 266 (32.4) | 118 (35.3) | 248 (30.1) | 96 (29.9) | 240 (29.3) | 107 (32.7) |
| Prior wrist fracture, n (%)* | 173 (21.1) | 75 (22.5) | 178 (21.6) | 69 (21.5) | 158 (19.3) | 69 (21.1) |
| No history of prior fracture, n (%)* | 307 (37.4) | 114 (34.1) | 305 (37.0) | 116 (36.1) | 308 (37.7) | 121 (37.0) |
*Lifetime history
BMD, bone mineral density; BMI, body mass index. Values for overall population are from Miller et al. [19]
Changes in BMD
BMD change from baseline: between-group differences
Between-group comparisons of BMD percent changes from baseline were prespecified exploratory analyses.
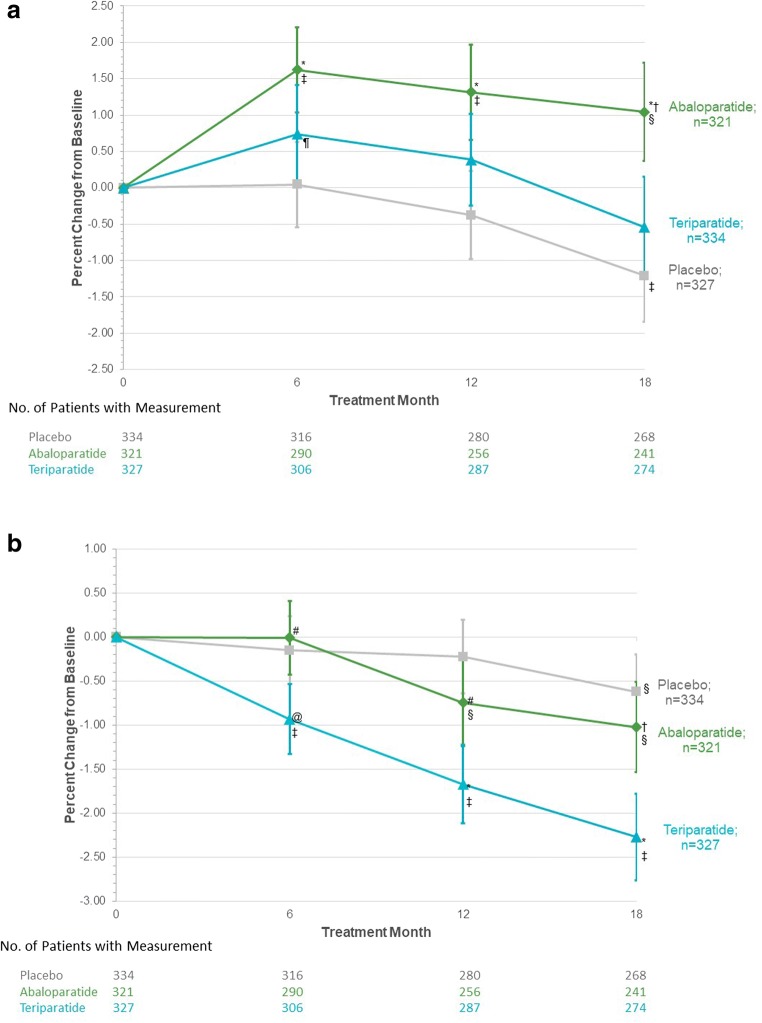
Increases from baseline in BMD at the ultradistal radius at 6 months were significantly greater for the abaloparatide group compared with placebo and remained higher for the duration of study (Fig. 2a). By 18 months, BMD change from baseline at the ultradistal site was 2.25 percentage points greater for the abaloparatide group compared with placebo and 1.54 percentage points greater for abaloparatide compared with teriparatide (Suppl Table 1). Ultradistal BMD change from baseline at 18 months was not significantly different for teriparatide compared with placebo.
Fig. 2.
Mean percent changes in bone mineral density (BMD) at (a) the ultradistal radius and (b) the 1/3 radius. Missing BMD data were imputed using the method of last observation carried forward. Errors bars in icate 95% confidence intervals. *p < 0.001 vs placebo; @p < 0.01 vs placebo; †p < 0.001 vs teriparatide; #p < 0.01 vs teriparatide; ‡p < 0.001 vs baseline; §p < 0.01 vs baseline; ¶p < 0.05 vs baseline
BMD decreases at the 1/3 radius were comparable between placebo and abaloparatide, but greater for teriparatide compared with placebo, at each time point (Fig. 2b). At 18 months, 1/3 radius BMD decreased 0.42% compared with placebo (p = 0.19, not significant) but the decline with abaloparatide was significantly lower than that seen with teriparatide (difference 1.22 percentage points, p < 0.001, Suppl Table 2). BMD losses at the 1/3 radius at 18 months were also greater for teriparatide compared with placebo (difference 1.66 percentage points, p< 0.001).
Within-group BMD change from baseline
BMD at the ultradistal radius increased above baseline at 6 months for the abaloparatide and teriparatide groups (Fig. 2a). Mean percent change from baseline at the ultradistal radius at 6 months was 1.62% for the abaloparatide group and 0.74% for the teriparatide group (Suppl Table 1). In the placebo group at 6 months, mean percent change from baseline at the ultradistal radius was 0.05%. By 18 months, mean percent change was 1.04% above baseline for abaloparatide but was similar to baseline at 18 months for teriparatide (Suppl Table 1). In the placebo group at 18 months, ultradistal BMD had declined below baseline.
At the 1/3 radius, mean BMD at 6 months was similar to baseline for abaloparatide and placebo but decreased from baseline for teriparatide. The mean percent change from baseline at 6 months was −0.01% in the abaloparatide group, − 0.15% in the placebo group and − 0.93% in the teriparatide group (Suppl Table 2). By 18 months, BMD at the 1/3 radius decreased compared with baseline within each treatment group (Fig. 2b). The mean percent change from baseline was − 1.02% in the abaloparatide group, − 0.62% in the placebo group, and − 2.27% in the teriparatide group (Suppl Table 2).
Wrist fractures
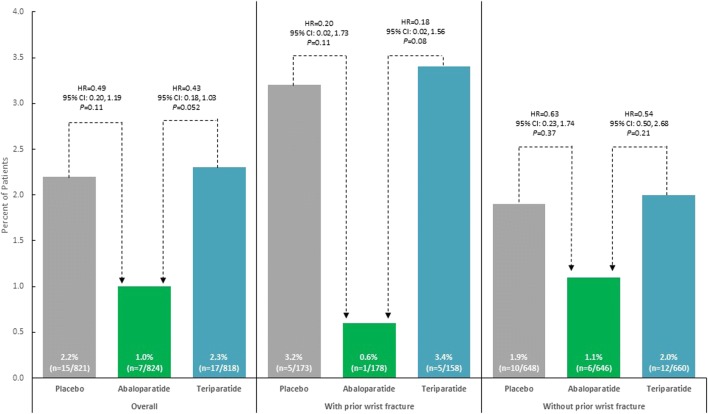
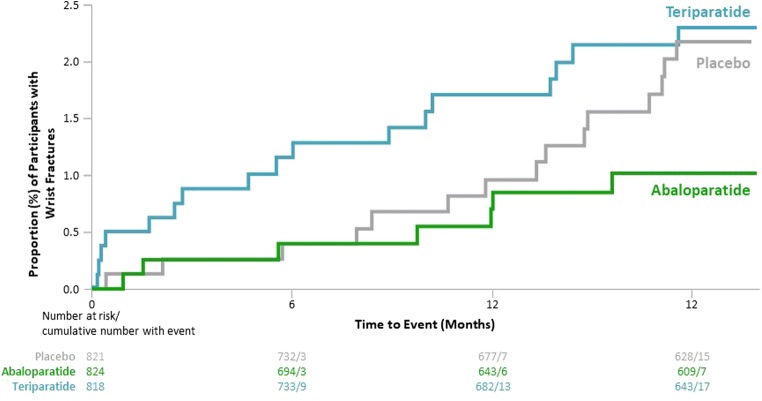
In the total cohort, there were 7 women with incident wrist fractures in the abaloparatide group (Kaplan-Meier estimate, 1.0%), 15 in the placebo group (2.2%), and 17 in the teriparatide group (2.3%; Fig. 3). All were minimal trauma fragility fractures except 2 in the placebo group that were assessed as due to trauma. There was a numerically, although not statistically significant, lower incidence of wrist fracture with abaloparatide compared with teriparatide (HR = 0.43, 95% CI 0.18, 1.03, p = 0.052) and abaloparatide compared with placebo (HR = 0.49, 95% CI 0.20, 1.19, p = 0.11). A similar trend was observed among the subgroup of women with wrist BMD measurements (abaloparatide 2.1%; placebo 3.4%; teriparatide 2.9%), although differences were not statistically significant (Suppl Fig. 1). In the full ACTIVE cohort, there were 509 women who had a history of prior wrist fracture. After 18 months of treatment, the incidence of new wrist fractures was numerically higher for women with versus without prior wrist fracture (Fig. 3). Of those women with a prior wrist fracture, there was 1 woman with a new wrist fracture in the abaloparatide group (Kaplan-Meier estimate, 0.6%), 5 in the placebo group (3.2%), and 5 in the teriparatide group (3.4%). The Kaplan-Meier curve for time to first wrist fracture suggests an early separation between abaloparatide and teriparatide and a longer-term separation between abaloparatide and placebo; however, results should be interpreted with caution since numbers of wrist fractures at these time points were small (Fig. 4).
Fig. 3.
Wrist fractures following 18 months of treatment, by prior history of wrist fracture. The percent of wrist fractures was calculated using cumulative Kaplan-Meier estimates at 19 months. CI, confidence interval; HR, hazard ratio
Fig. 4.
Kaplan-Meier curve for time to first wrist fracture. CI, confidence interval; HR, hazard ratio
Discussion
These analyses from the ACTIVE trial demonstrate that abaloparatide increased BMD at the predominantly trabecular ultradistal radius site more than teriparatide at the early time point (6 months) and the increase with abaloparatide was sustained over 18 months. These results are consistent with greater early increases in lumbar spine BMD observed with abaloparatide compared with teriparatide in the ACTIVE trial [19]. BMD at the cortical 1/3 radius site declined similarly for abaloparatide and placebo but decreased significantly more with teriparatide. We hypothesize that this is due to differences in stimulation of cortical remodeling for the two treatments. Several studies of teriparatide have shown evidence of increased cortical porosity in animal models [21, 22] and clinical studies [23, 24]. Although confirmation with clinical studies is required, abaloparatide has not shown evidence of cortical porosity in rodent or primate animal models [16, 17].
Consistent with the BMD findings, over 19 months, there were fewer wrist fractures with abaloparatide compared with both teriparatide and placebo. The Kaplan-Meier curve for time to first wrist fracture suggests an early separation between abaloparatide and teriparatide, consistent with a greater initial anabolic effect of abaloparatide, as well as perhaps a lesser increase in bone remodeling with abaloparatide. However, these results should be interpreted with caution since numbers of wrist fractures at these time points were small. The lower incidence of wrist fractures seen with abaloparatide (in both women with and without a prior history of wrist fracture) is similar to results observed for nonvertebral, clinical, and major osteoporotic fractures in the ACTIVE study [19].
The combination of BMD changes at the ultradistal radius and lower number of wrist fractures is noteworthy. The 2015 Official Positions of the International Society for Clinical Densitometry (ISCD) recommended against use of any forearm region except the 1/3 radius for diagnosis of osteoporosis [11]. While both the geometric properties of the cortical shell and the mineral densities of trabecular bone have been shown to be of value in predicting fracture risk, BMD at the ultradistal site is an independent predictor of the load needed to cause a fracture [25]. The occurrence of Colles’ fracture is correlated with BMD decrease at the ultradistal radius [26–28], and habitual loading at the ultradistal radius is correlated with improved BMD and bone mineral content (BMC) [20]. More recently, BMD at the ultradistal radius and the femoral neck was shown to improve prediction of hip fractures compared with femoral neck BMD alone, likely due to the combined trabecular and cortical bone parameters [12]. The results of the current analyses are consistent with these observations and suggest a potential role for ultradistal radius BMD assessment in predicting forearm fracture risk.
Teriparatide is effective in reducing vertebral and nonvertebral fractures. Furthermore, there were numerically fewer wrist fractures with teriparatide compared with placebo in the teriparatide registration trial [29]. Some studies predicted that teriparatide treatment increased mechanical strength at the distal radius using geometric parameters [30] and others showed no change in bone strength using finite element analysis [24, 31, 32]. Zanchetta et al. compared parameters of cortical bone quality at the predominantly cortical 15% (mid-distal) radius site using peripheral quantitative computed tomography (pQCT) cross-sectionally after 18 months of placebo, teriparatide 20 μg, or teriparatide 40 μg daily in 38 patients. There were no differences in cortical BMD or cortical thickness among treatment groups [30]. Several groups used high resolution pQCT at the distal radius over 12 to 24 months in small studies of 11 to 30 women receiving teriparatide. The findings from these studies were inconsistent, with one showing a significant decrease in total BMD and trabecular thickness and trends for decreased trabecular bone volume ratio and cortical BMD [24], one showing decreased cortical density and increased cortical porosity (but increased cortical thickness) [31], and one showing decreased cortical BMD and increased cortical porosity [32].
Although conclusions may be limited regarding the effects of teriparatide on these compartments of the wrist, given the small sample size of the studies, the ACTIVE study allows a direct comparison of teriparatide to abaloparatide in a large population of women with postmenopausal osteoporosis [19]. The differences between these two drugs may relate to differential PTH1 receptor binding of abaloparatide compared with teriparatide, allowing for a higher dose of abaloparatide (80 mcg/d) compared with teriparatide (20 mcg/d) and leading to greater stimulation of bone formation and less stimulation of bone resorption [13]. The different effects of these two agents on BMD at the ultradistal radius and 1/3 radius, and on wrist fracture, may relate to increased trabecular BMD and less intracortical bone resorption with abaloparatide compared with teriparatide. Several studies have observed an increase in cortical porosity with teriparatide treatment related to an increase in remodeling [21, 30, 33]. Given the exploratory nature of the current analyses, confirmation of these hypotheses awaits future studies directly comparing the effects of abaloparatide and teriparatide on compartments of trabecular and cortical bone.
There are limitations to these analyses. It is important to note that DXA measurement can be especially challenging at the ultradistal site because bone density changes substantially along the length of the forearm [34]. All analyses are exploratory, and results are therefore hypothesis-generating rather than confirmatory. Teriparatide was administered in an open-label format during ACTIVE, because it could be administered only via its trademarked injection pen. Wrist fracture incidence was low in all treatment arms, and although there were numerical differences, these were not statistically significant. There was a substantially higher proportion of Asian women in the study compared with other ethnicities. Furthermore, there was a patient dropout rate of approximately 25%; and the low incidence of wrist fractures and lack of forearm BMD in the full cohort precludes any assessment of the relationship between BMD changes and fracture risk in this study.
In conclusion, treatment with abaloparatide resulted in greater BMD increases at the more trabecular ultradistal radius compared with both the placebo and teriparatide groups and ultradistal radius BMD was better maintained with abaloparatide compared with teriparatide at the more cortical 1/3 site. These data are consistent with the numerically lower incidence of wrist fracture observed in women treated with abaloparatide compared with both teriparatide and placebo.
Electronic supplementary material
(DOCX 77 kb)
Acknowledgments
We thank Radius Health, Inc., for sponsoring this study. We also thank John L. Stock, MD, for providing medical writing support, funded by Radius Health, Inc.; and Ted Everson, PhD, an employee of Radius Health, Inc., for providing additional medical writing and editorial support.
Compliance with ethical standards
Conflict of interest disclosures
NBW: Founder, Osteodynamics, Speaker, Amgen, Speaker, Shire, Consultant, Abbvie, Consultant, Amgen, Consultant, Janssen Pharmaceuticals, Consultant, Radius Health, Inc., Consultant, Sanofi; LAF, GCW, GH: Employee and company stock, Radius Health, Inc.; YW: Employee, Radius Health, Inc.; PDM: Medical Advisory Board Member, Amgen, Medical Advisory Board Member, AgNovos, Medical Advisory Board Member, Lilly USA, LLC, Medical Advisory Board Member, Merck & Co., Medical Advisory Board Member, Radius Health, Inc., Medical Advisory Board Member, Roche Pharmaceuticals, Medical Advisory Board Member, Ultragenyx, Researcher, Alexion, Researcher, Amgen, Researcher, Boehringer Ingelheim, Researcher, Immunodiagnostics, Researcher, Eli Lilly & Company, Researcher, Merck & Co., Researcher, Merck Serono, Researcher, National Bone Health Alliance, Researcher, Novartis Pharmaceuticals, Researcher, Radius Health, Inc., Researcher, Roche Diagnostics, Researcher, Regeneron, Researcher, Daiichi Sankyo, Researcher, Ultragenyx. FC: Advisor, Lilly USA, LLC; Speaker, Lilly USA, LLC; Researcher, Lilly USA, LLC; Advisor, Amgen; Speaker, Amgen; Researcher, Amgen; Advisor, Radius Health, Inc.; Speaker, Radius Health, Inc., Advisor, Merck & Co.; Consultant, Tarsa.
Ethical review committee statement
The original trial upon which this study is based (Clinicaltrials.gov Identifier: NCT01343004) was performed in accordance with the ethical standards in the 1964 declaration of Helsinki and with relevant regulations of the US Health Insurance Portability and Accountability Act (HIPAA).
Footnotes
Radius Health, Inc., performed the work related to this study.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/30/2020
The original version of this article, published on 21 March 2019, unfortunately contained a mistake.
Change history
6/12/2020
The original version of this article, published on 21 March 2019, unfortunately contains some typos in Figs.��2, 3, 4, and Supplemental Fig.��1. The corrected figures are given below.
References
- 1.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. JBMR. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BJ, Song J, Dunlop DD, Fink HA, Cauley JA. Functional decline after incident wrist fractures-study of osteoporotic fractures: prospective cohort study. BMJ. 2010;341:c3324. doi: 10.1136/bmj.c3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litwic A, Lekarz, Warwick D, Dennison E. Distal radius fracture: cinderella of the osteoporotic fractures. Orthopedic Muscul Syst. 2014;3:162. [Google Scholar]
- 5.Rozental TD, Branas CC, Bozentka DJ, Beredjiklian PK. Survival among elderly patients after fractures of the distal radius. J Hand Surg. 2002;27A:948–952. doi: 10.1053/jhsu.2002.36995. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA) Osteoporos Int. 2008;19:607–613. doi: 10.1007/s00198-007-0508-8. [DOI] [PubMed] [Google Scholar]
- 7.Crandall CJ, Hovey KM, Cauley JA, Andrews CA, Curtis JR, Wactawski-Wende J, Wright NC, Li W, LeBoff MS. Wrist fracture and risk of subsequent fracture: findings from the Women’s Health Initiative Study. J Bone Miner Res. 2015;30:2086–2095. doi: 10.1002/jbmr.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuddihy MT, Gabriel SE, Crowson CS, O'Fallon WM, Melton LJ., III Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int. 1999;9:469–475. doi: 10.1007/s001980050172. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb CA, Yin Y, Gilula LA, Fisher AJ, Boyer MI. Wrist fractures: what the clinician wants to know. Radiology. 2001;219:11–28. doi: 10.1148/radiology.219.1.r01ap1311. [DOI] [PubMed] [Google Scholar]
- 10.Schlenker RA, von Seggen WW. The distribution of cortical and trabecular bone mass along the lengths of the radius and ulna and its implications for in vivo bone mass measurements. Calcif Tissue Res. 1976;20:41–52. doi: 10.1007/BF02546396. [DOI] [PubMed] [Google Scholar]
- 11.International Society for Clinical Densitometry. 2015 Official positions. Available at https://www.iscd.org/official-positions/. Accessed November 20, 2017
- 12.Biver E, Durosier-Izart C, Chevalley T, van Rietbergen B, Rizzoli R, Ferrari S. Evaluation of radius microstructure and areal bone mineral density improves fracture prediction in postmenopausal women. J Bone Miner Res. 2018;33:328–337. doi: 10.1002/jbmr.3299. [DOI] [PubMed] [Google Scholar]
- 13.Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016;157:141–149. doi: 10.1210/en.2015-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leder BZ, O’Dea LSL, Zanchetta JR, Kumar P, Banks K, McKay K, Lyttle CR, Hattersley G. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2014;100:697–706. doi: 10.1210/jc.2014-3718. [DOI] [PubMed] [Google Scholar]
- 15.Bahar H, Gallacher K, Downall J, Nelson CA, Shomali M, Hattersley G. Six weeks of daily abaloparatide treatment increased vertebral and femoral bone mineral density, microarchitecture and strength in osteopenic ovariectomized rats. Calcif Tissue Int. 2016;99:489–499. doi: 10.1007/s00223-016-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyle N, Varela A, Haile S, Guldberg R, Kostenuik PJ, Ominsky MS, Smith SY, Hattersley G. Abaloparatide, a novel PTH receptor agonist, increased bone mass and strength in ovariectomized cynomolgus monkeys by increasing bone formation without increasing bone resorption. Osteoporos Int. 2018;29:685–697. doi: 10.1007/s00198-017-4323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varela A, Chouinard L, Lesage E, Smith SY, Hattersley G. One year of abaloparatide, a selective activator of the PTH1 receptor, increased bone formation and bone mass in ovariectomized osteopenic rats without increasing bone resorption. J Bone Miner Res. 2017;32:24–33. doi: 10.1002/jbmr.3003. [DOI] [PubMed] [Google Scholar]
- 18.Bilezikian JP, Hattersley G, Fitzpatrick LA, Harris AG, Shevroja E, Banks K, Leder BZ, Zanchetta JR, Hans D. Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): a 24-week randomized clinical trial. Osteoporos Int. 2018;29:323–328. doi: 10.1007/s00198-017-4304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C, Study Investigators ACTIVE. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316:722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 20.Bareither ML, Grabiner MD, Troy KL. Habitual site-specific upper extremity loading is associated with increased bone mineral of the ultradistal radius in young women. J Women’s Health. 2008;17:1577–1581. doi: 10.1089/jwh.2008.0888. [DOI] [PubMed] [Google Scholar]
- 21.Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM. Intermittently administered human parathyroid hormone (1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2001;16:157–165. doi: 10.1359/jbmr.2001.16.1.157. [DOI] [PubMed] [Google Scholar]
- 22.Sato M, Westmore M, Ma YL, Schmidt A, Zeng QQ, Glass EV, Vahle J, Brommage R, Jerome CP, Turner CH. Teriparatide [PTH(1-34)] strengthens the proximal femur of ovariectomized nonhuman primates despite increasing porosity. J Bone Min Res. 2004;19:623–629. doi: 10.1359/JBMR.040112. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–1941. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int. 2011;22:357–362. doi: 10.1007/s00198-010-1226-1. [DOI] [PubMed] [Google Scholar]
- 25.Augut P, Reeb H, Claes LE. Prediction of fracture load at different skeletal sites by geometric properties of the cortical shell. J Bone Miner Res. 1996;11:1356–1363. doi: 10.1002/jbmr.5650110921. [DOI] [PubMed] [Google Scholar]
- 26.Eastell R, Riggs BL, Wahner HW, O'Fallon WM, Amadio PC, Melton LJ., III Colles’ fracture and bone density of the ultradistal radius. J Bone Miner Res. 1989;4:607–613. doi: 10.1002/jbmr.5650040419. [DOI] [PubMed] [Google Scholar]
- 27.Kanterewicz E, Yañez A, Pérez-Pons A, Codony I, Del Rio L, Díez-Pérez A. Association between Colles’ fracture and low bone mass: age-based differences in postmenopausal women. Osteoporos Int. 2002;13:824–828. doi: 10.1007/s001980200114. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Poest Clement E, Patka P, Vandormael K, Haarman H, Lips P. The effect of alendronate on bone mass after distal forearm fracture. J Bone Miner Res. 2000;15:586–593. doi: 10.1359/jbmr.2000.15.3.586. [DOI] [PubMed] [Google Scholar]
- 29.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 30.Zanchetta JR, Bogado CE, Ferretti JL, Wang O, Wilson MG, Sato M, Gaich GA, Dalsky GP, Myers SL. Effects of teriparatide [recombinant human parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18:539–543. doi: 10.1359/jbmr.2003.18.3.539. [DOI] [PubMed] [Google Scholar]
- 31.Hansen S, Hauge EM, Jensen J-EB, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zolendronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28:736–745. doi: 10.1002/jbmr.1784. [DOI] [PubMed] [Google Scholar]
- 32.Tsai JN, Uihlein AV, Burnett-Bowie SM, Neer RM, Derrico NP, Lee H, Bouxsein ML, Leder BZ. Effects of two years of teriparatide, denosumab, or both on bone microarchitecture and strength (DATA-HRpQCT study) J Clin Endocrinol Metab. 2016;101:2023–2030. doi: 10.1210/jc.2016-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano T, Burr DB, Turner CH, Sato M, Cain RL, Hock JM. Anabolic effects of human biosynthetic parathyroid hormone fragment (1-34), LY 333334, on remodeling and mechanical properties of cortical bone in rabbits. J Bone Miner Res. 1999;14:536–545. doi: 10.1359/jbmr.1999.14.4.536. [DOI] [PubMed] [Google Scholar]
- 34.Augat P, Fuerst T, Genant HK. Quantitative bone mineral assessment at the forearm: a review. Osteoporos Int. 1998;8:299–310. doi: 10.1007/s001980050068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 77 kb)