Summary
The narwhal (Monodon monoceros) is a highly specialized endemic Arctic cetacean, restricted to the Arctic seas bordering the North Atlantic. Low levels of genetic diversity have been observed across several narwhal populations using mitochondrial DNA and microsatellites. Despite this, the global abundance of narwhals was recently estimated at ∼170,000 individuals. However, the species is still considered vulnerable to changing climates due to its high specialization and restricted Arctic distribution. We assembled and annotated a genome from a narwhal from West Greenland. We find relatively low diversity at the genomic scale and show that this did not arise by recent inbreeding, but rather has been stable over an extended evolutionary timescale. We also find that the current large global abundance most likely reflects a recent rapid expansion from a much smaller founding population.
Subject Areas: Biological Sciences, Genetics, Evolutionary Biology
Graphical Abstract
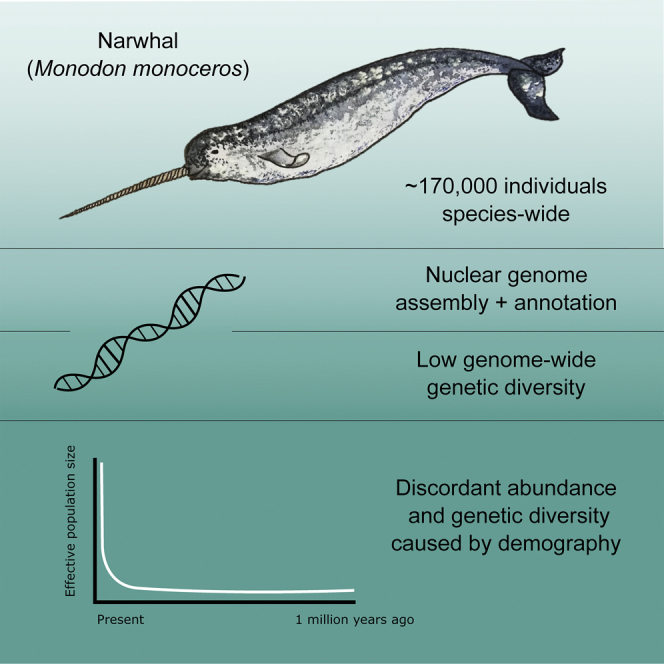
Highlights
-
•
Assembled and annotated narwhal nuclear genome
-
•
Low genetic diversity despite large global abundance
-
•
Lack of signs of inbreeding
-
•
Demography is the driving force behind the low diversity
Biological Sciences; Genetics; Evolutionary Biology
Introduction
Adaptive potential in the form of genetic diversity is assumed to be essential (Reed and Frankham, 2003, Ellegren and Galtier, 2016), especially during periods of rapid climatic change. The Arctic and its biodiversity are highly vulnerable to these shifts (Masson-Delmotte et al., 2018, Kovacs et al., 2011). The narwhal (Monodon monoceros) is the most specialized endemic Arctic cetacean, well known for its elongated caniniform “tusk” and deep diving capabilities (Heide-Jørgensen, 2009, Heide-Jørgensen and Dietz, 1995, Best, 1981). Narwhals are distributed in the Arctic seas bordering the North Atlantic (Figure 1A) and make annual migrations between coastal summering grounds and offshore ice-covered wintering grounds. Low levels of genetic diversity have been observed across several narwhal populations using mitochondrial DNA (mtDNA) and microsatellites (Palsbøll et al., 1997, Petersen et al., 2011), despite the global abundance of narwhals being relatively high. A recent global abundance estimate of ∼170,000 individuals (NAMMCO, 2018) resulted in a change in the category of narwhals in the International Union for Conservation of Nature red list of threatened species, where the species has been downgraded from “near threatened” to “least concern” in 2017 (Lowry et al., 2017). This classification, however, did not take genetic diversity and the future genetic adaptability of the narwhal into account. Therefore despite the downgrade in conservation status, the narwhal should still be considered one of the most vulnerable Arctic marine mammals to ongoing rapid climate changes, especially due to its high specialization and restricted Arctic-Atlantic distribution (Laidre et al., 2008).
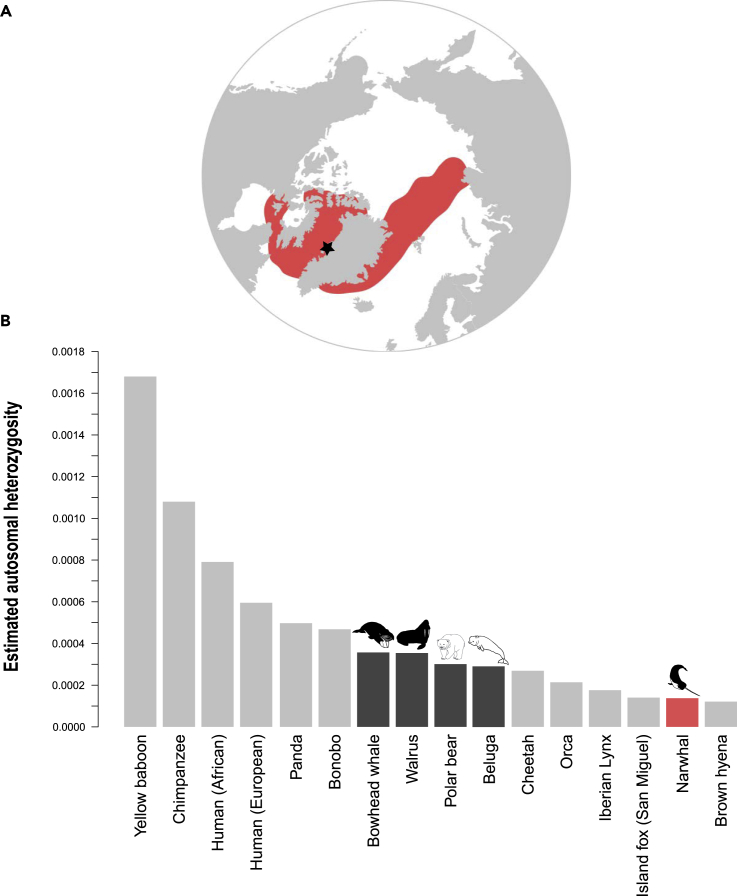
Figure 1.
Sampling Location of the Narwhal and Comparative Genome-wide Diversity of 16 Mammalian Species
(A) Map of the narwhal distribution range based on International Union for Conservation of Nature distribution data (Lowry et al., 2017).
(B) Average genome-wide autosomal heterozygosity values of a range of available mammalian genomes. y axis represents the average proportion of sites within the autosomes that are heterozygous. Light gray bar, non-Arctic endemics; dark gray bar, Arctic endemics; red bar, narwhal. Black star in (A) indicates sample location.
The finding of low genetic diversity despite large global abundance appears to contradict the neutral theory of molecular evolution (Kimura, 1968). The neutral theory states that neutral processes (e.g., genetic drift) are the main determinants of genetic diversity. If this is indeed the case, then the larger the population, the lower the chance that genetic drift will randomly remove genetic diversity from the gene pool. This therefore would suggest that genetic diversity is directly proportional to effective population size (Ne). Although this appears not the case in the narwhal, low genetic diversity despite large abundance could be explained by other factors, including (1) the number of breeding individuals, and thereby Ne, being very small despite the species' large abundance; (2) high levels of inbreeding; or (3) a recent population expansion from a small founding population. The first option seems unlikely as this infers that most adults from the ∼170,000 individuals do not reproduce. To investigate options 2 and 3, we assembled and annotated a nuclear genome from a narwhal from West Greenland. We find that the low diversity did not arise by recent inbreeding, but rather has been stable over an extended evolutionary timescale. Our analyses show that the current large global abundance most likely arose due to a recent rapid population expansion around the onset of the Last Glacial Maximum and that genetic diversity may not have had time to increase accordingly.
Results and Discussion
Narwhal Genome Assembly
We assembled 2,350,959,615 base pairs (bp) of the narwhal nuclear genome (2,156,711,577 bp excluding missing data [N's]) with a scaffold N50 of 1,483,363 bp and contig N50 of 10,481 bp, in a total of 21,007 scaffolds. We used ∼100× coverage of multiple short insert and ∼32× of mate paired Illumina libraries and in silico mate paired libraries (Grau et al., 2018) constructed using the beluga reference genome (Jones et al., 2017) (Genbank: GCA_002288925.2) (Table S1). Investigations into the completeness of the assembly using Benchmarking Universal Single-Copy Orthologs (BUSCO) analyses and the mammalian BUSCO gene set showed a high level of complete BUSCO scores (93%) (Table S2), indicating a fairly complete and high-quality genome. Repeat profiling results showed that our assembled genome consists of 37.9% repetitive elements (Table S3). We identified a total of 21,785 protein-coding genes through genome annotations with MAKER2 (Holt and Yandell, 2011). These protein-coding genes spanned a total of ∼612.8 Mb of the assembled narwhal genome, with exons accounting for ∼31.5 Mb.
Genome-wide Genetic Diversity
To investigate genome-wide levels of genetic diversity in the narwhal, we estimated autosomal heterozygosity across the genome using site allele frequency likelihoods with the software, analyzing next generation sequencing data (ANGSD) (Korneliussen et al., 2014). The estimated heterozygosity value of 0.000138 was very low, when compared with available heterozygosity estimates from 11 other mammalian genomes (Westbury et al., 2018) and three additional endemic Arctic marine mammals: beluga (Delphinapterus leucas), bowhead whale (Balaena mysticetus), and walrus (Odobenus rosmarus) (Figure 1B). This result is unexpected, owing to the large census size of narwhals (Lowry et al., 2017, NAMMCO, 2018). The genome-wide low heterozygosity level is, however, in agreement with previous findings based on mtDNA and microsatellites (Palsbøll et al., 1997, Petersen et al., 2011). Low genetic diversity despite wide distribution ranges and large population sizes has been reported in other large mammals (e.g., the brown hyena, Westbury et al., 2018; orca, Hoelzel et al., 2002), and may become a more common finding as more genomic datasets become available.
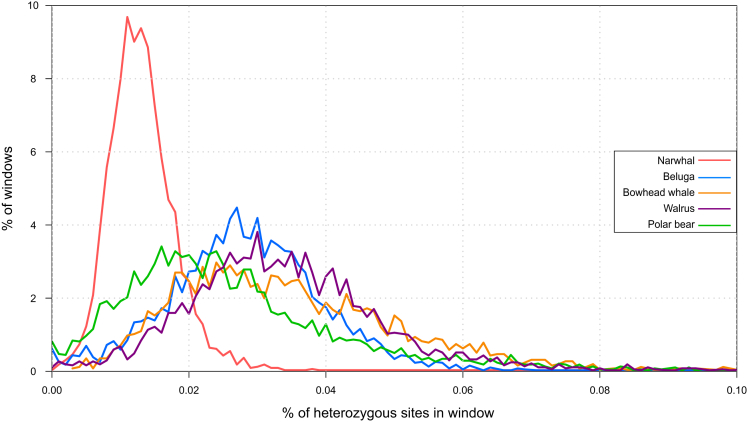
To investigate whether adaptive potential (i.e., genetic diversity) may be present in certain regions of the narwhal genome, we estimated heterozygosity levels across the genome using 500-kb non-overlapping windows. A relatively high number of regions of high heterozygosity among a general genome-wide pattern of low diversity could suggest that these regions are tied to the adaptive potential of the species and therefore need to retain these higher levels of diversity for the species to persist. We find that heterozygosity follows a normal distribution with very little variation (SD = 0.0000643), indicating an even distribution of diversity across the genome (Figure 2). To uncover whether the evenly distributed genome-wide low diversity is unique to the narwhal, or whether it is somehow associated with its Arctic existence, we compared this distribution to that of four other endemic Arctic marine mammal species (beluga, bowhead whale, walrus, and polar bear [Ursus maritimus]) (Figure 2). We find relatively less variability in the distribution of heterozygosity across the narwhal genome (Table S4). This pattern is unique to the narwhal, as the other species have more similar variations in heterozygosity across their genomes. This finding is consistent regardless of whether our narwhal or the beluga genome (Jones et al., 2017) was used as the mapping reference (Figure S1).
Figure 2.
The 500-kb Sliding Window Heterozygosity across the Genomes of Five Arctic Endemic Marine Mammal Species
x axis represents the percentage of the window that is heterozygous, and y axis represents the percentage of the total windows.
Inbreeding has been shown to be a major driver in the loss of genetic diversity following population bottlenecks (Palkopoulou et al., 2015, Abascal et al., 2016), and is a well-known factor in the reduction of survivability and reproductive success in naturally outbreeding species (Frankham, 2005). We investigated the 500-kb sliding windows for any indications of inbreeding. We find that only 0.03% of the 500-kb windows lack heterozygosity, indicating that inbreeding is most likely not the cause of low heterozygosity in the narwhal. To further validate this, we manually investigated for runs of homozygosity across the five largest scaffolds and found no contiguous stretches of homozygosity (Figure S2). Thus, it appears that, even though the genetic diversity of the narwhal is low, it may have arisen in a manner that allowed the species to slowly adapt over an extended time period, rather than rapidly through inbreeding. This could have implications for the long-term survivability of the species. Inbreeding can rapidly purge alleles from a gene pool that has adaptive potential. However, the slower decrease in diversity in the narwhal could have given the species time to adapt to the decreasing diversity by selectively removing alleles that had little to no influence on the adaptive potential of the species. Our findings appear to reflect the current large abundance of the narwhal, rather than the low levels of genomic diversity. Moreover, similar findings of no inbreeding despite low diversity have previously been reported in other species (Westbury et al., 2018), indicating that this may be an important method of adaptation in multiple species.
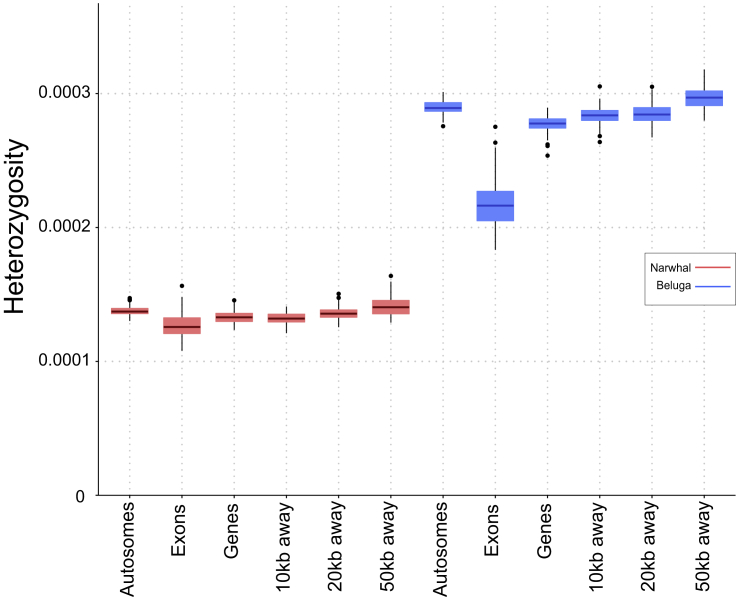
To investigate whether the narwhal exhibited higher diversity in regions of putatively higher selective pressure, as opposed to putatively neutral regions, we compared heterozygosity in protein-coding regions (exons) versus gene regions (introns plus exons) and non-coding flanking regions 10, 20, and 50 kb up- and downstream of protein-coding genes (Figure 3, Table S5). To elucidate whether this pattern is unique to the narwhal lineage, we ran the same suite of analyses on the beluga, its sister species (Figure 3, Table S6). The significance of the differences detected in these regions was tested using an unpaired two-sample t test. Significant differences in average heterozygosity between the exons and all other regions tested were found in both the narwhal (Table S7) and the beluga (Table S8). This finding is as expected, as coding regions are more conserved than non-coding regions, due to higher selective pressures. However, even though the p scores suggested significant differences in coding regions compared with non-coding regions in both the narwhal and the beluga, we note that the t scores uncovered in the narwhal are much lower than those found while comparing the same genomic regions in the beluga. This may imply a level of retention of diversity in coding regions relative to the non-coding regions. Such retained diversity could help to maintain some adaptive potential in the narwhal, enabling the species to adapt to changes in its environment, despite low genome-wide diversity levels. The overall low levels of heterozygosity and lack of variation in diversity levels between coding and non-coding regions across the narwhal genome, relative to the beluga, may suggest that heterozygosity levels are at a diversity stasis across the genome. Hence, in the narwhal, any further decrease in genomic diversity could lead to survivability problems.
Figure 3.
Box Plot Graphs Showing the Proportions of Heterozygosity across Different Genomic Regions in the Narwhal and Beluga
Data were based on sampling and resampling 10% of the windows in the stated region 100 times randomly.
Demographic History
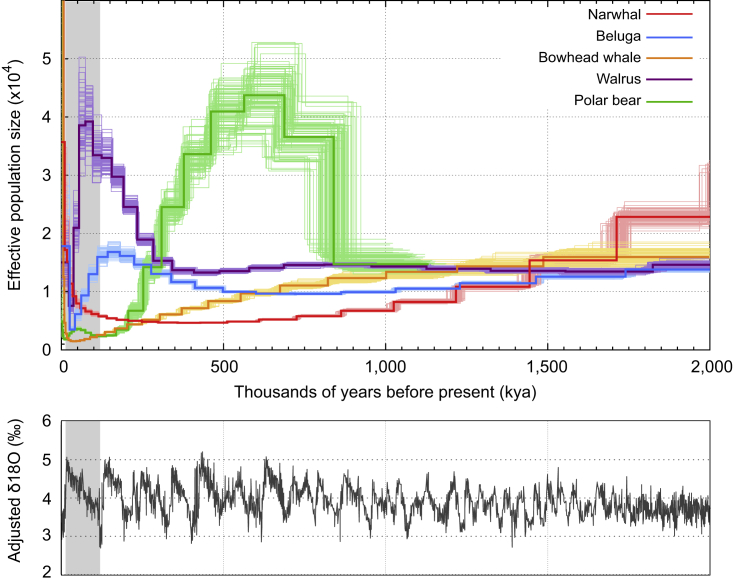
A recent bottleneck could offer an explanation for the current low genetic diversity in the narwhal. We therefore estimated the demographic history of the narwhal genome using the pairwise sequentially Markovian coalescent (PSMC) model (Li and Durbin, 2011) (Figure 4). We find no drastic recent bottlenecks, but rather find an ancient, gradual but constant decline in Ne beginning at ∼2.0 million years ago (Ma). The trajectory culminated in a persistently low Ne of ∼5,000 individuals starting ∼600 thousand years ago (kya), followed by an increase in Ne ∼100 kya and a subsequent rapid expansion ∼30–40 kya. The initial expansion coincides with the onset of the last glacial period ∼115 kya (Rasmussen et al., 2014), suggesting an environmental driver, possibly linked to an increase in Arctic sea ice. Similar patterns of expansion ∼30–40 kya have been documented in Arctic terrestrial mammals, e.g., mammoth, horse, and reindeer (Lorenzen et al., 2011), suggesting a significant ecological shift across the Arctic region at this time. The rapid recent increase in narwhal Ne is in agreement with previous hypotheses based on population genetic analysis of mtDNA control region sequences. Inferences suggested a rapid recent expansion from a small founding population at the end of the last glaciation (∼25 kya) (Palsbøll et al., 1997). The pattern of a long steady decline followed by constant low population size could explain the low genetic diversity in current populations. However, differentiating true changes in population size from changes in population structure and gene flow is difficult based on a single genome (Mazet et al., 2015). Therefore caution should be exercised when interpreting these demographic results from a single individual. Further work involving multiple individuals from several populations will provide essential additional information about the findings of the current study.
Figure 4.
Demographic History of Five Arctic Marine Mammal Species
Pairwise Sequentially Markovian Coalescent model of the narwhal and four other endemic Arctic marine mammal species and δ18O levels through time based on data found in Zachos et al. (2001). x axis represents thousands of years before present (kya), upper y axis represents effective population size (×10,000), lower y axis represents δ18O (‰), and faded lines represent bootstrap values. Shaded gray area represents the last glacial period.
To investigate whether the demographic trajectory is unique to the narwhal, or is associated with an Arctic lifestyle, we investigated the demographic history of the published genomes of beluga, bowhead whale, walrus, and polar bear. We find each species had its own unique demographic trajectory (Figure 4). This result is not unexpected, as each species has its own particular ecology, which would influence its demographic history. However, this result does suggest that simply living in the Arctic environment is in itself not the main driver behind the low genetic diversity seen in the narwhal.
Comparative History of the Narwhal and Beluga
The narwhal and its closest living relative, the beluga, are the only toothed whales found in the Arctic year round. The molecularly dated divergence time between the two species based on nuclear and mitochondrial markers is estimated at ∼5.5 Ma (Steeman et al., 2009). However, despite this deep divergence time, the narwhal and beluga share some similarities. Both species are well adapted to life in the high Arctic, have long lifespans (narwhal up to ∼100 years, Garde et al., 2007; beluga ∼75 years, Stewart et al., 2006), and have similar body sizes (Garde et al., 2007), reproductive strategies, and generation times (Heide-Jørgensen, 2009, O’corry-Crowe, 2009, Garde et al., 2015). Hybridization between the two species has been reported (Heide-Jørgensen and Reeves, 1993), but the reproductive success of the hybrid offspring remains unknown.
To gain further insight into the demographic history of the narwhal and its evolutionary relationship to the beluga, we estimated the timing of when viable admixture between the two species may have ceased. We used an F1 hybrid PSMC (hPSMC) model. Results from the hPSMC model and simulation analysis suggest that gene flow between narwhal and beluga, at least in the populations that these genomes come from, ceased between 1.25 and 1.65 Ma (Figure S3). This result suggests that viable gene flow between the two species continued for a long time after divergence (∼4 Ma), but the time frame also suggests that any contemporary hybrids are likely unable to reproduce, or have such high selective pressures against them that they fail to add anything to the parental species' gene pools. The narwhal in this study was sampled in West Greenland, and the beluga individual was sampled in Hudson Bay, Canada, where the species ranges do not overlap. Investigating narwhal and beluga individuals from populations where their ranges do overlap (e.g., West Greenland), may uncover more recent dates.
As more genomes become available, the notion that genetic diversity is directly associated with a species' long-term viability is being called into question. The demographic history of a species has been shown to be an important factor in long-term survival, despite seemingly detrimental low diversity (Westbury et al., 2018, Xue et al., 2015, Robinson et al., 2016). As narwhals are found only in the Atlantic sector of the Arctic, and not in the Pacific (Figure 1A), unlike the two other endemic Arctic cetaceans, the beluga and the bowhead whale, the limited geographic range may increase the species' vulnerability (Laidre et al., 2008). However, previous studies investigating wild species diversity using large genomic datasets found no detectable influence of geographic range on genomic diversity estimates (Romiguier et al., 2014), therefore the limited range most likely does not offer a suitable explanation for the low diversity. The same study also reported significant correlations between body mass, longevity and reproductive strategy, and genetic diversity (Romiguier et al., 2014). However, narwhals and belugas share many similarities in these traits (Heide-Jørgensen, 2009, O’corry-Crowe, 2009, Garde et al., 2015, Garde et al., 2007, Stewart et al., 2006), albeit the narwhal still has a much lower estimated genetic diversity. Hence these do not appear to be the main forces driving low diversity in the narwhal and indicate that somatic growth and life history parameters of the two species alone cannot explain the observed pattern of genetic diversity.
Although life history does not offer a definite explanation as to the low genetic diversity in narwhal, demographic history could offer a viable explanation. Previous studies have suggested that long-term, slow declines in population size (Westbury et al., 2018) or long-term low population size (Xue et al., 2015, Robinson et al., 2016) can allow a species to persist despite low diversity, by reducing the strain of deleterious recessive alleles. The narwhal has exhibited both of these demographic characteristics. Furthermore, our findings of an increase in Ne starting ∼100 kya offers an explanation for the current abundance, despite the low genetic diversity in the species today. The low contemporary genetic diversity may in part reflect the longevity and long generation time in narwhals (Garde et al., 2015), which could slow the increase of genomic diversity.
The sequencing and assembly of a narwhal nuclear genome provides insights into genome-wide genetic diversity patterns and the demographic history of the species. In a comparison with 14 other mammalian species, including four endemic Arctic marine mammals, we find the narwhal to have low genome-wide diversity, and a unique demographic history not shared with any other endemic Arctic marine mammals. Our analyses reveal that demographic history has been an important factor influencing patterns of genetic diversity in the narwhal and offers an explanation for the low diversity in this individual despite the large global abundance of the species. We do not find evidence that the low diversity was caused by rapid bottlenecks or inbreeding, both of which are common mechanisms used to explain this pattern. Rather, we propose that low diversity has been present in narwhals for an extended period of time. The species may have adapted to cope with this at a genome-wide level over time and has potentially reached a stasis as to how low its genetic diversity can go before influencing the long-term survival of the species. Our study sets the groundwork for future studies into the evolutionary history of narwhals, which will hopefully uncover more details as to the causes of low diversity and how it influences the long-term population survival of the species.
Limitations of the Study
All inferences of the narwhal at a species level were based on a single individual. Although our inferences were based on the entire nuclear genome, which consists of many independently evolving loci, which may have been present in multiple populations in the past, there could be differences between contemporary narwhal populations that could not be uncovered from our individual. To fully uncover whether the conclusions drawn in this article are representative of the current species as a whole, further studies using genomic data from multiple narwhal individuals and populations will need to be investigated.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
DNA sequencing of the narwhal genome was supported by Dronning Margrethes og Prins Henriks Fond, grant no. 2015/2-II-13. The work was further supported by Villum Fonden Young Investigator Programme, grant no. 13151; and Carlsberg Foundation Distinguished Associate Professor Fellowship, grant no. CF16-0202. We would like to thank Binia De Cahsan for drawing the animal images in Figure 1B and the narwhal illustration in the graphical abstract. The collection of samples from the Inuit hunt of narwhals was funded and conducted by the Greenland Institute of Natural Resources. Finally, we would like to thank Rheon Slade for his assistance in the design and layout of the graphical abstract.
Author Contributions
The project was conceptualized by E.D.L. Genome assembly, annotation, and analyses were performed by M.V.W. and B.P. Sample acquisition and information on the said sample was provided by E.G. and M.P.H.J. The manuscript was written by M.V.W. with significant input from E.D.L. All co-authors read and agreed on the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: May 1, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.03.023.
Contributor Information
Michael V. Westbury, Email: m.westbury@snm.ku.dk.
Eline D. Lorenzen, Email: elinelorenzen@snm.ku.dk.
Data and Software Availability
The narwhal assembly and short reads are available under the GenBank BioProject ID PRJNA508363.
Supplemental Information
References
- Abascal F., Corvelo A., Cruz F., Villanueva-Cañas J.L., Vlasova A., Marcet-Houben M., Martínez-Cruz B., Cheng J.Y., Prieto P., Quesada V. Extreme genomic erosion after recurrent demographic bottlenecks in the highly endangered Iberian lynx. Genome Biol. 2016;17:251. doi: 10.1186/s13059-016-1090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best R.C. The tusk of the narwhal (Monodon monoceros L.): interpretation of its function (Mammalia: Cetacea) Can. J. Zool. 1981;59:2386–2393. [Google Scholar]
- Ellegren H., Galtier N. Determinants of genetic diversity. Nat. Rev. Genet. 2016;17:422–433. doi: 10.1038/nrg.2016.58. [DOI] [PubMed] [Google Scholar]
- Frankham R. Genetics and extinction. Biol. Conserv. 2005;126:131–140. [Google Scholar]
- Garde E., Heide-Jørgensen M.P., Hansen S.H., Nachman G., Forchhamme M.C. Age-specific growth and remarkable longevity in narwhals (Monodon monoceros) from west Greenland as estimated by aspartic acid racemization. J. Mammal. 2007;88:49–58. [Google Scholar]
- Garde E., Hansen S.H., Ditlevsen S., Tvermosegaard K.B., Hansen J., Harding K.C., Heide-Jørgensen M.P. Life history parameters of narwhals (Monodon monoceros) from Greenland. J. Mammal. 2015;96:866–879. [Google Scholar]
- Grau J.H., Hackl T., Koepfli K.P., Hofreiter M. Improving draft genome contiguity with reference-derived in silico mate-pair libraries. GigaScience. 2018;7 doi: 10.1093/gigascience/giy029. giy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide-Jørgensen M.P. N - narwhal: Monodon monoceros. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. Second Edition. Academic Press; 2009. pp. 754–758. [Google Scholar]
- Heide-Jørgensen M.P., Dietz R. Some characteristics of narwhal, Monodon monoceros, diving behaviour in Baffin Bay. Can. J. Zool. 1995;73:2120–2132. [Google Scholar]
- Heide-Jørgensen M.P., Reeves R.R. Description of an anomalous monodontid skull from west Greenland: a possible hybrid? Marine Mammal Science. 1993;9:258–268. [Google Scholar]
- Hoelzel A.R., Natoli A., Dahlheim M.E., Olavarria C., Baird R.W., Black N.A. Low worldwide genetic diversity in the killer whale (Orcinus orca): implications for demographic history. Proc. Biol. Sci. 2002;269:1467–1473. doi: 10.1098/rspb.2002.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C., Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC bioinformatics. 2011;12:491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.J.M., Taylor G.A., Chan S., Warren R.L., Hammond S.A., Bilobram S., Mordecai G., Suttle C.A., Miller K.M., Schulze A. The genome of the beluga whale (Delphinapterus leucas) Genes (Basel) 2017;8:378. doi: 10.3390/genes8120378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Evolutionary rate at the molecular level. Nature. 1968;217:624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Korneliussen T.S., Albrechtsen A., Nielsen R. ANGSD: analysis of next generation sequencing data. BMC Bioinformatics. 2014;15:356. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs K.M., Lydersen C., Overland J.E., Moore S.E. Impacts of changing sea-ice conditions on Arctic marine mammals. Marine Biodiversity. 2011;41:181–194. [Google Scholar]
- Laidre K.L., Stirling I., Lowry L.F., Wiig O., Heide-Jørgensen M.P., Ferguson S.H. Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol. Appl. 2008;18(2 Suppl):S97–S125. doi: 10.1890/06-0546.1. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493–496. doi: 10.1038/nature10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen E.D., Nogués-Bravo D., Orlando L., Weinstock J., Binladen J., Marske K.A., Ugan A., Borregaard M.K., Gilbert M.T., Nielsen R. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature. 2011;479:359–364. doi: 10.1038/nature10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry L., Laidre K., Reeves R. Monodon monoceros. The IUCN Red List of Threatened Species 2017: e.T13704A50367651. 2017. Available at: [DOI]
- Masson-Delmotte V., Zhai P., Pörtner H.-O., Roberts D., Skea J., Shukla P.R., Pirani A., Moufouma-Okia W., Péan C., Pidcock R. Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Intergovernmental panel Clim. Change (ipcc) 2018 [Google Scholar]
- Mazet O., Rodríguez W., Chikhi L. Demographic inference using genetic data from a single individual: Separating population size variation from population structure. Theor. Popul. Biol. 2015;104:46–58. doi: 10.1016/j.tpb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- NAMMCO . NAMMCO Scientific Publications; 2018. Report of the NAMMCO Global Review of Monodontids.https://nammco.no/topics/sc-working-group-reports/ Available at: [Google Scholar]
- O’corry-Crowe G.M. Beluga whale: Delphinapterus leucas. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. Second Edition. Academic Press; 2009. pp. 108–112. [Google Scholar]
- Palkopoulou E., Mallick S., Skoglund P., Enk J., Rohland N., Li H., Omrak A., Vartanyan S., Poinar H., Götherström A. Complete genomes reveal signatures of demographic and genetic declines in the woolly mammoth. Curr. Biol. 2015;25:1395–1400. doi: 10.1016/j.cub.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsbøll P.J., Heide-Jørgensen M.P., Dietz R. Population structure and seasonal movements of narwhals, Monodon monoceros, determined from mtDNA analysis. Heredity. 1997;78(Pt 3):284–292. doi: 10.1038/hdy.1997.43. [DOI] [PubMed] [Google Scholar]
- Petersen S.D., Tenkula D., Ferguson S.H. Population genetic structure of narwhal (Monodon monoceros), Fisheries and Oceans Canada. Science. 2011 [Google Scholar]
- Rasmussen S.O., Bigle M., Blockley S.P., Blunier T., Buchardt S.L., Clausen H.B., Cvijanovic I., Dahl-Jensen D., Johnsen S.J. A stratigraphic framework for abrupt climatic changes during the Last Glacial period based on three synchronized Greenland ice-core records: refining and extending the INTIMATE event stratigraphy. Quat. Sci. Rev. 2014;106:14–28. [Google Scholar]
- Reed D.H., Frankham R. Correlation between fitness and genetic diversity. Conserv. Biol. 2003;17:230–237. [Google Scholar]
- Robinson J.A., Ortega-Del Vecchyo D., Fan Z., Kim B.Y., vonHoldt B.M., Marsden C.D., Lohmueller K.E., Wayne R.K. Genomic flatlining in the endangered Island fox. Curr. Biol. 2016;26:1183–1189. doi: 10.1016/j.cub.2016.02.062. [DOI] [PubMed] [Google Scholar]
- Romiguier J., Gayral P., Ballenghien M., Bernard A., Cahais V., Chenuil A., Chiari Y., Dernat R., Duret L., Faivre N. Comparative population genomics in animals uncovers the determinants of genetic diversity. Nature. 2014;515:261–263. doi: 10.1038/nature13685. [DOI] [PubMed] [Google Scholar]
- Steeman M.E., Hebsgaard M.B., Fordyce R.E., Ho S.Y., Rabosky D.L., Nielsen R., Rahbek C., Glenner H., Sørensen M.V., Willerslev E. Radiation of extant cetaceans driven by restructuring of the oceans. Syst. Biol. 2009;58:573–585. doi: 10.1093/sysbio/syp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R.E.A., Campana S.E., Jones C.M., Stewart B.E. Bomb radiocarbon dating calibrates beluga (Delphinapterus leucas) age estimates. Can. J. Zool. 2006;84:1840–1852. [Google Scholar]
- Westbury M.V., Hartmann S., Barlow A., Wiesel I., Leo V., Welch R., Parker D.M., Sicks F., Ludwig A., Dalén L. Extended and continuous decline in effective population size results in low genomic diversity in the world’s rarest Hyena species, the brown Hyena. Mol. Biol. Evol. 2018;35:1225–1237. doi: 10.1093/molbev/msy037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Prado-Martinez J., Sudmant P.H., Narasimhan V., Ayub Q., Szpak M., Frandsen P., Chen Y., Yngvadottir B., Cooper D.N. Mountain gorilla genomes reveal the impact of long-term population decline and inbreeding. Science. 2015;348:242–245. doi: 10.1126/science.aaa3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos J., Pagani M., Sloan L., Thomas E., Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.