Abstract
Background
In advanced cancer, patients want to know how their care options may affect survival and quality of life, but the impact of outpatient specialty palliative care on these outcomes in cancer is uncertain.
Purpose
To estimate the impact of outpatient specialty palliative care programs on survival and quality of life in adults with advanced cancer.
Methods
Following PRISMA guidelines, we conducted a systematic review and meta-analysis of randomized controlled trials comparing outpatient specialty palliative care with usual care in adults with advanced cancer. Primary outcomes were 1 year survival and quality of life. Analyses were stratified to compare preliminary studies against higher-quality studies. Secondary outcomes were survival at other endpoints and physical and psychological quality-of-life measures.
Results
From 2,307 records, we identified nine studies for review, including five high-quality studies. In the three high-quality studies with long-term survival data (n = 646), patients randomized to outpatient specialty palliative care had a 14% absolute increase in 1 year survival relative to controls (56% vs. 42%, p < .001). The survival advantage was also observed at 6, 9, 15, and 18 months, and median survival was 4.56 months longer (14.55 vs. 9.99 months). In the five high-quality studies with quality-of-life data (n = 1,398), outpatient specialty palliative care improved quality-of-life relative to controls (g = .18, p < .001), including for physical and psychological measures.
Conclusions
Patients with advanced cancer randomized to receive outpatient specialty palliative care lived longer and had better quality of life. Findings have implications for improving care in advanced cancer.
Keywords: Palliative care, Mortality, Quality of life, Meta-analysis, Behavioral medicine
Adults with advanced cancer who were assigned to receive palliative care experienced clinically meaningful improvements in physical and emotional quality of life and lived longer.
Introduction
In advanced cancer, where survival is commonly <1 year [1, 2] and symptoms and side effects are prevalent and often debilitating [3, 4], the fundamental challenge is to provide care to extend survival while simultaneously supporting quality of life [5–9]. Specialty palliative care programs are comprised of multidisciplinary teams of clinicians who meet with patients to support their quality of life, physically and psychologically, using specialized knowledge and skills to relieve the symptoms, side effects, and stress of serious illnesses [10–12]. Historically, specialty palliative care programs were confined to inpatient units [13–15] and focused on supporting the needs of patients near death. Acknowledging that patients’ quality-of-life concerns emerge much earlier in the illness trajectory, oncology has transitioned over the past two decades toward providing patients with palliative care early after the diagnosis of advanced cancer, on an outpatient basis, while patients continue to receive treatment [12]. Under the emerging paradigm of outpatient specialty palliative care, the palliative care team largely focuses on the assessment and management of symptoms and helping patients to cope with their illness [16]. Undoubtedly, the goal of improving quality of life in advanced cancer through the provision of outpatient specialty palliative care is worthwhile, but in making informed medical decisions patients with advanced cancer and their families also want to know how their care options may positively or negatively affect survival.
In fact, when patients, families, and clinicians discuss referrals to specialty palliative care, the elephant in the room is a collective fear that there could be tradeoffs between length and quality of life [17]. Patients, families, and clinicians often view palliative care as synonymous with, if not fostering, death, characterizing it variously as the grim reaper service [18, 19], giving up [20, 21], abandonment [22, 23], death row [24], and a death panel [25, 26]. Consequently, most referrals to outpatient specialty palliative care occur late in the disease course, if at all, and underutilization harms quality of life [15, 27–29]. In contrast to this climate of fear of hastening death, the landmark Temel et al. [1] study showed that among patients with advanced lung cancer, those randomized to outpatient specialty palliative care had better quality of life and lived >2 months longer than those receiving usual care. Yet, the generalizability of those findings to other studies of outpatient specialty palliative care in cancer has not been tested, so patients, families, and clinicians still lack evidence needed to make informed decisions. In the backdrop of this fear and uncertainty, the American Society of Clinical Oncology—the world’s largest professional oncology organization—now recommends outpatient specialty palliative care early after the diagnosis of advanced cancer, but this is based solely on a narrative literature review rather than a rigorous analysis of the potential impact on quality of life and survival duration [12].
There have been no published meta-analyses of randomized controlled trials (RCTs) examining the impact of outpatient specialty palliative care on survival and quality of life in advanced cancer, despite that this could have implications for both outcomes for over a half million patients annually. A prior meta-analysis [30], which should be lauded for its broad scope, included RCTs involving patients with heterogeneous serious illnesses (i.e., cancer, heart failure, dementia, HIV, multiple sclerosis, stroke, COPD, and renal disease) and found that palliative care programs improved quality of life without apparent effect on survival. Yet, there is a need for a targeted meta-analysis focusing in depth on outpatient palliative cancer care. First, that meta-analysis examined 3 month survival, and it is possible that a survival benefit could occur later given that patients with cancer may only meet with palliative care teams approximately monthly [16]. A longer-term endpoint, such as 1 year survival, would be clinically meaningful. Second, subgroup analyses of cancer patients combined results from inpatient and outpatient studies, so there remains a need for analyses examining the impact of the emerging model of outpatient specialty palliative care on survival in cancer. Three, the prior meta-analysis happened to include a potentially problematic study in the main analyses. The largest and most highly weighted study [31] was a cluster RCT with six units of randomization (i.e., six clinics). Patients at the three clinics receiving palliative care had more aggressive cancer diagnoses, biasing outcomes against the palliative care group. In addition to that meta-analysis, a second meta-analysis replicated the quality-of-life benefit of palliative care but did not examine survival [32]. Accordingly, a meta-analysis accounting for both outcomes that matter to patients with advanced cancer and clinicians—longer-term survival and quality of life—is needed to guide evidence-based oncology care [33].
The present meta-analysis was designed to examine the impact of outpatient specialty palliative care on survival and quality of life, pooling across published RCTs involving adults with advanced cancer and accounting for study quality. Primary outcomes were 1 year survival and quality of life during all follow-up assessments, and secondary outcomes included survival at other endpoints and distinguished between physical and psychological domains of quality of life.
Methods
Study Eligibility Criteria
A standardized protocol adhering to PRISMA guidelines was registered with PROSPERO (CRD42016046925) and used to conduct this meta-analysis. Eligibility criteria spanned the Participants, Interventions, Comparators, Outcomes, and Study designs (PICOS) of the investigations. Participants were adult (≥18 years old) outpatients with advanced cancer. Studies involving cancer samples or subsamples were eligible. RCTs were eligible, including standard, fast-track, and adaptive designs, using individual-patient or cluster randomization.
Eligible interventions were restricted to those offered through outpatient care. Studies solely implemented in other settings, for example, inpatient, day hospitals, and home care [11], were excluded; those combining outpatient with other models (e.g., outpatient with continuity to inpatient) remained eligible. For multifaceted interventions, patients were not required to utilize all intervention components. In accordance with the National Consensus Project [10], eligible specialty palliative care interventions had to have been offered via an “interdisciplinary team of skilled palliative care professionals,” operationalized here as involving clinicians spanning two or more disciplines, with at least one identified as a palliative care specialist; interventions restricted to a single discipline (e.g., cognitive behavior therapy offered by a psychologist) were excluded.
Outcomes included survival and quality of life. Survival outcomes included the proportion of the sample surviving at 1 year, as well as quarterly through 2 years, and median survival. Although quality of life is sometimes construed narrowly to focus solely on physical well-being, consistent with psychological theory [3, 34], we operationalized quality of life broadly to include physical measures (physical or functional well-being and physical symptom burden), psychological measures (depression, anxiety, emotional, social, or cognitive functioning), and global quality of life measures (e.g., the sum of two or more physical and psychological scales). We excluded single-item measures, though this did not result in the exclusion of any study.
Data Sources and Search Strategy
A systematic search strategy was used to review palliative care studies indexed in PubMed and EMBASE from inception through 4/24/17. The search used MeSH terms and keywords to identify studies mentioning (a) palliative or palliative care or palliative medicine or terminal care or hospice care or supportive care and (b) cancer or neoplasm or malignancies (Supplementary Table A1). We also manually screened references in review articles.
Selection of Studies
Two reviewers independently screened titles and abstracts of each citation to exclude obviously ineligible articles. Any article not excluded by both reviewers proceeded to full-text review, where two independent reviewers read each article and indicated whether it was eligible or recorded the reason for exclusion. Any disagreements were resolved by consensus.
Data Extraction
Standardized data extraction forms were used to record study characteristics, elements of study quality, and outcomes. Using prespecified criteria, we classified studies as providing high-quality evidence if they met both of two basic methodological criteria that distinguish among outpatient specialty palliative cancer care RCTs; alternatively, if studies met only one or none of our basic standards, they were classified as providing only preliminary evidence. Foremost, studies needed to include randomization procedures reasonable for avoiding a high risk of confounding, operationalized here as using either individual-patient randomization or cluster-randomization with at least 10 clusters. Out of convenience, cluster RCTs in supportive oncology have sometimes randomized by clinic, and therefore by cancer type (e.g., breast cancer clinic), so clinic-related differences could confound observed differences in survival and quality of life when the number of clusters is small. For example, if patients in the breast cancer clinic were assigned to palliative care, and patients in the lung cancer clinic assigned to usual care, survival and quality of life would be favorably biased in the palliative care group due to confounding by diagnosis. Although acknowledging the utility of power analyses and that often a much higher number of clusters are needed, a floor of approximately 10 clusters [35–37] is recommended as a minimum to provide remotely equivalent groups and therefore support causal inferences about between-group differences in survival and quality of life. As a second quality criterion, studies needed to include a sizable number of patients who were diagnosed with cancer, operationalized here as a cancer sample or subsample of at least 100. If studies included patients with heterogeneous illnesses, interventions could be insufficiently tailored to the cancer experience if including few patients with cancer diagnoses [38], thus offering only a weak test of efficacy. As well, even in cancer-specific studies, those with small samples are at an elevated risk of bias and contribute limited information when two or more large studies exist [39]. These basic criteria determined subgroups for analyses. For descriptive purposes, we also recorded Cochrane [40] and other quality features emphasized by methodologists and funders, detailed descriptions of the palliative care programs, and detailed participant eligibility criteria. In a post hoc analysis, we also extracted PEDro [41] quality criteria.
Data were also extracted on between-group difference in survival. Data were drawn from reported statistics or Kaplan–Meier curves. Data extracted from reported statistics included samples sizes, the number of participants or probability of surviving at time points from 3 to 24 months, the number of censored cases, and p values. For Kaplan–Meier curves, survival probabilities were extracted for each coordinate on the survival curve using digital extraction software (DigitizeIt 2.2.2, Germany) validated for this purpose [42]; this yields estimates of survival for each time point (e.g., 1 year) and yields robust multistudy survival curves that elegantly account for censoring. As well, three blinded research assistants demonstrated excellent inter-rater reliability (ICC = .99) extracting survival data from article versions that masked group assignment, recording statistics from text, tables, or manual quarterly estimates from survival curves. Values extracted using the DigitizeIt software demonstrated excellent convergent validity with corresponding values extracted by hand (r = .99) and were used where available.
The standardized mean difference, Hedges’ g [43], was used to compare quality of life outcomes among those randomized to palliative care versus control conditions. We extracted data on sample sizes, the number of censored cases, p values, effect sizes, means, and standard deviations (or proportions, if assessing quality of life categorically, e.g., presence of depression). Where possible, we extracted data from longitudinal mixed models that accounted for between-group differences over multiple follow-up assessments of quality of life simultaneously, as this increases power. If no such model was reported, we extracted follow-up data reported from each unique time point at least 1 month postrandomization. When articles reported multiple sensitivity analyses, analyses using multiple imputation to account for missing data were prioritized, followed by single imputation, followed by complete cases. As well, those accounting for potential confounders were prioritized over unadjusted models due to providing more precise estimates of effect. Three blinded research assistants demonstrated excellent inter-rater reliability (ICC = .99) extracting quality of life data from article versions that masked group assignment, with inconsistencies resolved by consensus.
Data Synthesis
Data were synthesized using Comprehensive Meta-Analysis 3.0 and R 3.3.3. For survival, the primary outcome was the risk difference in 1 year survival between groups. For quality of life, the primary outcome was the standardized mean difference (Hedges’ g) in quality of life between the outpatient specialty palliative care and control groups across available follow-ups. Analyses used the DerSimonian and Laird (random-effects) model to provide generalizable estimates of the observed effects. Analyses were stratified by study quality, with the Q and I2 statistics used to examine heterogeneity. All analyses accounted for censored cases. Effect size estimates were accompanied by forest plots, 95% confidence intervals, and p values. The authors are available to assist with replication efforts upon reasonable request.
For the risk difference in 1 year survival, we conducted sensitivity analyses examining the observed effect when excluding any single study, commonly called influence analysis. As secondary outcomes for survival, we examined the risk difference in survival quarterly from 3 to 24 months as well as median survival and hazard ratios derived from Kaplan–Meier curves. Analyses of Kaplan–Meier curves used Guyot’s [42] method; briefly, survival curve data were digitally extracted from published articles (see Data Extraction) and combined with data on censored cases to reconstruct patient-level data (i.e., time and status) needed to develop composite Kaplan–Meier curves combining data from available studies. The approach is validated [42, 44, 45] and yielded results comparable to less sophisticated methods that did not account for censoring. For all analyses, we conducted additional post hoc sensitivity analyses allowing a lone study with a small cancer subsample to be grouped with the high-quality studies, though all findings were comparable.
For quality of life, estimates were first pooled within-study [46] across available quality of life measures and time points of observation. This requires imputing an estimate of the intercorrelation among varying indicators of changes in quality of life. Based on a review of prior studies [47, 48], we estimated a value of r = .30 among quality of life change scores and conducted sensitivity analyses using plausible values from r = .00 to .60. Next, estimates were pooled across studies using the DerSimonian and Laird model, with sensitivity analyses examining observed effects when excluding any individual study, that is, influence analysis. As secondary outcomes for quality of life, subgroup analyses were used to examine effect sizes separately for physical, psychological, and global measures of quality of life.
Results
Study Selection
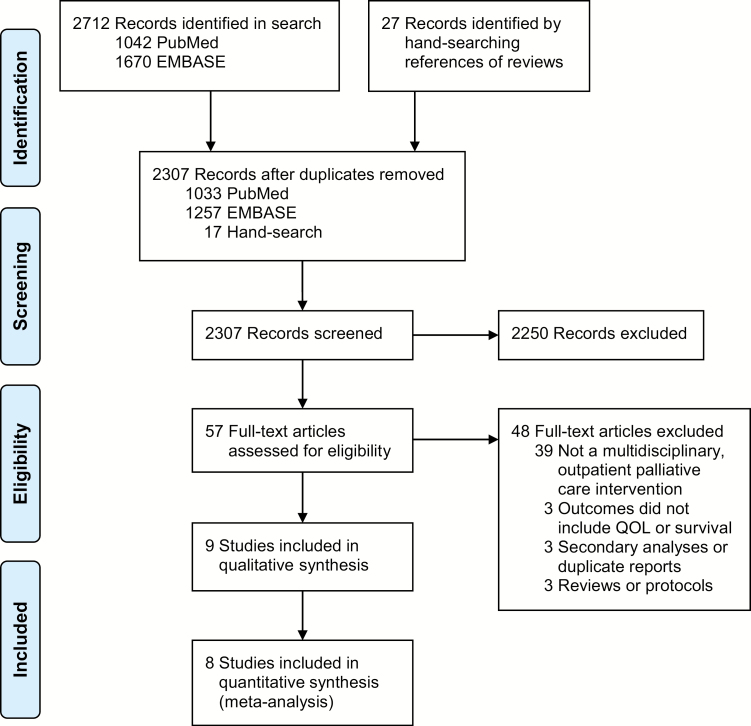
The search strategy identified 2,307 articles (Fig. 1). In screening abstracts, 57 articles were identified for full-text review, yielding nine studies for qualitative synthesis (Table 1). One study [49] involving a heterogeneous sample did not report results specific to the cancer subsample and was excluded from quantitative synthesis. The eight RCTs included in the meta-analysis were published from 2001 to 2017.
Fig. 1.
Flow diagram of study selection.
Table 1.
Description of RCTs of outpatient specialty palliative care for patients with advanced cancer identified in systematic review
| Evidencea | Study | Design | Intervention | Control | Demographics | Cancer site | QOL measures | Survival |
|---|---|---|---|---|---|---|---|---|
| High-quality evidence | Temel (2017) USA (MA) N = 350 |
RCT | Outpatient consultation team n = 175 |
Usual care n = 175 |
Age: M(SD) = 65 (11) Gender: 46% female Race: 92% white |
55% lung, 45% GI | FACT-G, PHQ-9, HADS-D, HADS-A | 3 and 6 months |
| Bakitas (2015) USA (NH, VT) N = 207 |
Fast-track RCT | Outpatient consultation and manualized telehealth support, symptom management, care coordination, and life review n = 104 |
Usual care for 3 months, then delayed access to the intervention n = 103 |
Age: M(SD) = 64 (10) Gender: 47% female Race: 97% white |
43% lung, 24% GI, 11% breast, 22% other | FACIT-Palliative, QUAL-E, CES-D | 3, 6, 9, 12 month, and Kaplan–Meier | |
| Zimmermann (2014) Canada N = 461 |
24-Cluster RCT | Outpatient consultation team and access to on-call, inpatient, and home services n = 228 |
Usual care n = 233 |
Age: M(SD) = 61 (12) Gender: 57% female Race: Not reported |
30% GI, 22% lung, 17% GU, 16% breast, 15% gynecologic | ESAS, QUAL-E, FACIT-Spiritual | 3 months | |
| Temel (2010) USA (MA) N = 151 |
RCT | Outpatient consultation team n = 77 |
Usual care n = 74 |
Age: M(SD) = 65 (10) Gender: 52% female Race: 97% white |
100% lung | FACT-L, PHQ-9, HADS-D, HADS-A | 3, 6, 9, 12, 15, 18, 21, and 24 months, and Kaplan–Meier | |
| Bakitas (2009) USA (NH, VT) N = 322 |
RCT | Manualized telehealth support, symptom management, care coordination, and shared medical appointments n = 161 |
Usual care n = 161 |
Age: M(SD) = 65 (11) Gender: 42% female Race: 99% white |
41% GI, 36% lung, 12% GU, 10% breast | ESAS, FACIT-Palliative, CES-D | 3, 6, 9, 12, 15, 18, 21, and 24 months, and Kaplan–Meier | |
| Preliminary evidence | McCorkle (2015) USA (CT) N = 146 |
4-Cluster RCT | Multidisciplinary outpatient consultation team n = 66 |
Usual care n = 80 |
Age: M = 60 Gender: 56% female Race: 85% white |
36% GI, 20% gynecologic, 18% head/neck, 25% lung | FACT-G, ESDS, HADS-A, PHQ-9, HDS, SDS | 3 months |
| Higginson (2014) UK (England) N = 21 (105)b |
Fast-track RCT | Multidisciplinary outpatient consultation team and home assessment of breathlessness n = 11 (53)b |
Usual care for 6 weeks, then delayed access to the intervention n = 10 (52)b |
Age: M(SD) = 67 (10) Gender: 42% female Race: Not reported |
62% lung, 14% hematologic, 10% GU, 14% other | None reported | 3 and 6 months | |
| Jordhøy (2001) Norway N = 434 |
6-Cluster RCT | Multidisciplinary outpatient consultation team n = 235 |
Usual care n = 199 |
Age: Median = 70 Gender: 47% female Race: Not reported |
42% GI, 16% GU, 15% breast/gyn, 12% lung, 15% other | EORTC QLQ-C30 | 3, 6, and 24 months | |
| Excluded from meta-analyses | Rabow (2004) USA (CA) N = 30 (90)b |
2-Cluster RCT | Multidisciplinary outpatient consultation team n = 13 (50)b |
Usual care n = 17 (40)b | Age: M(SD) = 69 (13) Gender: 64% female Race: 53% white |
Not reported | None reported | None reported |
CES-D Center for Epidemiological Studies Depression Scale; EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire; ESAS Edmonton Symptom Assessment Scale; ESDS Enforced Social Dependency Scale; FACIT Functional Assessment of Chronic Illness Therapy; FACT Functional Assessment of Cancer Therapy (G = General, L = Lung); GI gastrointestinal; GU genitourinary; HADS Hospital Anxiety and Depression Scale; HD Health Distress Scale; PHQ-9 Patient Health Questionnaire; QOL Quality of Life; QUAL-E Quality of Life at End of Life scale; SDS Symptom Distress Scale.
aHigh-quality studies had to have randomization procedures reasonable for avoiding confounding and involve ≥100 cancer patients (see Methods for details).
bParenthetical values indicate samples sizes for all study participants, including those without cancer.
Study Quality
There were five high-quality outpatient palliative cancer care studies identified for meta-analysis, including three standard RCTs [1, 50, 51], a fast-track RCT [2], and a 24-cluster RCT [52], with sample sizes from 151 to 461 cancer patients. As well, three preliminary studies provided lower-quality evidence. Two of these were cluster RCTs with <10 clusters; one study [31] had six clusters with baseline imbalance on time since diagnosis and cancer type, and the other study [53] had four clusters with baseline imbalance on cancer type, age, gender, and multimorbidity. Another study [54] provided only preliminary evidence relevant to oncology as few patients had cancer (n = 21) in a broader study of heterogeneous respiratory patients. Higher-quality studies met most Cochrane and other quality criteria assessed (Supplementary Table A2), aside from a lack of blinding, which is less practical in behavioral trials.
Outpatient Specialty Palliative Care Programs
The outpatient specialty palliative care programs often differed in format (breadth of the clinical team, frequency of visits, and format of visits) but covered similar content (for a detailed summary, see Supplementary Table A3). All outpatient specialty palliative care programs were led by clinicians with in-depth specialized training in palliative care consisting of board certification in palliative care, study-specific advanced training in palliative care, or both. Across the nine studies in the systematic review, the palliative care programs included nurses (k = 8 studies), physicians (k = 8), social workers (k = 4), clergy (k = 2), physiotherapists (k = 2), and other clinicians (k = 5, including psychologists, nutritionists, pharmacists, and others) with specialized palliative care training. The palliative care clinicians met with patients on a monthly to weekly basis, often for a half hour. Visits were most commonly in person, and all interventions included an in-person component, though three included a substantive phone-based component. Despite these differences in the surface-level appearance of outpatient specialty palliative care programs, the key content elements were often similar. In most of the interventions, palliative care clinicians focused on two key hallmarks (a): assessing and managing symptoms and side effects, and (b) assessing psychosocial concerns and helping patients to cope with advanced illness. Often, palliative care clinicians also helped with treatment decision making, advance care planning, care coordination, communication and family issues, and illness understanding.
Sample Characteristics
In total 2,092 patients with advanced cancer participated in the eight RCTs included in the meta-analysis, with 71.3% (n = 1,491) in the five high-quality RCTs (Table 1; for more detail on participant eligibility, see Supplementary Table A4). Participants were often in their 60s and 70s (Table 1). Gender was balanced evenly, with 49% in high-quality studies being female. Samples were mainly white, or race data were unreported. In the high-quality studies, participants were recruited from study sites a maximum of 430 miles (700 km) apart, with four conducted in the New England area of the USA. All studies identified eligible patients with advanced cancer based on carefully delineated diagnostic characteristics, with several studies further narrowing the sample using physician estimates of prognosis (Supplementary Table A4). Advanced lung (44%) and gastrointestinal (32%) cancers were the more common diagnoses in high-quality studies (Table 1). One year survival data were available from three studies, all high quality, whereas most studies tracked shorter-term survival. The validated FACT/FACIT [34] scales were most commonly used to assess quality of life (k = 6 studies).
Survival
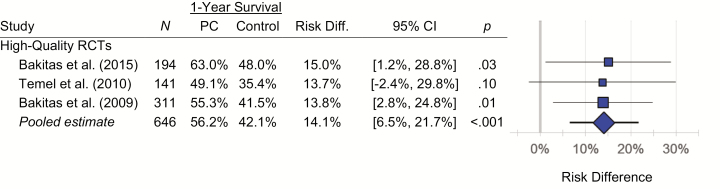
Relative to usual care, outpatient specialty palliative cancer care was associated with a 14.1% (95% CI, 6.5% to 21.7%; p < .001) absolute increase in 1 year survival based on three high-quality studies (Fig. 2), with no 1 year data from the other studies. Namely, 42.1% of advanced cancer patients receiving usual care were alive at 1 year follow-up, but 56.2% of those receiving outpatient specialty palliative care were alive. There was no meaningful heterogeneity in the survival difference across the three studies (Q = 0.02; df = 2; p = .99; I2 = 0.0%). Effects were comparable in sensitivity analyses excluding any one of the three studies (risk differences of 13.8% to 14.5%; p’s from .007 to .001).
Fig. 2.
Forest plots for RCTs examining the impact of outpatient specialty palliative care (PC) on 1 year survival in advanced cancer. Results were confined to high-quality RCTs as other studies did not track this outcome. Risk-difference estimates were consistent across the three studies (Q = 0.02; df = 2; I2 = 0.0%; p = .99).
Turning to other time points, no significant between-group differences in survival were observed at 3 months or 24 months regardless of study quality (for all survival endpoints, see Supplementary Table A5). At 6 months, study quality accounted for substantial heterogeneity in the observed survival benefit (between-group Q = 7.24; df = 1; p = .006; I2 = 46.7%). In high-quality studies (k = 4 studies), randomization to palliative care was associated with an absolute increase of 6.2% (95% CI, 1.0% to 11.4%; p = .02) in 6 month survival relative to usual care, with no meaningful heterogeneity across these studies (Q = 1.60; df = 3; p = .66; I2 = 0.0%). Only high-quality studies included survival data from 9 to 21 months. Participants randomly assigned to palliative care also experienced a survival advantage at 9 months (k = 3 studies; risk difference = 11.1%; 95% CI, 3.7% to 18.5%; p = .003), 15 months (k = 2; risk difference = 10.0%; 95% CI, 0.8% to 19.1%; p = .03), and 18 months (k = 2; risk difference = 12.7%; 95% CI, 0.3% to 25.0%; p = .04), with a trend at 21 months (k = 2; risk difference = 14.9%; 95% CI, −0.7% to 30.4%; p = .06).
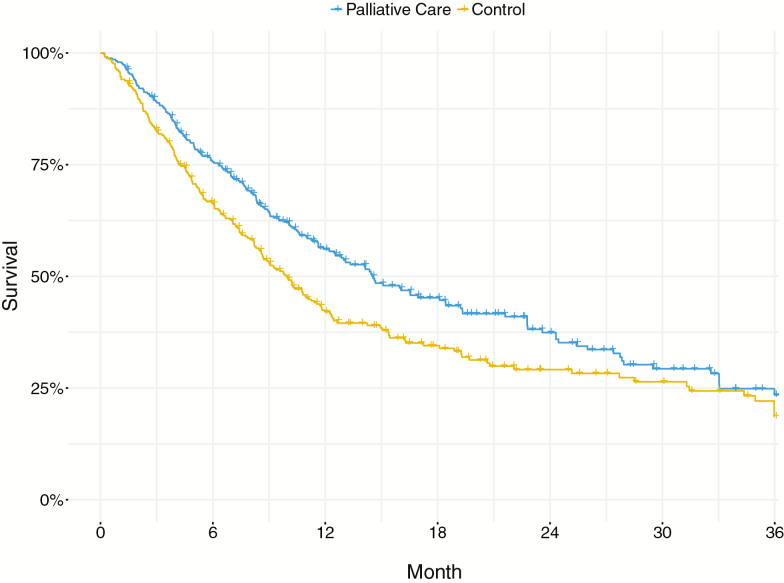
Kaplan–Meier curves were provided in three studies, all of high quality [1, 2, 50]. Analyses indicated a median survival advantage of 4.56 months (χ2 = 9.04; df = 1; p = .0026; Fig. 3) for those randomly assigned to palliative care (median survival = 14.55 months; 95% CI, 12.50 to 19.20 months), relative to controls (median survival = 9.99 months; 95% CI, 8.59 to 11.40 months). As indicated by a hazard ratio of 0.743 (95% CI, 0.612 to 0.902, Z = −2.996, p = .0027), patients randomized to palliative care had a 25.7% slower rate of mortality than controls.
Fig. 3.
Pooled Kaplan–Meier curves showing survival probabilities for patients (n = 680) from three high-quality RCTs (Bakitas et al., 2009, 2015; Temel et al., 2010) who were randomized to outpatient specialty palliative care versus control. Median survival was 4.56 months greater (χ2 = 9.04; df = 1; p = .0026) for patients randomized to outpatient specialty palliative care (median survival = 14.55 months; 95% CI, 12.50 to 19.20 months) than for patients in the control condition (median survival = 9.99 months; 95% CI, 8.59 to 11.40 months). Individual-patient data were reconstructed from published Kaplan–Meier curves to yield pooled curves [42].
Quality of Life
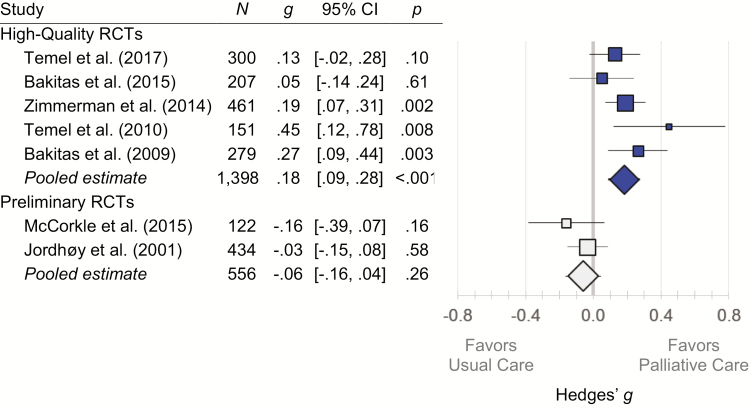
Study quality accounted for considerable cross-study heterogeneity in quality of life outcomes (between-group Q = 11.69; df = 1; p < .001; I2 = 70.8%) with the observed benefit of outpatient specialty palliative cancer care on quality of life confined to high-quality studies (Fig. 4). There were no significant effects for the preliminary studies, two cluster RCTs with an insufficient number of clusters needed to avoid confounding. In the five high-quality studies, randomization to palliative care was associated with better quality of life (g = .18; 95% CI, .09 to .28; p < .001). The modest heterogeneity present within the high-quality studies was not statistically significant (Q = 5.87; df = 4; p = .21; I2 = 31.9%). The effects observed in high-quality studies were comparable in sensitivity analyses imputing different estimates of the within-study outcome inter-correlation (g’s from .18 to .19; p’s < .001) and in influence analyses excluding any individual study (g’s from .16 to .21; p’s from .007 to <.001). In high-quality studies, effects were statistically significant for physical (k = 2 studies; g = .27; 95% CI, .08 to .46; p = .006), psychological (k = 5 studies; g = .19; 95% CI, .07 to .30; p = .001), and global (k = 5 studies; g = .20; 95% CI, .08 to .32; p = .001) measures of quality of life (Supplementary Table A6).
Fig. 4.
Forest plots for RCTs examining the impact of outpatient specialty palliative cancer care on quality of life. High-quality studies had adequate randomization procedures and sample sizes, whereas preliminary studies were both cluster RCTs with an insufficient number of clusters to avoid confounding by diagnosis. Study quality explained heterogeneity in observed effects (Q = 11.69; df = 1; p < .001; I2 = 70.8%), with the beneficial impact of outpatient specialty palliative care on quality of life observed exclusively in high-quality studies. CI confidence interval; Hedges’ g standardized mean difference; RCT randomized controlled trial.
Discussion
We found that patients with advanced cancer randomized to outpatient specialty palliative care experienced improved 1 year survival and quality of life relative to controls in this meta-analysis of RCTs. Analyses spanned eight RCTs, focusing on five high-quality RCTs [1, 2, 50–52] involving >1,400 patients with advanced cancer (Table 1). These findings build on the evidence summarized in narrative reviews [55–57], practice guidelines [12, 58], and prior meta-analyses that focused on shorter-term survival outcomes [30] or only examined quality of life [32]. Findings have implications for improving care for patients with advanced cancer.
Of profound importance to patients, families, oncology clinicians, and payers, we found that outpatient specialty palliative care improved survival for patients with advanced cancer. Based on three high-quality RCTs of patients with heterogeneous advanced cancer diagnoses [1, 2, 50], 1 year survival for patients receiving usual care was 42.1%, but this increased to 56.2% for those randomized to outpatient specialty palliative care, an absolute increase of 14.1% (Fig. 2) or about 1 in 7 additional patients alive at that endpoint. The increase in 1 year survival was consistent across these studies (13.7%–15.0%), and a survival benefit was also observed at the quarterly follow-up periods from 6 to 18 months (Supplementary Table A5). In fact, although median survival was 9.99 months for the usual care patients, this increased by 45.6% (4.56 months) to 14.55 months for the palliative care patients (Fig. 3). This survival benefit rivaled what one might observe in the context of a breakthrough oncology pharmaceutical trial [9]. In terms of public health impact, in the USA and Canada, where most of the outpatient specialty palliative care research has been conducted, >680,000 patients died of cancer in 2017 alone [59, 60]. While acknowledging the need for a future cost-effectiveness analysis, if outpatient specialty palliative care could offer a similar survival benefit at the population level, this would be equivalent to adding ≈250,000 person-years of life annually (4.56/12 × 680,000 = 258,400) in those two countries alone.
To avoid confusion, we clarify how these findings differ from that of a prior meta-analysis on palliative care and survival [30]. That study examined survival through 3 months, and like that study, we found no difference that early. Most patients only receive outpatient specialty palliative care semiweekly to monthly (Supplementary Table A3), so the cumulative “dose” may be too low at 3 months. As we also found no survival difference at our farthest endpoints of 21 and 24 months (Fig. 3 and Supplementary Table A5), there may be a critical window between 6 and 18 months where psychosocial interventions could increase the likelihood of surviving with advanced cancer.
In addition to the findings on survival, in five high-quality RCTs [1, 2, 50–52] patients randomized to receive outpatient specialty palliative cancer care experienced better quality of life than those receiving usual care (Fig. 4), consistent with the mission of palliative care. Palliative care improved both physical and psychological outcomes, with consistent findings across high-quality studies. Patients, families, oncology clinicians, and payers should be encouraged by the robustness of this finding. With our results, three teams [30, 32] have now conducted meta-analyses during similar time frames making independent methodological decisions, involving different data abstractors and analysts, and all agree that palliative care improves quality of life. Moreover, although outpatient specialty palliative care programs differ with respect to several surface features, such as the format of the intervention and composition of the team, it has been noted [12] that they focus on most of the same key elements [16], especially symptom management and coping (Supplementary Table A3), and achieve similar quality of life outcomes.
These findings on quality of life highlight the need for greater attention to measurement and outcome evaluation in palliative care research. The observed effect size for the quality of life benefit is often characterized as “small,” but the statistics employed in palliative care studies may underestimate the real benefit. This is because patients present with heterogeneous symptoms but the outcome measures and analyses commonly used in palliative care studies combine relevant-improving symptoms with irrelevant-low-stable symptoms, washing out observed benefits (for a detailed example, see Supplementary Fig. A1). More idiographic approaches, such as those commonly used in the psychotherapy outcomes literature, may also be useful for quantifying change in this context [61, 62].
Nonetheless, our conclusions are tempered by several limitations. This meta-analysis identified five high-quality studies of outpatient specialty palliative cancer care, led by three investigators. Many of the patients had lung and gastrointestinal cancers and were white, in their 60s and 70s, and treated in a geographically restricted northeastern region of North America. Thus, these results may not generalize to all patients with advanced cancer, nor patients with other serious illnesses. As well, due to the small number of known studies to date, we could not test for publication bias using conventional methods such as funnel plots. In addition to further replication of these findings, it would be important to investigate potential mechanisms implicated in improved survival and quality of life to develop stronger causal inferences.
An important opportunity in future studies of palliative care will be the increased integration of theory. As researchers and clinicians often wonder what is in the palliative care “syringe,” there have been several attempts to describe key elements of palliative care visits (see Supplementary Table A3; also Refs. 16, 63, and 64). Further theoretical development can provide a stronger justification for defining core elements of palliative care interventions, suggest mediating and moderating mechanisms, and guide the choice of outcome measures. Few studies of palliative care have examined potential mediators and moderators [16, 65, 66], including factors as simple as the length of the palliative care visit or timing. In our review of individual RCTs, the link between elements of the intervention and the choice of particular survey outcome measures was commonly underspecified, perhaps dampening effects. The opportunity for greater theoretical integration is not unique to palliative care and has proven useful in other areas of behavioral medicine [67–69].
In summary, outpatient specialty palliative care increased survival and improved quality of life for patients with advanced cancer. These findings may help destigmatize palliative care by reassuring patients, families, clinicians, and payers that palliative care can reduce suffering without compromising longevity.
Supplementary Material
Acknowledgments
This study was supported by National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health (USA) and the Louisiana Board of Regents. The authors conducted the research independently of the funders.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Michael Hoerger, Graceanne R. Wayser, Gregory Schwing, Ayako Suzuki, and Laura M. Perry declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1. Temel JS, Greer JA, Muzikansky A, et al. . Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 2. Bakitas MA, Tosteson TD, Li Z, et al. . Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobsen PB, Andrykowski MA. Tertiary prevention in cancer care: understanding and addressing the psychological dimensions of cancer during the active treatment period. Am Psychol. 2015;70:134–145. [DOI] [PubMed] [Google Scholar]
- 4. Moens K, Higginson IJ, Harding R; EURO IMPACT Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48:660–677. [DOI] [PubMed] [Google Scholar]
- 5. Meropol NJ, Egleston BL, Buzaglo JS, et al. ; CONNECT Study Research Group. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thatcher N, Girling DJ, Hopwood P, Sambrook RJ, Qian W, Stephens RJ. Improving survival without reducing quality of life in small-cell lung cancer patients by increasing the dose-intensity of chemotherapy with granulocyte colony-stimulating factor support: Results of a British medical research council multicenter randomized trial. Medical research council lung cancer working party. J Clin Oncol. 2000;18:395–404. [DOI] [PubMed] [Google Scholar]
- 7. Hamilton DW, Bins JE, McMeekin P, et al. . Quality compared to quantity of life in laryngeal cancer: A time trade‐off study. Head Neck. 2016;38:631–637. [DOI] [PubMed] [Google Scholar]
- 8. Malhotra C, Xiang L, Ozdemir S, Kanesvaran R, Chan N, Finkelstein EA. A comparison of attitudes toward length and quality of life between community-dwelling older adults and patients with advanced cancer. Psychooncology. 2017;26:1611–1617. [DOI] [PubMed] [Google Scholar]
- 9. Salas-Vega S, Iliopoulos O, Mossialos E. Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol. 2017;3:382–390. [DOI] [PubMed] [Google Scholar]
- 10. National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 3rd ed Pittsburgh, PA:National Consensus Project for Quality Palliative Care; 2013. [Google Scholar]
- 11. El Osta B, Bruera E. Models of palliative care delivery. In: Bruera E, Higginson I, von Gunten C, Morita T, eds. Textbook of Palliative Medicine. Boca Raton, FL: CRC Press; 2015:275–286. [Google Scholar]
- 12. Ferrell BR, Temel JS, Temin S, et al. . Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:96–112. [DOI] [PubMed] [Google Scholar]
- 13. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: A status report. J Palliat Med. 2016;19:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes MT, Smith TJ. The growth of palliative care in the United States. Annu Rev Public Health. 2014;35:459–475. [DOI] [PubMed] [Google Scholar]
- 15. Hui D, Elsayem A, De la Cruz M, et al. . Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoerger M, Greer JA, Jackson VA, et al. . Defining the elements of early palliative care that are associated with patient-reported outcomes and the delivery of end-of-life care. J Clin Oncol. 2018;36:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoerger M, Perry LM, Gramling R, Epstein RM, Duberstein PR. Does educating patients about the early palliative care study increase preferences for outpatient palliative cancer care? Findings from Project EMPOWER. Health Psychol. 2017;36:538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kavalieratos D, Mitchell EM, Carey TS, et al. . “Not the ‘grim reaper service’”: An assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Am Heart Assoc. 2014;3:e000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Omilion-Hodges LM, Swords NM. The grim reaper, hounds of hell, and Dr. Death: The role of storytelling for palliative care in competing medical meaning systems. Health Commun. 2017;32:1272–1283. [DOI] [PubMed] [Google Scholar]
- 20. Lo B, Quill T, Tulsky J. Discussing palliative care with patients. ACP-ASIM end-of-life care consensus panel. American College of Physicians-American Society of Internal Medicine. Ann Intern Med. 1999;130:744–749. [PubMed] [Google Scholar]
- 21. Horlait M, Chambaere K, Pardon K, Deliens L, Van Belle S. What are the barriers faced by medical oncologists in initiating discussion of palliative care? A qualitative study in Flanders, Belgium. Support Care Cancer. 2016;24:3873–3881. [DOI] [PubMed] [Google Scholar]
- 22. Han PK, Arnold RM. Palliative care services, patient abandonment, and the scope of physicians’ responsibilities in end-of-life care. J Palliat Med. 2005;8:1238–1245. [DOI] [PubMed] [Google Scholar]
- 23. Buckley de Meritens A, Margolis B, Blinderman C, et al. . Practice patterns, attitudes, and barriers to palliative care consultation by gynecologic oncologists. J Oncol Pract. 2017, 13:e703–e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zimmermann C, Swami N, Krzyzanowska M, et al. . Perceptions of palliative care among patients with advanced cancer and their caregivers. CMAJ. 2016:cmaj. 151171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meirick PC. Motivated misperception? Party, education, partisan news, and belief in “death panels”. Journal Mass Commun Q. 2013, 90:39–57. [Google Scholar]
- 26. Meier DE, Casarett DJ, von Gunten CF, Smith WJ, Storey CP. Palliative medicine: Politics and policy. J Palliat Med. 2010;13:141–146. [DOI] [PubMed] [Google Scholar]
- 27. Cheng WW, Willey J, Palmer JL, Zhang T, Bruera E. Interval between palliative care referral and death among patients treated at a comprehensive cancer center. J Palliat Med. 2005;8:1025–1032. [DOI] [PubMed] [Google Scholar]
- 28. Osta BE, Palmer JL, Paraskevopoulos T, et al. . Interval between first palliative care consult and death in patients diagnosed with advanced cancer at a comprehensive cancer center. J Palliat Med. 2008;11:51–57. [DOI] [PubMed] [Google Scholar]
- 29. Kumar P, Casarett D, Corcoran A, et al. . Utilization of supportive and palliative care services among oncology outpatients at one academic cancer center: Determinants of use and barriers to access. J Palliat Med. 2012;15:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kavalieratos D, Corbelli J, Zhang D, et al. . Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA. 2016;316:2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jordhøy MS, Fayers P, Loge JH, Ahlner-Elmqvist M, Kaasa S. Quality of life in palliative cancer care: Results from a cluster randomized trial. J Clin Oncol. 2001;19:3884–3894. [DOI] [PubMed] [Google Scholar]
- 32. Gaertner J, Siemens W, Meerpohl JJ, et al. . Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: Systematic review and meta-analysis. BMJ. 2017;357:j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montori VM, Guyatt GH. Progress in evidence-based medicine. JAMA. 2008;300:1814–1816. [DOI] [PubMed] [Google Scholar]
- 34. Cella D, Stone AA. Health-related quality of life measurement in oncology: Advances and opportunities. Am Psychol. 2015;70:175–185. [DOI] [PubMed] [Google Scholar]
- 35. Taljaard M, Teerenstra S, Ivers NM, Fergusson DA. Substantial risks associated with few clusters in cluster randomized and stepped wedge designs. Clin Trials. 2016;13: 459–463. 1740774516634316. [DOI] [PubMed] [Google Scholar]
- 36. Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: Variance components and intra-cluster correlation of commonly used psychosocial measures. Psychooncology. 2013;22:1738–1747. [DOI] [PubMed] [Google Scholar]
- 37. Hemming K, Girling AJ, Sitch AJ, Marsh J, Lilford RJ. Sample size calculations for cluster randomised controlled trials with a fixed number of clusters. BMC Med Res Methodol. 2011;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. [DOI] [PubMed] [Google Scholar]
- 39. Turner RM, Bird SM, Higgins JP. The impact of study size on meta-analyses: Examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8:e59202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins JPT, Green S.. Cochrane Handbook for Systematic Reviews of Interventions. (Vol. Version 5.1.0). Chichester (UK):John Wiley & Sons, Ltd; 2011. [Google Scholar]
- 41. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 42. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hedges LV. Estimation of effect size from a series of independent experiments. Psychol Bull. 1982, 92:490–499. [Google Scholar]
- 44. Uno H, Claggett B, Tian L, et al. . Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32:2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siemieniuk RA, Agoritsas T, Manja V, et al. . Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: Systematic review and meta-analysis. BMJ. 2016;354:i5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borenstein M, Hedges LV, Higgins J, Rothstein HR. Multiple outcomes or time‐points within a study. Introduction to Meta-Analysis. Chichester (UK):John Wiley & Sons, Ltd;2009:225–238. [Google Scholar]
- 47. Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002;11:207–221. [DOI] [PubMed] [Google Scholar]
- 48. Bush SH, Parsons HA, Palmer JL, Li Z, Chacko R, Bruera E. Single- vs. multiple-item instruments in the assessment of quality of life in patients with advanced cancer. J Pain Symptom Manage. 2010;39:564–571. [DOI] [PubMed] [Google Scholar]
- 49. Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: A controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164:83–91. [DOI] [PubMed] [Google Scholar]
- 50. Bakitas M, Lyons KD, Hegel MT, et al. . Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Temel JS, Greer JA, El-Jawahri A, et al. . Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol. 2017;35:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zimmermann C, Swami N, Krzyzanowska M, et al. . Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. [DOI] [PubMed] [Google Scholar]
- 53. McCorkle R, Jeon S, Ercolano E, et al. . An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: A cluster randomized trial. J Palliat Med. 2015;18:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Higginson IJ, Bausewein C, Reilly CC, et al. . An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: A randomised controlled trial. Lancet Respir Med. 2014;2:979–987. [DOI] [PubMed] [Google Scholar]
- 55. El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol. 2011;9:87–94. [DOI] [PubMed] [Google Scholar]
- 56. Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families?Cancer J. 2010;16:423–435. [DOI] [PubMed] [Google Scholar]
- 57. Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: A systematic review. JAMA. 2008;299:1698–1709. [DOI] [PubMed] [Google Scholar]
- 58. Oncology Nursing Society. Palliative Care for People with Cancer Available at https://www.ons.org/advocacy-policy/positions/practice/palliative-care. Accessibility verified August 20, 2018.
- 59. Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017. Toronto, ON: Canadian Cancer Society; 2017. Available at http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/. Accessibility verified August 20, 2018. [Google Scholar]
- 60. American Cancer Society. Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessibility verified August 20, 2018. [Google Scholar]
- 61. Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. [DOI] [PubMed] [Google Scholar]
- 62. Haynes SN, Mumma GH, Pinson C. Idiographic assessment: Conceptual and psychometric foundations of individualized behavioral assessment. Clin Psychol Rev. 2009;29:179–191. [DOI] [PubMed] [Google Scholar]
- 63. Bischoff K, Yang E, Kojimoto G, et al. . What we do: Key activities of an outpatient palliative care team at an academic cancer center. J Palliat Med. 2018;21: 999–1004. [DOI] [PubMed] [Google Scholar]
- 64. Yoong J, Park ER, Greer JA, et al. . Early palliative care in advanced lung cancer: A qualitative study. JAMA Intern Med. 2013;173:283–290. [DOI] [PubMed] [Google Scholar]
- 65. Nipp RD, El-Jawahri A, Traeger L, et al. . Differential effects of early palliative care based on the age and sex of patients with advanced cancer from a randomized controlled trial. Palliat Med. 2018;32:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Greer JA, Jacobs JM, El-Jawahri A, et al. . Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. J Clin Oncol. 2018;36:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Masters KS. Introduction to the Special Section on Behavior Change Intervention Development: Theories, Methods, and Mechanisms. New York, NY:Oxford University Press; 2018. [DOI] [PubMed] [Google Scholar]
- 68. Klein WM, Rothman AJ, Cameron LD. Theoretical innovations in social and personality psychology and implications for health: Introduction to special issue. Health Psychol. 2013;32:457–459. [DOI] [PubMed] [Google Scholar]
- 69. Davis R, Campbell R, Hildon Z, Hobbs L, Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: A scoping review. Health Psychol Rev. 2015;9:323–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.