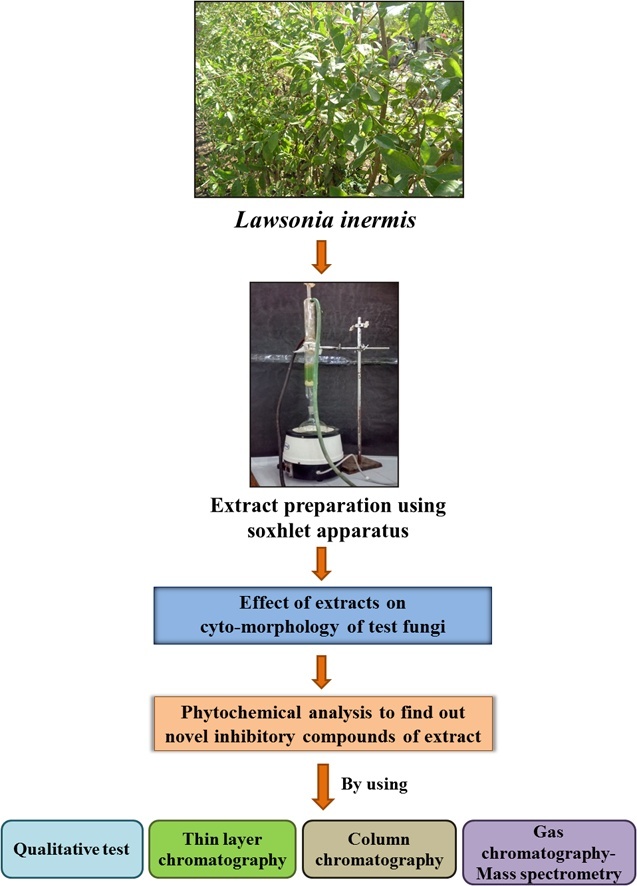
Graphical abstract
Abbreviations: PDA, Potato dextrose agar; CC, Column chromatography; TLC, Thin layer chromatography; HPLC, High performance liquid chromatography; DAD, Diode array detector; MFC, Minimum fungicidal concentration; GC–MS, Gas chromatography–mass spectrometry; PE, petroleum ether; RF, Retardation factor; SE, Standard error; CD, Critical difference; CV, Coefficient of variation; MIC, Minimum inhibitory concentration; PE, Petroleum ether; HCl, Hydrochloric acid; FeCl3, Ferric chloride; NaOH, Sodium hydroxide; H2SO4, Sulfuric acid
Keywords: Lawsonia inermis, Bioformulations, Curvularia lunata, Secondary metabolites, Chromatography
Highlights
-
•
Isolation of active principle compound was found maximum in acetone extract of L. inermis leaf.
-
•
Phytochemical tests suggest that carbohydrate, steroids, volatile oils, flavonoids, and tannins were found to be present in acetone extract of L. inermis leaf.
-
•
In vitro assay of antifungal activity of all column fractions fraction no. F1 which exhibited most significant antifungal activity against the test fungus.
-
•
GC–MS analysis of column fraction showed the occurrence of total 6 constituents.
-
•
The obtained constituents are hexacosane, octadecane, docosane, heptacosane methyl, octacosane, and tetracosane.
Abstract
Plants produce a high diversity of natural products with a prominent function in the protection against microbial pathogens on the basis of their toxic effect on growth and reproduction. In the present study, effect of partially purified acetone fraction of L. inermis leaves on various cytomorphological parameters i.e. mycelium width, conidial size, etc. of test fungi and fraction was subjected to confirming the presence of primary and secondary metabolites by rapid qualitative phytochemical tests, chromatographic methods such as TLC, column chromatography, GC–MS, etc. which were responsible for the inhibition of growth of test pathogen conidial size of Curvularia lunata decreased up to 64.76% at 0.039 μg/ml concentration of the extract. Mycelial width of C. lunata increased up to 55.91% at 0.312 μg/ml concentration of the extract. Carbohydrate, steroids, volatile oils, flavonoids, and tannins were found to be present in acetone extract of L. inermis leaf. Total of 7 bands were observed in TLC fingerprinting of L. inermis acetone fraction. Total of 10 fractions were collected from the column chromatography. Fractions which show the most significant antifungal activity against the test fungus was subjected to further GC–MS analysis for the separation and identification of active principle. GC–MS analyses show the presence of total 6 constituents i.e. hexacosane, octadecane, docosane, heptacosane methyl, octacosane, and tetracosane.
1. Introduction
The genus Curvularia represents the most widespread filamentous fungi in subtropical and tropical region of the world [1]. It causes brutal losses in the tropical regions but is a minor pathogen in temperate regions [[2], [3], [4]]. The cultural characteristic of hyphal growth of test fungi Curvularia lunata is black brown cottony growth in an appearance on the rich medium such as potato dextrose agar (PDA) [5]. The basis of fungal vegetative or hyphal growth is the continued and coordinated expansion of fungal cell/hyphae into a simple linear or complex structure. Inhibition in the hyphae of fungal cells is clearly visible in the Petri plates and can be measured as percent (%) inhibition in growth. As a result of hyphae inhibition reproductive structures also get inhibited. Sporulation is the most common mode of reproduction for diverse groups of fungi, which results in the production of large numbers of mitotically derived spores or conidia, the primary agents for infecting host plants for many plant pathogenic fungi [6]. When there is inhibition in the hyphal growth the number of reproductive structures also get reduced.
Growth and reproduction of microbes are usually denoted by the change in total population rather than an increase in the size or mass of an individual organism. The extreme growth of the pathogens result in various diseases, thus in order to treat a disease, it is very essential to inhibit the growth of microorganisms or their reproductive structures. Plants have an inbuilt capability to synthesize various secondary metabolites which act as main agents for plant defense actions against microorganisms, insects, and herbivores [7]. The use of various plant extracts and essential oils for growth inhibition has been reported by various authors [[8], [9], [10], [11], [12], [13], [14], [15], [16]].
The biological and molecular action of plant secondary metabolites induces various morphological, cytological changes in microorganisms. Banos and Lopez [17] reported that chitosan and various plant extracts induce alterations in conidia and mycelial morphology of Fusarium oxysporum, Penicillium digitatum, and Rhizopus stolonifer. The effect of leaf extracts of Cassia alata L., Cassia fistula L. and Cassia tora L. on mycelial morphology, conidial morphology, and germination of Microsporum gypseum has been reported by Phongpaichit et al. [18]. Toxigenic Aspergillus parasiticus also exhibits morphological and anatomical changes when exposed to neem (Azadirachta indica) leaf and seed aqueous extracts [19]. Berdicevsky et al. [20] reported the changes in hyphae and spore structure in dermatophytes due to severe destruction of in fungal cell coat by treatment with Inula viscosa extract. Goel and Sharma [21] reported a remarkable decrease in growth and reproduction, changes in cell number, mycelium width, conidial size and conidiophore size of Aspergillus fumigates by acetone extract of leaf and alcohol extract of inflorescence of Euphorbia pulcherrima. Sharma and Sharma [22] reported the remarkable effect of acetone fraction of L. inermis leaves and petroleum ether (PE) fraction of Corymbia citriodora leaf, their mixture and essential oils on various cytomorphological parameters i.e. mycelium width, conidial size, hyphal morphology, conidiophore size, etc. of test fungi Aspergillus flavus and Aspergillus parasiticus. Parveen and Sharma [23] also studied the effect of plant extract on growth and reproduction of Pythium aphanidermatum and Pythium myriotylum. Hada and Sharma [24] reported the inhibition of growth of Alternaria solani using cassia fistula fruit pulp extract.
In order to find out the active compound present in the active fraction of plant extract, the extract is initially subjected to thin layer chromatography (TLC) to find out the number of constituent compounds and then each compound is separated [25]. Separation of an individual compound can be done by various methods like column chromatography (CC), high-performance liquid chromatography (HPLC), gas chromatography (GC), etc. These fractions are further assayed for antifungal activity [26,27].
Many researchers have investigated the antimicrobial activity of active compounds isolated from plants. Ilic et al. [28] detected flavonoids from flowers of Linum capitatum using thin layer chromatography. Phenolic compounds were reported in the leaves of Cymbopogon citratus using TLC [29]. Vessal et al. [30] reported the detection of steroid and alkaloid glycosides by thin layer chromatographic in fruits of Winter Cherry (Physalis alkekengi). Lee [31] used silica gel column chromatography to evaluate the fungicidal activity of volatile compounds isolated from Acorus gramineus rhizome, against phytopathogenic fungi. Shiac et al. [32] separated the antioxidants from the extract of Taraxacum mongolicum by using HPLC. Gomez-Alonsoa et al. [33] also performed HPLC with UV–vis photodiode array detector (DAD) and fluorescence detection for examination of diverse grape and wine phenolics. Kartal et al. [34] isolated Echimidine N-oxide from the root of Symphytum sylvaticum and this compound was found to be inhibitory against Epidermophyton floccosum, Dreschlera rostrata, Microsporum canis, Nigrospora oryzae, Aspergillus niger, Allefsheira boydii, and Candida albicans. Drimane sesquiterpene was found to be inhibitory against Epidermophyton floccosum and Trichophyton rubrum isolated from Drimys brasiliensis [35].
In the present study, the effect of partially purified acetone fraction of L. inermis leaves on various cytomorphological parameters i.e. mycelium width, conidial size, etc. of test fungi and extract was subjected to rapid qualitative phytochemical tests for confirming the presence of primary and secondary metabolites which were responsible for the inhibition of growth of test pathogen and active fractions were isolated by chromatographic methods such as TLC, column chromatography, and GC–MS, and these fractions were further assayed for antifungal activity.
2. Materials and methods
2.1. Effect of extracts on cytomorphology of test fungi
Effect of selected extract on various morphological and cytological parameters of test pathogen was studied. Test fungi were treated with increasing concentrations of the extract up till minimum inhibitory concentration (MIC). A small fungal biomass consisting of mycelium, vesicle and conidia/spores were removed from each tube and microscopic examination was done after staining with cotton blue and mounting in lactophenol. Change in mycelium width and conidial size morphology were observed with the help of Olympus trinocular research microscope BX-51 and analyzed by Image analysis software Olysia Bioreport 3.2 of Olympus.
2.2. Phytochemical analysis of Lawsonia inermis leaf extract
Qualitative methods were used for the identification of different secondary metabolites or phytochemicals present in the plant extracts. Acetone fractions of leaf were subjected to qualitative test suggested by Kokate et al. [36]. The leaves were shade dried, crushed, powdered and extracted (100 g/ml). Various solvents (methanol, ethanol, hexane, chloroform, acetone, ethyl acetate, and distilled water) were used for extraction. The extract was kept in an orbital shaker for 5–6 days at 25 °C temperature. The supernatant was collected and analyzed for the presence and absence of alkaloids, steroids, volatile oils, carbohydrates, tannins, flavonoids, and saponins.
2.3. Tests for detection of secondary metabolites
2.3.1. Alkaloids
Alkaloids are compounds having one or more nitrogen which contain a heterocyclic ring. Wagner's test can be performed to find out the presence of alkaloids in the partially purified fractions [37]. After putting in 2 drops of diluted hydrochloric acid (HCl) in 1 ml amount of extract, it was stirred and filtered. Wagner's reagent results in the formation of a reddish brown precipitate. Various alkaloid reagents were tested on the filtrate and the development of colored precipitate was observed.
2.3.2. Volatile oils
Volatile or essential oils are the odorous volatile chemical constituents of plants. Sudan III test can be used to detect the presence of volatile oils. A modified method of Kokate et al. [37] was used for the detection of volatile oils. Development of red color on mixing with Sudan III would indicate the presence of volatile oils. A small amount of extract and Sudan III dye are mixed for the observation of the development of red color.
2.3.3. Tannins
Chemically, tannins contain a mixture of complex organic substances. Polyphenols are present in these substances. The presence of condensed tannins is indicated by the development of green color and the presence of hydrolyzable tannins is indicated by blue color. Examination of tannins was detected using the slightly modified method of Trease and Evans [38]. About 0.5 ml of extract was dissolved in 5.0 ml of distilled water. The mixture was treated with alcohol ferric chloride (FeCl3) solution and observed for the color development.
2.3.4. Saponin
Saponins are known to be complex glycoside compounds. In these compounds, aglycone is steroidal in nature. Foam test can be used to detect the presence of saponins. Detection of saponin was carried out by the slightly modified method of Kokate [39]. About 0.5 ml of extract was dissolved in 20 ml of distilled water and shaken in a graduated cylinder for 15 min. Formation of persistent frothing on warming indicates the presence of saponins. The ability of saponins to produce frothing in aqueous solution was used as a screening test for the sample.
2.3.5. Carbohydrates
Carbohydrates are widely distributed in plants. They can be detected by Molish’s test or Fehling’s test [37]. For Fehling’s test, 1 ml of extract mixed in 5 ml of distilled water and filtered after dissolution. A few drops of naphthol and concentrated sulfuric acid (H2SO4) were added to the filtrate, the color of the filtrate changed to purple. It indicates the presence of sugar. Similarly, when a small quantity of filtrate was heated with an equal amount of Fehling A and Fehling B solution, the color changed to brick red color. It indicates the presence of carbohydrates.
2.3.6. Flavonoids
Flavonoids usually occur in plants as glycosides. In this one or more of phenolic hydroxyl groups are combined with sugar residues. Alkaline reagent test is to be used to detect flavonoids. Detection of flavonoids was done by using the slightly modified method of Evans [40]. 0.5 ml of extract was dissolved in 5 ml of 10% aqueous sodium hydroxide (NaOH) solution. It results in the development of reddish brown color which shows the presence of flavonoids.
2.3.7. Sterols
Sterols are triterpenes and based on cyclopentane perhydroxy phenanthrene ring system. They are also called phytosterols. Liebermann's Burchard test is used for detection of phytosterols [41]. 1 ml of extract was mixed with 1 ml of acetic anhydride and 2 ml CHCl3 followed by the gradual addition of concentrated H2SO4 through the side of the test tube. Formation of a ring of brown color at the junction of two layers is the indication of the presence of sterols.
2.4. Isolation and characterization of the active principle from selected leaf extract by using various chromatography techniques
Isolation of active principle from selected acetone extract of L. inermis leaf was done by using chromatography techniques i.e. thin layer chromatographic fingerprinting, column chromatography and gas chromatography. Characterization of the active compound was done by mass spectrometry analysis of the extracted phytochemicals.
2.4.1. TLC fingerprinting of acetone fraction of Lawsonia inermis
For TLC fingerprinting, a 10 cm long TLC plate was cut and marked carefully. 10 μl of plant extract was spotted onto the marked plate with the help of a capillary tube or pipette. Acetone: n-hexane: benzene (2.5 ml: 8 ml: 1 ml) was used as mobile phase. The TLC plate was kept in a chromatographic chamber containing the respective solvent system and the chamber was covered with a glass plate to prevent the evaporation of the solvent. The plate was allowed to remain in the chamber until the solvent reached up to 9 cm distance. The plate was then observed in UV-florescence analysis cabinet at short and long wavelengths.
2.4.2. Visualization of TLC plate
The TLC fingerprinting plate was derivatized with anisaldehyde sulphuric acid reagent followed by heating at 100 °C till colored bands of various secondary metabolites appeared. The observations were taken before and after derivatization which visible in ultraviolet light.
Rf values were calculated as follows:
2.5. Column chromatography of partially purified acetone fraction of Lawsonia inermis leaf
10 gm of dried and partially purified acetone fraction of Lawsonia inermis leaf was dissolved in the mobile phase i.e. 25 ml acetone, 80 ml hexane and 1 ml benzene and this solution was subjected to column chromatography. Glass column (Merck: 100–200 mm) filled with 650 g of silica gel was used for column chromatography. According to the color band developed in column different fractions of the extract containing various secondary metabolites were collected. All fractions obtained from the column were dried in rotary vacuum evaporator under reduced pressure. These fractions were screened for their antifungal activity against C. lunata. The fraction showing best antifungal activity against C. lunata was subjected to further purification and characterization for active molecule via gas chromatography and mass spectrometry.
2.6. Identification and structure determination by gas chromatography/mass spectrometry (GC–MS)
The GC–MS analysis was performed on a GC (Perkin-Elmer) system coupled to Perkin- Elmer Turbo Mass MS. HP1-MS capillary column (30 m × 0.25 μm × 0.25 μm) was used under the following conditions: oven temperature programmed from 70 °C for 10 min, then gradually increased at 290 °C at 3 min; injector temperature, 250 °C; carrier gas Helium, flow rate 1 ml/min; volume of the injected sample was 0.4 μl; split ratio 1:60; ionization energy 70 eV: Run time 40 min. The relative amount of each component was calculated by comparing its average peak area to the total area. The identification of the separated volatile compounds was done through retention indices and mass spectrometry by comparing mass spectra of the unknown peaks with those stored in the Nist 98/Nbs 75 K GC–MS library.
3. Results and observations
3.1. Effect of extracts on the morphology of test fungi
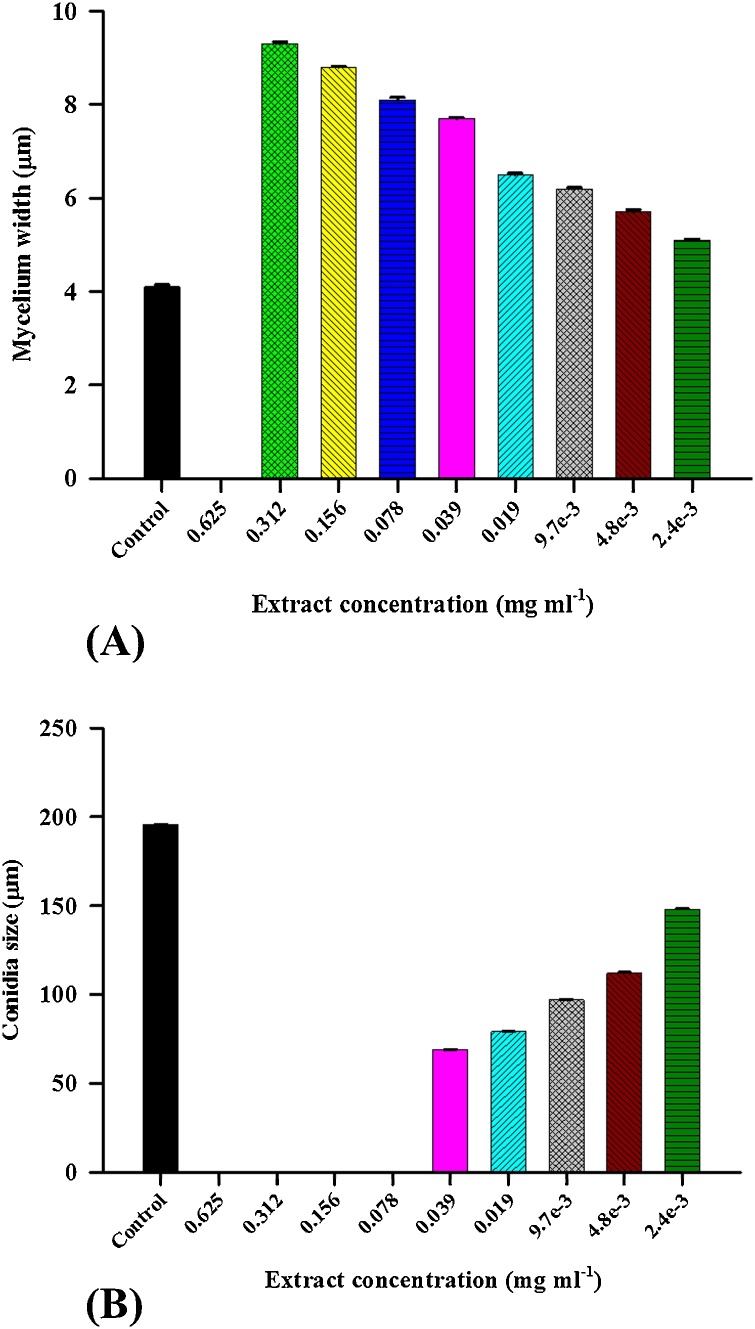
Study of leaf extracts effect of L. inermis on morphology and reproduction of test fungi are presented in Table 1, Table 2 and Figs. 1(A, B, C) & Fig. 2(A, B). The luxuriant growth of test fungi i.e. C. lunata was observed in control i.e. thick mycelial mat showed the presence of abundant conidiophores with a large number of conidia. A gradual decrease in conidia size and increase in mycelial width was observed with increasing concentration of the extract till minimum inhibitory concentration (MIC) at 0.625 mg/ml (Supplementary Fig. 1). Therefore, minimum fungicidal concentration (MFC) was also determined, and it was observed at 1.25 mg/ml (Supplementary Fig. 1).
Table 1.
Effect of different concentrations of acetone extract of Lawsonia inermis leaf on mycelium width of Curvularia lunata.
| S. No. | Extract concentration (mg/ml) | Mycelium width (μm) ± SD | % Increase in mycelium width |
|---|---|---|---|
| 1. | Control | 4.1 ± 0.05 | – |
| 2. | 0.625 | NF | – |
| 3. | 0.312 | 9.3 ± 0.04 | 55.91 |
| 4. | 0.156 | 8.8 ± 0.02 | 53.40 |
| 5. | 0.078 | 8.1 ± 0.05 | 49.38 |
| 6. | 0.039 | 7.7 ± 0.02 | 46.75 |
| 7. | 0.019 | 6.5 ± 0.03 | 36.92 |
| 8. | 0.0097 | 6.2 ± 0.03 | 33.87 |
| 9. | 0.0048 | 5.7 ± 0.05 | 28.07 |
| 10. | 0.0024 | 5.1 ± 0.02 | 19.60 |
NF: Not Found.
Table 2.
Effect of different concentrations of acetone extract of Lawsonia inermis leaf on conidial size of Curvularia lunata.
| S. No. | Extract concentration (mg/ml) | Conidial size (μm) (L × W) | Conidial size (μm) ± SD (Area) | % Reduction in conidial size |
|---|---|---|---|---|
| 1. | Control | 24.16 × 8.1 | 195.69 ± 0.17 | – |
| 2. | 0.625 | CNF | CNF | – |
| 3. | 0.312 | CNF | CNF | – |
| 4. | 0.156 | CNF | CNF | – |
| 5. | 0.078 | CNF | CNF | – |
| 6. | 0.039 | 14.80 × 4.66 | 68.96 ± 0.29 | 64.76 |
| 7. | 0.019 | 15.96 × 4.96 | 79.16 ± 0.19 | 59.54 |
| 8. | 0.0097 | 17.63 × 5.50 | 96.96 ± 0.28 | 50.45 |
| 9. | 0.0048 | 18.50 × 6.06 | 112.11 ± 0.39 | 42.71 |
| 10. | 0.0024 | 21.06 × 7.03 | 148.05 ± 0.18 | 24.34 |
CNF: Conidia Not Formed.
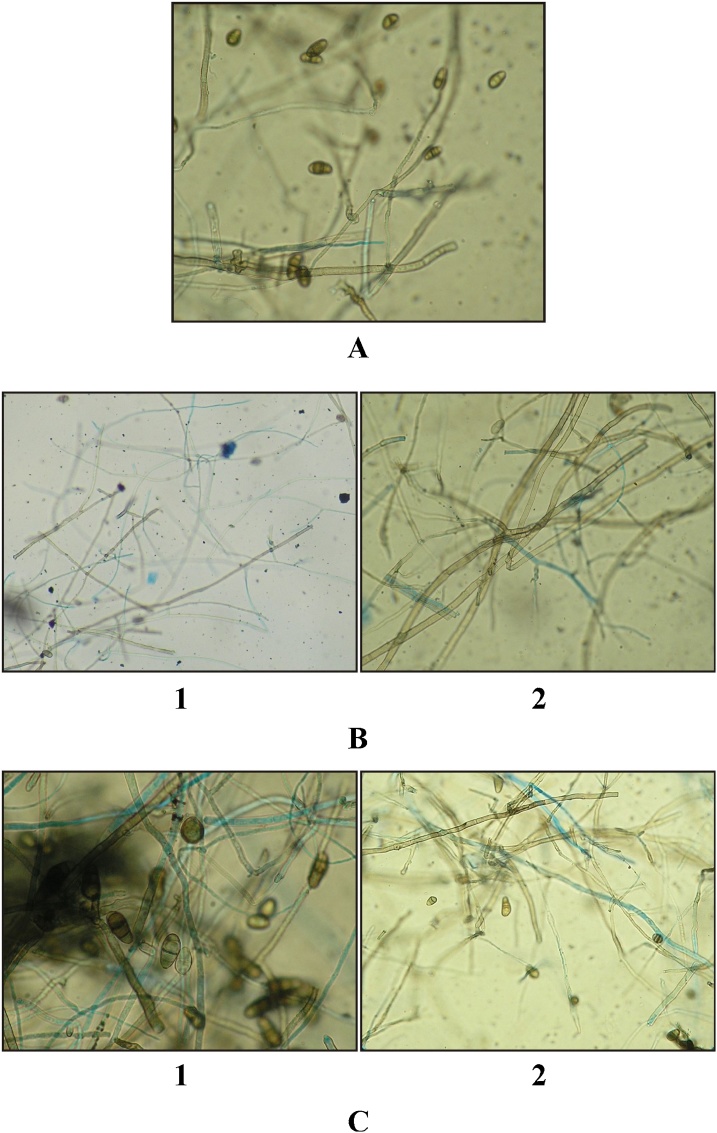
Fig. 1.
Morphological alterations in Curvularia lunata due to treatment with Acetone extract on different concentrations. (A) Mycelium and conidia of Curvularia lunata (Control at 400X). (B) (1) Normal mycelium (Control at 400X); (2) Mycelium showing increasing width (Control at 400X). (C) (1) Normal mycelium (Control at 400X); (2) Conidia showing a decrease in size (Control at 400X).
Fig. 2.
(A) Effect of different concentrations of acetone extract of Lawsonia inermis leaf on mycelium width of Curvularia lunata. (B) Effect of different concentrations of acetone extract of L. inermis leaf on conidia size of C. lunata.
A decrease in size of conidia was directly proportional to the increasing concentration of extract. Conidial size of C. lunata decreased up to 64.76% at 0.039 μg/ml concentration of the extract (Table 2). Increasing in mycelia width was directly proportional to the increasing concentration of extract. Mycelial width of C. lunata increased up to 55.91% at 0.312 μg/ml concentration of the extract (Table 1 & Supplementary Fig. 1).
3.2. Phytochemical analysis
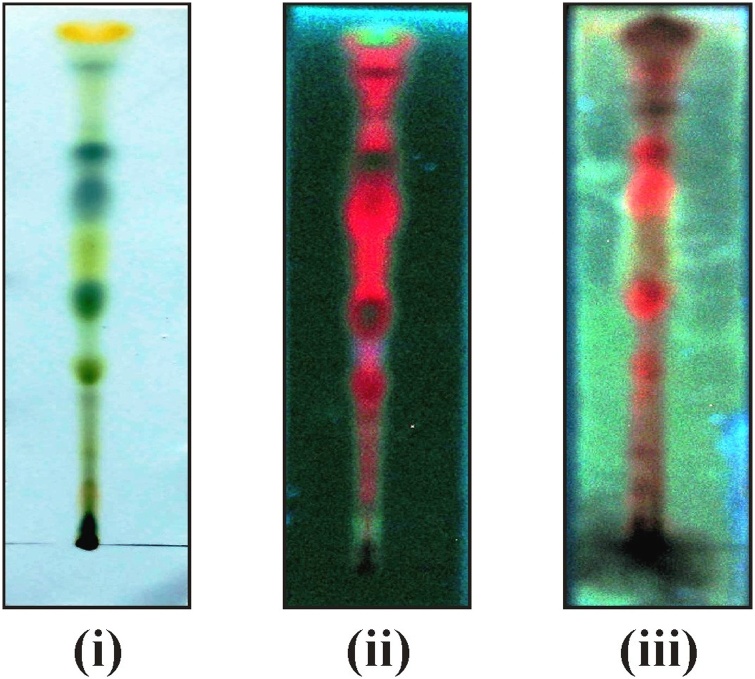
Phytochemical tests suggest that carbohydrate, steroids, volatile oils, flavonoids, and tannins were found to be present in acetone extract of L. inermis leaf. TLC plates showed clear distinct color bands. After derivatization i.e. spray of anis-aldehyde reagent on TLC plate. Changes in the color of these bands suggest the presence of different secondary metabolites in the extract (Fig. 3)
Fig. 3.
Thin layer chromatography (TLC) of various fractions of Lawsonia inermis leaf. (i) TLC of acetone fraction of leaf under visible light; (ii) TLC of acetone fraction of leaf under UV light (Before derivatization); (iii) TLC of acetone fraction of leaf under UV light (After derivatization).
The Rf values and color of bands on thin layer chromatography fingerprinting are given in Table 3 and Total 7 bands were observed in TLC fingerprinting of L. inermis acetone fraction. Rf values were calculated of these bands. The presences of different bands of different colors show the existence of various secondary metabolites in the fractions.
Table 3.
Rf values of TLC fingerprinting of acetone fraction of Lawsonia inermis leaf.
| Band number | Colour of bands |
Rf value | |
|---|---|---|---|
| Before derivatization | After derivatization | ||
| 1. | Green | Blue green | 0.16 |
| 2. | Greenish blue | Dark blue | 0.35 |
| 3. | Yellow | Yellow | 0.52 |
| 4. | Light yellow | Yellow blue | 0.63 |
| 5. | Blue | Purple | 0.70 |
| 6. | Dark blue | Light blue | 0.75 |
| 7. | Yellow | Dark yellow | 0.85 |
3.3. Column chromatography and GC–MS analysis of column fractions
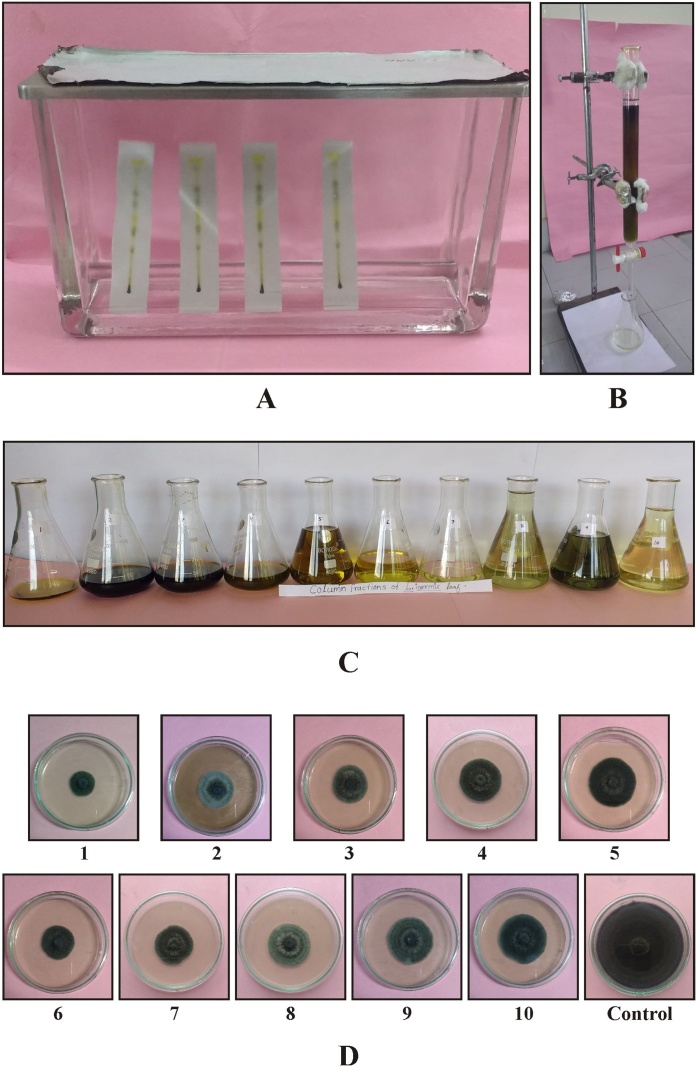
All the fractions were analyzed for further antifungal activity against pathogenic fungus C. lunata. Results of the antifungal activity of ten column fractions of acetone extract against C. lunata are presented in Table 4 and Fig. 4A-D. Column fraction no. F1 showed best maximum inhibition (75.00%) followed by fraction no. F6 (62.91%), F2 (57.66%), F3 (56.86%), F7 (54.84%), F8 (52.42%), F4 (50.00%), F9 (47.18%), F5 (43.95), and F10 (40.72%), respectively.
Table 4.
Antifungal activity of column fractions (1–10 numbers) of acetone extract of Lawsonia inermis leaf against Curvularia lunata.
| S. No. | Type of extract | Growth diameter after 7 days (mm) | % Mycelial growth inhibition |
|---|---|---|---|
| 1. | F1 | 20.66 ± 0.57 | 75.00 |
| 2. | F2 | 35.00 ± 1.00 | 57.66 |
| 3. | F3 | 35.66 ± 1.15 | 56.86 |
| 4. | F4 | 41.33 ± 1.52 | 50.00 |
| 5. | F5 | 46.33 ± 0.57 | 43.95 |
| 6. | F6 | 30.66 ± 0.57 | 62.91 |
| 7. | F7 | 37.33 ± 1.15 | 54.84 |
| 8. | F8 | 39.33 ± 0.57 | 52.42 |
| 9. | F9 | 43.66 ± 0.57 | 47.18 |
| 10. | F10 | 49.00 ± 1.73 | 40.72 |
| 11. | Control (Water) | 82.67 ± 0.57 | NI |
F: Fraction; NI: No Inhibition.
Fig. 4.
Phytochemical analysis of extracts. (A) Thin layer chromatographic chamber; (B) Column chromatography; (C) Lawsonia inermis leaf fractions collected from column chromatography; (D) Antifungal activity of column fractions (1–10) of acetone extract of Lawsonia inermis leaf against Curvularia lunata.
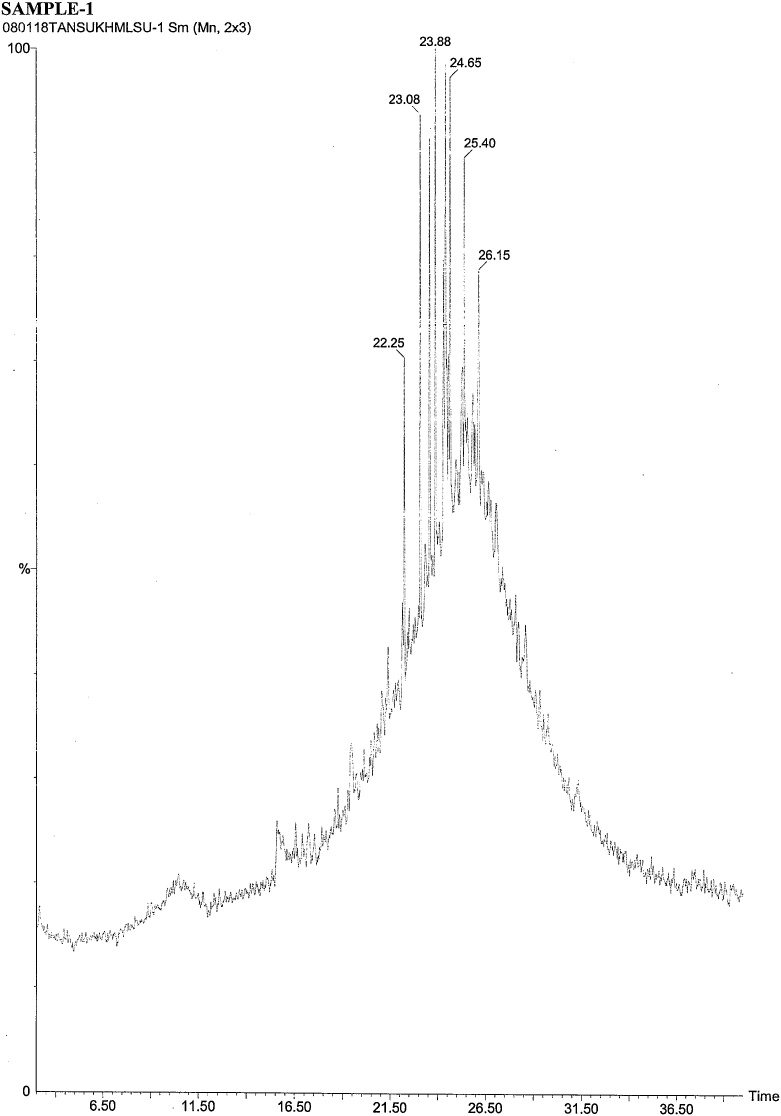
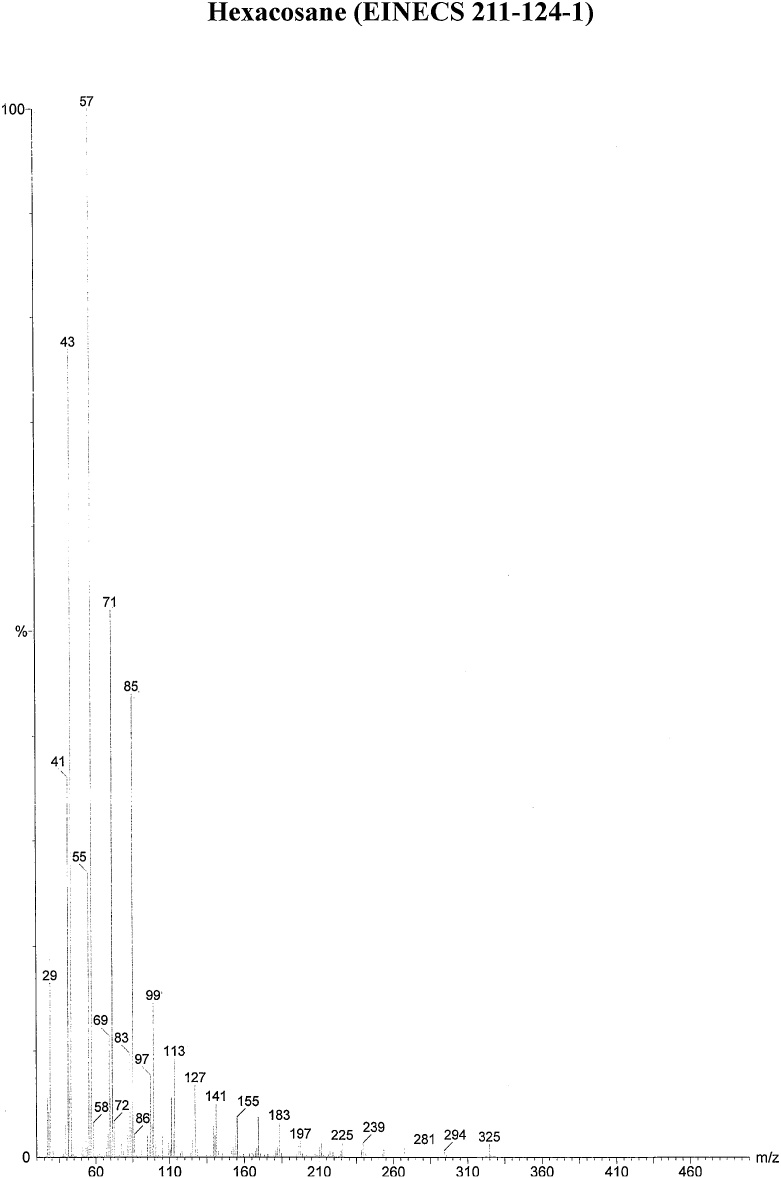
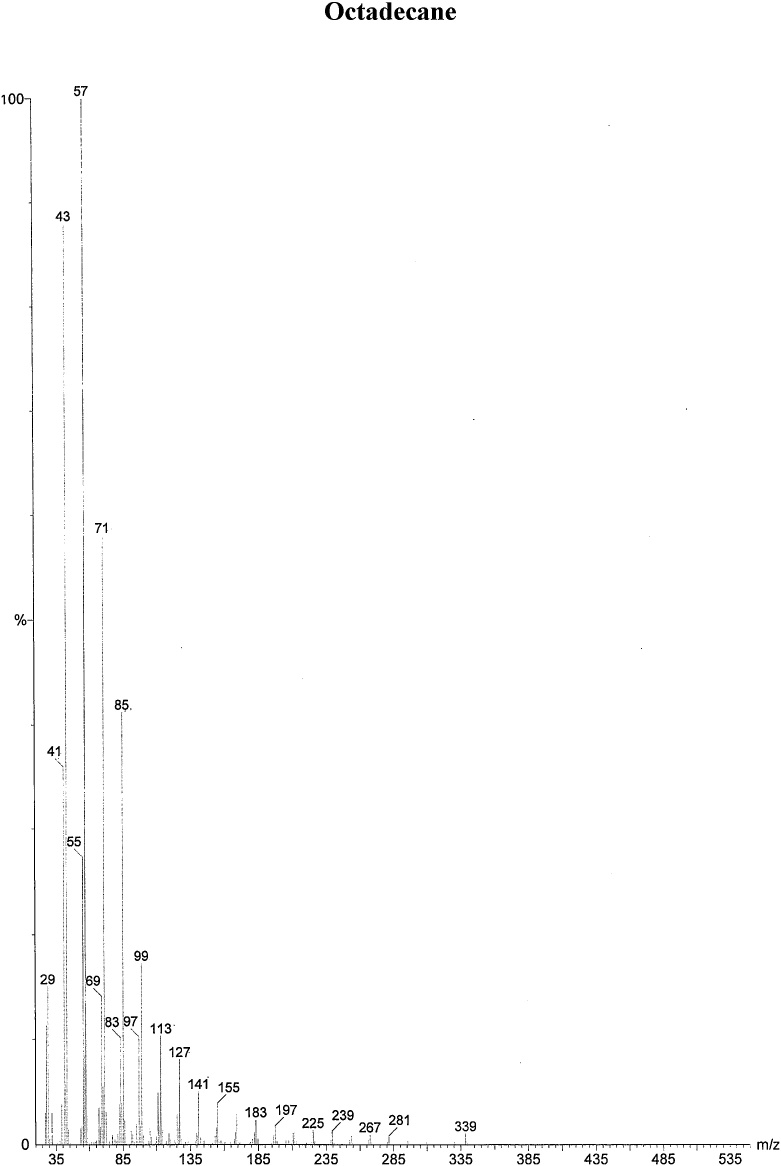
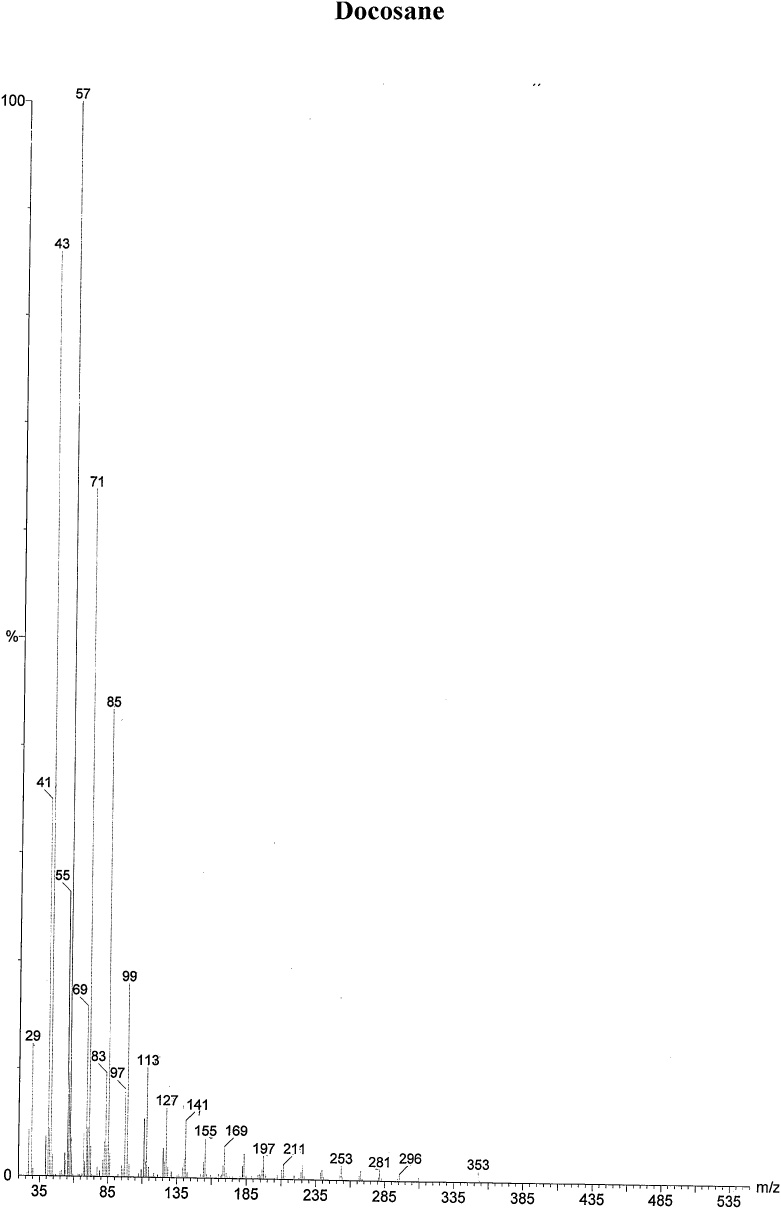
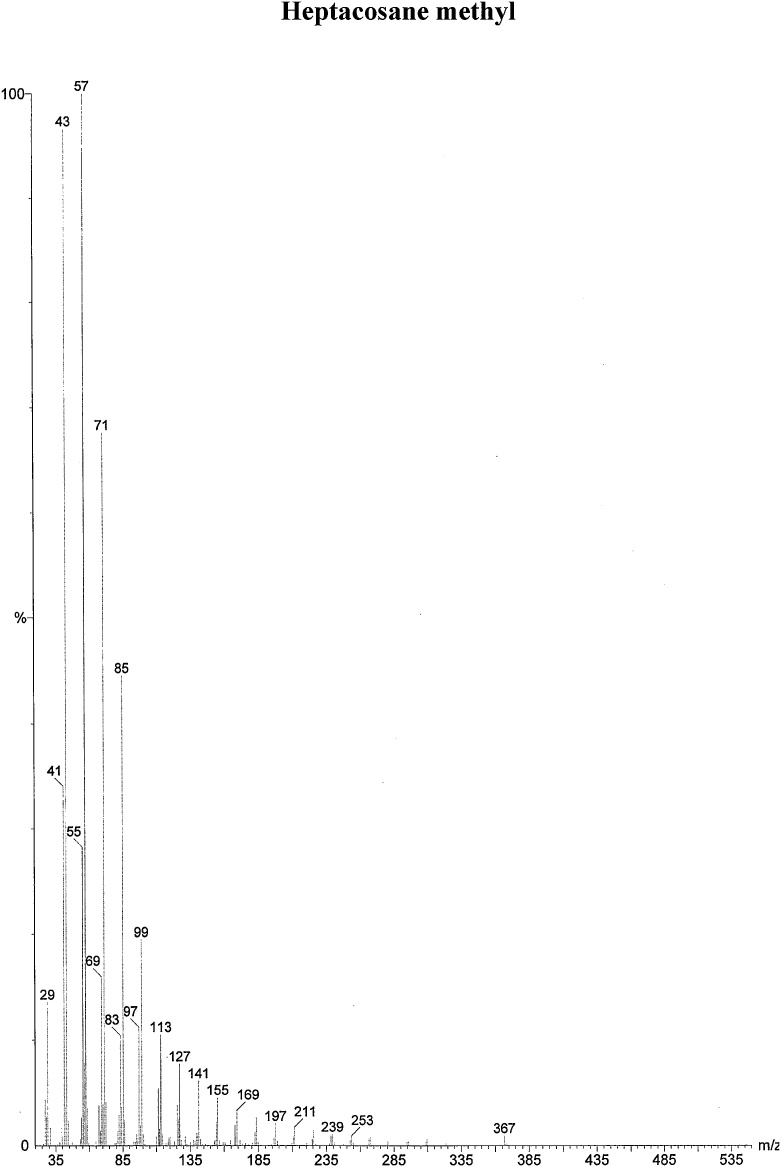
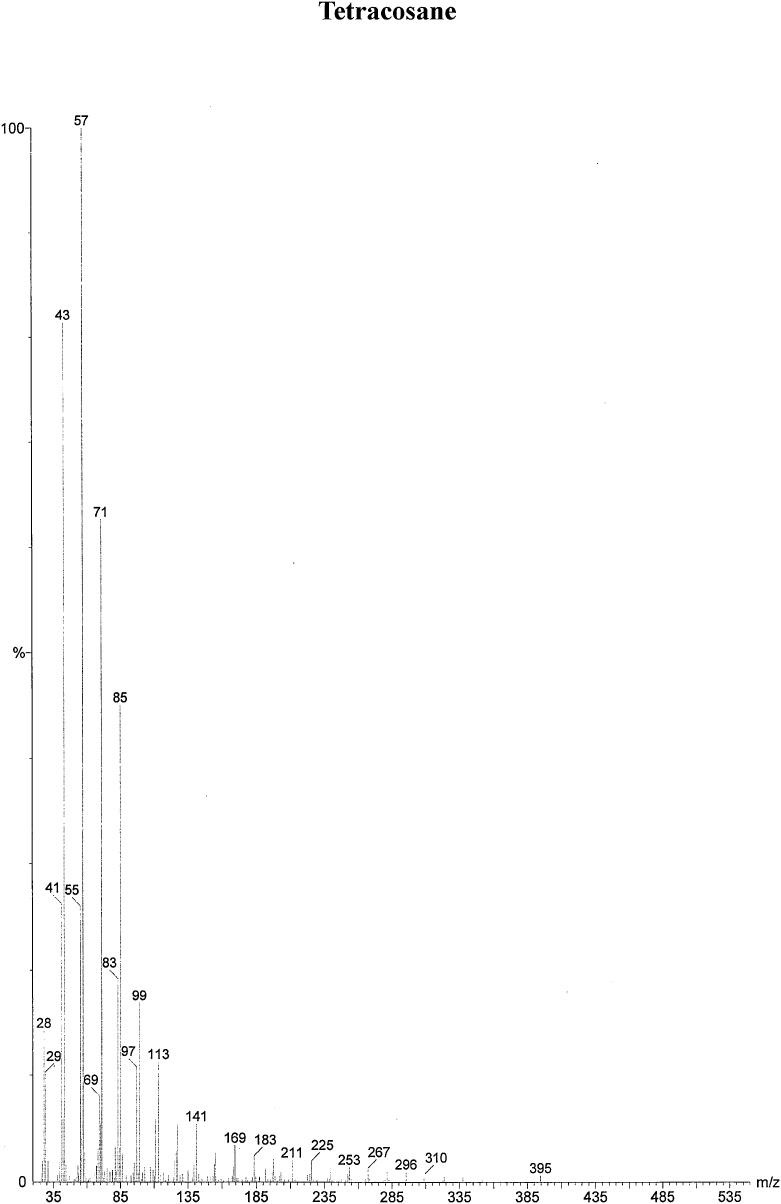
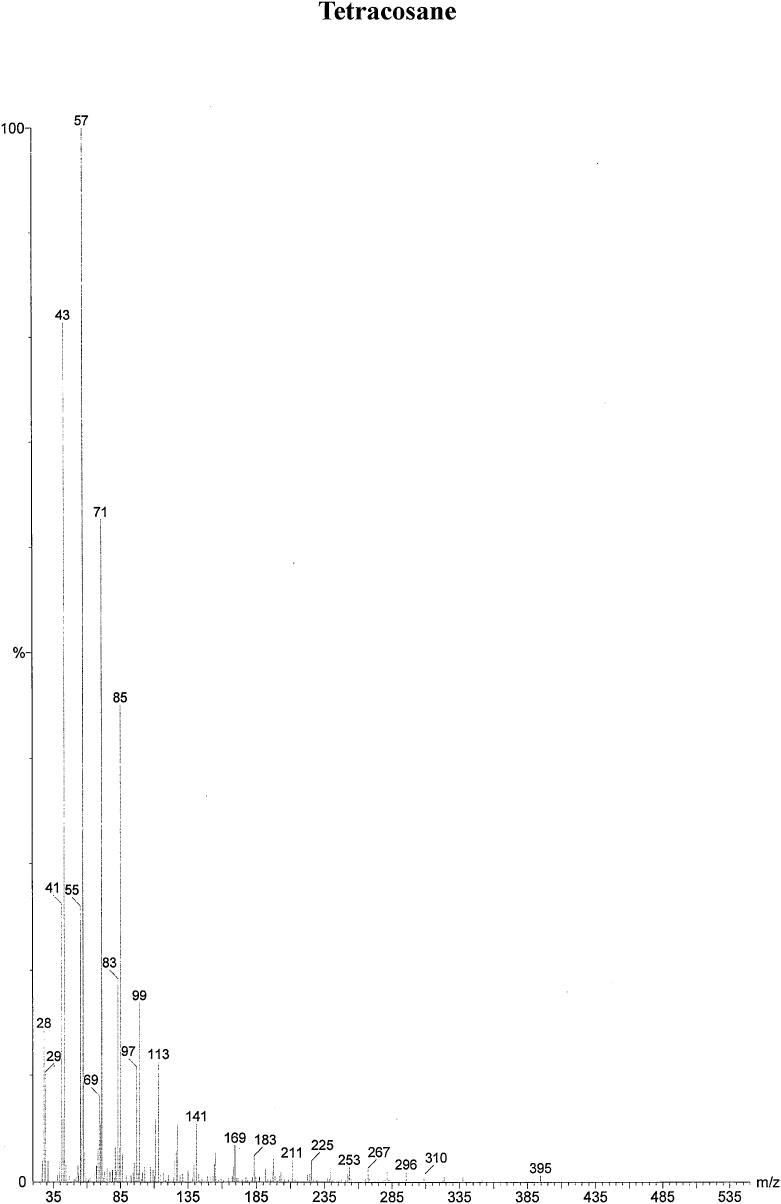
On the basis of in vitro assay of antifungal activity of all column fractions, fraction no. F1 which exhibited the most significant antifungal activity against the test fungus was subjected to further GC–MS analysis for the separation and identification of active principle. The chromatogram obtained in GC–MS analysis of L. inermis fraction is presented as in Fig. 4A. GC–MS analysis of column fraction showed the occurrence of total 6 constituents which are hexacosane (Fig. 4B), octadecane (Beilstein 1770570) (Fig. 4C), docosane (EINECS 211-121-5) (Fig. 4D), heptacosane methyl (14167-66-9) (Fig. 4E), octacosane (RN 630-02-4) (Fig. 4F) and tetracosane (Beilstein 1758462) (Fig. 4G).
4. Discussion
Microorganisms are able to reproduce tremendously within a relatively short time when they find favorable conditions such as an abundant supply of nutrients, optimum temperature, pH, etc. The extreme growth of the pathogens results in various diseases, thus in order to treat a disease, it is very essential to inhibit the growth of microorganisms. Use of plants as a source of medicine is as old as humanity. It is estimated that about 7500 plants are used in local health practices in India. Plants have an immense ability to synthesize aromatic substances which are the master agents for plant defense mechanisms against microorganisms, insects, and herbivores. Thus, plant extracts and their products are now being exploited as agents of disease control.
In this study, we have studied the effect of acetone extract of L. inermis on the growth and reproduction of test fungus C. lunata. Many researchers have been applied different plant extracts to study the effect on growth and reproduction of different pathogenic fungi but few reports are found on the effect of leaf extract of L. inermis on growth and reproduction of C. lunata which is the causal agent of leaf spot disease in maize. However, reports are available on the inhibitory effect of L. inermis plant extract on other plant pathogenic fungi. Sharma and Sharma [22] reported the remarkable effect of acetone fraction of L. inermis leaves and PE fraction of E. citriodora leaf, their mixture and essential oils on various cytomorphological parameters i.e. mycelium width, conidial size, conidiophore size, etc. of A. flavus and A. parasiticus. Martin et al. [42] studied the effect of alcoholic and aqueous extracts of jabuticaba (Myrciaria cauliflora), guava (Psidium guajava) and jambolan (Syzygium cumini L.) on different biological parameters against fungus Beauveria bassiana (Bals.) Vuill. Results showed concentration-dependent plant extract inhibition of fungal growth which may be due to increase in the concentration of secondary metabolites/active components on increasing the concentration. The same pattern was also reported by Bonjar [43], Maresa et al. [44], Thanaboripat et al. [8], Reddy et al. [9], Reddy et al. [10], and Goel and Sharma [21] (Fig. 5).
Fig. 5.
(A) Gas Chromatography–Mass spectrometry analysis of Fraction no.1 of acetone fraction of Lawsonia inermis leaf; (B) Mass spectrum of Hexacosane (EINECS 211-124-1); (C) Mass spectrum of Octadecane (Beilstein 1770570); (D) Mass spectrum of Docosane (EINECS 211-121-5); (E) Mass spectrum of Heptacosane methyl (14167-66-9); (F) Mass spectrum of Octacosane (RN 630-02-4); (G) Mass spectrum of Tetracosane (Beilstein 1758462).
The major changes observed due to extracting treatment were increased width of mycelium and decreased conidial size. This increase in width of hyphae, decreased conidial size may be because of the presence of secondary metabolite/s in the plant extract. These may inactivate the microbial adhesions, may change the permeability of the membrane allowing water to enter in and /or may get accumulated in the hyphae of test fungi that results in the increase in the width of hyphae and decreased conidial size, respectively.
Ya et al. [45] and Tsuchiya et al. [46] also reported that the plant secondary metabolites have the ability to inactivate microbial adhesions by disrupting fungal membrane, fungal enzymes, cell envelope and transport proteins which ultimately alter the arrangement of the membrane and hence change in the fungal cell/hyphae morphology as well as other structures. Abnormal filamentation in the fungus has been reported after treatment with plant extract by Rath et al. [47] and Pattnaik et al. [48]. Nakamura et al. [49] also suggested that change in the morphology of hyphae of pathogenic fungi Candida spp. could be due to the loss of integrity of the cell wall and the consequent effect on plasma membrane permeability by the active metabolites from Ocimum gratissimum. Pornsuriya et al. [50] reported that secondary metabolites from Chaetomium caused abnormal mycelial growth in P. aphanidermatum. He also reported abnormal swellings and increased the diameter of sporangium, oospores as well as the diameter of oogonia. Many scientists also have studied the effect of different plant extracts on growth and reproduction of different plant pathogenic fungi [[22], [23], [24],[51], [52], [53]] and presently it is a very interesting area for finding a novel inhibitory agent to control diseases in the eco-friendly ways.
Plants produce a huge miscellany of secondary compounds that are synthesized through secondary metabolism as protection against a wide variety of microorganisms (fungi, viruses, bacteria), herbivores (arthropods, vertebrates), and insect attack. Secondary metabolites such as alkaloids, essential oils (lower terpenoids and phenylpropanoids), coumarins, anthraquinones, flavonoids, terpenoids (cardenolides, diterpenes, monoterpenoids, iridoids, sesquiterpenoids, and triterpenoids) and steroids have a pervasive history of use as therapeutic metabolites [[54], [55], [56], [57], [58]]. Secondary metabolites are also useful for humans for controlling various diseases. Hence, there has been an amazing insurgence of interest in research of natural products over the past decade or so.
Phytochemical screening of extracts reveals the presence of various secondary metabolites in it. Several researchers have evaluated the phytochemical property of plant extracts by the qualitative methods [[59], [60], [61],23,[62], [63], [64], [65]]. Results of phytochemical testing of different fractions of L. inermis showed the presence of various secondary metabolites. Tannins, carbohydrate, saponins, and flavonoids were present in PE fraction of L. inermis leaf. Chloroform and benzene fraction of leaf showed the presence of flavonoids and tannins. Carbohydrate, steroids, volatile oils, flavonoids, and tannins were found to be present in acetone extract of L. inermis leaf. Methanol fraction of leaf gave positive results for tannins, steroids, carbohydrates, saponins, and flavonoids whereas aqueous fraction exhibited the presence of carbohydrates, tannins, and steroids.
The next steps include the purification and identification of secondary metabolites present in active fractions. It has been achieved by thin layer chromatography (TLC), column chromatography (CC), gas chromatography (GC), etc. TLC separates the ingredient fractions present in the extract [[66], [67], [68], [69], [70], [71]]. In the present study, TLC fingerprinting of acetone extract of L. inermis leaf was done. The Rf values and colors of the resolved bands of TLC fingerprinting of leaves are given in Table 3. After derivatization of the TLC plate, the obtained bands showed the presence of various secondary metabolites in the extract. TLC fingerprinting of acetone extract of L. inermis leaf showed the development of bands of light blue, dark blue colors which are characteristics of triterpenoids and pink and red color bands indicates the presence of flavonoids and tannins respectively.
Column fractions of acetone extract of L. inermis leaf were also collected. The fractions showed different colors which indicate the presence of different secondary metabolites. Thus, the phytochemical study reveals that a broad category or group of secondary metabolites is present in these plant extracts or fractions which may be responsible for its antifungal activity. The fractionation by TLC and column chromatography suggested the presence of triterpenoids, tannins, and flavonoids in leaf extract. Further separation and characterization of the active principle from column fractions of L. inermis using GC–MS analyses reveal the presence of hexacosane, octadecane, docosane, heptacosane methyl, octacosane, and tetracosane.
Hexacosane and heptacosane methyl belong to the class of organic compounds and show medicinal properties. Hexacosane has been detected from many plant species like Glycyrrhiza glabra and Vanilla planifolia but heptacosane methyl has been found only in G. glabra [72] and octadecane was identified as a volatile component in the extract of Korean Chamchwi (Aster scaber Thunb) [73]. Remaining three components i.e. octacosane, docosane and tetracosane are not identified as having medicinal properties. L. inermis also shows the vast medicinal properties and many other researchers also detected other compounds which show the medicinal properties. Mikhaeil et al. [74] reported that L. inermis contains p-coumaric acid, lawsone, apigenin, luteolin, 2-methoxy-3-methyl-1,4- naphthoquinone, cosmosiin and apiin. The antifungal activity of 1,4- α naphthoquinone and hydroxyl-carbons was reported by many researchers [22,75,76].
Author’s contributions
TSB: Planned and performed the experiments, wrote the manuscript and prepared the final version of the manuscript. MM: planned to design the manuscript format, data analyzed, analyzed the results, helped for statistical analysis and drafting the manuscript. KS: supervised the whole work. All authors have read and approved the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
Tansukh Barupal is thankful to University Grants Commission (UGC), New Delhi, Government of India, for the award of UGC-BSR fellowship. The Authors are also thankful to Professor S. S. Sharma, maize pathologist (AICRP-Maize, New Delhi), Department of Plant Pathology, RCA, MPUAT, for help in the identification of the fungus.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00335.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Bisht S., Kumar P., Srinivasanraghvan A., Purohit J. In vitro management of Curvularia leaf spot of maize using botanicals, essential oils and bio-control agents. Bioscan. 2013;8:731–733. [Google Scholar]
- 2.Huang J., Zheng L., Hsiang T. First report of leaf spot caused by Curvularia verruculosa on Cynodon sp. In Hubei. China. Plant Pathol. 2005;54(2):253. [Google Scholar]
- 3.Hua Li-Fu, Zhi Ye-Hua, Tao Wang-Yu, Xiong-Mian P. Research progress for maize Curvularia leaf spot disease. J. Maize Sci. 2006;12:97–101. [Google Scholar]
- 4.Akinbode O.A. Evaluation of antifungal efficacy of some plant extracts on Curvularia lunata, the causal organism of maize leaf spot. Afr. J. Environ. Sci. Technol. 2010;4:797–800. [Google Scholar]
- 5.Gadeeyya G., Kumar P.K. Isolation and characterization of mycoflora from infected weeds. J. Plant Prot. Res. 2014;6:1–19. [Google Scholar]
- 6.Yu J.H., Mah J.H., Seo J.A. Growth and developmental control in the model and pathogenic Aspergilli. Eukaryot. Cell. 2006;5:1577–1584. doi: 10.1128/EC.00193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan M.M. Plant products as antimicrobial agents. Cli. Microbiol. Rev. 1999;12:564. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thanaboripat D., Chareonsettasilp S., Pandee K., Udomwongsup K. Inhibitory effect of Kaffir lime, bitter cucmber and tobacco extract on the growth of Aspergillus flavus. KMITL Sci. Tech. J. 2006;6:18–24. [Google Scholar]
- 9.Reddy K.R.N., Reddy C.S., Muralidharan K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Cont. 2009;20:173–178. [Google Scholar]
- 10.Reddy K.R.N., Nurdijati S.B., Salleh B. Efficacy of aqueous medicinal plant extracts on growth and citrinin production by Penicillium citrinum isolated from rice grains. Afr. J. Microbiol. Res. 2010;4:2562–2565. [Google Scholar]
- 11.Dissanayake M.L.M.C., Jayasinghe J.A.N. Antifungal activity of selected medicinal plant extracts against plant pathogenic fungi; Rhizoctonia solani, Colletotrichum musea and Fusarium oxysporum. IJSIT. 2013;2:421–431. [Google Scholar]
- 12.Sesan T.E., Enache E., Beatrice M.I.B.M., Oprea M., Oancea F., Iacomi C. Antifungal activity of some plant extracts against Botrytis cinerea pers.iN the blackcurrant crop (Ribes nigrum L.) Acta. Sci. Pol. Hortorum. Cultus. 2015;14:29–43. [Google Scholar]
- 13.Devi K.B., Pavankumar P., Bhadraiah B. Antifungal activity of plant extracts against post-harvest fungal pathogens. Int. J. Curr. Microbiol. App. Sci. 2017;6:669–679. [Google Scholar]
- 14.Gakuubi M.M., Maina A.W., Wagacha J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int. J. Microbiol. 2017:7. doi: 10.1155/2017/8761610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meena M., Swapnil P., Zehra A., Aamir M., Dubey M.K., Upadhyay R.S. Beneficial microbes for disease suppression and plant growth promotion. In: Singh D., Singh H., Prabha R., editors. Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer; Singapore: 2017. pp. 395–432. [Google Scholar]
- 16.Meena M., Dubey M.K., Swapnil P., Zehra A., Singh S., Kumari P., Upadhyay R.S. The rhizosphere microbial community and methods of its analysis. In: Singh H.B., Sarma B.K., Keswani C., editors. Advances in PGPR Research. CAB International; 2017. pp. 275–295. [Google Scholar]
- 17.Banos S.B., Lopez M.H. Growth inhibition of selected fungi by chitosan and plant extracts. Rev. Mex. Fitopatol. 2004;22:178–186. [Google Scholar]
- 18.Phongpaichit S., Pujenjob N., Rukachaisirikul V., Ongsakul M. Antifungal activity from leaf extracts of Cassia alata L., Cassia fistula L. and Cassia tora L. Songklanakarin. J. Sci. Technol. 2004;26:741–748. [Google Scholar]
- 19.Abyaneh M.R., Allameh A., Tiraihi T., Ghahfarokhi M.S., Ghorbanian M. Morphological alterations in toxigenic Aspergillus parasiticus exposed to neem (Azadirachta indica) leaf and seed aqueous extracts. Mycopathologia. 2005;159:565–570. doi: 10.1007/s11046-005-4332-4. [DOI] [PubMed] [Google Scholar]
- 20.Berdicevsky I., Duek L., Neeman I., Maoz M. Antimycotic activity of tayunin-Inula viscose extract-SEM observations. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL, USA; 2001. pp. 16–19. Abstract Number J-98. [Google Scholar]
- 21.Goel A., Sharma K. Effect of Euphorbia pulcherrima leaf and inflorescence extract on various cytomorphological parameters of Aspergillus fumigates: World Academy of Science, Engineering and Technology. Int. J. Bio. Biom. Agri. Food & Biotech. Eng. 2013;7:516–519. [Google Scholar]
- 22.Sharma A., Sharma K. 2011. Study of Antifungal Activity of Plant Extracts Against Aflatoxin Producing Strains of Aspergillus species. A Thesis submitted to M.L.S.U., Udaipur. [Google Scholar]
- 23.Parveen T., Sharma K. 2015. Development of Herbal Biocontrol Agents Against Certain Ginger Rot Causing Pythium species. A Thesis submitted to M.L.S.U., Udaipur. [Google Scholar]
- 24.Hada D., Sharma K. Isolation and characterization of chemical compounds from fruit pulp of cassia fistula and their antimicrobial activity. J. Drug Deliv. Ther. 2018;8:15–20. [Google Scholar]
- 25.Phillipson J.D. Phytochemistry and pharmacognosy. Phytochemistry. 2007;68:2960–2972. doi: 10.1016/j.phytochem.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Proestos C., Sereli D., Komaitis M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006;95:44–52. [Google Scholar]
- 27.Hossain M.A., Ismail Z., Rahman A., Kang S.C. Chemical composition and anti-fungal properties of the essential oils and crude extracts of Orthosiphon stamineus Benth. Ind. Crops Prod. 2008;27:328–334. [Google Scholar]
- 28.Ilic S.B., Konstantinovic S.S., Todorovic Z.B. Flavonoids from flower of Linum capitatum kit. Phys. Chem. Technol. 2004;3:67–71. [Google Scholar]
- 29.Satya V.K., Radhajeyalakshmi R., Kavitha K., Paranidharan V., Bhaskaran R., Savchuk S., Fernando W.C.D. Effect of timing of application and population dynamics on the degree of biological control of Sclerotinia sclerotiorum by bacterial antagonistic. FEMS Microl. Ecol. 2005;49:379–388. doi: 10.1016/j.femsec.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Vessal M., Akmali M., Bambaee-Row N. Thin layer chromatographic detection of steroid and alkaloid glycosides in an ethanolic extract of winter cherry (Physalis alkengi) fruits. Arch. Iranian. Med. 2006;9:315–318. [Google Scholar]
- 31.Lee H.S. Fungicidal properties of active component derived from Acorus gramineus rhizome against phytopathogenic fungi. Elseweir Ltd. 2006:1324–1328. doi: 10.1016/j.biortech.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Shiac S., Zhaob Y., Zhouc H., Zhanga Y., Jianga X., Huanga K. Indentification of antioxidants from Taraxacum mongolicum by high-performance liquid chromatography-diode array detection-radial scavenging detection-electrospray ionization mass spectrometry and nuclear magnetic resonance experiments. J. Chromatogr. 2008;1209:145–152. doi: 10.1016/j.chroma.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Alonsoa S., Garcia-Romeroa E., Hermosin-Gutierrez I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Anal. 2007;20:618–626. [Google Scholar]
- 34.Kartal M., Kurucu S., Choudhary M.I. Antifungal activity of different extracts and Echimidine-N-oxide from Symphytum sylvatifcum Boiss. Subsp. Sepulcrale (Boiss & Bal.) Greuter and Burdet var. Sepulcrale. Truk. J. Med. Sci. 2001;31:487–492. [Google Scholar]
- 35.Malheiros A., Filho V.C., Schmitt C.B., Yunes R.A., Escalante A., Svetaz L., Lacchino S., Monache F.D. Antifungal activity of drimane sesquiterpenes from Drimys brasiliensis using bioassay guided fractionation. J. Pharm. Pharm. Sci. 2005;8:335–339. [PubMed] [Google Scholar]
- 36.Kokate C.K., Purohit A.P., Gokhale S.B. Analytic Pharmacognosy. 7th ed. Nirali Prakashan; Pune: 1990. Pharmacognogy; pp. 122–124. [Google Scholar]
- 37.Kokate C.K., Purohit A.P., Gokhale S.B. Niraliprakashan: 2006. 23rd ed. 2006. Pharmacognosy; pp. 493–497. [Google Scholar]
- 38.Trease G.E., Evans W.C. 14th edn. Bailliere Tindall Ltd.; London: 1996. A Textbook of Pharmacognosy; p. 612. 10:9780702018992. [Google Scholar]
- 39.Kokate C.K. Phytochemical methods. Phytotherapy. 1999;78:126–129. [Google Scholar]
- 40.Evans W.C. An index of medicinal plants. Textbook Pharmacognosy. 1997;7:12–14. [Google Scholar]
- 41.Huang T.C., Chen C.P., Wefler V., Raftery A. A stable reagent for the Liebermann-Burchard reaction. Application to rapid serum cholesterol determination. Anal. Chem. 1961;33(10):1405–1407. [Google Scholar]
- 42.Martin C.C., Alvesb L.F.A., Mamprimb A.P. Effect of plant extracts and a disinfectant on biological parameters and pathogenicity of the fungus Beauveria bassiana (Bals.) Vuill. (Ascomycota: Cordycipitaceae) Braz. J. Biol. 2016;76:420–427. doi: 10.1590/1519-6984.17914. [DOI] [PubMed] [Google Scholar]
- 43.Bonjar G.H.S. Evaluation of antibacterial properties of Iranian medicinal plants against Micrococcus luteus, Serratia marcescens, Klebsiella pneumoniae and Bordetella bronchoseptica. Asian J. Plant Sci. 2004;3:82–86. [Google Scholar]
- 44.Maresa D.B., Tosia F., Polib E., Andreottia C., Romagnolic Antifungal activity of on some phytopathogenic fungi: ultrastructural evidence on Pythium ultimum. Microbiol. Res. 2004;15:295–304. doi: 10.1016/j.micres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Ya C., Gaffney S.H., Lilley T.H., Haslam E. Carbohydrate polyphenol complexation. In: Hemingway R.W., Karchesy J.J., editors. Chemistry and Significance of Condensed Tannins. Plenum Press; New York: 1988. p. 553. [Google Scholar]
- 46.Tsuchiya H., Sato M., Miyazaki Y., Fujiwara S., Taniyaki S., Ohyama M., Tanaka T., Linuma M. Comparative study on the antibacterial activity of phytochemical flavones against methicillin resistant Staphylococcus aureus. J. Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 47.Rath C.C., Dash S.K., Mishra R.K., Charyulu J.K. Anti E. coli activity of turmeric (Curcuma longa L.) essential oil. Indian Drugs. 2001;38:106–111. [Google Scholar]
- 48.Pattnaik S., Subramanyam V.R., Rath C.C. Effect of essential oils on the viability and morphology of E. coli. Microbios. 1995;84:195–199. [PubMed] [Google Scholar]
- 49.Nakamura C.V., Ishida K., Faccin L.C., Filho B.P.D., Cortez D.A.G., Rozental S., de souza W., Nakamura U. In vitro activity of essential oil from Ocimum gratissimum L. against four Candida species. Res. Microbiol. 2004;155:579–586. doi: 10.1016/j.resmic.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Pornsuriya C., Soytong K., Kanokmedhakul S., Lin F.C. Efficacy of antifungal metaboilites from some antagonistic fungi against Pythium aphanidermatum. J. Agri. Tech. 2010;6:299–308. [Google Scholar]
- 51.Mesa-Arango A.C., Trevijano-Contador N., Roman E., Sanchez-Fresneda R., Casas C., Herrero E. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014;58:6627–6638. doi: 10.1128/AAC.03570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meena M., Swapnil P., Upadhyay R.S. Isolation, characterization and toxicological potential of tenuazonic acid, alternariol and alternariol monomethyl ether produced by Alternaria species phytopathogenic on plants. Sci. Rep. 2017;7:8777. doi: 10.1038/s41598-017-09138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meena M., Swapnil P., Zehra A., Dubey M.K., Upadhyay R.S. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathology Plant Protect. 2017;50:13–14. [Google Scholar]
- 54.Silva L., Gloria I.K., Soo L., Douglas A., Kinghorn . Special problems with the extraction of plants. In: Corrado C., editor. Methods in Biotechnology, Natural Product Isolation. Humana Press Inc; Totowa, New Jersey: 1998. [Google Scholar]
- 55.Cannell J.P.R. How to approach the isolation of natural products. In: Cannell J.P., Richard, editors. Methods in Biotechnology: Natural Products Isolation. Humana Press Inc.; Totowa, New Jersey: 1998. [Google Scholar]
- 56.Ezekiel J.S., Adamu H.M., Chindo I.Y., Garba I.H. Phytochemical profile and antioxidant activities of solvent-solvent fractions of Haematostaphis barteri Hook F. (Anacardiaceae) stem bark extracts. Int. J. Pharmacogn. Phytochem. Res. 2016;8:51–56. [Google Scholar]
- 57.Meena M., Aamir M., Vikas K., Swapnil P., Upadhyay R.S. Evaluation of morpho-physiological growth parameters of tomato in response to Cd induced toxicity and characterization of metal sensitive NRAMP3 transporter protein. Environ. Exper. Bot. 2018;148:144–167. [Google Scholar]
- 58.Meena M., Swapnil P., Zehra A., Dubey M.K., Aamir M., Patel C.B., Upadhyay R.S. Virulence factors and their associated genes in microbes. In: Singh H.B., Gupta V.K., Jogaiah S., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2019. pp. 181–208. [Google Scholar]
- 59.Babayi H., Kolo I., Okogun J.I., Lijah U.J.J. The antimicrobial activities of methanolic extracts of Eucalyptus camaldulen and Terminalia catappa against some pathogenic microorganisms. Biokemistri. 2004;16:106–111. [Google Scholar]
- 60.Solis C., Bocerra J., Flores C., Robledo J., Silva M. Antibacterial and antifungal terpenes from Pilgerodendron uviferum (D. Don) Florin. J. Chil. Chem. Soc. 2004;49:157–161. [Google Scholar]
- 61.Ayandele A.A., Abebiyi A.O. The phytochemical analysis and antimicrobial screening of extracts of Olax subscorpioidea. Afr. J. Biotechnol. 2007;6:868–870. [Google Scholar]
- 62.Okore V.C., Ugwu C.M., Oleghe P.O., Akpa P.A. Selective anti candidal action of crude aqueous pod extract of Lecaniodiscus cupanioides: a preliminary study on Candida albicans obtained from an AIDS patients. Sci. Res. Essays. 2007;2:043–046. [Google Scholar]
- 63.Usman H., Osuji J.C. Phytochemical and in vitro antimicrobial assay of the leaf extract of Newbouldia leaves. Afr. J. Trad. CAM. 2007;4:476–480. [PMC free article] [PubMed] [Google Scholar]
- 64.Usman H., Musa Y.M., Ahmadu A.A., Tijjani M.A. Phytochemical and antimicrobial effects of Chrozophora senegalensis. Afr. J. Trad. CAM. 2007;4:488–494. [PMC free article] [PubMed] [Google Scholar]
- 65.Meena M., Zehra A., Dubey M.K., Aamir M., Gupta V.K., Upadhyay R.S. Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (TeA, AOH, and AME) Front. Plant Sci. 2016;7:1408. doi: 10.3389/fpls.2016.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karou D., Savodago A., Canini A., Yaamego S., Montesano C., Simpore J., Colizzi V., Traore A.S. Antibacterial activity of alkaloids from Sida acuta. Afr. J. Biotechnol. 2006;5:195–200. [Google Scholar]
- 67.Sokovic M., Marin P.D., Brkic D., Griensven L.J.L.D.V. Chemical composition and antibacterial activity of essential oils of ten aromatic plants against human pathogenic bacteria. Food. 2007;1:1–7. [Google Scholar]
- 68.Bluma V.R., Etcheverry M.G. Application of essential oils in maize grain: impact on Aspergillus section Flavi growth parameters and aflatoxin accumulation. Food Microbiol. 2008;25:324–334. doi: 10.1016/j.fm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Vilela G.R., Almeida G.S., D’Arce M.A.B.R., Moraes M.H.D., Brito J.O., Silva M.F., Das G.F., Silva S.C., Piedade S.M., de S., Domnigues M.A.C., Gloria E.M. Activity of essential oil and its major compound, 1-8, cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J. Stored Prod. Res. 2009;45:108–111. [Google Scholar]
- 70.Kumar A., Shukla R., Singh P., Dubey N.K. Biodeterioration of some herbal raw materials by storage fungi and assessment of Cymbopogon flexuosus essential oil and its components as antifungal. Int. Biodeter. Biodegr. 2009;63:712–716. [Google Scholar]
- 71.Zuzarte M., Goncalves M.J., Cavaleiro C., Dinis A.M., Canhoto J.M., Salqueiro L.R. Chemical composition and antifungal activity of the essential oils of Lavandula pedunculata (Miller) Cav. Chem. Biodiverse. 2009;6:1283–1292. doi: 10.1002/cbdv.200800170. [DOI] [PubMed] [Google Scholar]
- 72.Duke . US Dept. Agric., Agric Res. Service; 2017. Phytochemical and Ethnobotanical Databases Hexacosane.https://phytochem.nal.usda.gov/phytochem/search [Google Scholar]
- 73.Chung T.Y., Eiserich J.P., Shibamoto T. Volatile compounds isolated from edible Korean Chamchwi (Aster scaber Thunb) J. Agric. Food Chem. 1993;41:1693–1697. [Google Scholar]
- 74.Mikhaeil B.R., Badria F.A., Maatooq G.T., Amer M.M.A. Antioxidant and immunomodulatory constituents of heena leaves. Z. Naturforsch. 2004;59:468–476. doi: 10.1515/znc-2004-7-803. [DOI] [PubMed] [Google Scholar]
- 75.Ambrogi V., Artini D., Carneri I.D., Castellino S., Dradi A., Logemann W., Meinradi G., Somma M.D., Tosolini G. Studies on the antibacterial and antifungal properties of 1,4α naphthoquinones. Br. J. Pharmac. 1970;40:871–880. doi: 10.1111/j.1476-5381.1970.tb10662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Babu P.D., Subhasree R.S. Antimicrobial activities of Lawsonia inermis - A review. Acad. J. Plant Sci. 2009;2:231–232. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.