Abstract
There is an inherent need to identify differentially expressed genes (DEGs), characterize these genes and provide functional enrichment analysis to the publicly available lung cancer datasets, primarily coming from next-generation sequencing data or microarray gene expression studies. The risk of lung cancer in patients with smokers is manifold, and with chronic obstructive pulmonary disease (COPD) it is 2- to 5-fold greater, compared with smokers without COPD. In the present study, differential expression analysis and gene functional enrichment analysis of lung cancer gene expression datasets obtained from NCBI-GEO were performed. The result identifies a significant number of DEGs which have at least a 2-fold change in their expression. Among them, six genes were found to have a 4-fold change in the expression level, and 47 genes exhibited a 3-fold change in the expression. It was also observed that most of the genes were upregulated and few genes were downregulated.
Keywords: lung cancer, COPD, differential expression analysis, microarray analysis
Introduction
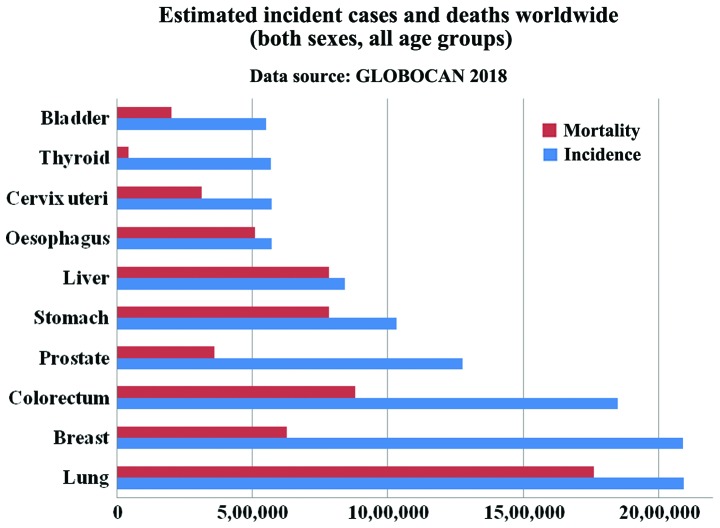
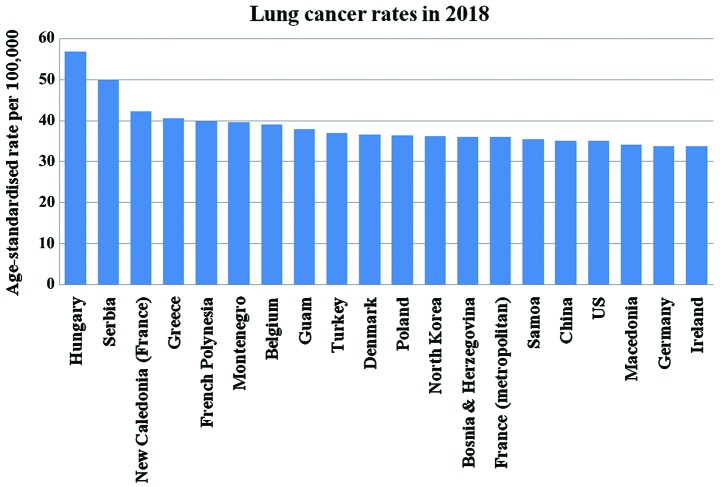
Lung cancer is one of the most common cancer types occurring in both men and women. According to the American Institute for Cancer Research (AICR), approximately 2 million new cases of lung cancer were reported in the year 2018 (1,2). As per the GLOBOCAN report of 2018, lung and breast cancer have the highest incidence rate, with lung cancer (Fig. 1) being the leading cause of mortality (2) consistent with other reports (3,4,5). A list of the top 20 countries with the highest rate of lung cancer in 2018 is presented in Fig. 2 (1). Strong evidence suggests that arsenic-containing drinking water and high-dose of beta-carotene augment the risk of lung cancer. In addition, consuming red meat and alcoholic may increase the risk (6). Lung cancer begins in the lungs as a mutation in oncogenes and proliferates as primary tumor and may spread to lymph nodes or other organs in the body by metastases. It is classified as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Of the two, NSCLSC accounts for approximately 85% among all the lung cancer cases. The major subtypes of NSCLC are adenocarcinoma (40%), squamous cell carcinoma (30%), and large cell carcinoma (15%) (7). Smoking is the main causative agent of lung cancer. For a non-smoker, exposure to passive smoking also causes lung cancer. In general, exposure to a carcinogen increases the risk of developing lung cancer, which includes asbestos, arsenic, chromium, nickel, radon, tobacco, benzene, cadmium, formaldehyde and crystalline silica (8). It has been reported that there is approximately 16% chance for 5-year survival (9).
Figure 1.
Estimated incident cases and deaths for the top 10 cancer types (2).
Figure 2.
Top 20 countries affected by lung cancer (1).
As far as lung cancer is concerned, the chronic obstructive pulmonary disease (COPD) is a significant risk factor which can be associated with the patient's susceptibility to cigarette smoking. In fact, severe inflammation induced due to toxic gases trigger COPD and lung cancer (10). The most common COPD are emphysema and chronic bronchitis. Bronchitis is inflammation of the bronchi. Emphysema causes damage to the alveoli, the air sacs in the lungs. The walls of the damaged alveoli become stretched out and make it difficult for diffusion. COPD is primarily caused by smoking and long-term exposure and contact with harmful pollutants that include certain chemicals, dust, or fumes and rarely, by alpha-1-antitrypsin and deficiency or a genetic condition.
COPD is measured by spirometry grading systems and one of them is GOLD classification. The GOLD classification is used for determining COPD severity and helps in prognosis and treatment plan. Based on spirometry testing, COPD and is graded as: mild (grade 1), moderate (grade 2), severe (grade 3) and very severe (grade 4). It is dependent on the result of the spirometry test of a patient's FEV1, i.e., the volume of air one may breathe out of the lungs in the first one second of a forced expiration. As FEV1 decreases, the severity increases. With the progress in time, the patient is more susceptible to various complications, including respiratory infections, heart problems, high blood pressure in lung arteries (pulmonary hypertension), flu, colds, pneumonia, depression, anxiety, and lung cancer.
In fact, COPD and lung cancer are linked in a number of ways, one being that smoking is the most common risk factor; others include passive smoke or exposure to chemicals or other fumes in the workplace. It has been estimated that between 40 and 70% of individuals with lung cancer also have COPD and it is concluded that COPD is a risk factor for lung cancer (11,12). By contrast, a study by Durham and Adcock (9) suggested that COPD is a driving factor in lung cancer. COPD is the leading cause of mortality projected to rank 3rd in 2020 (13) and comes under the environmental factors such as smoking (14). Exacerbation of COPD exhibits various symptoms that include cough, production of sputum or shortness of breath. It can be caused either by bacterial or viral infections or inhaled particles. The genetic factor can also be helpful in determining the frequency of this disease (15).
Gene expression studies are an important tool for transcriptomic analysis of an organism that helps to quantify expression level genes in both disease and normal conditions. Gene expression profiles of two different conditions (disease versus normal) can be compared to reveal potential key regulators or differentially expressed genes (DEGs), or co-regulated genes, either up- or downregulated (16). The key regulators or DEGs may be possible gene biomarker responsible for the disease condition (17,18). A few gene expression studies on COPD and lung cancer (14,15) are available; however, our aim is to identify DEGs and determine their functional analysis. The present study presents a systems biology perspective to decipher DEGs in lung cancer using microarray gene expression profiles and determine their functional analysis.
Materials and methods
Datasets
In order to identify DEGs, i.e., key gene biomarkers, two types of samples with multiple replicas were required: lung cancer tissue samples and healthy lung tissue samples. On studying these samples, factors that could be the reason for COPD or lung cancer were identified. These factors were genetic or environmental. COPD may be an emphysema type. In emphysema, air sacs are damaged and the patient does not get the oxygen required. Exacerbation of COPD can be diagnosed on the basis of symptoms including cough, shortness of breath, and generation of sputum.
In the present study, publicly available gene expression profiles were obtained from Gene Expression Omnibus (GEO accession no. GSE1650) where data referable to patients were properly anonymized by submitters and informed consent was obtained by the investigators during the original data collection. The following information labels were available and collected for each sample: sample GSM number, status (public on month/day/year), title (number letter) sample type (RNA), source name (lung tissue), organism (Homo sapiens), extracted molecule (total RNA), and description (lung tissue and resected lung taken from smokers).
Of the 30 patients, 18 samples belong to severe emphysema patients and the remaining 12 samples belong to patients having mild or no emphysema. A comparison was made of the expression profiles of severely emphysematous tissue and normal/mildly emphysematous lung tissue from smokers with nodules suspicious of lung cancer. The comparison provides insights into the pathogenetic mechanisms of COPD.
Methodology
The adopted methodologies are presented in Fig. 3 and described as follows:
Figure 3.
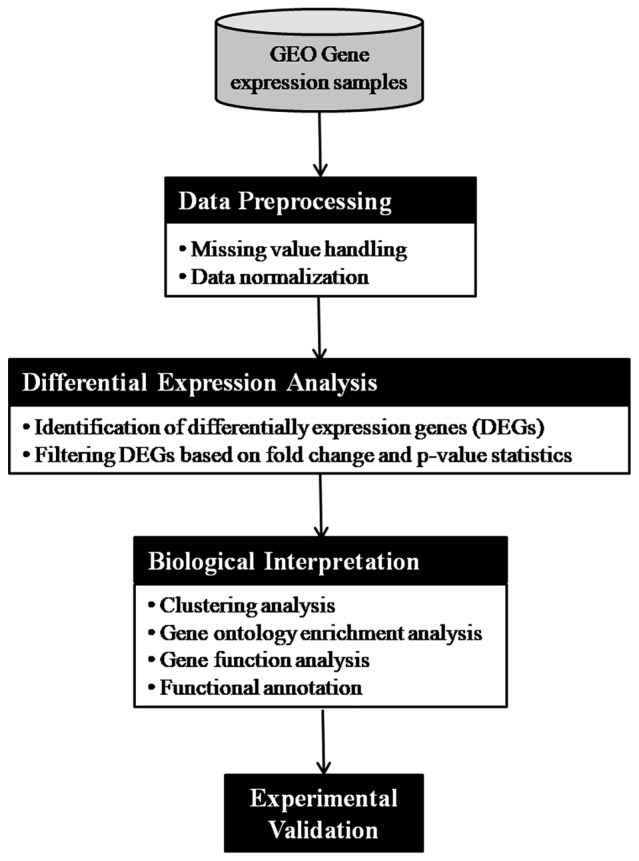
Flowchart of the gene expression analysis pipeline.
Data preprocessing: the microarray data were originally available as a CEL file, which is quantified and converted to gene expression values. After conversion into gene expression values, it is further quality checked and normalized to reduce variance among the data.
Differential expression analysis: the analysis of DEGs was performed using GEO2R tool available at NCBI-GEO. It is a user-friendly and interactive web-based tool that helps the researcher to compare groups of samples for the purpose of identifying DEGs across experimental conditions. We used adjusted P-value with Benjamini and Hochberg (19) false discovery rate and log fold-change as statistical metrics for evaluation purpose.
Results and Discussion
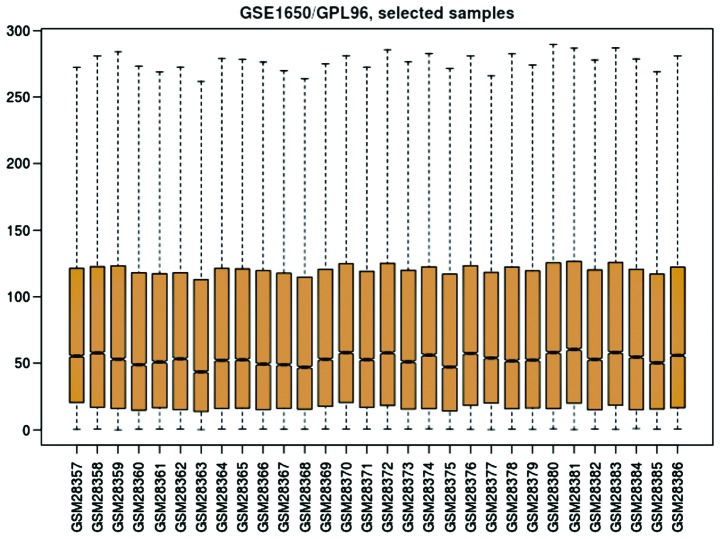
The considered datasets comprising 30 patients, out of which 18 samples belong to severe emphysematous tissue and 12 patients have normal/mildly emphysematous lung tissue from smokers suspicious of lung cancer. In order to understand the distribution of gene expression data among these two groups of samples, we depicted boxplot as shown in Fig. 4. It is observed from the boxplot (Fig. 4) that the values of gene expression lie between 0 and 300, while their 2nd quartile (mean) fluctuates around 50. Thus, the gene expression data are uniformly distributed.
Figure 4.
Boxplot of the 30 samples (18 samples from severe emphysematous tissue and 12 samples from normal/mildly emphysematous lung tissue from smokers suspicious of lung cancer).
The Heatmap diagram shows the combined with clustering group genes and/or samples based on gene expression similarity pattern, which is helpful for the identification of commonly regulated genes, or gene signature associated with a disease. The heatmap diagram of our considered dataset is shown in Fig. 5, where rows represent genes and column represents samples. The changes of gene expression are depicted as color intensity; for instance, green color represents downregulated genes, red presents upregulated genes, and black represents no changes in the expression. It is observed from Fig. 5 that the majority of the genes are regulated, either down- or up-regulated.
Figure 5.
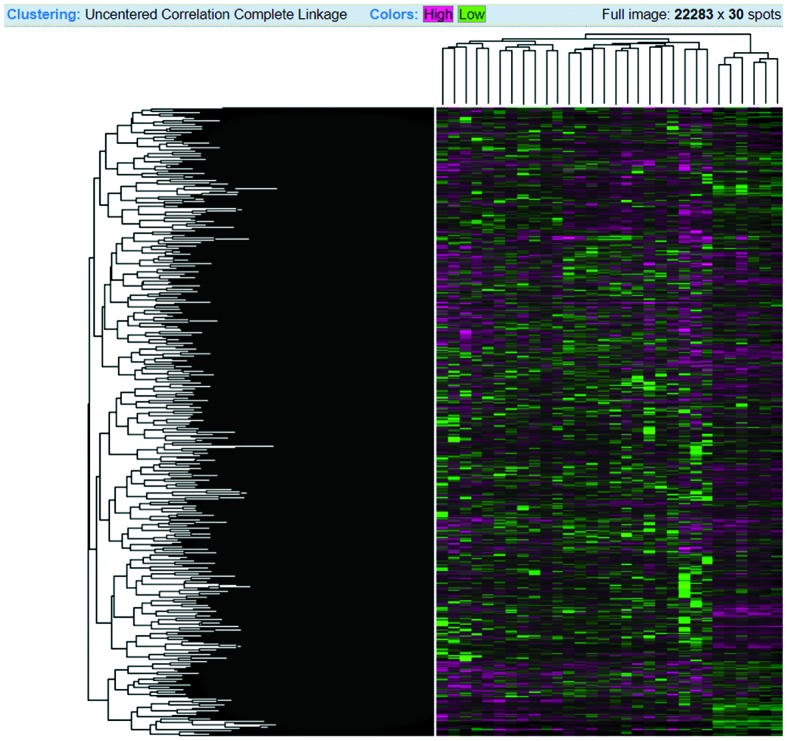
Heatmap diagram of the 30 samples. Rows represent genes, and columns represent samples. Red, upregulated genes; green, downregulated genes; black, unchanged gene expression.
Differential expression analysis. We performed the differential expression analysis (DEGs) between the two samples, i.e., between severe emphysematous lung tissue and normal/mildly emphysematous from smokers suspicious of lung cancer. We filtered DEGs with a significance level of 5% (P-value < =0.05) and had fold-change (FC) ≥2. In this way, we obtained 623 DEGs which had FC ≥2 in the expression level between the two samples. Out of 623 DEGs, 6 genes have a 4-fold change in the expression level, while 47 DEGs have a 3-fold change in their expression level (Fig. 6). The list of DEGS show 3- and 4-fold change in the expression level, along with other statistics such as adjusted P-value, P-value, moderated t-statistics, B-statistics, log FC and FC (Table I).
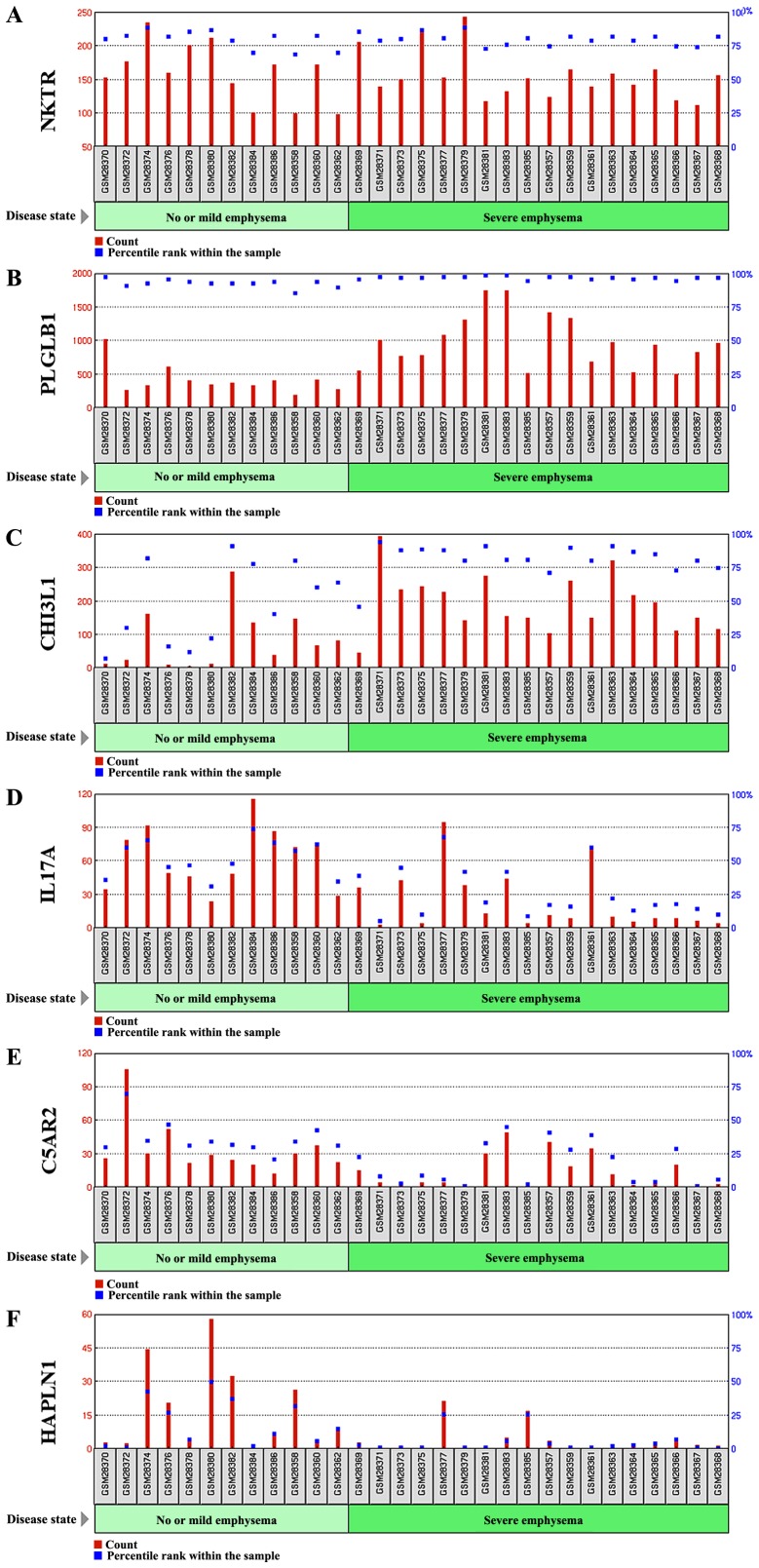
Figure 6.
Gene expression profile graph of (A-F) six DEGs with a 4-fold change in the expression level.
Table I.
List of differentially expressed genes along with their various scores.
| Gene name | Adjusted P-value | P-value | t-statistics | B-statistics | logFC | FC | Gene description |
|---|---|---|---|---|---|---|---|
| NKTR | 0.1544 | 0.0002 | 4.1685 | 0.4851 | 2.1583 | 4.4639 | Natural killer cell triggering receptor |
| PLGLB1/PLGLB2 | 0.2440 | 0.0019 | 3.4003 | −1.2650 | 2.1000 | 4.2872 | Plasminogen-like B1/B2 |
| CHI3L1 | 0.1384 | 0.0002 | −4.2923 | 0.7747 | −2.0635 | 4.1801 | Chitinase 3 like 1 |
| IL17A | 0.1264 | 0.0001 | 4.5501 | 1.3809 | 2.0311 | 4.0873 | Interleukin 17A |
| C5AR2 | 0.1885 | 0.0005 | 3.8610 | −0.2268 | 2.0277 | 4.0775 | Complement component 5a receptor 2 |
| HAPLN1 | 0.1953 | 0.0008 | 3.7205 | −0.5476 | 2.0119 | 4.0332 | Hyaluronan and proteoglycan link protein 1 |
| LOXL1 | 0.1264 | 0.0001 | −4.5643 | 1.4142 | −1.9436 | 3.8466 | Lysyl oxidase like 1 |
| BRF1 | 0.1895 | 0.0007 | 3.7798 | −0.4127 | 1.9285 | 3.8067 | BRF1, RNA polymerase III transcription initiation factor 90 kDa subunit |
| CSF3 | 0.3087 | 0.0046 | 3.0529 | −2.0141 | 1.9089 | 3.7552 | Colony stimulating factor 3 |
| PPM1A | 0.1885 | 0.0004 | 3.9522 | −0.0170 | 1.9064 | 3.7487 | Protein phosphatase, Mg2+/Mn2+ dependent 1A |
| UBR2 | 0.1351 | 0.0001 | −4.3783 | 0.9764 | −1.8816 | 3.6849 | Ubiquitin protein ligase E3 component n-recognin 2 |
| CLCA3P | 0.2905 | 0.0031 | 3.2114 | −1.6766 | 1.8733 | 3.6637 | Chloride channel accessory 3, pseudogene |
| MYH7/MYH6 | 0.1885 | 0.0006 | 3.8079 | −0.3485 | 1.8698 | 3.6549 | Myosin, heavy chain 7, cardiac muscle, beta/myosin heavy chain 6 |
| ZNF214 | 0.2418 | 0.0018 | 3.4102 | −1.2431 | 1.8664 | 3.6462 | Zinc finger protein 214 |
| PCSK1 | 0.1544 | 0.0002 | 4.2011 | 0.5612 | 1.8579 | 3.6248 | Proprotein convertase subtilisin/kexin type 1 |
| PTGER3 | 0.1885 | 0.0006 | 3.8053 | −0.3544 | 1.8309 | 3.5575 | Prostaglandin E receptor 3 |
| GYS2 | 0.2458 | 0.0020 | 3.3823 | −1.3045 | 1.8290 | 3.5530 | Glycogen synthase 2 |
| BCAN | 0.2188 | 0.0014 | 3.5128 | −1.0156 | 1.8263 | 3.5462 | Brevican |
| KIF23 | 0.2012 | 0.0010 | 3.6177 | −0.7803 | 1.8148 | 3.5181 | Kinesin family member 23 |
| HTR2B | 0.1923 | 0.0007 | −3.7608 | −0.4560 | −1.7822 | 3.4396 | 5-hydroxytryptamine receptor 2B |
| BMP2K | 0.343 | 0.0087 | 2.8034 | −2.5282 | 1.7688 | 3.4077 | BMP2 inducible kinase |
| CHIT1 | 0.1544 | 0.0002 | −4.1743 | 0.4987 | −1.7674 | 3.4044 | Chitinase 1 |
| TNIP3 | 0.1885 | 0.0006 | 3.8506 | −0.2507 | 1.7650 | 3.3988 | TNFAIP3 interacting protein 3 |
| PHLPP1 | 0.3203 | 0.0056 | 2.9788 | −2.1690 | 1.7485 | 3.3601 | PH domain and leucine rich repeat protein phosphatase 1 |
| POSTN | 0.2692 | 0.0026 | 3.2736 | −1.5422 | 1.7481 | 3.3592 | Periostin |
| CCL20 | 0.3597 | 0.0126 | 2.6482 | −2.8359 | 1.7446 | 3.3511 | C-C motif chemokine ligand 20 |
| SMARCA2 | 0.3332 | 0.0074 | 2.8663 | −2.4007 | 1.7365 | 3.3323 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2 |
| TSC22D2 | 0.3475 | 0.0111 | 2.7019 | −2.7306 | 1.7225 | 3.3001 | TSC22 domain family member 2 |
| FOSB | 0.4467 | 0.0368 | 2.1818 | −3.6944 | 1.7159 | 3.2850 | FosB proto-oncogene, AP-1 transcription factor subunit |
| GGA2 | 0.262 | 0.0024 | 3.3140 | −1.4542 | 1.7134 | 3.2793 | Golgi associated, gamma adaptin ear containing, ARF binding protein 2 |
| KYNU | 0.3263 | 0.0067 | 2.9063 | −2.3189 | 1.7128 | 3.2779 | Kynureninase |
| CCDC88C | 0.3197 | 0.0053 | 3.0028 | −2.1190 | 1.7090 | 3.2693 | Coiled-coil domain containing 88C |
| MCF2 | 0.343 | 0.0087 | 2.8022 | −2.5305 | 1.7089 | 3.2692 | MCF.2 cell line derived transforming sequence |
| PAICS | 0.3197 | 0.0054 | 2.9931 | −2.1393 | 1.7001 | 3.2491 | Phosphoribo-sylaminoimidazole carboxylase; phosphoribosyla-minoimidazolesuccinocarbo-xamide synthase |
| CREBZF | 0.3047 | 0.0045 | 3.0666 | −1.9851 | 1.6783 | 3.2004 | CREB/ATF bZIP transcription factor |
| AFF2 | 0.2458 | 0.0019 | 3.3904 | −1.2868 | 1.6748 | 3.1927 | AF4/FMR2 family member 2 |
| XIST | 0.3861 | 0.0182 | −2.4926 | −3.1341 | −1.6726 | 3.1880 | X inactive specific transcript (non-protein coding) |
| BPESC1 | 0.3735 | 0.0153 | 2.5674 | −2.9922 | 1.6687 | 3.1793 | Blepharophimosis, epicanthus inversus and ptosis, candidate 1 (non-protein coding) |
| KRT17/JUP | 0.2473 | 0.0021 | −3.3627 | −1.3476 | −1.6661 | 3.1736 | Keratin 17/junction plakoglobin |
| CALCRL | 0.2458 | 0.0020 | 3.3835 | −1.3019 | 1.6656 | 3.1724 | Calcitonin receptor like receptor |
| SALL1 | 0.3352 | 0.0079 | 2.8388 | −2.4566 | 1.6603 | 3.1609 | Spalt like transcription factor 1 |
| GRIK2 | 0.3457 | 0.0100 | 2.7456 | −2.6440 | 1.6533 | 3.1455 | Glutamate ionotropic receptor kainate type subunit 2 |
| KLK13 | 0.2347 | 0.0017 | 3.4334 | −1.1918 | 1.6488 | 3.1358 | Kallikrein-related peptidase 13 |
| NOL4 | 0.1885 | 0.0005 | −3.9187 | −0.0943 | −1.6357 | 3.1073 | Nucleolar protein 4 |
| KCNV1 | 0.2012 | 0.0010 | 3.6356 | −0.7400 | 1.6335 | 3.1027 | Potassium voltage-gated channel modifier subfamily V member 1 |
| GTSE1 | 0.2692 | 0.0025 | 3.2897 | −1.5071 | 1.6184 | 3.0703 | G2 and S-phase expressed 1 |
| SPON1 | 0.1885 | 0.0004 | −3.9605 | 0.0023 | −1.6153 | 3.0637 | Spondin 1 |
| CST1 | 0.1264 | 0.0001 | −4.6287 | 1.5662 | −1.6132 | 3.0593 | Cystatin SN |
| TSPAN2 | 0.456 | 0.0406 | 2.1368 | −3.7713 | 1.6114 | 3.0556 | Tetraspanin 2 |
| PLD1 | 0.2628 | 0.0024 | 3.3081 | −1.4670 | 1.6076 | 3.0473 | Phospholipase D1 |
| CDHR5 | 0.2692 | 0.0026 | 3.2810 | −1.5259 | 1.5982 | 3.0276 | Cadherin related family member 5 |
| SULF1 | 0.2188 | 0.0014 | −3.5003 | −1.0433 | −1.5974 | 3.0260 | Sulfatase 1 |
| SLC12A4 | 0.2922 | 0.0035 | 3.1634 | −1.7797 | 1.5933 | 3.0175 | Solute carrier family 12 member 4 |
The profile graph of the six DEGs having a 4-fold change in the expression, i.e., NKTR, PLGLB1, CHI3L1, IL17A, C5AR2, and HAPLN1 are depicted in Fig. 6.
We further performed the Gene Ontology (GO) functional enrichment analysis of six DEGs found to have a 4-fold change in their expression (Table II). From our DEGs analysis, it can be inferred that the NKTR gene was upregulated 4-fold. This gene is expressed in natural killer cells as a multi-domain structure (20) with a peptidyl-prolyl cis-trans isomerase activity in oligopeptides assisting protein folding (21) and a putative tumor-recognition complex participating in NK cells function (20). PLGLB1 is a 4-fold upregulated gene expressed a plasminogen-like protein B found to bind to lysine binding sites present in the kringle structures of plasminogen (22). Similarly, CHI3L1 expression by approximately 4-fold plays an important role in tissue remodeling, and helps to cope with the changes in environment, T-helper cell type 2 inflammatory response and interleukin-3 induced inflammation, as well as inflammatory cell apoptosis (23,24).
Table II.
GO enrichment analysis of six differentially expressed genes.
| Gene name | GO molecular function | GO biological process | Cellular component | PMID |
|---|---|---|---|---|
| NKTR | Cyclosporin A binding, peptidyl-prolyl cis-trans isomerase activity, unfolded protein binding | Protein peptidyl-prolyl isomerization, protein refolding | Cytosol, mitochondrion, nucleoplasm | 20676357, 20676357, 21873635 |
| PLGLB1/PLGLB2 | –– | –– | Extracellular region | UniProt |
| CHI3L1 | Carbohydrate binding, chitin binding, extracellular matrix structural constituent | Apoptotic process, carbohydrate metabolic process, cartilage development, cellular response to tumor necrosis factor, lung development | Endoplasmic reticulum | 12775711, 9492324, 8245017, 18403759, 16234240 |
| IL17A | Cytokine activity | Apoptotic process, cell-cell signalling, cell death, cytokine- mediated signalling pathway, immune response, inflammatory response | Extracellular region, extracellular space | 7499828, 8390535 |
| C5AR2 | Complement component C5a receptor activity, G protein-coupled receptor activity | Chemotaxis, complement receptor mediated signaling pathway, inflammatory response, negative regulation of tumor necrosis factor production | Basal plasma membrane, plasma membrane | 21873635, 16204243, 22960554 |
| HAPLN1 | Extracellular matrix structural constituent conferring compression resistance, hyaluronic acid binding | Cell adhesion, central nervous system development, extracellular matrix organization, skeletal system development | Collagen-containing extracellular matrix, extracellular matrix | 20551380, 21873635, 23979707 |
In conclusion, COPD is a lung disease ranked third as a reason for mortality worldwide (13) This disease is influenced by both genetic and environmental factors. Cigarette smokers are the topmost risk factor in the western world. COPD constitutes the leading cause of mortality related to environmental factors such as smoking. Exacerbation of COPD exhibits various symptoms that include cough, production of sputum or shortness of breath. It can be caused either by bacterial or viral infections or inhaled particles. The genetic factor can also be helpful in determining the frequency of this disease. In this study, we performed differential gene expression analysis of 30 samples belonging to two different tissue types - severe emphysematous tissue and normal/mildly emphysematous lung tissue from smokers suspicious of lung cancer. We identified approximately 623 DEGs having 2- or more fold-change in their expression level, out of which 6 genes have 4-fold change, and 47 genes have a 3-fold change in the expression. We also performed GO enrichment analysis which uncovers fruitful knowledge that can be further validated from wet lab.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GJ conceived and designed the study. MB provided study materials or patients and was responsible for the collection and assembly of data, data analysis and interpretation. Both authors were involved in writing the manuscript. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethics review submission for approval is not required for this work. There is no identifiable more than minimal risk for the following reasons: i) This study does not contain human participants or animals procedures performed by any of the authors; ii) the data were taken from publicly available resource (GEO Datasets) where data referable to patients were properly anonymized by submitters and informed consent was obtained by the investigators during the original data collection; and iii) any active dissemination, in addition to the intention to submit findings for publication is purely an academic discussion of the study topic, i.e., method vis-à-vis analysis of gene expression.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
References
- 1.AICR, corp-author. American Institute for Cancer Research, World Cancer Research Fund: Lung Cancer Statistics. https://www.wcrf.org/dietandcancer/cancer-trends/lung-cancer-statistics. [Feb;2019 ];2018
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2018;124:2785–2800. doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, et al. Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, Soerjomataram I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J Cancer. 2019;144:49–58. doi: 10.1002/ijc.31664. [DOI] [PubMed] [Google Scholar]
- 6.AICR, corp-author. American Institute for Cancer Research, World Cancer Research Fund. http://www.aicr.org/continuous-update-project/lung-cancer.html. [Feb;2019 ];Continuous Update Project Report: Lung Cancer. 2018
- 7.Zappa C, Mousa SA. Non-small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33:681–703. doi: 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer. 2015;90:121–127. doi: 10.1016/j.lungcan.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekine Y, Katsura H, Koh E, Hiroshima K, Fujisawa T. Early detection of COPD is important for lung cancer surveillance. Eur Respir J. 2012;39:1230–1240. doi: 10.1183/09031936.00126011. [DOI] [PubMed] [Google Scholar]
- 11.Dai J, Yang P, Cox A, Jiang G. Lung cancer and chronic obstructive pulmonary disease: From a clinical perspective. Oncotarget. 2017;8:18513–18524. doi: 10.18632/oncotarget.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ytterstad E, Moe PC, Hjalmarsen A. COPD in primary lung cancer patients: Prevalence and mortality. Int J Chron Obstruct Pulmon Dis. 2016;11:625–636. doi: 10.2147/COPD.S101183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZH, Kim HP, Ryter SW, Choi AM. Identifying targets for COPD treatment through gene expression analyses. Int J Chron Obstruct Pulmon Dis. 2008;3:359–370. doi: 10.2147/COPD.S1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow JD, Qiu W, Chhabra D, Rennard SI, Belloni P, Belousov A, Pillai SG, Hersh CP. Identifying a gene expression signature of frequent COPD exacerbations in peripheral blood using network methods. BMC Med Genomics. 2015;8:1. doi: 10.1186/s12920-014-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raza K. IGI Global; Pennsylvania, PA: 2016. Analysis of microarray data using artificial intelligence based techniques. In: Computational Intelligence Applications in Bioinformatics. Dash S and Subudhi B (eds) pp. 216–239. [Google Scholar]
- 17.Jabeen A, Ahmad N, Raza K. Differential expression analysis of ZIKV infected human RNA sequence reveals potential biomarkers. bioRxiv. 2018:498295. [Google Scholar]
- 18.Raza K. Reconstruction, topological and gene ontology enrichment analysis of cancerous gene regulatory network modules. Curr Bioinform. 2016;11:243–258. doi: 10.2174/1574893611666160115212806. [DOI] [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 20.Anderson SK, Gallinger S, Roder J, Frey J, Young HA, Ortaldo JR. A cyclophilin-related protein involved in the function of natural killer cells. Proc Natl Acad Sci USA. 1993;90:542–546. doi: 10.1073/pnas.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis TL, Walker JR, Campagna-Slater V, Finerty PJ, Paramanathan R, Bernstein G, MacKenzie F, Tempel W, Ouyang H, Lee WH, et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010;8:e1000439. doi: 10.1371/journal.pbio.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissbach L, Treadwell BV. A plasminogen-related gene is expressed in cancer cells. Biochem Biophys Res Commun. 1992;186:1108–1114. doi: 10.1016/0006-291X(92)90861-E. [DOI] [PubMed] [Google Scholar]
- 23.Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, Hrebicek M, Aerts JM. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.