Highlights
-
•
There is a close interaction between insomnia and pain severity/disability.
-
•
CBT-I can improve sleep and pain interference in chronic spinal pain.
-
•
Clinical implication 1: combined approach may improve pain, sleep and function.
-
•
Clinical implication 2: may lead to new treatment guidelines for professionals.
Keywords: Chronic pain, Low back pain, Neck pain, Randomized controlled trial
Abstract
Background
Insomnia is a highly prevalent and debilitating comorbidity that is often not addressed in therapy for chronic spinal pain (CSP). Given the close interaction between insomnia and CSP severity and related disability, targeting sleep problems during therapy could improve treatment outcomes in these patients.
Objective
Can cognitive behavioral therapy for insomnia (CBT-I) combined with the modern neuroscience approach (i.e. pain neuroscience education and cognition-targeted exercise therapy) reduce pain and improve sleep, physical activity and function in people with CSP and comorbid insomnia?
Methods
Participants: One-hundred-twenty participants with chronic spinal pain and comorbid insomnia Intervention: CBT-I combined with the modern neuroscience approach (experimental) compared to the modern neuroscience approach alone (control). Both interventions start with three sessions of pain neuroscience education, followed by six sessions of CBT-I and nine sessions of cognition-targeted exercise therapy in the experimental group, or 15 sessions of cognition-targeted exercise therapy in the control group.
Measurements
Primary outcome measure: self-reported pain severity (Brief Pain Inventory). Secondary outcome measures: pain sensitivity (pressure pain thresholds, and online questionnaires), sleep-related outcomes (home-based polysomnography and online questionnaires), physical activity (actigraphy), and function (online questionnaires). Online questionnaires will be completed at baseline, directly post-treatment, and at 3, 6 and 12 months post-treatment. Polysomnography, pressure pain thresholds and actigraphy will be carried out at baseline, post-treatment and at 12 months follow-up.
Discussion
Findings may provide (1) a novel therapeutic approach for people with CSP and comorbid insomnia to improve pain, sleep, physical activity and function, and (2) new treatment guidelines for professionals.
Trial registration
Clinicaltrials.gov NCT03482856 (https://clinicaltrials.gov/ct2/show/NCT03482856).
Introduction
Chronic spinal pain (CSP) includes chronic low back pain, nonspecific failed back surgery syndrome (i.e. anatomically successful operation conducted more than 3 years ago, without symptom disappearance), chronic whiplash, or chronic non-traumatic neck pain. CSP is a major public health problem worldwide, and is associated with high rates of disability, high socioeconomic impact and costs, and low quality of life.1, 2, 3 Despite the scientific progress in the treatment of CSP, current treatments do not address prevalent comorbidities like insomnia.4 If present, insomnia contributes substantially to CSP severity and related disability.4 Research findings show that sleep disturbances have a bidirectional relation with CSP,5, 6 and that sleep problems may act as both a precipitating and a perpetuating factor.7 This suggests that improving night-time sleep may be of added value for successful CSP treatment in those patients with comorbid insomnia. If left untreated, insomnia may be a major barrier to effective CSP management.7, 8 Conservative and pharmacological strategies often do not address insomnia,9, 10, 11 urging the need for studies that examine the value of addressing insomnia in CSP during physical therapy treatment.
Cognitive behavioral therapy for insomnia (CBT-I) is the standard evidence-based care for treating chronic primary insomnia in general,12 and in people with CSP.7 CBT-I typically includes education about sleep, changing negative thoughts and beliefs about sleep, sleep hygiene (i.e. promoting good sleep and lifestyle habits and optimizing sleep environments), stimulus control, sleep restriction therapy, and relaxation exercises.7, 12, 13 Importantly, a recent systematic review demonstrated that specially trained physical therapists can deliver such behavioral interventions and were able to reduce pain and improve disability and quality of life in people with low back pain, which demonstrates feasibility of implementation.14
Although evidence supporting the use of CBT-I in people with CSP is scarce, a proof-of-concept study found that CBT-I was successful (moderate to large effect sizes) for improving sleep and the extent to which pain interfered with daily functioning in people with CSP.7 However, the study sample was small (n = 9), treatment arms were unbalanced, and only subjective measures of sleep were used. Therefore, replication in a larger, multi-center trial is required. In addition, CBT-I is not a standalone treatment for CSP, but should be integrated within the available evidence-based treatment for CSP.15 Preliminary evidence to support the combination of CBT-I with cognition-targeted treatment for chronic pain comes from two small-scale pilot studies (n = 21 and 20 respectively) that found significant long-term improvements in sleep, disability, pain interference, depression and fatigue.5, 16 These studies were conducted on samples of patients with disparate chronic pain conditions hampering the generalizability to people with CSP.
This will be the first randomized controlled trial with balanced treatment arms to examine the added value of CBT-I to current best evidence physical therapy for people with CSP and insomnia. The primary scientific objective of this study is to examine whether CBT-I combined with the modern neuroscience approach (i.e. pain neuroscience education plus cognition-targeted exercise therapy [CTET])15 is superior to the modern neuroscience approach alone to reduce pain (primary outcome measure). The effectiveness of the modern neuroscience approach alone was recently established.15 The secondary scientific objective is to examine if the experimental treatment is also more effective in this population on a range of: pain-related outcomes, sleep-related outcomes, physical activity and function (secondary outcome measures) in people with CSP and comorbid insomnia.
Methods
Trial design
This study will be a multi-center randomized controlled trial, organized at the University Hospital Ghent and University Hospital Brussels. Results from this randomized controlled trial will be reported according to the CONSORT guidelines.17
Participants
One-hundred-twenty patients with CSP and comorbid insomnia will be recruited. Participants will be recruited from the participating hospitals, primary care, advertisements and announcements in local newspapers, pharmacies, and (online or printed) publications from patient support groups. Inclusion and exclusion criteria can be found in Table 1. People living or working outside a radius of 50 km around the therapy location will be excluded to avoid drop-out.
Table 1.
In- and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
| Nonspecific spinal pain for at least 3 months’ duration, at least 3 days/week | Severe underlying sleep pathology (identified through baseline data of polysomnography) |
| Aged between 18 and 65 years | Neuropathic pain |
| Seeking care because of neck pain or low back pain | Chronic widespread pain syndromes |
| Native Dutch speaker | Shift workers |
| Having insomnia: in the absence of other intrinsic sleep disorders and shift work, insomnia is defined as >30 min of sleep latency and/or minutes awake after sleep onset for >3 days/week for >6 months | Being pregnant or pregnancy (including given birth) in the preceding year |
| Not starting new treatments or medication and continuing their usual care 6 weeks prior to and during study participation (to obtain a steady state) | Thoracic pain in absence of neck or low back pain |
| Refraining from analgesics, caffeine, alcohol or nicotine in the previous 48 h of the assessments | History of specific spinal surgery (i.e. surgery for spinal stenosis) |
| Nonspecific failed back surgery >3 years are permitted | Body Mass Index over 30 |
| Not undertaking exercise (>three metabolic Equivalents) 3 days before the assessments | Presence of a current clinical depression diagnosed by a doctor |
Randomization
Participants will be randomized into the control or experimental group. Randomization lists will be prepared by the Biostatistics Unit of Ghent University, which has no other involvement in this study. Randomization lists will be made available separately for both treatment centers (Ghent and Brussels), and be stratified for: sex (male or female) and dominant pain problem (neck pain or low back pain). Randomization will be concealed using opaque, closed envelopes. An independent researcher not involved in the treatment or assessment of the participants will perform the allocation of participants.
Outcome measures
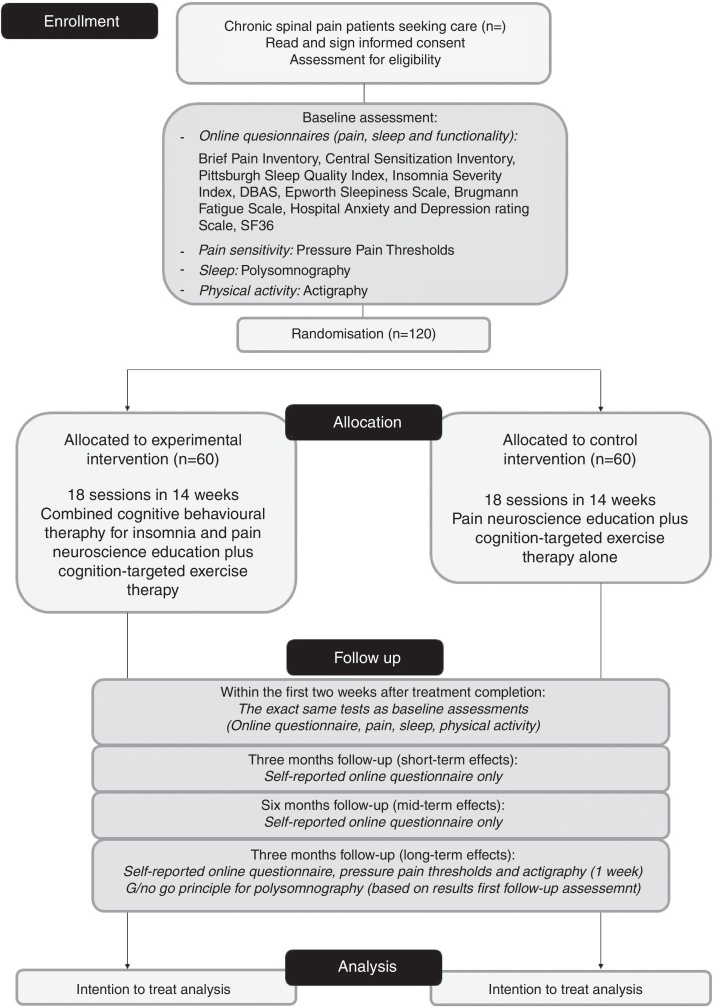
All outcomes will be assessed in line with IMMPACT/OMERACT recommendations,18 and will be carried out at baseline, within 2 weeks after treatment completion, and at 3, 6 and 12 months after the end of treatment (for detailed overview, see Fig. 1). For the final polysomnography assessment, we will apply a Go/No-Go principle: if no significant changes are found in polysomnography directly after treatment (compared to baseline), the polysomnography will not be repeated at 12 months follow-up. All assessments will be performed by the same researcher (TB), who will be blinded to the maximal extent possible to the group allocation and who will have no involvement in the treatment. The assessor will be extensively trained by researchers (MM, OM, JN, AM), who have broad experience applying the outcome measures.
Figure 1.
CONSORT flow chart.
Primary outcome measure
Pain was chosen as primary outcome measure as this is the most relevant outcome measure (and primary care demand) in people suffering from CSP and insomnia.19
The Brief Pain Inventory (BPI) allows patients to rate (1) the intensity of their pain and (2) the impact of pain on functioning.20 Among other things, this brief questionnaire contains four questions investigating pain intensity (the worst pain in the last 24 h, the least pain in the last 24 h, the average pain and pain now). Since chronic pain is a fluctuating condition, the question “please rate your pain by circling the one number that best describes your pain on the AVERAGE” is used as primary outcome measure to evaluate pain intensity on an 11-point (0–10) numeric rating scale. The BPI is recommended as a core outcome measure in clinical trials evaluating chronic pain treatments,18 and its reliability and validity are well-established.21, 22
Secondary outcome measures
Pain related outcomes will be evaluated using online questionnaires and pressure pain thresholds. Besides pain intensity, the BPI also evaluates the pain interference related to general activity, walking, work, mood, enjoyment of life, relation with others and sleep on separate 11-point numeric rating scales. The Central Sensitization Inventory (CSI) will be used to assess self-reported signs of central sensitization and its overlapping symptoms, using 25 statements related to current health symptoms, indicative of central sensitization (scored on a five-point Likert scale ranging from zero to four). A total score of 40 is the cut off value to indicate the presence of central sensitization and the CSI has proven psychometric strength.23, 24, 25
Pressure pain thresholds (i.e. the point of minimum pressure that induces an unpleasant sensation) will be determined using digital pressure algometry (Wagner instruments) applied both at symptomatic levels (trapezius muscle in neck pain patients, lumbar paravertebral muscles in low back pain patients) and at remote sites (web between the thumb and the index, and the proximal one third of the calf).26, 27, 28 The order of test sites will be randomized. The threshold is determined as the mean of two consecutive (30 s in between) measurements.
Sleep related outcomes will be evaluated using six questionnaires and polysomnography. Polysomnography (assessed at home using the portable Alice PDX system, Philips Respironics Inc™) will be used to exclude comorbid sleep disorders (e.g. apnea, restless leg syndrome, etc.) and will provide following parameters: time in bed, total sleep time, sleep onset latency, wake duration after sleep onset, early morning awakening, sleep staging, sleep efficiency, sleep fragmentation, respiratory parameters, cardiac and myoclonic activity. Self-reported sleep will be evaluated using the Pittsburg Sleep Quality Index, the Insomnia Severity Index, the Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-16), the Epworth Sleepiness Scale (to assess sleep propensity), the Brugmann Fatigue Scale (to assess rest propensity), and the Hospital Anxiety and Depression rating Scale (to assess affective symptoms like anxiety and depression, which impact sleep). The Alice PDx and all questionnaires possess satisfactory measurement properties.29, 30, 31, 32, 33, 34, 35
Physical activity (i.e. rest/activity cycles) and function will be assessed using three-axis accelerometer activity monitors (GT9X-BT, Actigraph) and the Short Form Health Survey-36 (SF-36). Actigraphy has been well-validated for the estimation of rest/activity cycles, and the psychometric properties of the SF-36 are well-established.36, 37
Interventions
All interventions will be given by physical therapists with a master's degree. They will receive extensive training in delivery of all intervention components. Pain neuroscience education and CTET will be taught by two researchers with broad clinical and scientific experience in the matter (JN and AM). CBT-I training will be led by a practicing somnologist with extensive experience (OM).
All participants will receive 18 sessions of therapy over a period of 14 weeks (for details see Table 2). Sessions will last 30 min and will be individual (one-on-one) sessions, except for the first group education session, which will take up to 1 h. Participants from different intervention groups (experimental and control) will not be mixed. The content, but not the therapy/therapist exposure time will differ between the intervention groups (see section on ‘Treatment contrast’).
Table 2.
Organization of therapeutic sessions.
| Week | Experimental treatment | Control treatment |
|---|---|---|
| Weeks 1–2 | Session 1–3: Pain Neuroscience Education (one group session, one online session, one individual session) |
Session 1–3: Pain Neuroscience Education (one group session, one online session, one individual session) |
| Weeks 3–14 | Session 4–18: CTET (9 sessions) plus CBT-I (6 sessions) |
Session 4–18: CTET alone (15 sessions) |
CTET = Cognition-targeted exercise therapy; CBT-I = Cognitive behavioral therapy for insomnia.
Experimental intervention
The experimental intervention will comprise three sessions of pain neuroscience education, nine sessions of CTET and six sessions of CBT-I. The combination of pain neuroscience education with CTET will further on be referred to as ‘the modern neuroscience approach’.
Pain neuroscience education aims to reconceptualize the patients’ pain beliefs, to increase the patients’ knowledge of pain and to decrease its threat value. The content will be based on current knowledge of the neurophysiology of pain according to Wall and Melzack,38 ‘Pijneducatie, een praktische handleiding voor de (para)medici’ by Van Wilgen and Nijs,39 and ‘Explain Pain’ by Butler and Moseley,40 including following topics in laymen's terms: (1) the neuron, (2) the synapse, (3) descending nociceptive inhibition and facilitation, (4) peripheral sensitization, and (5) central sensitization. More details on the content and application of pain neuroscience education, can be found elsewhere.15, 41
Individual CBT-I sessions aim to improve sleep (quality), and includes sleep restriction therapy, stimulus control instructions, relaxation techniques, sleep hygiene instructions and cognitive therapy.7 The details on the content of the sleep management program are described in Table 3 5, 7, 12, 42 and elsewhere.13 The content of these sessions will be individually-tailored to the specific needs and case of the patient.
Table 3.
Content of the sleep management program.
| Component | Specific content |
|---|---|
| General sleep education | Explaining the association between pain and sleep, sleep architecture, Processes that regulate sleep, interindividual differences, factors contributing to the development of insomnia, vicious cycle of insomnia. |
| Sleep restriction therapy | The manipulation of homeostatic sleep drive to facilitate sleep initiation and consolidation using sleep restriction, i.e. limiting the amount of time spent in bed to an amount equal to the average sleep time. Once sleep becomes more efficient and robust, total sleep time will be increased incrementally on a week-to-week basis. |
| Stimulus control instructions | Based on the principles of operant and classical conditioning: • Restriction of bedroom behaviors to sleep and sex • Limiting the amount of time spent awake in bed or in the bedroom • Promoting counter-conditioning by ensuring that bed and bedroom environment are tightly coupled with sleepiness and sleep • Promoting a strong association between the bedroom and sleep by allowing sleep to occur uniquely in association with the bedroom |
| Sleep hygiene instructions (i.e. promoting good sleep and lifestyle habits) |
Instructions include: • The replacement of sleep-interfering behaviors with sleep-promoting behaviors through sleep hygiene education and behavior change counseling • Optimizing sleep environments |
| Sleep specific cognitive therapy | Includes: • Modifying maladaptive sleep-related cognitions • Changing negative thoughts about sleep • Decatastrophization to address the perception of dire consequence of sleep loss |
| Relaxation training | Learning to cope better with stress, and ruminating thoughts |
| Self-monitoring of daily sleeping patterns | Daily self-monitoring of time in bed, sleep onset latency, wake after sleep onset, and total sleep time and daytime consequences by using a sleep diary. |
The main principles of CTET include: (1) a time-contingent approach to exercise, (2) continuously targeting the cognitions, beliefs and perceptions of the patient regarding his/her symptoms and the outcome of each exercise, and (3) gradual progression toward feared, avoided, challenging, stressful and functional movements and activities. Details regarding this approach are described elsewhere.43 The specific content of the exercises will be individually-tailored based on the ‘feared activities form’. This form will allow participants to indicate which movements are feared and/or avoided, and will allow the therapist to create a clear hierarchy in the exercises, movements and activities offered. Like in CBT-I, communication techniques are crucial and will be aligned with the content of the pain neuroscience education sessions: exercise as brain therapy rather than a modality to correct a biomechanical deficit. The major part of exercises and movements used in the CTET-program will also be delivered as home exercises. During the physical therapy sessions, during the home exercises and during activities in daily life, it is crucial that the participants avoid all ‘safety behavior’, to focus on a normal and functional way of moving.
Control intervention
The control intervention will comprise the modern neuroscience approach alone, i.e. three session of pain neuroscience education (organized identical to the experimental intervention), and 15 sessions of cognition-targeted exercise therapy. Additional in-house exercise therapy sessions will be added to balance the volume of treatment across the two arms (i.e. 18 therapy sessions in both intervention groups). Like in the experimental treatment, exercises will also be performed at home. This way, the control intervention will be similar to the therapy that was delivered in a previous study on chronic spinal pain,15 comprising 15 sessions of cognition-targeted exercise therapy. This approach was found effective to improve pain, disability, pain cognitions, and physical and mental functioning.15
Treatment contrast
The main difference between both intervention groups is the inclusion of six sessions of CBT-I in the experimental group. Both interventions consist of an equal total number of sessions (i.e. 18), in which the experimental intervention will cover the modern neuroscience approach plus CBT-I (3 sessions education, 9 sessions CTET and 6 sessions CBT-I), while the control group covers only the modern neuroscience approach (3 sessions education plus 15 sessions CTET). Therefore, the main difference is the time spent on CTET and the additional CBT-I content in the experimental group (see Table 2).
Sample size calculation
Based on the design including two treatment arms, a sample size of 120 subjects is needed in the study to observe a moderate significant between-group effect (Cohen's f = 0.25, based on the moderate effect size reported in the pilot study7). This sample size calculation accounts for the primary outcome measure (pain) and a 20% loss to follow-up after 1 year, which is the loss to follow up of a successful previous study that examined the modern neuroscience approach alone compared to usual care physical therapy.15 Calculations were based on two-tailed testing (alpha = 0.05). Allocation ratio (N2/N1) was defined as 1, resulting in 60 patients in the experimental group and 60 in the control group (n = 120).
Data management plan
The collection of personal and demographic characteristics will be carried out once at baseline. All personal identifiable information and clinical trial data will be separated. Clinical trial data will be identified by a unique participant ID, and the link between personal identifiable data and this ID will be stored securely and separately from trial data.
This study will collect and analyze new (quantitative) data only. Raw data will be analyzed and expressed as graphs, tables and annotated images, some of which are expected to be published in the future. Electronic records of the data will be generated and saved to the university server (which is automatically backed up daily). Data generated will be stored in various formats and sizes of datasets, all of which will be accessible using common software allowing easy access and long-term validity during and after the project, thus facilitating data sharing. However, all collected data will be handled confidentially.
The responsible person to preserve the data during and at least 5 years after the end of the research will be the Project Coordinator (AM), who will be closely supervised by the Principal Investigator (BC). In case the Project Coordinator does not continue working at the Vrije Universiteit Brussel or Ghent University in the 5 years after the end the research, the principal investigator will take over the role as responsible person. Along with the Project Coordinator and the Principle Investigator, members of the project team will have responsibility for study-wide data management, data security and quality assurance of data. The University Research Data Management team will be able to advise on best practice in data management and security. Electronic data will be protected by a dual-password “barrier” and that pen-and-paper data will be protected in a closed cabinet within the closed office of the PI.
Data analysis
Baseline data will provide cross-sectional results on pain measures, pain-related measures, sleep measures, physical activity and function measures for the complete CSP group and comparisons between possible subgroups.
AN(C)OVA repeated measures analyses will be used to evaluate and compare therapy effects. Data analysis will be conducted following intention to treat principles. Effect sizes as well as 95% confidence intervals and clinically significant differences will be calculated. Statistical significant differences will be defined at alpha 0.05. In addition, numbers needed to treat will be calculated.
Blinding
Randomization and group allocation will be completely masked for the statistician. The study participants and the outcome assessor will be blinded to the maximal extent possible. Therefore, study participants will be asked not to communicate with the assessor about the intervention received. Furthermore, the interventions will take place at different times during the day to minimize the contamination between groups. Study participants receiving different interventions will not see each other in the hospital waiting rooms. The therapists providing the experimental treatment will not be involved in providing the control intervention and vice versa.
After the final assessment (i.e. 12-month follow-up), the success of the assessor blinding will be examined by asking whether the assessor thinks the participant has received the experimental or control intervention, including the percentage of certainty (i.e. 50% certainty indicating a pure guess).
Ethics
Approval to conduct this study was granted by the ethics committee of the Ghent University Hospital (2018/0277) and the University Hospital Brussels (2018/077), and the full study protocol was also registered at clinicaltrials.gov (no. NCT03482856). This trial will be conducted in compliance with the Declaration of Helsinki (1964 and amendments) and Good Clinical Practice. Patients will give their written informed consent prior to the start of any study-related procedure. All personal information collected from the participants will be stored and analyzed confidentially.
This study is funded by Fonds Wetenschappelijk Onderzoek – Toegepast Biomedisch Onderzoek (FWO-TBM, grant no. T001117N). The funder will have no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, approval, or submission of any manuscript that will emerge from this study.
Discussion
If this study indicates that the combination of CBT-I with the modern neuroscience approach (i.e. pain neuroscience education and CTET) is more effective than the modern neuroscience approach alone for reducing pain and improving sleep and physical activity or function in people with CSP and comorbid insomnia, the combined therapy should be applied as the new standard conservative treatment for these patients. Therefore, this study has great potential to significantly impact (1) the patient with CSP and comorbid insomnia, and (2) the professional.
The most important contribution to the physical therapy and rehabilitation profession will be the development of novel treatment guidelines for people with CSP and comorbid insomnia. These results will also contribute to understanding the relation between (changes in) pain-related factors, sleep-related factors, physical activity and functioning.
With the inclusion of 120 people with CSP and comorbid insomnia, this will be the largest study to investigate the effectiveness of combining two well-founded conservative treatment strategies, being CBT-I12 and the modern neuroscience approach.15 Furthermore, the multi-centered design of the study increases the external validity of the study findings, as it involves treatment by different physical therapists in different settings. Other strengths include the large predetermined sample, the well-validated and reliable outcome measures, the balanced-treatment arms, the randomized controlled study design, and the blinded outcomes assessments up to 1-year follow-up.
Conflicts of interest
The authors declare no conflicts of interest. This study was funded by Fonds Wetenschappelijk Onderzoek – Toegepast Biomedisch Onderzoek (FWO-TBM, grant no. T001117N). The funder will have no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, approval, or submission of any manuscript that will emerge from this study. Jo Nijs has co-authored a Dutch book for clinicians on pain neuroscience education, but the royalties for that book are collected by the Vrije Universiteit Brussel and not him personally.
References
- 1.Murray C.J.L., Vos T., Lozano R. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad A.H., Jaber S., Aziz M.I.A. The state of health in the Arab world, 1990–2010: an analysis of the burden of diseases, injuries, and risk factors. Lancet. 2014;383(9914):309–320. doi: 10.1016/S0140-6736(13)62189-3. [DOI] [PubMed] [Google Scholar]
- 3.Balague F., Mannion A.F., Pellise F., Cedraschi C. Non-specific low back pain. Lancet (Lond, England) 2012;379(9814):482–491. doi: 10.1016/S0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- 4.Tang N.K.Y., Wright K.J., Salkovskis P.M. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16(1):85–95. doi: 10.1111/j.1365-2869.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 5.Pigeon W.R., Moynihan J., Matteson-Rusby S. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: a pilot study. Behav Res Ther. 2012;50(11):685–689. doi: 10.1016/j.brat.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCracken L.M., Iverson G.L. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7(2):75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 7.Jungquist C.R., O’Brien C., Matteson-Rusby S. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010;11(3):302–309. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N.K.Y., Sanborn A.N. Better quality sleep promotes daytime physical activity in patients with chronic pain? A multilevel analysis of the within-person relationship. PLoS One. 2014;9(3):e92158. doi: 10.1371/journal.pone.0092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams A.C., de C., Eccleston C., Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henschke N., Ostelo R.W., van Tulder M.W. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010;(7):CD002014. doi: 10.1002/14651858.CD002014.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung C.W., Qiu Q., Choi S.-W., Moore B., Goucke R., Irwin M. Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Phys. 2014;17(5):401–414. [PubMed] [Google Scholar]
- 12.Finan P.H., Buenaver L.F., Coryell V.T., Smith M.T. Cognitive-behavioral therapy for comorbid insomnia and chronic pain. Sleep Med Clin. 2014;9(2):261–274. doi: 10.1016/j.jsmc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nijs J., Mairesse O., Neu D. Sleep disturbances in chronic pain: neurobiology assessment, and treatment in physical therapist practice. Phys Ther. 2018 doi: 10.1093/ptj/pzy020. [DOI] [PubMed] [Google Scholar]
- 14.Hall A., Richmond H., Copsey B. Physiotherapist-delivered cognitive-behavioural interventions are effective for low back pain, but can they be replicated in clinical practice? A systematic review. Disabil Rehabil. 2018;40(1):1–9. doi: 10.1080/09638288.2016.1236155. [DOI] [PubMed] [Google Scholar]
- 15.Malfliet A., Kregel J., Coppieters I. Effect of pain neuroscience education combined with cognition-targeted motor control training on chronic spinal pain: a randomized clinical trial. JAMA Neurol. 2018;75(7):808–817. doi: 10.1001/jamaneurol.2018.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N.K.Y., Goodchild C.E., Salkovskis P.M. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: a pilot randomised controlled trial. Behav Res Ther. 2012;50(12):814–821. doi: 10.1016/j.brat.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dworkin R.H., Turk D.C., Farrar J.T. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2) doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Chiarotto A., Boers M., Deyo R.A. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain. 2018;159(3):481–495. doi: 10.1097/j.pain.0000000000001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanhope J. Brief Pain Inventory review. Occup Med (Chicago IL) 2016;66(6):496–497. doi: 10.1093/occmed/kqw041. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza T., Mayne T., Rublee D., Cleeland C. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10(4):353–361. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Keller S., Bann C.M., Dodd S.L., Schein J., Mendoza T.R., Cleeland C.S. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Cuesta-Vargas A.I., Neblett R., Chiarotto A. Dimensionality and reliability of the central sensitization inventory in a pooled multicountry sample. J Pain. 2018;19(3):317–329. doi: 10.1016/j.jpain.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Scerbo T., Colasurdo J., Dunn S., Unger J., Nijs J., Cook C. Measurement properties of the central sensitization inventory: a systematic review. Pain Pract. 2017 doi: 10.1111/papr.12636. [DOI] [PubMed] [Google Scholar]
- 25.Kregel J., Vuijk P.J., Descheemaeker F. The Dutch central sensitization inventory (CSI): factor analysis, discriminative power, and test-retest reliability. Clin J Pain. 2016;32(7):624–630. doi: 10.1097/AJP.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 26.Chesterton L.S., Sim J., Wright C.C., Foster N.E. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23(9):760–766. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 27.Cathcart S., Pritchard D. Reliability of pain threshold measurement in young adults. J Headache Pain. 2006;7(1):21–26. doi: 10.1007/s10194-006-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer A.A. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30(1):115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 29.Nilius G., Domanski U., Schroeder M. A randomized controlled trial to validate the Alice PDX ambulatory device. Nat Sci Sleep. 2017;9:171–180. doi: 10.2147/NSS.S133789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Morin C.M., Vallieres A., Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin C.M., Belleville G., Belanger L., Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendzerska T.B., Smith P.M., Brignardello-Petersen R., Leung R.S., Tomlinson G.A. Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med Rev. 2014;18(4):321–331. doi: 10.1016/j.smrv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Mairesse O., Damen V., Newell J., Kornreich C., Verbanck P., Neu D. The Brugmann Fatigue Scale: an analogue to the Epworth sleepiness scale to measure behavioral rest propensity. Behav Sleep Med. 2017 October:1–2. doi: 10.1080/15402002.2017.1395336. [DOI] [PubMed] [Google Scholar]
- 35.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 36.Martin J.L., Hakim A.D. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McHorney C.A., Ware J.E.J., Raczek A.E., The M.O.S. 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wall P., Melzack R. 4th ed. Churchill Livingston; Edinburgh UK: 1999. Textbook of Pain. [Google Scholar]
- 39.van Wilgen C.P., Nijs J. Bohn Stafleu van Loghum; Houten: 2010. Pijneducatie – Een Praktische Handleiding Voor (Para)Medici. [Google Scholar]
- 40.Butler D.S., Moseley G.L. NOI Group Publishing; Adelaide: 2003. Explain Pain. [Google Scholar]
- 41.Malfliet A., Kregel J., Meeus M. Applying contemporary neuroscience in exercise interventions for chronic spinal pain: treatment protocol. Braz J Phys Ther. 2017;21(5) doi: 10.1016/j.bjpt.2017.06.019. [accepted for publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritterband L.M., Bailey E.T., Thorndike F.P., Lord H.R., Farrell-Carnahan L., Baum L.D. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21(7):695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malfliet A., Kregel J., Meeus M. Applying contemporary neuroscience in exercise interventions for chronic spinal pain: treatment protocol. Braz J Phys Ther. 2017;21(5) doi: 10.1016/j.bjpt.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]