Abstract
Post‐exertional malaise and delayed recovery are hallmark symptoms of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Studies on repeated cardiopulmonary exercise testing (CPET) show that previous exercise negatively affects oxygen uptake (VO 2) and power output (PO) in ME/CFS. Whether this affects arterial lactate concentrations ([Laa]) is unknown. We studied 18 female patients (18–50 years) fulfilling the Canadian Consensus Criteria for ME/CFS and 15 healthy females (18–50 years) who underwent repeated CPETs 24 h apart (CPET 1 and CPET 2) with [Laa] measured every 30th second. VO 2 at peak exercise (VO 2peak) was lower in patients than in controls on CPET 1 (P < 0.001) and decreased in patients on CPET 2 (P < 0.001). However, the difference in VO 2peak between CPETs did not differ significantly between groups. [Laa] per PO was higher in patients during both CPETs (P interaction < 0.001), but increased in patients and decreased in controls from CPET 1 to CPET 2 (P interaction < 0.001). Patients had lower VO 2 (P = 0.02) and PO (P = 0.002) at the gas exchange threshold (GET, the point where CO 2 production increases relative to VO 2), but relative intensity (%VO 2peak) and [Laa] at GET did not differ significantly from controls on CPET 1. Patients had a reduction in VO 2 (P = 0.02) and PO (P = 0.01) at GET on CPET 2, but no significant differences in %VO 2peak and [Laa] at GET between CPETs. Controls had no significant differences in VO 2, PO or %VO 2peak at GET between CPETs, but [Laa] at GET was reduced on CPET 2 (P = 0.008). In conclusion, previous exercise deteriorates physical performance and increases [Laa] during exercise in patients with ME/CFS while it lowers [Laa] in healthy subjects.
Keywords: Elevated lactate, exercise intolerance, metabolism, oxygen uptake, post‐exertional malaise
Introduction
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a complex, multisystem and often debilitating disorder of unknown etiology (Institute of Medicine 2015). Common symptoms include fatigue, post‐exertional malaise (PEM), widespread pain, sleep disturbances, cognitive dysfunction, sensory hypersensitivity, orthostatic intolerance, and gastrointestinal discomfort. There are no reliable diagnostic markers or clinical findings that can verify the diagnosis, which is based on self‐reported symptoms. There is no widely accepted agreement regarding diagnostic criteria. The Canadian Consensus Criteria (CCC) (Carruthers et al. 2003) and The International Consensus Criteria (ICC) (Carruthers et al. 2011) both appear to identify a subgroup of patients with more severe functional impairments, as well as marked physical and cognitive symptoms (Jason et al. 2013). PEM is defined as a substantial worsening of symptoms after mild to moderate physical, mental, or emotional exertion. PEM and impaired recovery after exertion are mandatory criteria in both the CCC and the ICC.
Cardiopulmonary exercise testing (CPET) provides an accurate and objective assessment of functional capacity (ATS/ACCP Statement on cardiopulmonary exercise testing, 2003; Arena et al. 2007). Repeated CPET have demonstrated that ME/CFS patients are unable to reproduce oxygen uptake and power output at peak exercise (VO2peak) and/or at the gas exchange threshold defined by the V‐slope method, when tested on two consecutive days (Vermeulen et al. 2010; Snell et al. 2013; Keller et al. 2014; Nelson et al. 2019). The gas exchange threshold is the point where VCO2 increases relative to VO2, and has traditionally been viewed as a transition from aerobic to anaerobic energy production, coinciding with the onset of lactate accumulation. However, the notion of tissue hypoxia as the main cause of lactate accumulation is no longer accepted (Brooks 2018). Lactate is always the end result of glycolysis, also under aerobic conditions (Rogatzki et al. 2015). Glycolysis increases during exercise, and lactate is rapidly cleared from the circulation, mainly through oxidation and gluconeogenesis. During an incremental exercise test, blood lactate accumulation occurs when the rate of lactate appearance exceeds the rate of lactate disposal (Brooks 2018). Trained individuals have a lower blood lactate concentration and increased lactate metabolic clearance rate for an absolute exercise intensity, compared to untrained individuals. However, the rate of lactate appearance has been found to be similar in trained and untrained subjects at the same relative intensity defined as percent of VO2peak (%VO2peak) (Bergman et al. 1999; Messonnier et al. 2013).
Previous studies have not demonstrated consistent results with regard to physical performance and lactate accumulation in ME/CFS (Riley et al. 1990; Wong et al. 1992; Gibson et al. 1993; Lane et al. 1998; Sargent et al. 2002; Jammes et al. 2005), but lactate accumulation during repeated exercise testing has not been examined. The aim of this study was therefore to examine if VO2 and arterial plasma lactate concentrations at various exercise intensities differed between patients with ME/CFS and healthy controls by performing two CPETs 24 h apart.
Materials and Methods
Approvals
The study protocol was approved by the Regional Committee for Medical and Health Research Ethics in Norway (no. 2012/571‐1), and is registered with ClinicalTrials.org (ID NCT02970240).
Subjects
We used social network and media coverage to invite potential participants, and we received a large number of requests to participate in this study. All requests were pre‐screened with regard to age (18–50 years), gender (females), place of living (in the study area), health status, medication, and current level of physical activity. Eligible candidates were interviewed consecutively on telephone or in person. All included patients fulfilled the CCC for ME/CFS (Carruthers et al. 2003). We excluded patients who were pregnant, bedridden or had comorbidities that could interfere with CPET results, that is, lung‐ and heart disorders, or used medication known to affect physical performance. The controls had no known previous or current serious illness, did not use any regular medication (oral contraceptives were allowed), had no first‐grade relatives with ME/CSF and exercised less than twice weekly on a regular basis. To minimize PEM due to traveling to the study site, the patients were offered to stay at the study site during the test period. Eighteen female patients with ME/CFS and 15 female healthy controls participated in the study after signing informed consent forms.
Exercise testing
All tests were performed between 8 and 11 am at the Glittre Clinic, a rehabilitation hospital for patients with chronic pulmonary diseases. The participants were asked to refrain from physical exertion 72 h prior to the first CPET and were tested after an overnight fasting, allowing free consumption of water. Weight (to the nearest 0.5 kg) and height (to the nearest 1.0 cm) were recorded and a spirometry was performed at baseline the first day (Oxycon Pro, Erich Jaeger GmbH, Wurzburg, Germany). Baseline electrocardiogram (ECG) was monitored on both days (CS‐200, Schiller, Baar, Switzerland). Prior to each test we inserted a catheter (BD Arterial Cannula 20G 1.1 × 45 mm, Franklin Lakes, NJ) into the radial artery for blood sampling.
The participants performed two maximal incremental ramp tests (CPET1 and CPET2), 24 h apart, on a cycle ergometer (Ergometrics 900, Ergoline, Bitz, Germany). Gas exchange and ventilation were measured breath‐by‐breath (Oxycon Pro). The flow sensor was calibrated with a 3 L syringe prior to each test (Hans Rudolph, Shawnee, KS), and the gas analyzer for O2 and CO2 was calibrated against commercial standards. The increment rate was set individually, with the aim of reaching VO2peak within 8 to 12 min of exercise. The rate was based on previous and current level of activity, physical examination, age, height and weight, and ranged from 10 to 24 W/min for the ME/CFS patients, and 15–30 W/min for the controls. The protocol included a 2‐min resting phase and 2 min of unloaded pedaling at a rate of 60–75 rpm, followed by a linear increase in power output until volitional exhaustion or until the participant was unable to maintain a cycling frequency above 45 rpm. They were given strong vocal encouragement during the tests. ECG was monitored continuously. Criteria to terminate the exercise test were any signs of distress, such as pallor or dizziness, chest pain, significant arrhythmias, or signs of ischemia on ECG. After reaching VO2peak, the participants continued to breathe through the facemask for a 3‐min recovery period.
Analyses of biochemical markers
Arterial blood samples were collected at rest before each CPET and every 30th second during exercise. Test tubes contained an antiglycolytic agent to prevent continued glycolysis in the blood samples (2.0 mL sodium fluoride, potassium oxalate BD Vacutainer® Plus, Franklin Lakes, NJ). The samples were immediately placed in iced water and centrifuged at 1500g for 15 min at 21°C within 30 min (Sigma 2‐7, Osterode am Harz, Germany). Plasma was separated and put on dry ice before storing at −80°C until analyzed. Arterial lactate concentration ([Laa]) was determined by spectrophotometer (MaxMat PL, Montpellier, France). We added 4‐chlorophenol, lactate oxidase, peroxidase and 4‐aminophenazone (Lactate, Spinreact, Girona, Spain) to plasma and the tubes were mixed at 37°C for 10 min. The absorbance of samples and standards was read at 505 nm against the blank, with a detection limit of 0.044 mmol/L and a linearity limit of 16.85 mmol/L. Control sera (SPINTROL H Normal and Pathologic, Spinreact, Girona, Spain) were used to monitor the performance of assay procedures, as recommended by the manufacturer. Arterial blood samples for analyses of hemoglobin concentration were collected at rest before each CPET and within 90 sec after peak exercise had been achieved, and analyzed within 1 minute after sampling (ABL835 version 6.16 Flex; Radiometer, Copenhagen, Denmark).
Analyses of exercise variables
Variables measured breath‐by‐breath were averaged over a 30‐sec sampling time. The gas exchange threshold was visually identified by the V‐slope method (Schneider et al. 1993), independently by two investigators. Relative exercise intensity was defined as per cent of VO2peak (%VO2peak). The point where [Laa] started to accumulate was defined as the lactate turnpoint (LT) and was determined by log‐log transformation of [Laa] versus power output, that is, at the intersection of their regression lines, as described by Beaver et al. (1985), and using a computer script designed for this purpose. Power output with its linear increase during test, was preferred above oxygen uptake in order to avoid the need of smoothing the oxygen curves prior to log‐log transformation, given that oxygen uptake not necessarily increases in a strictly linear fashion (Beaver et al. 1985; Goodwin et al. 2007). We also estimated power output for a fixed [Laa] of 4 mmol/L, often referred to as the onset of blood lactate accumulation (OBLA) (Goodwin et al. 2007). A fitted line for [Laa] during each individual CPET was visually inspected to find the corresponding power output (to the nearest 0.5 W).
Sample size
According to a reproducibility study on CPET, a difference in VO2peak of 10% represents a real change in exercise tolerance (Hansen et al. 2004). In a previous study on repeated CPET in subjects with ME/CFS, mean (SD) VO2peak decreased with 6 (7.5) % from CPET1 to CPET2, corresponding to −1.33 (1.68) mL/kg/min (Vermeulen et al. 2010). To demonstrate a difference of 10% between the two tests, corresponding to 2.23 mL/kg/min, with a two‐sided significance level of 5% and 80% power; our study would require a sample size of 18 participants in the ME/CSF group.
Statistics
Data are presented as mean (SD). Two‐sample and paired t‐tests were used for all CPET variables. Due to somewhat skewed distributions, [Laa] at rest, at gas exchange threshold and at LT were also analyzed after log10 transformation, but this did not change the results (data not shown). Mixed model analysis for repeated measurements with random intercept and random effect of power output was applied for [Laa] during the tests. Both power output and the square of power output were included in the model. Group and day were modeled as fixed effects. We tested for interaction between group and test, and between group and power output and the square of power output (i.e. whether there were group differences in [Laa] accumulation with increasing power output). To detect whether the difference in [Laa] curves from CPET1 to CPET2 was significantly different between the groups, we calculated the change in [Laa] for each power output per kilo body weight (POkg) in every participant. We performed mixed model analyses on the calculated change in [Laa], with fixed intercept, random effect of POkg, and with group modeled as fixed effect, to test for interaction between group and POkg. P‐values less than 0.05 were considered statistically significant. Statistical analyses were performed with SPSS (IBM SPSS Statistics version 22, Armonk, NY).
Results
Subject characteristics
The inclusion process is illustrated in Figure 1, and the subject characteristics of the 18 patients and 15 controls that completed both CPETs with arterial plasma samples for lactate analyses are reported in Table 1. Age and height were similar in the two study groups, but the patients had higher body weight and BMI than the controls. Spirometry prior to CPET was within normal limits in both groups and did not indicate any obstructive or restrictive lung disorders. The hemoglobin concentration was also similar between the groups at CPET1 and CPET2.
Figure 1.
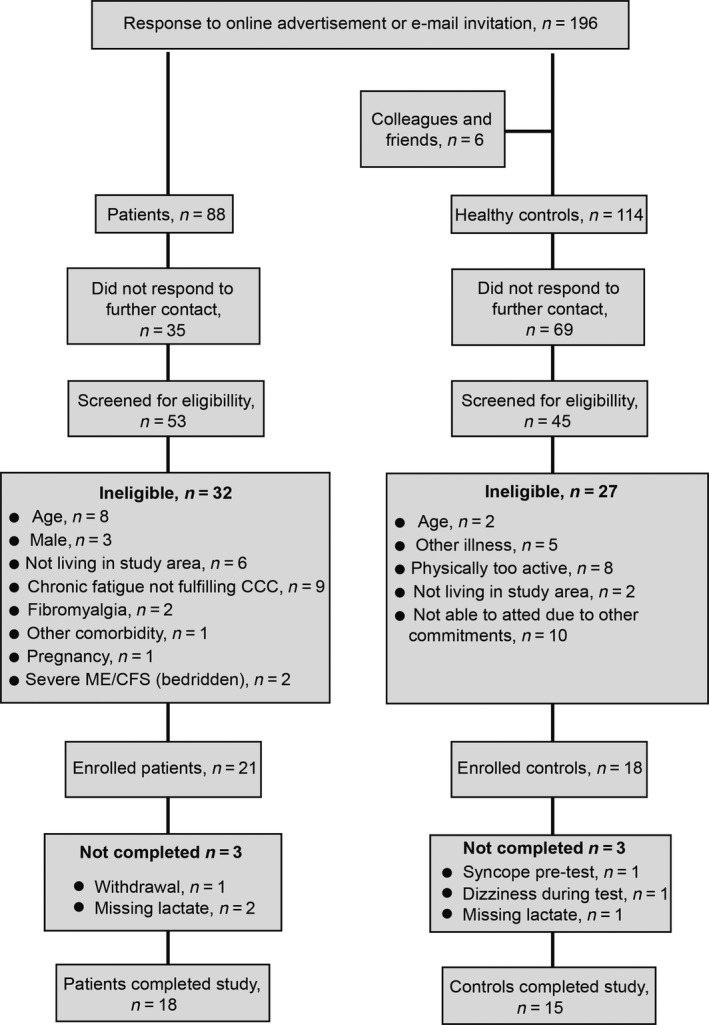
Flowchart of the inclusion of participants.
Table 1.
Baseline characteristics of the two study groups
| Characteristic | ME/CFS (n = 18) | Controls (n = 15) | P‐value |
|---|---|---|---|
| Age (years; range) | 38 (21–49) | 34 (21–49) | 0.14 |
| Height (m) | 1.71 (0.08) | 1.69 (0.07) | 0.46 |
| Weight (kg) | 73 (13) | 63 (11) | 0.02 |
| BMI (kg/m2) | 25.2 (5.0) | 22.0 (3.2) | 0.03 |
| FVC (L) | 4.43 (0.57) | 4.41 (0.66) | 0.95 |
| FEV1 (L) | 3.51 (0.46) | 3.55 (0.56) | 0.83 |
| FEV1/FVC (%) | 79.4 (5.0) | 80.5 (6.0) | 0.56 |
| Predicted FVC (%) | 121 (17) | 121 (16) | 0.97 |
| Predicted FEV1 (%) | 111 (17) | 112 (15) | 0.88 |
| Hb day 1 (g/dL) | 13.2 (0.9) | 12.7 (0.8) | 0.19 |
| Hb day 2 (g/dL) | 12.5 (0.9) | 12.0 (0.8) | 0.18 |
Values are mean (SD) unless otherwise reported.
Peak exercise responses
VO2peak was significantly lower in patients than controls on both CPETs (Fig. 2A). VO2peak was further reduced from CPET1 to CPET2 in patients, but the difference in peak VO2peak between CPETs did not differ significantly between the two groups (Fig. 2B). Maximum heart rate and respiratory exchange rate did not differ significantly between the groups or between the two CPETs (Fig. 2C and D). Power output at peak exercise (POpeak) was significantly lower in patients than controls on both CPETs (Fig. 3A), also when analyzed for power output per body weight (POkg) (Fig. 3B). Both groups had a significant reduction in POpeak from CPET1 to CPET2. The difference in POpeak between the two CPETs was not significantly different between the groups (Fig. 3C).
Figure 2.
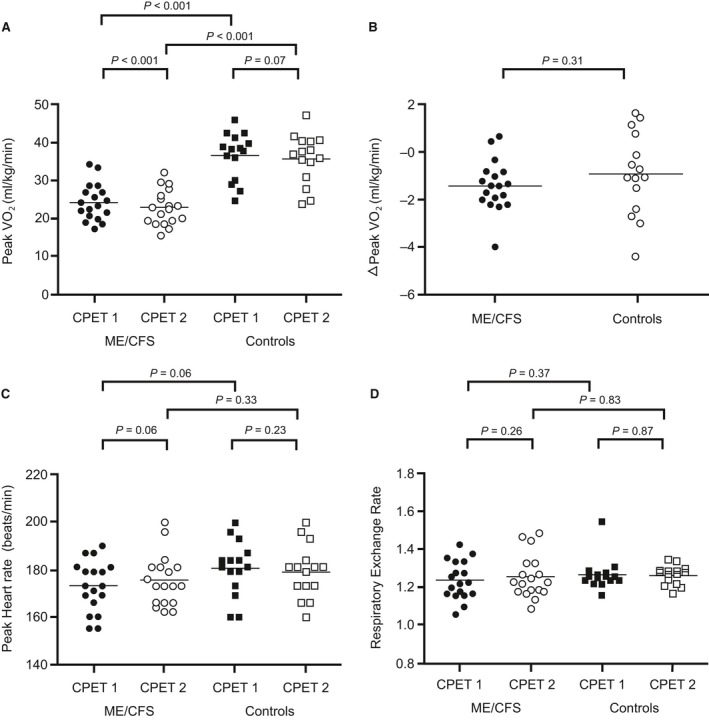
Peak exercise responses. Data points represent individual measures for each participant and horizontal lines are mean values. (A) shows VO2peak for both CPETs and both study groups, while (B) shows the difference (Δ) in VO2peak between CPET1 and CPET2 for both study groups. (C) shows peak heart rate for both CPETs and for both study groups, and (D) shows respiratory exchange rate at peak exercise for both CPETs and for both study groups.
Figure 3.
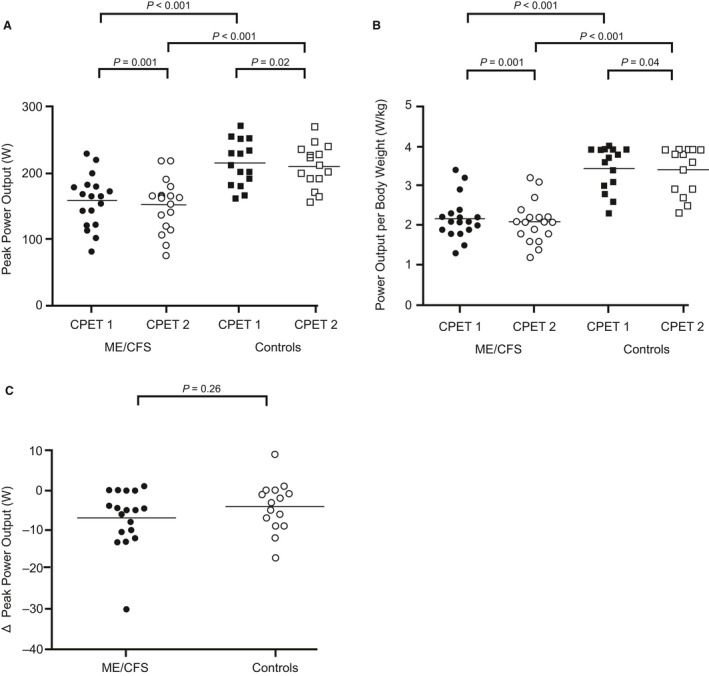
Power output at peak exercise. Data points represent individual measures for each participant and horizontal lines are mean values. (A) shows peak power output for both CPET1 and CPET2 and for both study groups, (B) shows peak power output adjusted for body weight, and (C) shows the difference (Δ) in peak power output between CPET1 and CPET2 for both study groups.
Lactate concentrations at baseline and during tests
Resting [Laa] did not differ between patients and controls before CPET1, but were significantly different before CPET2 (Fig. 4A). In the mixed model analyses, [Laa] per power output and POkg were significantly higher in patients than in controls on both CPETs (P interaction < 0.001; Fig. 4B and C). Furthermore, the difference in [Laa] per power output and POkg between the groups increased from CPET1 to CPET2 (P interaction < 0.001; Fig. 4B and C). The [Laa] curve for CPET2 was shifted significantly to the left in patients (P < 0.001) and to the right in controls (P < 0.001), compared to the [Laa] curves for CPET1 (Fig. 4B and C).
Figure 4.
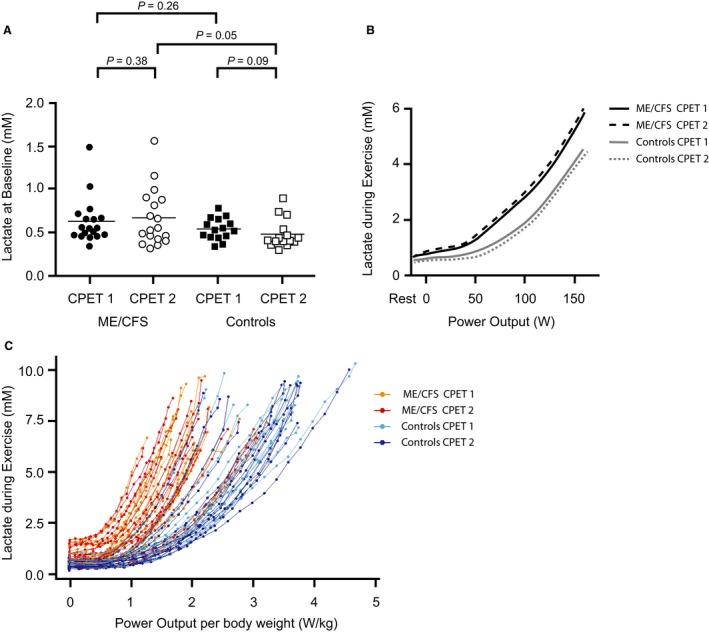
[Laa] at baseline and during exercise. Data points represent individual measures for each participant and horizontal lines are mean values in (A), and shows resting [Laa] at baseline prior to each CPET for both study groups. (B) shows mean [Laa] curves for both study groups on both days up to 150 W, as this was the mean peak power output in the patient group. (C) shows the raw data of all [Laa] samples and data point represents individual [Laa] measures for each participant per power output per body weight. Lines represent individual curves of [Laa] per power output per body weight.
Gas exchange threshold
VO2 at gas exchange threshold (GET) was significantly lower in patients than controls on both CPETs (Fig. 5A). VO2 at GET was further reduced in patients from CPET1 to CPET2, but the difference in VO2 at GET did not differ significantly between groups (Fig. 5B). The power output at GET was significantly lower in patients compared to controls on both CPETs, and was further reduced on CPET2 (Fig. 5C). The difference in power output at GET was significantly different between groups (Fig. 5D). The relative exercise intensity (%VO2peak) did not differ significantly between patients and controls on either CPET (Fig. 5E and F). The [Laa] at GET was not significantly different between groups on CPET1, but was significantly reduced in controls on CPET2 (Fig. 5G), and the difference in [Laa] at GET from CPET1 to CPET2 was significantly different between patients and controls (Fig 5H). Neither the respiratory exchange rate nor the heart rate at GET differed significantly between groups or the CPETs (data not shown).
Figure 5.
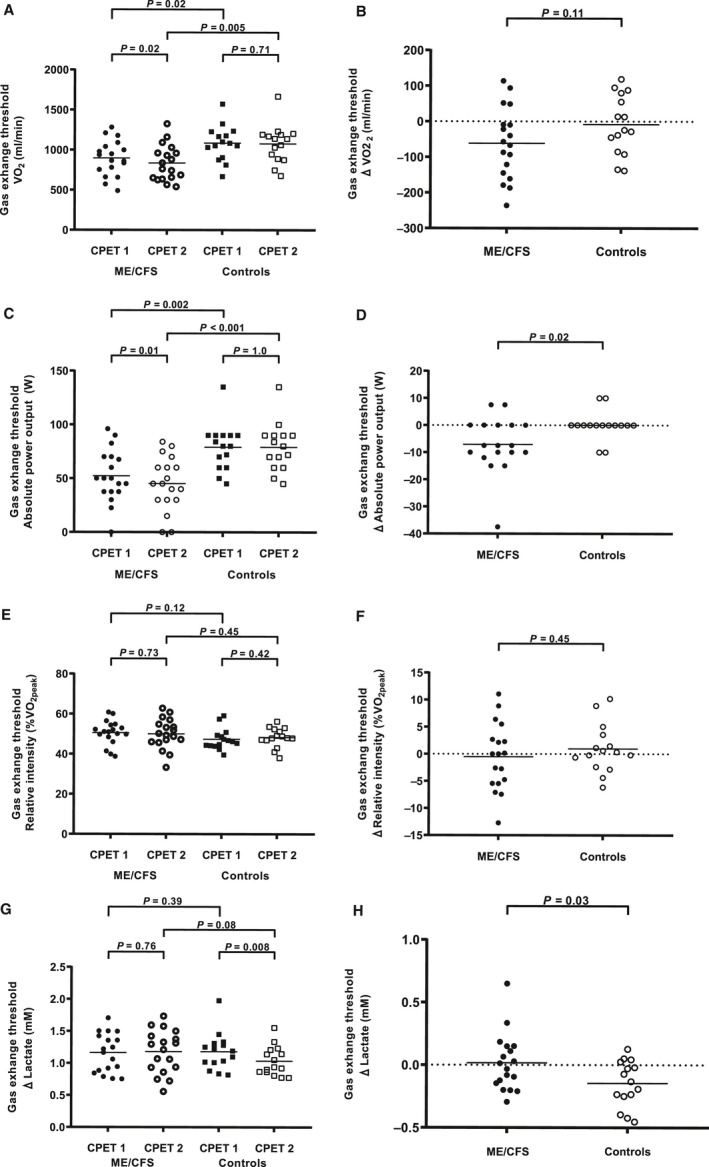
Exercise responses and [Laa] at the gas exchange threshold (GET) defined by the V‐slope method. Data points represent individual measures for each participant and horizontal lines are mean values. (A) shows VO2 at GET for both CPETs and both study groups. (B) shows the difference (Δ) in VO2 at GET between CPET1 and CPET2 for both study groups. (C) shows the absolute power output at GET for both CPETs and both study groups. (D) shows the difference (Δ) in absolute power output at GET between CPET1 and CPET2 for both study groups. (E) shows the relative exercise intensity as %VO2peak at GET for both CPETs and both study groups. (F) shows the difference (Δ) in %VO2peak at GET between CPET1 and CPET2 for both study groups. (G) shows [Laa] at GET for both CPETs and both study groups. H shows the difference (Δ) in [Laa] at GET between CPET1 and CPET2 for both study groups.
The lactate turnpoint and onset of blood lactate accumulation
The lactate turnpoint (LT) occurred at a significantly lower power output in patients on both CPETs, but neither group had any significant difference in power output between CPETs (Fig 6A and B). The [Laa] at LT was not significantly different between groups on CPET1, but was significantly reduced in controls from CPET1 to CPET2 (Fig. 6C). The difference in [Laa] at LT between CPETs was significantly different between the groups (Fig. 6D). The onset of blood lactate accumulation (OBLA) occurred at a significantly lower power output in patients compared to controls on both CPETs. The power output at OBLA increased significantly in controls and decreased significantly in patients from CPET1 to CPET2 (Fig. 6E), and the difference in power output at OBLA from CPET1 to CPET2 was significantly different between groups (Fig. 6F).
Figure 6.
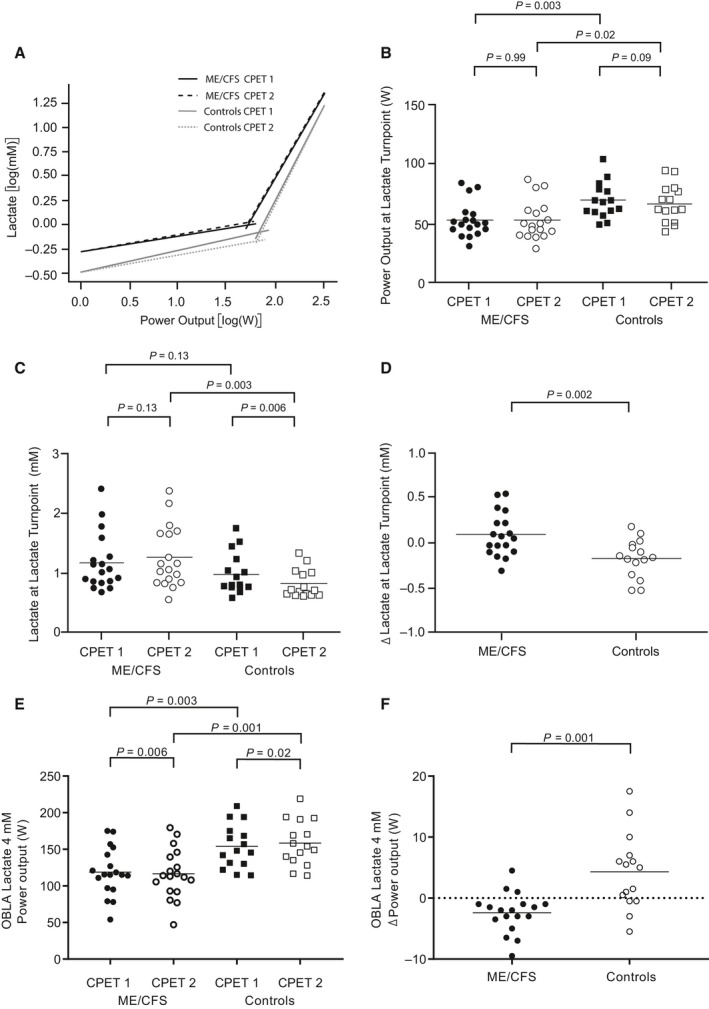
Power output and [Laa] at the lactate turnpoint (LT) and at the onset of blood lactate accumulation of 4 mmol/L (OBLA). (A) shows the determination of LT by log‐log transformation of [Laa] versus power output for both CPETs and both study groups. The intersection of the two regression lines defines the LT. Data points in (B) to (F) represent individual measures for each participant and horizontal lines are mean values. (B) shows the power output at LT for both CPETs and both study groups. (C) shows the [Laa] at LT for both CPETs and both study groups, while (D) shows the difference (Δ) in [Laa] between CPET1 and CPET2 for both study groups. (E) shows the power output at OBLA for both CPETs and both study groups, and (F) shows the difference (Δ) in power output at OBLA between CPET1 and CPET2 for both study groups.
Discussion
In this study of repeated CPET in ME/CFS patients and healthy controls, VO2peak and VO2 at GET decreased significantly in patients, but not in controls, from CPET1 to CPET2, but the differences in VO2peak and VO2 at GET between the two CPETs did not differ significantly between the two study groups. However, the patients had elevated [Laa] for any absolute power output compared with the healthy controls, and both GET and LT occurred at a significantly lower VO2 and absolute power output in patients. Both study groups had similar relative exercise intensity (%VO2peak) at GET on both CPETs. When retested after 24 h, controls had no significant reduction in the power output at GET, but the [Laa] at GET was significantly reduced. In contrast, patients had a significant reduction in the power output at GET on CPET2, while no significant reduction in [Laa] at GET. Power output at OBLA demonstrated the same pattern, with a decrease in patients and an increase in controls from CPET1 to CPET2. Pre‐exercise [Laa] was also significantly different between the two study groups before CPET2. Whereas previous exercise (CPET1) led to lower [Laa] per absolute power output (right curve shift) on CPET2 in controls, [Laa] per absolute power output increased and the curve was shifted to the left on CPET2 in patients.
Repeated measurements of cardiopulmonary exercise test variables have generally shown good reproducibility in patients with moderate exercise impairment due to chronic disease, and can also be reliably and reproducibly assessed in patients with severe exercise intolerance (Hansen et al. 2004). Although our patients had a reduced activity level compared to their pre‐illness capacity, none of them were bedridden or severely physically restricted. All participants had normal hemoglobin concentration, normal spirometry values prior to the CPETs, as well as a normal to high breathing reserve at peak exercise. Blood sampling during CPET1 amounted to approximately 150–200 mL blood which could explain the reduction in resting hemoglobin concentrations between the two CPETs (Schaffartzik et al. 1993). Mean respiratory exchange ratio and maximum heart rate were similar in both groups on both CPETs, and the findings are therefore not likely explained by deliberate underperformance or lack of effort. Patients and healthy controls were similar in gender, age and height, but patients were heavier than controls. We therefore also reported results for power output per body weight, but this did not affect the results.
As opposed to what others have found, the ability to reproduce VO2peak did not differ significantly between the two study groups. Although VO2peak decreased significantly in patients, the absolute change was too small, in our opinion, to indicate any major change in exercise tolerance or explain PEM. Furthermore, the decrease in resting hemoglobin concentration of 0.7 g/dL in both study groups from CPET1 to CPET2 should be taken into consideration. As an average, a reduction in hemoglobin concentration of 1 g/dL accounts for a reduction in peak VO2 of 0.97 mL/kg/min (Agostoni et al. 2010). In our study, this would equal an expected mean reduction in peak VO2 of 0.68 mL/kg/min from CPET1 to CPET2.
The test protocol did show differences in [Laa] between ME/CFS patients and healthy controls on both CPETs, and this difference increased on CPET2. Untrained subjects will rapidly improve their lactate clearance ability if they engage in regular endurance training (Donovan and Brooks 1983; Phillips et al. 1995), thus lowering blood lactate concentrations for a given work load (Holloszy and Coyle 1984; Yoshida et al. 1992). Such a right shift of the lactate curve can be demonstrated as early as 24 h after prior exercise (Neary and Wenger 1985), and fits well with the findings in our control group on CPET2. [Laa] per absolute power output is elevated in untrained compared to trained subjects (MacRae et al. 1992), and can explain the difference in [Laa] between patients and controls on CPET1. However, one would still have expected a right shift on the second day in patients as well; similar to what was observed in the controls.
GET occurred at a similar relative exercise intensity defined as % of VO2peak for each CPET in both patients and controls. On CPET1 patients had a lower power output at GET than controls, but [Laa] was similar between the groups. On CPET2 patients had a reduction in power output at GET, but similar [Laa], whereas controls had similar power output at GET, but reduced [Laa]. This inability to reproduce power output at GET seems to be a consistent finding in patients with CFS/ME (VanNess et al. 2010; Vermeulen et al. 2010; Snell et al. 2013; Keller et al. 2014; Hodges et al. 2018). If this was caused by deconditioning alone, one would expect to find similar results in other conditions where deconditioning, low exercise tolerance and fatigue are prevalent. However, patients with sarcoidosis (Braam et al. 2013) and multiple sclerosis (Hodges et al. 2018) are able to reproduce their power output at GET, despite a low exercise capacity.
LT occurred at a lower absolute power output in patients than in controls. The power output at LT did not differ between the two CPETs in either group, contrary to our findings when we applied the V‐slope method. One explanation might be that some patients probably reached their LT during unloaded pedaling on CPET2, at which point we did not have lactate measures every 30th second. This would affect the computed regression lines. Nonetheless, [Laa] showed the same pattern, with similar [Laa] on CPET1 and significant differences in [Laa] between the two study groups on CPET2.
A left shift of the lactate curve in ME/CFS patients was suggested by Lane et al. (1994), but subsequent studies have not been conclusive. For example, maximal exercise testing of healthy subjects and ME/CFS patients diagnosed according to the Fukuda criteria did not show any abnormalities in maximal oxygen uptake, lactate accumulation or differences in LT determined by the log‐log method (Sargent et al. 2002), or in venous lactate versus VO2 (Jammes et al. 2005). Others have found elevated lactate accumulation on submaximal levels of exertion in patients diagnosed according to the CDC 1988 working case definition (Holmes et al. 1988; Riley et al. 1990), as well as in a proportion of ME/CFS patients fulfilling the rather unspecific Oxford criteria, where subjects with normal lactate concentrations were more likely to suffer from psychiatric comorbidities (Lane et al. 1995, 1998). Two other studies did not find any differences in resting or maximal lactate concentrations, but found significantly lower postexercise lactate concentrations in patients (Gibson et al. 1993; Georgiades et al. 2003). The discrepancies may be explained by different diagnostic criteria and different exercise protocols. To our knowledge, repeated exercise testing with lactate profiles on two consecutive days has not been studied in ME/CFS patients fulfilling case definitions where PEM is a mandatory symptom.
LT seems to correspond to a limitation in the metabolic clearance rate where lactate appearance exceeds lactate disposal, regardless of training status, and lactate appearance seems to be closely related to %VO2peak. Furthermore, blood lactate concentrations at same relative intensity are similar in trained and untrained (Messonnier et al. 2013). Training increases intramuscular lactate clearance primarily by increasing oxidation by upregulation of mitochondrial proteins, and reduces net lactate production in muscle due to facilitated lactate exchange between glycolytic and oxidative fibers. Patients and controls had similar [Laa] at similar relative exercise intensity on CPET1, but controls had a reduction in [Laa] at both relative and absolute intensity on CPET2 that was not seen in patients. We propose that the previous exercise led to an improved lactate disposal in controls on CPET2, as their [Laa] was reduced both at rest, at the gas exchange threshold and at the lactate turnpoint, and as the onset of blood lactate accumulation at 4 mmol/L occurred at a higher absolute power output.
Disturbed energy metabolism in ME/CFS has been demonstrated by several investigators. Myoblasts grown in presence of serum from severe ME/CFS patients show increased mitochondrial respiration and increased lactate secretion (Fluge et al. 2016). Peripheral blood mononuclear cells (PBMC) from ME/CFS patients have an impaired maximal respiration capacity compared to healthy PBMCs, suggesting an inability to adequately increase the respiratory rate in response to elevated metabolic stress (Tomas et al. 2017). In vitro electric pulse stimulation of muscle cells as a model to investigate metabolic changes during exercise has shown impaired AMPK phosphorylation and glucose uptake in cells from ME/CFS patients and diminished release of IL‐6 compared to healthy muscle cells (Brown et al. 2015). Exercise leads to a transient upregulation of pyruvate dehydrogenase kinase (PDK), particularly during recovery, but returns to resting values within 24 h (Pilegaard and Neufer 2004). PBMCs from ME/CFS patients show upregulated expression of PDK (Fluge et al. 2016), proposing a disturbed PDK regulation which could limit the pyruvate flux with the potential to affect the clearance of lactate through oxidation. Patients with ME/CFS have elevated lipopolysaccharide (LPS) levels in blood compared to healthy controls (Giloteaux et al. 2016). Exercise leads to increased bacterial translocation in ME/CFS patients and is proposed as a possible cause for PEM (Shukla et al. 2015). LPS could affect metabolism, either through inflammation and/or elevated catecholamines. Several conditions are associated with elevated lactate, and elevated lactate might be an attempt to mitigate the effects of injury and stress rather than causing it (Brooks 2018).
This study was performed on female patients with mild to moderate degree of ME/CFS. Diagnosing patients based on clinical criteria rather than a valid biomarker carries the risk of including a less representative group. However, no such biomarker exists today. Ideally one should have patients and controls with similar activity level, as well as similar body weight. The strengths of this study are that patients fulfilled the Canadian criteria with PEM as a required symptom, and all patients were evaluated by the same physician prior to inclusion. All included participants reached predefined test criteria for maximal exercise testing and arterial lactate was sampled every 30th second. All tests were supervised by the same staff, and the testing conditions were similar for all participants, including time of day, metabolic state prior to tests (fasting), and that most patients stayed at the test facility in order to eliminate additional exertion between the tests. It should be noted that the patients experienced considerable symptom exacerbation after the tests, often lasting for weeks.
Exercise intolerance, PEM and delayed recovery are prominent symptoms in ME/CFS. Intriguingly, this study indicates that previous exercise increases lactate accumulation in ME/CFS as opposed to the reduction seen in healthy controls, although the mechanism for this has yet to be established. We do not know whether this finding points to a central pathophysiological mechanism in ME/CFS, or if it is a secondary phenomenon. It might even be an attempt to alleviate the negative impact that exercise seems to have in these patients. Further research is needed in order to elucidate the causes of this abnormal response to exertion.
Conflict of Interest
None declared.
Lien K., Johansen B., Veierød M. B., Haslestad A. S., Bøhn S. K., Melsom M. N., Kardel K. R., Iversen P. O.. Abnormal blood lactate accumulation during repeated exercise testing in myalgic encephalomyelitis/chronic fatigue syndrome. Physiol Rep, 7 (11), 2019, e14138, 10.14814/phy2.14138
Funding Information
The Norwegian Extra Foundation and The Norwegian ME‐Association.
References
- Agostoni, P. , Salvioni E., Debenedetti C., Vignati C., Cattadori G., Contini M., et al. 2010. Relationship of resting hemoglobin concentration to peak oxygen uptake in heart failure patients. Am. J. Hematol. 85:414–417. [DOI] [PubMed] [Google Scholar]
- Arena, R. , Myers J., Williams M. A., Gulati M., Kligfield P., Balady G. J., et al. 2007. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 116:329–343. [DOI] [PubMed] [Google Scholar]
- ATS/ACCP Statement on cardiopulmonary exercise testing . 2003. Am. J. Respir. Crit. Care Med. 167:211–277. [DOI] [PubMed] [Google Scholar]
- Beaver, W. L. , Wasserman K., and Whipp B. J.. 1985. Improved detection of lactate threshold during exercise using a log‐log transformation. J. Appl. Physiol. 59:1936–1940. [DOI] [PubMed] [Google Scholar]
- Bergman, B. C. , Wolfel E. E., Butterfield G. E., Lopaschuk G. D., Casazza G. A., Horning M. A., et al. 1999. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 87:1684–1696. [DOI] [PubMed] [Google Scholar]
- Braam, A. W. , de Haan S. N., Vorselaars A. D., Rijkers G. T., Grutters J. C., van den Elshout F. J., et al. 2013. Influence of repeated maximal exercise testing on biomarkers and fatigue in sarcoidosis. Brain Behav. Immun. 33:57–64. [DOI] [PubMed] [Google Scholar]
- Brooks, G. A. 2018. The science and translation of lactate shuttle theory. Cell Metab. 27:757–785. [DOI] [PubMed] [Google Scholar]
- Brown, A. E. , Jones D. E., Walker M., and Newton J. L.. 2015. Abnormalities of AMPK activation and glucose uptake in cultured skeletal muscle cells from individuals with chronic fatigue syndrome. PLoS ONE 10:e0122982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers, B. M. , Jain A. K., De Meirleir K. L., Peterson D. L., Klimas N. G., Lerner A. M., et al. 2003. Myalgic encephalomyelitis/chronic fatigue syndrome. J. Chronic Fatigue Syndr. 11:7–115. [Google Scholar]
- Carruthers, B. M. , van de Sande M. I., De Meirleir K. L., Klimas N. G., Broderick G., Mitchell T., et al. 2011. Myalgic encephalomyelitis: international consensus criteria. J. Intern. Med. 270:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, C. M. , and Brooks G. A.. 1983. Endurance training affects lactate clearance, not lactate production. Am. J. Physiol. 244:E83–E92. [DOI] [PubMed] [Google Scholar]
- Fluge, O. , Mella O., Bruland O., Risa K., Dyrstad S. E., Alme K., et al. 2016. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight 1:e89376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, K. , Straus S. E., Hickie I., Sharpe M. C., Dobbins J. G., and Komaroff A.. 1994. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 121:953–959. [DOI] [PubMed] [Google Scholar]
- Georgiades, E. , Behan W. M., Kilduff L. P., Hadjicharalambous M., Mackie E. E., Wilson J., et al. 2003. Chronic fatigue syndrome: new evidence for a central fatigue disorder. Clin. Sci. (Lond.) 105:213–218. [DOI] [PubMed] [Google Scholar]
- Gibson, H. , Carroll N., Clague J. E., and Edwards R. H.. 1993. Exercise performance and fatiguability in patients with chronic fatigue syndrome. J. Neurol. Neurosurg. Psychiatry 56:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloteaux, L. , Goodrich J. K., Walters W. A., Levine S. M., Ley R. E., and Hanson M. R.. 2016. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, M. L. , Harris J. E., Hernandez A., and Gladden L. B.. 2007. Blood lactate measurements and analysis during exercise: a guide for clinicians. J. Diabetes Sci. Technol. 1:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, J. E. , Sun X. G., Yasunobu Y., Garafano R. P., Gates G., Barst R. J., et al. 2004. Reproducibility of cardiopulmonary exercise measurements in patients with pulmonary arterial hypertension. Chest 126:816–824. [DOI] [PubMed] [Google Scholar]
- Hodges, L. D. , Nielsen T., and Baken D.. 2018. Physiological measures in participants with chronic fatigue syndrome, multiple sclerosis and healthy controls following repeated exercise: a pilot study. Clin. Physiol. Funct. Imaging 38:639–644. [DOI] [PubMed] [Google Scholar]
- Holloszy, J. O. , and Coyle E. F.. 1984. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 56:831–838. [DOI] [PubMed] [Google Scholar]
- Holmes, G. P. , Kaplan J. E., Gantz N. M., Komaroff A. L., Schonberger L. B., Straus S. E., et al. 1988. Chronic fatigue syndrome: a working case definition. Ann. Intern. Med. 108:387–389. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) . 2015. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. The National Academies Press,Washington, DC. [PubMed] [Google Scholar]
- Jammes, Y. , Steinberg J. G., Mambrini O., Bregeon F., and Delliaux S.. 2005. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J. Intern. Med. 257:299–310. [DOI] [PubMed] [Google Scholar]
- Jason, L. A. , Brown A., Evans M., Sunnquist M., and Newton J. L.. 2013. Contrasting chronic fatigue syndrome versus myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue 1:168–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, B. A. , Pryor J. L., and Giloteaux L.. 2014. Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO(2)peak indicates functional impairment. J. Transl. Med. 12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. J. , Woodrow D., and Archard L. C.. 1994. Lactate responses to exercise in chronic fatigue syndrome. J. Neurol. Neurosurg. Psychiatry 57:662–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. J. , Burgess A. P., Flint J., Riccio M., and Archard L. C.. 1995. Exercise responses and psychiatric disorder in chronic fatigue syndrome. BMJ 311:544–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. J. , Barrett M. C., Woodrow D., Moss J., Fletcher R., and Archard L. C.. 1998. Muscle fibre characteristics and lactate responses to exercise in chronic fatigue syndrome. J. Neurol. Neurosurg. Psychiatry 64:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae, H. S. , Dennis S. C., Bosch A. N., and Noakes T. D.. 1992. Effects of training on lactate production and removal during progressive exercise in humans. J. Appl. Physiol. 72:1649–1656. [DOI] [PubMed] [Google Scholar]
- Messonnier, L. A. , Emhoff C. A., Fattor J. A., Horning M. A., Carlson T. J., and Brooks G. A.. 2013. Lactate kinetics at the lactate threshold in trained and untrained men. J. Appl. Physiol. 114:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary, P. J. , and Wenger H. A.. 1985. The effects of prior exercise on the lactate and ventilatory thresholds. J. Sports Sci. 3:189–195. [DOI] [PubMed] [Google Scholar]
- Nelson, M. J. , Buckley J. D., Thomson R. L., Clark D., Kwiatek R., and Davison K.. 2019. Diagnostic sensitivity of 2‐day cardiopulmonary exercise testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Transl. Med. 17:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, S. M. , Green H. J., Tarnopolsky M. A., and Grant S. M.. 1995. Increased clearance of lactate after short‐term training in men. J. Appl. Physiol. 79:1862–1869. [DOI] [PubMed] [Google Scholar]
- Pilegaard, H. , and Neufer P. D.. 2004. Transcriptional regulation of pyruvate dehydrogenase kinase 4 in skeletal muscle during and after exercise. Proc. Nutr. Soc. 63:221–226. [DOI] [PubMed] [Google Scholar]
- Riley, M. S. , O'Brien C. J., McCluskey D. R., Bell N. P., and Nicholls D. P.. 1990. Aerobic work capacity in patients with chronic fatigue syndrome. BMJ 301:953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatzki, M. J. , Ferguson B. S., Goodwin M. L., and Gladden L. B.. 2015. Lactate is always the end product of glycolysis. Front. Neurosci. 9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent, C. , Scroop G. C., Nemeth P. M., Burnet R. B., and Buckley J. D.. 2002. Maximal oxygen uptake and lactate metabolism are normal in chronic fatigue syndrome. Med. Sci. Sports Exerc. 34:51–56. [DOI] [PubMed] [Google Scholar]
- Schaffartzik, W. , Barton E. D., Poole D. C., Tsukimoto K., Hogan M. C., Bebout D. E., et al. 1993. Effect of reduced hemoglobin concentration on leg oxygen uptake during maximal exercise in humans. J. Appl. Physiol. 75:491–498; discussion 489–490. [DOI] [PubMed] [Google Scholar]
- Schneider, D. A. , Phillips S. E., and Stoffolano S.. 1993. The simplified V‐slope method of detecting the gas exchange threshold. Med. Sci. Sports Exerc. 25:1180–1184. [PubMed] [Google Scholar]
- Shukla, S. K. , Cook D., Meyer J., Vernon S. D., Le T., Clevidence D., et al. 2015. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS ONE 10:e0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell, C. R. , Stevens S. R., Davenport T. E., and Van Ness J. M.. 2013. Discriminative validity of metabolic and workload measurements for identifying people with chronic fatigue syndrome. Phys. Ther. 93:1484–1492. [DOI] [PubMed] [Google Scholar]
- Tomas, C. , Brown A., Strassheim V., Elson J. L., Newton J., and Manning P.. 2017. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE 12:e0186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanNess, J. M. , Stevens S. R., Bateman L., Stiles T. L., and Snell C. R.. 2010. Postexertional malaise in women with chronic fatigue syndrome. J. Women's Health 19:239–244. [DOI] [PubMed] [Google Scholar]
- Vermeulen, R. C. , Kurk R. M., Visser F. C., Sluiter W., and Scholte H. R.. 2010. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J. Transl. Med. 8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, R. , Lopaschuk G., Zhu G., Walker D., Catellier D., Burton D., et al. 1992. Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest 102:1716–1722. [DOI] [PubMed] [Google Scholar]
- Yoshida, T. , Udo M., Ohmori T., Matsumoto Y., Uramoto T., and Yamamoto K.. 1992. Day‐to‐day changes in oxygen uptake kinetics at the onset of exercise during strenuous endurance training. Eur. J. Appl. Physiol. Occup. Physiol. 64:78–83. [DOI] [PubMed] [Google Scholar]


