Highlights
-
•
Dry needling (DN) is commonly used for Orofacial pain treatment.
-
•
DN is better than other interventions for pain in the short-term.
-
•
DN is better than sham therapy on pressure pain threshold in the short-term.
-
•
Due to the very low quality of evidence and the small effect size, caution is needed.
-
•
Randomized controlled trials of high methodological quality are needed.
Keywords: Dry needling, Orofacial pain, Temporomandibular disorder, Myofascial pain, Trigger point
Abstract
Background
Orofacial pain of myofascial origin is often associated with temporomandibular joint dysfunction, affects chewing muscles and may lead to functional limitations. Dry needling is an intervention commonly used for inactivating myofascial pain trigger points.
Objective
To systematically review the effects of dry needling on orofacial pain of myofascial origin in patients with temporomandibular joint dysfunction.
Methods
This systematic review has pain intensity as primary outcome. Searches were conducted on April 13th, 2018 in eight databases, without publication date restrictions. We selected randomized controlled trials published in English, Portuguese, or Spanish, with no restrictions regarding subject ethnicity, age or sex.
Results
Seven trials were considered eligible. There was discrepancy among dry needling treatment protocols. Meta-analysis showed that dry needling is better than other interventions for pain intensity as well as than sham therapy on pressure pain threshold, but there is very low-quality evidence and a small effect size. There were no statistically significant differences in other outcomes.
Conclusion
Clinicians can use dry needling for the treatment of temporomandibular joint dysfunction, nevertheless, due the low quality of evidence and high risk of bias of some included studies, larger and low risk of bias trials are needed to assess the effects of dry needling on orofacial pain associated with temporomandibular joint dysfunction.
Introduction
Orofacial pain is a condition that affects both the soft and mineralized tissue of the oral cavity and face.1 Myofascial pain is the second most recurrent type of orofacial pain, it is estimated that 33% of people have symptoms in the face and chewing muscles.1 This condition is frequently associated with temporomandibular joint dysfunction (TMD), which also involves chewing muscles, periauricular area and related structures.2 The incidence of TMD in the United States is estimated in 3.9% per year,3 and the prevalence of pain related TMD in the Brazilian population is 25.6%.2
It is generally assumed that myofascial pain related to TMD4 may originate from trigger points (TP), which are characterized by hypersensitivity of a palpable nodule or taut band and pain due to local muscle contraction, which can reduce range of motion.5
The treatment of myofascial pain is often based on inactivating the TP,6 and therefore, a number of non-invasive methods have been used, including ischemic compression, passive stretching, transcutaneous electrostimulation nerve stimulation, massage, biofeedback, ultrasound, infrared laser, and cognitive behavioral therapy.7, 8
In addition to these methods, a minimally invasive technique called dry needling (DN) has been developed in recent decades.7 DN involves applying sterile monofilament needles that penetrate the skin and muscles, stimulating points underlying the TP region in order to regulate neuromuscular pain and movement deficits.1
Despite the widespread use of DN, there is no conclusive evidence of its effectiveness for treating orofacial pain related to the chewing muscles.9 We aimed to systematically review to investigate the effects of DN on orofacial pain of myofascial origin in patients with TMD.
Methods
To summarize the evidence about DN and orofacial pain, a systematic review of randomized controlled trials was conducted. We followed the recommendations of Cochrane Handbook for Systematic Reviews of Interventions10 as well as the tutorial of systematic reviews of the Brazilian Journal of Physical Therapy.11 This systematic review was prospectively registered on the PROSPERO database (CRD42017048449).
Eligibility criteria
To be considered eligible for this systematic review, the studies had to be randomized controlled trials comparing DN with placebo/sham therapy or other interventions, published in English, Spanish or Portuguese, and had to address the effects of DN on orofacial pain of myofascial pain origin, without restrictions on publication date or on subject age, sex or ethnicity. Exclusion criteria included acupuncture or wet needling in the intervention group, the inclusion of subjects with neurologic, rheumatic, vascular, metabolic or neoplastic diseases or the involvement of surgical procedures in the orofacial region.
Search strategy
The search was conducted on April 13th, 2018 in MEDLINE (via Pubmed), LILACS (via BVS), CINAHL (via EBSCO), Physiotherapy Evidence Database (PEDro), The Cochrane Central Register of Controlled Trials (by using Cochrane CENTRAL), SCOPUS, Web of Science and ProQuest.
Initially, a search was performed in Pubmed using the following search strategy: ((((((acup*) OR needles[MeSH Terms]) OR dry needling) AND facial pain[MeSH Terms]) OR temporomandibular joint disorder[MeSH Terms]) OR trigger points[MeSH Terms]) OR myofascial pain syndrome[MeSH Terms] AND (randomized controlled trial[Publication Type] OR (randomized[Title/Abstract] AND controlled[Title/Abstract] AND trial[Title/Abstract])). After this trial, the search strategy was deemed adequate for use in the other databases.
Study selection
In order to identify potentially eligible studies, two reviewers (MBA and MLN) independently selected the articles. After the articles had been selected according to title, the abstracts were analyzed, and articles meeting the eligibility criteria were read in full. The reviewers compared the studies and, in case of disagreement, a third reviewer (MAB) arbitrated. Any disagreements about a study's eligibility were decided by a consensus meeting.
Data collection process
Two reviewers extracted the data from eligible studies independently using a standard form for data extraction. All analyses were conducted in Review Manager (RevMan) software (version 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark).12 The following information was extracted: authors; year of publication; study objective, characteristics of the participants (sex, age, diagnosis, symptom length); description of the intervention and control groups; description of outcomes (orofacial pain assessment instruments, pressure pain threshold (PPT) and pain-free maximum mouth opening (MMO)), type of comparison, description of the results, follow-up and studies included and excluded in the meta-analysis. The data extracted from all included studies are shown in Table 1.
Table 1.
Summary of randomized clinical trials included in the review.
| Study, year | Participants | Intervention group | Intervention protocol | Comparison group | Outcome measure | Results | Follow-up | Meta-analysis |
|---|---|---|---|---|---|---|---|---|
| Gonzalez-Perez et al., 201520 | n = 48; 19F; 5M Age: 18–65 Pain: ≥6 m | DN lateral pterygoid. | LTN: 1 min Freq: 1 p/w Duration: 3 w |
Methocarbamol; paracetamol. | Pain intensity. MMO. |
Pain intensity 28 d (p < 0.001); 70 d (p = 0.011). MMO (p > 0.05). | 28/70 d | |
| Silva et al., 201221 | n = 20; 20F Age: NS Pain: NS | DN masseter. | LTN: NS Freq: 1 |
DN; lidocaine injection. | Pain intensity. PPT. | Pain intensity (p > 0.05). PPT (p > 0.05). |
24 h 7/15 d 21/30 d |
|
| Itoh et al., 201222 | n = 16; 5F; 11M Age: 19–24 Pain: ≥6 m | DN masseter, temporalis, lateral pterygoid, DM, SM, SCM, TM. | LTN: 30 min Freq: 1 p/w Duration: 5 w |
Sham DN. | Pain intensity. MMO. | Pain intensity (p = 0.003). MMO (p = 0.236). | 5/10 w | X |
| Diraçoglu et al., 201216 | n = 52; 45F; 7M Age: 18–57 Pain: ≥1 m e ½ | DN masseter, temporalis. Pain education. |
LTN: NS Freq: 1 p/w Duration: 3 w |
Sham DN. Pain education. |
Pain intensity. PPT. MMO. |
Pain intensity (p = 0.478). PPT (p < 0.001). MMO (p = 0.411). |
10 w | |
| Fernandez et al., 201017 | n = 12F Age: 20–41 Pain: ≥6 m | DN masseter. | LTN: NS. Freq: 1 p/w Duration: 2 w |
Sham DN. | Pain intensity. PPT. MMO. |
Pain intensity (p < 0.001). PPT (p < 0.001). MMO (p < 0.001). |
X | |
| McMillian et al., 199718 | n = 30F Age: 20–50 Pain: ≥3 m | DN masseter; sham procaine injection. | LTN: 1–2 min. Freq: 1 p/w Duration: 3 w |
G1: Procaine injection; sham DN. G2: Sham DN; sham procaine injection. |
Pain intensity. PPT. |
Pain intensity (p > 0.05). PPT (p > 0.05) |
X | |
| Uemoto et al., 201319 | n = 21F Age: 20–52 Pain: NS | DN right masseter; lidocaine injection; SOM 10's. | LTN: NS Freq: 3 (72/48/72 h) |
G1: Laser masseter right (do: 4 J/cm2); left (do: 8 J/cm2); SOM 10's. G2: placebo laser; SOM 10's. |
Pain intensity. PPT. MMO. |
There was no comparison between groups. | X | X |
m, months; NS, no specified; DM, digastric muscle; SM, splenius muscle; SCM, sternocleidomastoid muscle; TM, trapezius muscle; LTN, length of time of the needle; Freq, frequency; p/, per; w, week; D, day; do, dose; G, comparison group; SOM, stretching open mouth; PPT, pressure pain threshold; MMO, pain-free mouth opening.
Risk of bias in individual and across studies
The studies were evaluated individually for their risk of bias. The risk of bias was assessed using the Cochrane Collaboration risk of bias assessment tool, which rates studies as high, low or unclear risk of bias.10, 13 Two reviewers (CV and MBA) assessed randomization, allocation, blinding, incomplete data outcome, selective reporting and other bias, the assessment was standardized following the Cochrane Handbook for Systematic Reviews of Interventions.10 In case of any disagreement, it solved by discussion, if the disagreement persists, a third assessor (MAB) arbitrated.
Thereafter, the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was used to measure the quality of evidence of each outcome. The GRADE system consists of five items: (1) study limitations (risk of bias); (2) inconsistency of results (heterogeneity); (3) indirectness of evidence; (4) imprecision of the effect estimates and (5) reporting bias. The quality of the evidence was classified into four categories: high, moderate, low and very low.14 The assessment were made by two reviewers (CV and MBA) and if any disagreement were found, it was discussed, if there was no agreement between the reviewers, a third reviewer (MAB) arbitrated. The criteria used to downgrade the quality of evidence was based on informations given by the GRADE system and on the recommendations made by Atkins et al.15 and Balshem et al.14
Summary measures
For this systematic review the pain intensity was considered as primary outcome, PPT and pain-free MMO were considered as secondary outcomes.
Synthesis of results
Data analyses were performed to determine the treatment effects of DN compared to sham therapy or other interventions. When necessary, the outcome measures were converted to a 10-point scale of orofacial pain intensity, Newtons or pounds were converted to kg/cm2 for PPT and MMO was converted to a 100 mm scale. The mean differences (MD) and 95% Confidence Interval (CI) were then computed. Analyses were carried out at 3 assessment points, with data from the included studies classified according to the following intervals: (1) short-term follow-up (up to 3 months), (2) medium-term follow-up (3–6 months), and (3) long-term follow-up (beyond 6 months). Results with ρ-values lower than 0.05 were considered statistically significant. Clinical relevance was determined according to the effect size: small = MD < 1 (i.e. 1 point in NPRS or 10% of 10-mm VAS), medium = MD < 2 and large = MD ≥ 2.
Meta-analysis
Meta-analysis of data across trials was conducted when the studies reported the same outcomes and used similar outcome measures that could be transformed to an equivalent unit measure. Review Manager (RevMan) software (version 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark)12 was used to conducted the meta-analysis.
Heterogeneity of studies
Heterogeneity among studies was reported using the I2 statistic, defined in the Cochrane handbook as follows: 0–40% might not be important; 30–60% may represent moderate heterogeneity; 50–90% may represent substantial heterogeneity; 75–100% represents considerable heterogeneity.
Results
Study selection
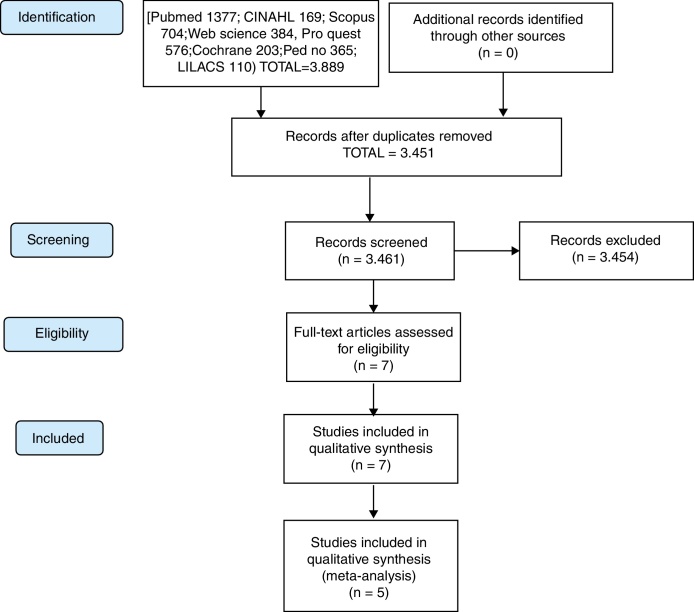
As summarized in Fig. 1, a total of 3889 studies were found and 428 duplicates were removed. The 3461 remaining studies were filtered by title and abstract, of which seven articles were selected for full reading. Thus, seven16, 17, 18, 19, 20, 21, 22 articles met the eligibility criteria and were included in this review, although only five16, 21 were included in the meta-analysis, since the data in two studies19, 22 was insufficient. All authors were contacted by email to clarify questions about their data, although, unfortunately, none responded.
Figure 1.
Flow diagram of included articles.
Study characteristics
The studies were published between 1997 and 2015. Their sample sizes ranged from 12 to 52 individuals (pooled n = 199) whose ages ranged from 18 to 65 years. Myofascial TMD was diagnosed using the following instruments: Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD),17, 20, 21, 23 HELKIMO – Clinical Dysfunction for Temporomandibular Disorders Index I e III,22 the International Headache Society's myofascial pain classifications18 and algometry.16 The duration of orofacial pain symptoms ranged from 1½ months to 5 years. Follow-up ranged from 24 h to 10 weeks.
The studies involved several types of DN protocols. The duration of DN treatment was detailed in only three studies (1–2 min18, 20 and 30 min22). The response to needle manipulation was only specified in three studies,17, 20, 22 i.e. therapist-verified involuntary local muscle contraction after TP needling. DN intervention lasted one,21 three,16, 18, 20 four19 or five22 sessions, with one weekly session being the most prevalent frequency.16, 20, 21, 22 Unfortunately, the poor description of interventions is very common in papers, but it must be avoided and the researchers should start to use TIDiER checklist to do not forget any information about their interventions.24
All studies assessed pain intensity with the Visual Analogue Scale (VAS)16, 18, 20, 21, 22, 23 or the Numeric Pain Rating Scale (NPRS).17 PPT was measured with algometry, although two studies20, 22 did not evaluate this outcome. Pain-free MMO measurement was performed with a millimeter ruler16, 17, 20, 22 or caliper,19 although two studies18, 21 did not assess MMO.
Only five studies16, 22 compared DN with sham/placebo. DN was also compared with an methocarbamol (380 mg) and paracetamol (300 mg),20 lidocaine injection21 and procaine injection.18 Uemoto et al.,19 who used infrared laser in their control group, did not compare it with DN and were thus excluded from the meta-analysis.
All study characteristics and the results of the individual studies are shown in Table 1.
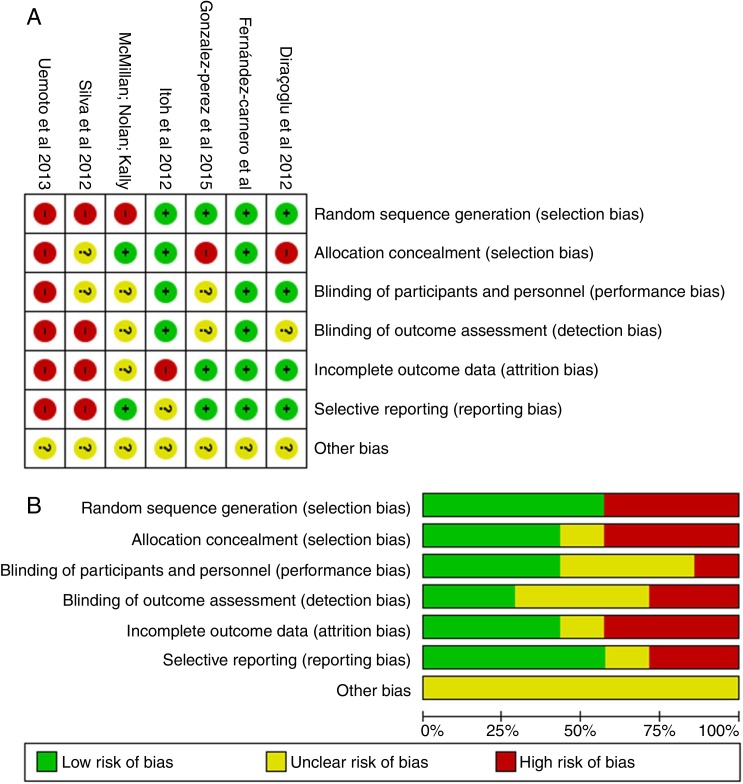
Risk of bias within studies
The risk of bias in the included articles was considered high, as shown in Fig. 2(A). The main risk of bias was related to the blinding of outcome assessment.
Figure 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study (A) and about each risk of bias item presented as percentages across all included studies (B).
Risk of bias across studies
The risk of bias across studies is shown in Fig. 2(B). Random sequence generation, allocation concealment and incomplete outcome data were the most prevalent risk of bias across the eligible trials.
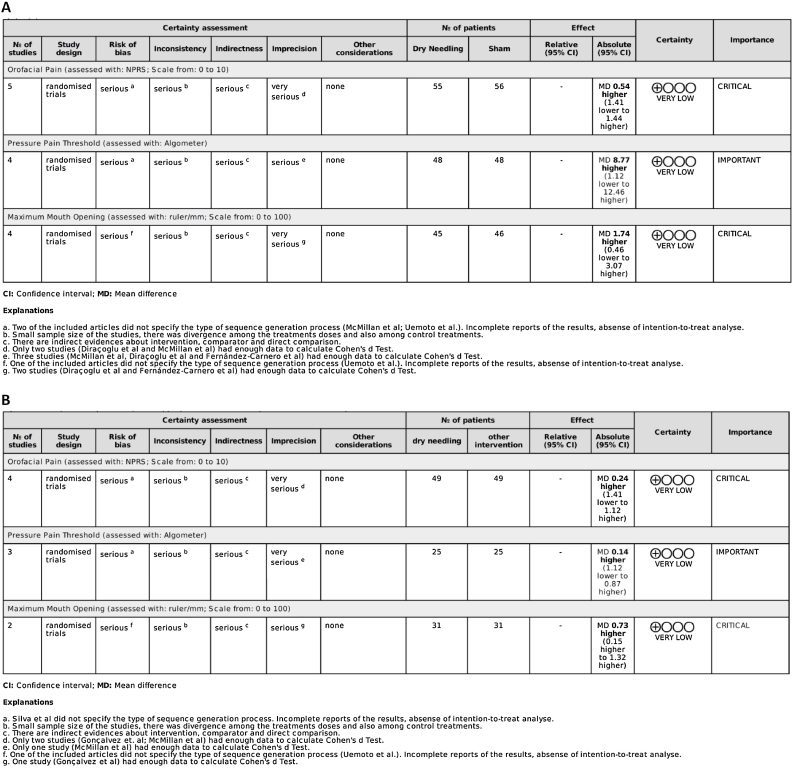
Quality of evidence
Quality assessment is shown in Fig. 3. Study quality was assessed using the GRADE system, which indicated very low quality with serious or very serious methodological problems. Due to missing data, it was not possible to use all the articles to assess any single outcome; at least two were removed in each case (Fig. 3).
Figure 3.
Quality of evidence (GRADE) between Dry Needling versus Sham (A), and Dry Needling versus Other Interventions (B).
Synthesis of the results
Dry needling vs. sham
Pain intensity
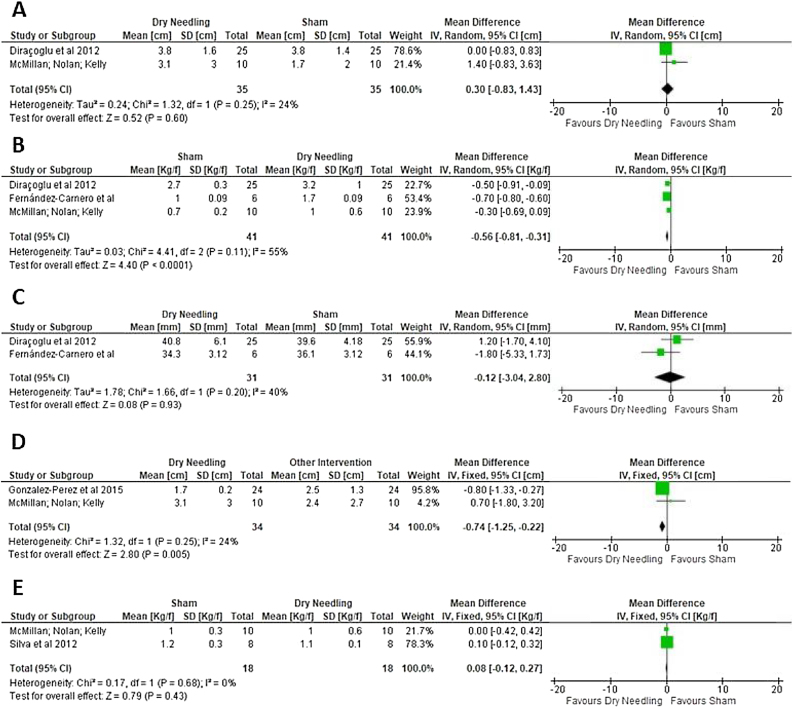
There is very low quality evidence that no statistically significant difference was found between DN and sham for short-term orofacial pain (two trials, pooled n = 70; MD = 0.30; 95% CI = −0.83 to 1.43; I2 = 24%) (Fig. 4A). It was not possible to conduct a meta-analysis of the medium- and long-term effects. Other 2 studies22, 25 assessed this outcome but they were not part of meta-analyses due inconsistency of data, one of these studies22 finding a significant reduction in pain (ρ = 0.003) in the DN group compared to sham.
Figure 4.
Effect of Dry Needling vs. Sham Therapy on Pain (A), Pressure Pain Threshold (B) and Pain-free Maximum Mouth Opening (C), and effect of Dry Needling vs. Other Interventions on Pain (D) and Pressure Pain Threshold (E).
Pressure pain threshold
There is very low quality evidence that DN was better than sham therapy for PPT in the short-term (three trials, pooled n = 82; MD = 0.56; 95% CI = 0.31 to 0.81; I2 = 55%) (Fig. 4B), however, the effect size in favor of DN was considered small (MD < 1). It was not possible to conduct a meta-analysis of medium-term and long-term PPT effects because no study evaluated them. Itoh et al.,22 did not assess this outcome.
Pain-free maximum mouth opening
There is very low quality evidence that no between-group difference was found in short-term pain-free MMO (2 trials, pooled n = 62; MD = 0.12; 95% CI = −3.04 to 2.80; I2 = 40%) (Fig. 4C). Medium- and long-term meta-analysis were not possible to be calculated due any study assessed these follow-ups. Itoh et al.,22 assessed pain-free MMO although they did not find difference between groups (p > 0.05). McMillan et al.,18 did not assessed this outcome.
Dry needling vs. other interventions
Pain intensity
There is very low quality evidence that DN was better than other interventions for short-term pain (2 trials, n = 68; MD = −0.74; 95% CI = −1.25 to −0.22; I2 = 24%) (Fig. 4D), however, the effect size of the treatment was considered small (MD < 1). Meta-analysis for medium- and long-term effects was not possible due to the lack of data. Another two trials19, 21 assessed this outcome, however due the lack of data, they were not included in the meta-analysis, Uemoto et al.,19 found an improvement in pain intensity after DN (p = 0.03), nonetheless Silva et al.,21 did not find significant improvement in this outcome (p > 0.05).
Pressure pain threshold
There is very low quality evidence that there was no statistically significant difference between DN and other interventions for short-term PPT (two trials, pooled n = 36; MD = −0.08; 95% CI = −0.27 to 0.12; I2 = 0%) (Fig. 4E). Meta-analysis was not performed for medium- or long-term effects due to the lack of data on these time-points. One trial19 also assessed PPT but it was not included in the meta-analysis due to the lack of data. Gonzalez-Perez et al.,20 did not assessed this outcome.
Pain-free maximum mouth opening
Meta-analysis of DN and other interventions in the short-term, medium-term and long-term for pain-free MMO was impossible. One study20 assessed this outcome but due lack of data it was impossible to run a meta-analysis. The quality evidence of this outcome was also classify as “very low quality” by the GRADE.
Discussion
This systematic review and meta-analysis of 7 trials (pooled n = 199 individuals) investigated the effectiveness of DN compared to sham therapy,16, 19, 20, 21, 22 other interventions (lidocaine injection,20, 21, 22 procaine injection)18 or combination drug therapy (methocarbamol and paracetamol)20 for orofacial pain, PPT, disability and pain-free MMO in subjects with TMD-related myofascial pain. We found very-low-quality evidence that: (1) compared with sham therapy, DN increases PPT in the short-term, and (2) DN is more effective than other interventions at decreasing pain intensity.
Nevertheless, meta-analysis regarding DN and other interventions could only be applied at short-term follow ups. No meta-analysis was possible for disability and pain-free MMO due to insufficient data. No statistically significant differences were found between DN and sham therapy in the short-term for pain or pain-free MMO. When DN was compared with other interventions for PPT, the same result was found. However, significant differences were found for PPT when DN was compared with sham therapy and for pain intensity when DN was compared with other interventions. These results suggest that DN increases PPT compared with sham therapy and decreases pain intensity compared to other interventions (lidocaine injection and combination drug therapy (methocarbamol and paracetamol)). Finally, no meta-analysis could be conducted for medium- or long-term effects in any outcome because none study assessed medium or long-term follow-up.
Studies in this review that used sham therapy applied needles near the TP site. There is evidence that DN applied in the muscle belly or in the subcutaneous region of the fascia has effects on pain modulation in myofascial syndrome.26, 27, 28 However, it is not clear whether DN is superior to placebo.7 Similarly, studies using minimal/sham acupuncture procedures also suggest that sham acupuncture needles evoke a physiological response.29, 30 Another aspect that may explain why DN did not significantly decrease pain intensity compared to sham therapy may be the poor methodological quality of the included studies and their heterogeneity of protocols.16, 18
The literature reports greater efficacy for laser therapy than dry needling. It has been suggested that laser therapy causes improvement in microcirculation, which would increase the oxygen supply to hypoxic cells and help remove cell metabolism waste products, thus breaking the vicious cycle of pain, muscle spasm and further pain. While laser therapy is the method of choice for patients with a fear of needles and healthcare professionals inexperienced with dry needling techniques, further controlled studies are still needed to conclusively demonstrate the greater efficacy of this method.23
Moreover, all but one of the studies recruited patients with chronic orofacial pain. Although it is known that treating chronic orofacial pain is complex and a biopsychosocial approach is necessary, only one study applied pain education in addition to DN. A systematic review on musculoskeletal conditions31 and another on myofascial TPs32 found similar results about pain intensity and PPT, and both studies also found low-quality-evidence in their included studies.
Conclusion
The studies included in this systematic review suggest that DN is better than sham therapy for PPT and better than other interventions for pain intensity in the short-term. Nevertheless, due to the very low quality of evidence, DN cannot be strongly recommended over sham therapy or other interventions.
To date, there is insufficient data to draw strong conclusions about DN for the treatment of orofacial pain associated with TMD. Randomized controlled trials of low risk of bias are strongly needed.
Conflicts of interest
Marcos Lisboa Neves and Marina Barbosa de Almeida use dry needling therapy in clinical practice and provide postgraduate training programs. The others authors declare no conflicts of interest.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.5th ed. Quintessence Publishing Co, Inc; Chicago: 2013. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. [Google Scholar]
- 2.Gonçalves D.A.d.G., Dal Fabbro A.L., Campos J.A.D.B., Bigal M.E., Speciali J.G. Symptoms of temporomandibular disorders in the population: an epidemiological study. J Orofac Pain. 2010;24(3):270–278. http://www.ncbi.nlm.nih.gov/pubmed/20664828 Accessed 14.05.18. [PubMed] [Google Scholar]
- 3.Slade G.D., Bair E., Greenspan J.D. 2013. Signs and Symptoms of First-Onset TMD and Sociodemographic Predictors of Its Development: The OPPERA Prospective Cohort Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalichman L., Vulfsons S. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med. 2010;23(5):640–646. doi: 10.3122/jabfm.2010.05.090296. [DOI] [PubMed] [Google Scholar]
- 5.Travell J.G., Simons D.G. Williams & Wilkins; Baltimore: 1983. Myofascial Pain and Dysfunction: The Trigger Point Manual. [Google Scholar]
- 6.Zhuang X., Tan S., Huang Q. Understanding of myofascial trigger points. Chin Med J (Engl) 2014;127(24):4271–4277. [PubMed] [Google Scholar]
- 7.Cummings T.M., White A.R. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil. 2001;82(7):986–992. doi: 10.1053/apmr.2001.24023. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Reyes M., Uyanik J.M. Orofacial pain management: current perspectives. J Pain Res. 2014;99(February) doi: 10.2147/JPR.S37593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sport Phys Ther. 2013;43(9):620–634. doi: 10.2519/jospt.2013.4668. [DOI] [PubMed] [Google Scholar]
- 10.Wiley-Blackwell; Chichester, England; Hoboken, NJ: 2008. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 11.Mancini M.C., Cardoso J.R., Sampaio R.F. Tutorial for writing systematic reviews for the Brazilian Journal of Physical Therapy (BJPT) Braz J Phys Ther. 2014;18(6):471–480. doi: 10.1590/bjpt-rbf.2014.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Review Manager, (RevMan) [Computer program] Version 5.3. 2014.
- 13.Higgins J.P.T., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(October (2)):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balshem H., Helfand M., Schünemann H.J. GRADE guidelines: 3 Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Atkins D., Best D., Briss P.A. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dıraçoğlu D., Vural M., Karan A., Aksoy C. Effectiveness of dry needling for the treatment of temporomandibular myofascial pain: a double-blind, randomized, placebo controlled study. J Back Musculoskelet Rehabil. 2012;25(4):285–290. doi: 10.3233/BMR-2012-0338. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Carnero J., La Touche R., Ortega-Santiago R. Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J Orofac Pain. 2010;24(1):106–112. [PubMed] [Google Scholar]
- 18.McMillan A.S., Nolan A., Kelly P.J. The efficacy of dry needling and procaine in the treatment of myofascial pain in the jaw muscles. J Orofac Pain. 1997;11(4):307–314. http://www.ncbi.nlm.nih.gov/pubmed/9656906. [PubMed] [Google Scholar]
- 19.Uemoto L., Garcia M.A.C., Gouvêa C.V.D., Vilella O.V., Alfaya T.A. Laser therapy and needling in myofascial trigger point deactivation. J Oral Sci. 2013;55(2):175–181. doi: 10.2334/josnusd.55.175. http://www.ncbi.nlm.nih.gov/pubmed/23748458. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Perez L., Infante-Cossio P., Granados-Nunez M., Urresti-Lopez F., Lopez-Martos R., Ruiz-Canela-Mendez P. Deep dry needling of trigger points located in the lateral pterygoid muscle: efficacy and safety of treatment for management of myofascial pain and temporomandibular dysfunction. Med Oral Patol Oral y Cir Bucal. 2015;(January):e326–e333. doi: 10.4317/medoral.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva R.O.F.d., Conti P.C.R., Araújo C.d.R.P., Silva R.d.S. Evaluation of dry needling and 0.5% lidocaine injection therapies in myofascial pain trigger points in masticatory muscles. Dental Press J Orthod. 2012;17(2):113–118. [Google Scholar]
- 22.Itoh K., Asai S., Ohyabu H., Imai K., Kitakoji H. Effects of trigger point acupuncture treatment on temporomandibular disorders: a preliminary randomized clinical trial. J Acupunct Meridian Stud. 2012;5(2):57–62. doi: 10.1016/j.jams.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Uemoto L., de Azevedo R.N., Alfaya T.A., Jardim Reis R.N., Depes de Gouvea C.V., Cavalcanti Garcia M.A. Myofascial trigger point therapy: laser therapy and dry needling. Curr Pain Headache Rep. 2013;17(9):357. doi: 10.1007/s11916-013-0357-4. [DOI] [PubMed] [Google Scholar]
- 24.Yamato T., Maher C., Saragiotto B. The TIDieR checklist will benefit the physical therapy profession. Braz J Phys Ther. 2016;20(3):191–193. doi: 10.1590/bjpt-rbf.2014.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Carnero J., La Touche R., Ortega-Santiago R. Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J Orofac Pain. 2010;24(1):106–112. http://www.ncbi.nlm.nih.gov/pubmed/20213036. [PubMed] [Google Scholar]
- 26.Short-term improvement following dry needle stimulation of tender points in fibromyalgia. Rheumatol Int. 2014;34(6):861–866. doi: 10.1007/s00296-013-2759-3. [DOI] [PubMed] [Google Scholar]
- 27.Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6(1):83–90. doi: 10.1016/0304-3959(79)90142-8. [DOI] [PubMed] [Google Scholar]
- 28.Neal B.S., Longbottom J. Is there a role for acupuncture in the treatment of tendinopathy? Acupunct Med. 2012;30(4):346–349. doi: 10.1136/acupmed-2012-010208. [DOI] [PubMed] [Google Scholar]
- 29.Lundeberg T., Lund I., Sing A., Näslund J. Is placebo acupuncture what it is intended to be? Evid-Based Complement Altern Med. 2011;2011:1–5. doi: 10.1093/ecam/nep049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White A.R., Filshie J., Cummings T.M. Clinical trials of acupuncture: consensus recommendations for optimal treatment, sham controls and blinding. Complement Ther Med. 2001;9(4):237–245. doi: 10.1054/ctim.2001.0489. [DOI] [PubMed] [Google Scholar]
- 31.Gattie E., Cleland J.A., Snodgrass S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: a systematic review and meta-analysis. J Orthop Sport Phys Ther. 2017;47(3):133–149. doi: 10.2519/jospt.2017.7096. [DOI] [PubMed] [Google Scholar]
- 32.Espejo-Antúnez L., Tejeda J.F.-H., Albornoz-Cabello M. Dry needling in the management of myofascial trigger points: a systematic review of randomized controlled trials. Complement Ther Med. 2017;33:46–57. doi: 10.1016/j.ctim.2017.06.003. [DOI] [PubMed] [Google Scholar]