Abstract
The aim of this study was to examine the impact of iodine on the development of thyroid cancer cells and to detect the underlying mechanisms. It was observed that proliferation was promoted and apoptosis was inhibited in cells treated with iodine at a specific concentration. This treatment group was then selected for further analysis, to investigate how iodine affects the development of thyroid cancer cells. It was reported that sperm protein associated with the nucleus, X-linked, family member A1 (SPANXA1) expression in iodine-treated cells was significantly upregulated. Furthermore, downregulation of SPANXA1 inhibited cell proliferation, migration and invasion, and promoted cell apoptosis. These results suggested that SPANXA1 played an important role in iodine-treated thyroid cancer cells. Novel associations between SPANXA1 and thyroid cancer were described in the current study. In addition, SPANXA1 gene silencing resulted in the downregulation of PI3K and phosphorylated (p)AKT expression in iodine-treated thyroid cancer cells, whereas iodine treatment alone resulted in upregulated PI3K and p-AKT expression. Inhibiting PI3K further suppressed cell proliferation and contributed to apoptosis, even in the presence of SPANXA1 at high levels. As a consequence, PI3K/AKT may be one of the key signalling pathways by which iodine promotes thyroid cancer development in association with SPANXA1. In addition, our results further suggested that patients with thyroid cancer may need to avoid high-iodine intake.
Keywords: thyroid cancer, sperm protein associated with the nucleus, X-linked, family member A1, PI3K/AKT signalling pathway, iodine
Introduction
Thyroid cancer is one of the most common endocrine cancers and includes anaplastic carcinoma, medullary carcinoma, follicular carcinoma and papillary carcinoma (1). Among these, the most common type of thyroid carcinoma is papillary thyroid carcinoma (PTC), which accounts for about 85% of all thyroid cancers (2). Thyroid cancer incidence has been reported to have increased rapidly worldwide, with the PTC incidence increasing the most (3,4). Between 1975 and 2009, the incidence of thyroid cancer has nearly tripled from 4.9 to 14.3 per 100,000 individuals, and the increase was attributable to PTC, the incidence of which increased from 3.4 to 12.5 per 100,000 individuals (5). Although thyroid cancer typically has low mortality and high survival rates, disease recurrence is high and a subgroup of patients with tumours exhibits aggressive characteristics (6). Therefore, it is important to examine the factors and underlying molecular mechanisms of thyroid cancer development to further assist in guiding the lifestyle choices of patients with thyroid cancer.
Numerous studies have investigated potential prognostic factors of thyroid cancer (7–9); a high prevalence of BRAF gene mutations (10) and significantly increased expression levels of the sodium/iodide symporter in thyroid cancer have been reported (11). Although the exact mechanism remains unknown, it is unlikely that genetic factors alone can explain the increase in thyroid cancer incidence in recent years therefore, an association with other factors has been proposed (12,13). Previous epidemiological surveys have indicated that high iodine intake may play an important role in the promotion of thyroid cancer (14–16).
Iodine is an essential trace element and affects the function of the thyroid gland (17). Both deficient and excessive levels of iodine may lead to thyroid diseases (18). However, the effects of iodine in thyroid cancer are controversial and whether iodine promotes or prevents the progression of thyroid cancer remains unknown. A previous study demonstrated that iodine can induce apoptosis and prevent the progress of thyroid cancer development (19). It was further reported that iodine prevents the transformation from PTC to anaplastic thyroid cancer (20,21). Gerard et al (22) suggested that thyroid cancer benefits from iodine deficiency through an angiogenic reaction via vascular endothelial growth factor (VEGF) induction. However, it was further reported that iodine may promotes the thyroid cancer incidence rate (14). An increase in the prevalence of thyroid cancer has been observed following the introduction of universal salt iodization (14). The papillary-to-follicular incidence rate ratios increased significantly from 3.98 between 1980 and 1984 to 9.88 between 2005 and 2009 (23). Therefore, increased attention has focused on the mechanism by which iodine affects thyroid cancer development. For instance, a high iodine diet promotes thyroid cancer development by upregulating p14ARF and p16INK4a expression (24). An association between high iodine intake and the T1799A BRAF mutation in PTC has been described (25). Therefore, high iodine intake may be a risk factor in thyroid cancer development; however, the underlying mechanisms have not been clearly elucidated and the key genes and pathways involved remain unknown.
In the present study, the effects of varying extra amounts of iodine on thyroid cancer cells were examined. In addition, high throughput RNA-sequencing and RT-qPCR were used to identify the key genes through which iodine affected thyroid cancer cells. The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signalling pathway in a series of in vitro assays was examined. The results revealed that SPANXA1 may serve an important oncogenic role in thyroid cancer cells pre-treated with extra-low doses of iodine via the PI3K/AKT signalling pathway.
Materials and methods
Cell culture and experimental groups
Human thyroid cancer cell line BCPAP was purchased from Jennio Biotech Co., Ltd. The genetic alteration most often found in thyroid cancer is the mutant BRAF gene; BCPAP cells harbour the BRAF mutation (26). Therefore, this cell line was selected to examine the impacts of iodine on the development of thyroid cancer. Cells cultured in Roswell Park Memorial Institute (RPMI)-1640 (Corning, Inc.), containing 10% foetal bovine serum (FBS; Corning, Inc.) was referred to as the control group. A significant increase in the prevalence of thyroid cancer had been observed after universal salt iodization (15), with potassium iodate (KIO3) being the major additive material in the process of salt iodization (27). Therefore, KIO3 was dissolved in RPMI-1640 to adjust the concentration of iodine. LY294002 was used to inhibit the PI3K/AKT signalling pathway, which may serve an important role in the effects of iodine on thyroid cancer cell growth. According to the amount of KIO3 added in to RPMI-1640 medium, the iodine concentrations in the media were 1.0×10−3, 1.0×10−4, 1.0×10−5, 1.0×10−6, 1.0×10−7 and 1.0×10−8 mol/l. The cells (3×105 cells/well) were cultured in iodine-enriched environment with 5% CO2 at 37°C to examine the impact of iodine. pH values at 37°C of all culture mediums supplemented with iodine at 1.0×10−3, 1.0×10−4, 1.0×10−5, 1.0×10−6, 1.0×10−7 and 1.0×10−8 mol/l for 24 h were measured; KIO3 did not change the pH value of the culture medium (data not shown). Iodine supplementation at 1.0×10−6 mol/l promoted cell proliferation and inhibited cell apoptosis the most previously compared with control group, therefore, this concentration was used for subsequent experiments. To detect the expression of PI3K and p-AKT affected by downregulating SPANXA1, the cells treated with siRNA-SPANXA1 were compared with control group treated with siRNA-NC and blank group treated without siRNAs.
Cell proliferation assay
BCPAP cells (3×103) were seeded in 96-well plates in triplicates, and were cultured in 100 µl RPMI-1640 containing 10% FBS. MTT (Sigma-Aldrich; Merck KGaA) was added into each well at a final concentration of 5 mg/ml. After 4 h of MTT incubation with 5% CO2 at 37°C on days 0, 1, 2 and 3, dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added to dissolve the insoluble formazan reduced from MTT, and was subsequently injected into each well. The absorbance was measured using the Infinite® 200 pro NanoQuant spectrophotometer (Tecan Group, Ltd.) at a wavelength of 490 nm to determine cell proliferation.
Apoptosis analysis
BCPAP cells (3×105) were seeded into 6-well plates with RPMI-1640 complete culture medium. After 48 h of treatment, the cells were washed with PBS, suspended in binding buffer (Beyotime Institute of Biotechnology) and serum-deprived for 24 h prior to FACS. The cells were stained with 5 µl Annexin V-fluorescein isothiocyanate (FITC) and 5 µl propidium iodide (Vazyme Biotech Co., Ltd.) and incubated at room temperature for 15 min, according to the manufacturer's protocol. After staining, the proportion of apoptotic cells was analysed using a flow cytometer (BD Biosciences).
High-throughput RNA-sequencing
High-throughput RNA-sequencing was conducted (Seqhealth Technology Co., Ltd.) to screen differentially expressed genes between BCPAP cells incubated in ordinary medium and the ones treated with extra-lower iodine. Total RNA was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and purified using an RNeasy Plus Mini kit (Qiagen). Each group was sequenced on one sequencing lane of an Illumina Genome Analyzer II system (Illumina). The paired-end reads were trimmed with the Illumina standard adapter (‘AGATCGGAAGAGC’) low quality sequence 3end by using Trim Galore (version 3, http://www.bioinformatics.babraham.ac.uk/projects/trim_galore) and mapped to the human genome (version h19; ftp://ftp.ensembl.org/pub/release-75/gtf/homo_sapiens/Homo_sapiens.GRCh37.75.gtf.gz) using Tophat (version 2.1.0, http://ccb.jhu.edu/software/tophat/index.shtml). The expression levels of each gene were analysed using Cufflinks (version 2.21, http://cole-trapnell-lab.github.io/cufflinks). The mapped reads were used to quantify the transcripts from the RefSeq reference database. For the functional annotation analysis of genes, the Database for Annotation, Visualization and Integrated Discovery (DAVID, http://david.ncifcrf.gov/home.jsp) online tool was used.
SPANXA1-knockdown by transient RNA interference
BCPAP cells (3×105) were seeded into 6-well plates and incubated for 24 h. Cells were transiently transfected with synthesized SPANXA1 small interfering (si)RNAs (GCCTGCCACTGACATTGAA, 20 µM; Ribobio Co., Ltd.) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Red fluorescent protein (RFP) was used to detect transfection efficiency. Media were changed at 4–6 h. Cells were harvested 48 h after transfection, and reverse transcription-quantitative PCR (RT-qPCR) was performed to confirm transfection.
Wound healing assay
BCPAP cells in logarithmic growth phase were placed in 6-well plates with a density of 3×105 cells/well. Once the cells reached 80% confluence, a vertical scratch was made on the cell layer using a 10-µl pipette tip. Thereafter, the cells were incubated in serum-free RPMI-1640 with 5% CO2 at 37°C. Images of the plates were captured at 0, 24 and 48 h at ×20 magnification using an inverted microscope (Olympus Corporation, Tokyo, Japan).
Cell migration assay
Transwell assays were performed to evaluate the invasive capability of BCPAP cells. Transwell chambers with polyvinylidene difluoride (PVDF) filters (pore size 8.0-µm; Corning, Inc.) were pre-coated with 50 µl Matrigel (BD Biosciences) diluted 1:3 in serum-free RPMI-1640. Cells (3×104 cells/well) were suspended in 200 µl serum-free RPMI-1640 and placed into the upper chambers in triplicate. RPMI-1640 containing 600 µl FBS was added to the lower chamber as a chemotactic factor. Following 48-h incubation, the non-invading cells on the upper surface were carefully removed with cotton swabs. Cells that migrated through the pores and adhered to the lower surface were fixed with 4% paraformaldehyde for 30 min at room temperature and stained with 0.1% crystal violet for 30 min at room temperature, and images were captured under a fluorescent microscope (Olympus Corporation).
RT-qPCR analysis
Total RNA extraction from the cells was performed using TRIzol kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The obtained RNA was reversely transcribed into cDNA (10 µl) using a PrimeScript RT Reagent kit (Takara, Bio, Inc.). cDNA was used as the template for qPCR detection with primers and SYBR Master Mixture (Takara Bio, Inc.). The PCR was performed using the VIIA7 Real-Time PCR System (Thermo Fisher Scientific, Inc.), and the thermocycling conditions were as follows: Pre-denaturation at 95°C for 2 min, 40 cycles of denaturation at 95°C for 15 sec and annealing at 60°C for 15 sec, followed by final extension at 95°C for 15 sec. The relative mRNA expression levels in each sample were calculated using the 2−ΔΔCq method. The primers were synthesized by Generay Biotech Co., Ltd., and the sequences are listed in Table I. mRNA levels were normalized to the internal reference gene β-actin.
Table I.
Primer sequences used in the present study.
| Gene | Primers (5′→3′) |
|---|---|
| BMS1P17 | F: GCACAGGCTGCATCTCCACTA |
| R: CCCACGGCATCAGACTAAAGG | |
| CALD1 | F: GTTTCCATCTGGGGTTTTAGTT |
| R: TATGTGGGAGAAAGGGAATGT | |
| H3F3BP1 | F: CTGGAAGGGAAGTCTGCGAAT |
| R: TACTGGAGGGGTGAAGAAACC | |
| IGKV10R-2 | F: GGAGTTTTCCTTGGTTTCTGC |
| R: AGTCTCCATCCTCCCTGTCTG | |
| MKI67 | F: AAGCCCTCCAGCTCCTAGTCCTA |
| R: GCCACTCTTTCTCCCTCCTCTCT | |
| SACS | F: CCAGGTGGTAAAGGAAGGAAA |
| R: GTGGGCGAGGGATCAGTAGTA | |
| VCAN | F: GTATTTGTAGCACTGCCCTTG |
| R: TGTCACTCTAATCCCTGTCGT | |
| SPANXA1 | F: TTCCTCCTGTAGCGAACCACT |
| R: TGCCACTGACATTGAAGAACC | |
| β-actin | F: CATGTACGTTGCTATCCAGGC |
| R: CTCCTTAATGTCACGCACGAT |
BMS1P17, BMS1, ribosome biogenesis factor pseudogene 17; CALD1, caldesmon 1; H3F3BP1, H3 histone, family 3B pseudogene 1; MKI67, marker of proliferation Ki-67; SACS, sacsin molecular chaperone; SPANXA1, sperm protein associated with the nucleus, X-linked, family member A1; VCAN, versican.
Western blot analysis
Total protein was extracted from BCPAP cells using RIPA lysis buffer (Beyotime Institute of Biotechnology), and its concentration was determined using a bicinchoninic acid assay. Extracted protein (~25 µg) in each group was denatured at 95°C for 10 min. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes (100V; 60 min). The membranes were blocked for 2 h in Tris-buffered saline with Tween-20 (TBST) with 5% non-fat milk at room temperature, and were subsequently incubated at 4°C overnight with primary antibodies against PI3K (cat. no. 4228; dilution, 1:1,000), phosphorylated-AKT (p-AKT; cat. no. 9271; dilution, 1:500), AKT (cat. no. 9272; dilution, 1:500) and GADPH (cat. no. 5174; dilution, 1:1,000). Following the incubation, the membranes were washed three times using TBST and incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G secondary antibody (cat. no. 5151; dilution, 1:2,000) at room temperature for 1 h. All antibodies were purchased from Cell Signaling Technology, Inc. The membranes were washed with TBST, and the blots were examined by enhanced chemiluminescence. Protein expression levels were semi-quantified by densitometry analysis using an imaging system (LI-COR, Inc.).
Statistical analysis
All statistical analysis was performed using SPSS 18.0 software (SPSS, Inc.). The results were presented as the mean ± standard deviation as appropriate. All calculated significances are based on the one-way analysis of variance test and the Tukey's post-hoc test. For direct comparisons between two groups, paired Student's t-test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Iodine at 1.0×10−6 mol/l significantly promotes cell proliferation and inhibits apoptosis
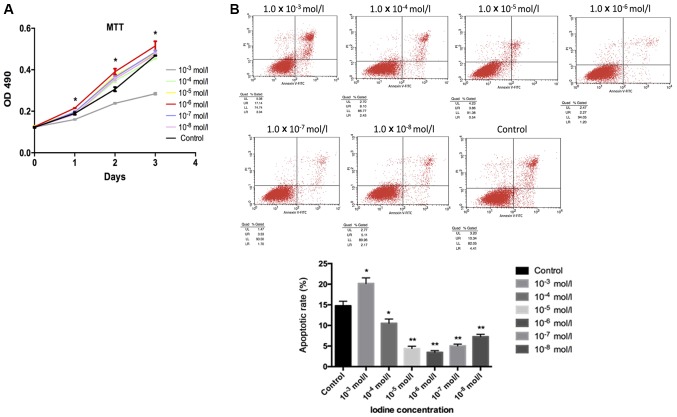
Cell proliferation, as evaluated by an MTT assay, suggested that iodine at 1.0×10−4, 1.0×10−5, 1.0×10−6, 1.0×10−7 and 1.0×10−8 mol/l enhanced cell proliferation when compared with the control group. Significant increases in cell proliferation compared with the control were observed following treatment with 1.0×10−6 mol/l. However, cells incubated with iodine at 1.0×10−3 mol/l presented notably decreased cell growth compared with the control group (Fig. 1A). For apoptosis, these results were reversed. Apoptosis in BCPAP cells incubated with iodine at 1.0×10−4, 1.0×10−5, 1.0×10−6, 1.0×10−7 and 1.0×10−8 mol/l was significantly decreased compared with the control group; the lowest degree of apoptosis was observed in response to 1.0×10−6 mol/l iodine. Apoptosis in samples treated with iodine at 1.0×10−3 mol/l significantly increased compared with the control (Fig. 1B).
Figure 1.
Cell proliferation evaluated by MTT assay and flow cytometry. (A) It was observed that iodine at lower concentrations (1.0×10−4, 1.0×10−5, 1.0×10−6, 1.0×10−7 and 1.0×10−8 mol/l) contributed to the proliferation of BCPAP cells, while iodine of a higher concentration (1.0×10−3 mol/l) had a negative effect. Cells treated with iodine at 1.0×10−6 mol/l exhibited the most pronounced changes. (B) BCPAP cell apoptosis was analysed by flow cytometry with different concentrations of iodine. BCPAP cell apoptosis, when incubated with lower concentrations of iodine (1.0×10−4, 1.0×10−5, 1.0×10−6, 1.0×10−7 and 1.0×10−8 mol/l) was significantly inhibited. Among these, the highest inhibitory effect was observed with 1.0×10−6 mol/l iodine. A higher concentration of iodine (1.0×10−3 mol/l) appeared to promote cell apoptosis. *P<0.05 and **P<0.01 vs. the control group. OD, optical density.
High throughput RNA-sequencing shows that iodine at 1.0×10−6 mol/l promotes thyroid cancer development via SPANXA1
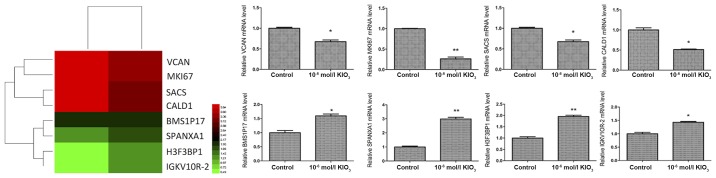
Our results indicated that iodine exhibits important roles in thyroid cancer, particularly at 1.0×10−6 mol/l. In order to investigate the molecular mechanism by which iodine affects thyroid cancer cells, high throughput RNA-sequencing was utilized to screen for differentially expressed genes in BCPAP cells incubated in ordinary medium (control group) and cells cultured in iodine-enriched medium (1.0×10−6 mol/l). The differential expression of genes was confirmed via RT-qPCR (Fig. 2). It was revealed that SPANXA1 mRNA expression was significantly upregulated in BCPAP cells when treated with iodine.
Figure 2.
High throughput RNA-sequencing data shown as a heat map and expression of different genes in BCPAP cells treated with iodine at 1.0×10−6 mol/l. Red represents high expression, and green represents low expression. *P<0.05 and **P<0.01 vs. the control group. KIO3, potassium iodate; BMS1P17, BMS1, ribosome biogenesis factor pseudogene 17; CALD1, caldesmon 1; H3F3BP1, H3 histone, family 3B pseudogene 1; MKI67, marker of proliferation Ki-67; SACS, sacsin molecular chaperone; SPANXA1, sperm protein associated with the nucleus, X-linked, family member A1; VCAN, versican.
Downregulation of SPANXA1 inhibits cell proliferation, migration and invasion, and promotes cell apoptosis in thyroid cancer cells incubated with an extra-low dose of iodine
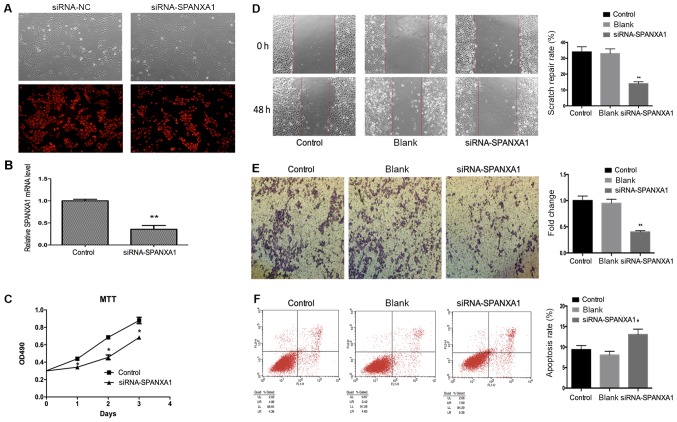
BCPAP cells incubated with iodine at 1.0×10-6 mol/l were selected to be transfected with 50 nmol/l siRNA-SPANXA1 to inhibit SPANXA1 expression (Fig. 3A). There was no notable difference in efficiency between siRNA-NC group and siRNA-SPANXA1 group. Therefore, the present study proposed that alterations in the parameters assessed may be induced by siRNA-SPANXA1. After transfection, SPANXA1 mRNA expression was downregulated significantly, which was evaluated by RT-qPCR (Fig. 3B). As presented in Fig. 3C, the proliferation of transfected BCPAP cells was lower when compared with the control group. The results demonstrated that migration (Fig. 3D) and invasion (Fig. 3E) of the siRNA-SPANXA1-transfected BCPAP cells were also significantly decreased compared with the control group. The apoptotic rate of the siRNA-SPANXA1-transfected BCPAP cells significantly increased when compared with the control group (13.05 vs. 9.35%), which implied that downregulating SPANXA1 may significantly induce BCPAP cell apoptosis (Fig. 3F).
Figure 3.
Effects of SPANXA1 on BCAP cell proliferation, migration, invasion and apoptosis. (A) Microscopy images of cells transfected with 50 nmol/l siRNA and control cells (magnification, ×400). (B) After transfection, SPANXA1 expression was decreased. (C) Proliferation of BCPAP cells treated with iodine at 1.0×10−6 mol/l as assessed by an MTT assay; SPANXA1-knockdown BCPAP cells exhibited suppressed proliferation. Migration of BCPAP cells with downregulated SPANXA1 was evaluated using (D) a Transwell assay and (E) invasion was analysed using a scratch-wound assay. Downregulation of SPANXA1 contributed to the inhibition of migration and invasion of iodine-treated BCPAP cells. Magnification, ×20. (F) Cell apoptosis results were obtained by flow cytometry and suggested that downregulation of SPANXA1 increased apoptosis in iodine-treated BCPAP cells (1.0×10−6 mol/l). *P<0.05 and **P<0.01 vs. the control group. NC, negative control; SPANXA1, sperm protein associated with the nucleus, X-linked, family member A1; si, small interfering; OD, optical density.
Iodine promotes thyroid cancer development via SPANXA1 through the PI3K/AKT signalling pathway
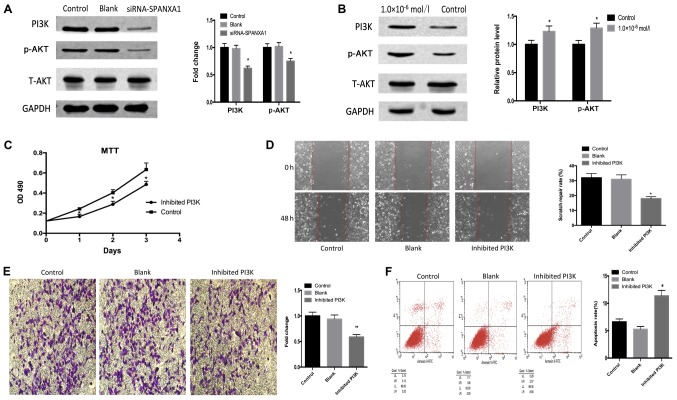
As presented in Fig. 4A, PI3K and p-AKT expression was downregulated following SPANXA1 knockdown. PI3K and p-AKT expression were detected in the control and iodine treatment (1.0×106 mol/l) groups. It was observed that PI3K and p-AKT expression levels were upregulated in the iodine treatment group compared with the control group (Fig. 4B). In addition, LY294002, a specific PI3K inhibitor, was added to the iodine-treated cells (1.0×10−6 mol/l; with high expression of SPANXA1). The effects of the inhibitor on the PI3K/AKT signalling pathway were confirmed by an MTT assay (Fig. 4C), scratch (Fig. 4D) and Transwell (Fig. 4E) assays, as well as by flow cytometry (Fig. 4F). Downregulation of the PI3K/AKT signalling pathway inhibited cell proliferation, migration, invasion and promoted cell apoptosis. Our results suggested that the inhibition of the PI3K/AKT signalling pathway reversed the SPANXA1-promoted development of BCPAP cells pre-treated with iodine.
Figure 4.
Effects of the PI3K/AKT signalling pathway. (A) Downregulation of SPANXA1 suppresses PI3K and p-AKT expression in iodine-treated BCPAP cells (1.0×10−6 mol/l). (B) PI3K and p-AKT protein expression were increased in BCPAP cells incubated with iodine at 1.0×10−6 mol/l. Applying PI3K inhibitor LY294002 to iodine-treated cells suppressed biological behaviours, including cell proliferation, invasion and migration, but promoted apoptosis, as demonstrated by (C) MTT, (D) wound healing and (E) Transwell assays, as well as (F) flow cytometry, respectively. Magnification, ×20. *P<0.05 and **P<0.01 vs. the control group. SPANXA1, sperm protein associated with the nucleus, X-linked, family member A1; p, phosphorylated; PI3K, phosphoinositide 3-kinase; t, total.
Discussion
Thyroid cancer is a common endocrine cancer and its etiological factors include radiation, the environment and genetics (1,10,13). An epidemiological study showed that high urinary iodine is a risk factor for thyroid tumorigenesis (28). Another study demonstrated that iodine regulates G2/M progression, induced by C-C motif ligand 21/C-C motif chemokine 7 interactions in primary cultures of thyroid cancer cells with RET/PTC expression (29). A study using rats revealed that low and high iodine diets reduced the expression of p14ARF and p16INK4a, and promoted thyroid cancer development (24). Therefore, the association between iodine and thyroid cancer has become a key area of interest within the field. However, the underlying mechanisms by which iodine affects thyroid cancer cells remain unclear.
The present study investigated how iodine affected the physiological features of thyroid cancer cells in vitro, including proliferation and apoptosis. The results indicated that iodine served a dual role in the proliferation and apoptosis of thyroid cancer cells. Compared with the control group, extra-high doses of iodine (1.0×10−3 mol/l) inhibited cell proliferation and promoted cell apoptosis, while extra-low doses of iodine (1.0×10−4−1.0×10−8 mol/l) exhibited opposing effects. In particular, proliferation in cells incubated with iodine at 1.0×10−6 mol/l increased significantly. The experimental outcomes suggested that extra-low doses of iodine may be unfavourable for patients with thyroid cancer. Therefore, iodine intake should be restricted, since extra iodine may expedite tumour progression.
Thyroid cancer cells cultured with iodine at 1.0×10−6 mol/l were selected for subsequent experiments, investigating prominent biological alternations induced by iodine. High throughput RNA-sequencing results indicated that the level of SPANXA1 was increased in thyroid cancer cells treated with this specific concentration of iodine. It should be noted that the effects of the SPANX gene family on tumour cells have been reported for various cancers, including melanoma (30), myeloma and haematological malignancies (31). High SPANX gene expression promotes the progression of various cancers under most circumstances. For instance, experimental evidence suggested that SPANX-A/C/D promotes breast cancer progression by regulating complementary cellular functions to induce its invasiveness (32). Furthermore, there may be a potential association between SPANX overexpression and prostate cancer development (33). To the best of our knowledge, less attention has been paid to the SPANXA1 gene in previous cancer research. In melanoma and glioblastoma cell lines, SPANXA1 is the most frequently expressed SPANX variant (34). Previous studies demonstrated that SPANXA1 expressed in thyroid tissue and thyroid cancer (35,36). Our findings regarding the connection between SPANXA1 and thyroid cancer complemented the exploration of thyroid cancer from a genic point of view.
To confirm the role of SPANXA1, its expression was downregulated in thyroid cancer cells through transfection. It was found that transfected thyroid cancer cells exhibited reduced cell proliferation, migration and invasion and increased thyroid cancer cell apoptosis. These findings indicated that SPANXA1 was one of the key genes, which enhanced the process of tumour growth in cells treated with an extra-low dose of iodine. High levels of SPANX in tumour cells have been detected in various cancers (32,33). The use of SPANX as a prognostic marker may be promising; however, little is known about the cellular behaviour during the regulation of SPANX expression. In this context, the tumour-suppressive role of downregulated SPANX was supported by transfection experiments. In conjunction with increased gene expression in thyroid cancer cells treated with iodine, these results may suggest the involvement of SPANXA1 in iodine-mediated thyroid cancer development, yet the potential effects of SPANXA1 on other tumour cells require further investigation.
After documenting the high prevalence of SPANXA1 in iodine-treated thyroid cancer, the signalling pathway by which SPANXA1 exerts its oncogenic action on thyroid cancer cells treated with extra-low doses of iodine was elucidated. PI3K/AKT, a classical signalling pathway, is involved in the regulation of various human cancers (37). Activating PI3K stimulates the phosphorylation of AKT and therefore influences the biological behaviour of tumours (38,39). It has been previously reported that the PI3K/AKT signalling pathway was connected to thyroid cancers (40,41). It was demonstrated that leptin is involved in thyroid cancer pathogenesis through the PI3K/AKT signalling pathway via the obesity receptor (42). Another study proposed that miR-34a regulates growth arrest specific 1 expression to promote proliferation and suppress apoptosis of thyroid cancer cells via the PI3K/AKT/BAD signalling pathway (43). A previous study established that p-53-inducible gene 3 plays an oncogenic role in thyroid cancer via the regulation of the PI3K/AKT/PTEN signalling pathway (44).
The PI3K/AKT signalling pathway is involved in the thyroid autoregulation induced by iodide (45), and therefore, it is proposed that the PI3K/AKT signalling pathway is highly likely to be involved in thyroid cancer development in iodine-rich environments. To confirm this, the association between SPANXA1 expression and protein targets of the PI3K/AKT signalling pathway were examined in thyroid cancer cells. PI3K and p-AKT exhibited increased expression in thyroid cancer cells treated with an extra-low dose of iodine. SPANXA1 gene silencing in these cells resulted in the downregulation of PI3K and p-AKT expression. These results revealed that the PI3K/AKT signalling pathway was involved in the progression of SPANXA1-mediated thyroid cancer cells, pre-treated with extra-low doses of iodine. Data analysis was further conducted to elucidate whether the PI3K/AKT signalling pathway is the key signalling pathway through which SPANXA1 affects thyroid cancer cells treated with extra-low doses of iodine. Inhibiting PI3K, without changing SPANXA1 expression, reduced cell proliferation, migration and invasion, and promoted apoptosis. This highlighted the importance of the PI3K signalling pathway in the development of iodine-treated thyroid cancer cells.
In summary, extra-low doses of iodine promoted the proliferation and inhibited the apoptosis of thyroid cancer cells, particularly at 1.0×10−6 mol/l. The molecular mechanisms behind this phenomenon were examined by a series of experiments using thyroid cancer cells treated with iodine at this specific concentration. The SPANXA1 gene was discovered to be responsible for the development of iodine-treated thyroid cancer cells. The high expression of SPANXA1 promoted cell proliferation and inhibited cell apoptosis. In addition, PI3K/AKT was proposed to be a key signalling pathway through which SPANXA1 mediates its effects. The results suggested that SPANXA1 may be a biomarker in thyroid cancer and may help in developing effective dietary plans for patients with thyroid cancer, in which patients should limit excessive iodine intake.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (grant no. 81673108 to HQ) and the Science and Technology Planning Project of Harbin, China (grant no. 2016RAXYJ088 to HQ).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HQ designed the present study. XY participated in study design and drafted the manuscript. JS and JH performed the literature research and statistical analysis. LS and HW performed the western blot experiments. DZ and QF analysed the data. JL performed the reverse transcription-quantitative PCR experiments.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 3.Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer ‘epidemic’-screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 4.Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, Lee DH, Lee KH, Community of Population-Based Regional Cancer Registries Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48:436–450. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 6.Solis OE, Mehta RI, Lai A, Mehta RI, Farchoukh LO, Green RM, Cheng JC, Natarajan S, Vinters HV, Cloughesy T, Yong WH. Rosette-forming glioneuronal tumor: A pineal region case with IDH1 and IDH2 mutation analyses and literature review of 43 cases. J Neurooncol. 2011;102:477–484. doi: 10.1007/s11060-010-0335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarkesh M, Zadeh-Vakili A, Akbarzadeh M, Fanaei SA, Hedayati M, Azizi F. The role of matrix metalloproteinase-9 as a prognostic biomarker in papillary thyroid cancer. BMC Cancer. 2018;18:1199. doi: 10.1186/s12885-018-5112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi C, Thi Thao Tran N, Van Ngu T, Park SW, Song MS, Kim SH, Bae YU, Ayudthaya PDN, Munir J, Kim E, et al. Promotion of tumor progression and cancer stemness by MUC15 in thyroid cancer via the GPCR/ERK and integrin-FAK signaling pathways. Oncogenesis. 2018;7:85. doi: 10.1038/s41389-018-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim M, Jeon MJ, Oh HS, Park S, Song DE, Sung TY, Kim TY, Chung KW, Kim WB, Shong YK, et al. Prognostic implication of N1b classification in the eighth edition of the tumor-node-metastasis staging system of differentiated thyroid cancer. Thyroid. 2018;28:496–503. doi: 10.1089/thy.2017.0473. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;63:4561–4567. [PubMed] [Google Scholar]
- 11.Kim S, Chung JK, Min HS, Kang JH, Park DJ, Jeong JM, Lee DS, Park SH, Cho BY, Lee S, Lee MC. Expression patterns of glucose transporter-1 gene and thyroid specific genes in human papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;48:91–97. doi: 10.1007/s13139-013-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitahara CM. New evidence on the association between prediagnostic thyroid-stimulating hormone levels and thyroid cancer risk. Cancer Epidemiol Biomarkers Prev. 2017;26:1163–1164. doi: 10.1158/1055-9965.EPI-17-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams D. Radiation carcinogenesis: Lessons from Chernobyl. Oncogene. 2008;27(Suppl 2):S9–S18. doi: 10.1038/onc.2009.349. [DOI] [PubMed] [Google Scholar]
- 14.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–2793. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS. Reply to: Papillary thyroid microcarcinoma in developing country scenario with endemic iodine deficiency. Surgery. 2017;162:191. doi: 10.1016/j.surg.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Zamrazil V, Cerovska J, Bílek R, Simecková A, Vrbíková J, Dvoráková M, Hníková O, Janecková M, Tomiska F. The effect of insufficient iodine intake on the size and function of the thyroid gland. Bratisl Lek Listy. 1995;96:609–612. (In Czech) [PubMed] [Google Scholar]
- 18.Fiore E, Latrofa F, Vitti P. Iodine, thyroid autoimmunity and cancer. Eur Thyroid J. 2015;4:26–35. doi: 10.1159/000371741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XH, Chen GG, Vlantis AC, van Hasselt CA. Iodine mediated mechanisms and thyroid carcinoma. Crit Rev Clin Lab Sci. 2009;46:302–318. doi: 10.3109/10408360903306384. [DOI] [PubMed] [Google Scholar]
- 20.Dijkstra B, Prichard RS, Lee A, Kelly LM, Smyth PP, Crotty T, McDermott EW, Hill AD, O'Higgins N. Changing patterns of thyroid carcinoma. Ir J Med Sci. 2007;176:87–90. doi: 10.1007/s11845-007-0041-y. [DOI] [PubMed] [Google Scholar]
- 21.Maier J, van Steeg H, van Oostrom C, Paschke R, Weiss RE, Krohn K. Iodine deficiency activates antioxidant genes and causes DNA damage in the thyroid gland of rats and mice. Biochim Biophys Acta. 2007;1773:990–999. doi: 10.1016/j.bbamcr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Gerard AC, Humblet K, Wilvers C, Poncin S, Derradji H, de Ville de Goyet C, Abou-el-Ardat K, Baatout S, Sonveaux P, Denef JF, Colin IM. Iodine-deficiency-induced long lasting angiogenic reaction in thyroid cancers occurs via a vascular endothelial growth factor-hypoxia inducible factor-1-dependent, but not a reactive oxygen species-dependent, pathway. Thyroid. 2012;22:699–708. doi: 10.1089/thy.2011.0387. [DOI] [PubMed] [Google Scholar]
- 23.Aschebrook-Kilfoy B, Grogan RH, Ward MH, Kaplan E, Devesa SS. Follicular thyroid cancer incidence patterns in the United States, 1980–2009. Thyroid. 2013;23:1015–1021. doi: 10.1089/thy.2012.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun R, Wang J, Li X, Li L, Yang J, Ren Y, Xi Y, Sun C. Effect of iodine intake on p14ARF and p16INK4a expression in thyroid papillary carcinoma in rats. Med Sci Monit. 2015;21:2288–2293. doi: 10.12659/MSM.893486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, Zhang Y, Shan Z, Teng W, Xing M. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612–1617. doi: 10.1210/jc.2008-2390. [DOI] [PubMed] [Google Scholar]
- 26.Saiselet M, Floor S, Tarabichi M, Dom G, Hébrant A, van Staveren WC, Maenhaut C. Thyroid cancer cell lines: An overview. Front Endocrinol (Lausanne) 2012;3:133. doi: 10.3389/fendo.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao X, Ma W, Liu L, Xu J, Wang H, Li X, Wang J, Zhang J, Wang Z, Gu Y. Analysis of potassium iodate reduction in tissue homogenates using high performance liquid chromatography-inductively coupled plasma-mass spectrometry. J Trace Elem Med Biol. 2015;32:1–6. doi: 10.1016/j.jtemb.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Wang Y, Wang L, Wang X, Sun C, Xing M, Zhao W. Strong association of high urinary iodine with thyroid nodule and papillary thyroid cancer. Tumour Biol. 2014;35:11375–11379. doi: 10.1007/s13277-014-2397-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YY, Liu ZB, Ye XG, Ren WM. Iodine regulates G2/M progression induced by CCL21/CCR7 interaction in primary cultures of papillary thyroid cancer cells with RET/PTC expression. Mol Med Rep. 2016;14:3941–3946. doi: 10.3892/mmr.2016.5686. [DOI] [PubMed] [Google Scholar]
- 30.Salemi M, Calogero AE, Vicari E, Migliore E, Zaccarello G, Cosentino A, Amore M, Tricoli D, Castiglione R, Bosco P, Rappazzo G. A high percentage of skin melanoma cells expresses SPANX proteins. Am J Dermatopathol. 2009;31:182–186. doi: 10.1097/DAD.0b013e3181978d6f. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Zhang Y, Liu H, Salati E, Chiriva-Internati M, Lim SH. Gene expression and immunologic consequence of SPAN-Xb in myeloma and other hematologic malignancies. Blood. 2003;101:955–960. doi: 10.1182/blood-2002-06-1930. [DOI] [PubMed] [Google Scholar]
- 32.Maine EA, Westcott JM, Prechtl AM, Dang TT, Whitehurst AW, Pearson GW. The cancer-testis antigens SPANX-A/C/D and CTAG2 promote breast cancer invasion. Oncotarget. 2016;7:14708–14726. doi: 10.18632/oncotarget.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salemi M, Calogero AE, Zaccarello G, Castiglione R, Cosentino A, Campagna C, Vicari E, Rappazzo G. Expression of SPANX proteins in normal prostatic tissue and in prostate cancer. Eur J Histochem. 2010;54:e41. doi: 10.4081/ejh.2010.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zendman AJ, Zschocke J, van Kraats AA, de Wit NJ, Kurpisz M, Weidle UH, Ruiter DJ, Weiss EH, van Muijen GN. The human SPANX multigene family: Genomic organization, alignment and expression in male germ cells and tumor cell lines. Gene. 2003;309:125–133. doi: 10.1016/S0378-1119(03)00497-9. [DOI] [PubMed] [Google Scholar]
- 35.Yu K, Ganesan K, Tan LK, Laban M, Wu J, Zhao XD, Li H, Leung CH, Zhu Y, Wei CL, et al. A precisely regulated gene expression cassette potently modulates metastasis and survival in multiple solid cancers. PLoS Genet. 2008;4:e1000129. doi: 10.1371/journal.pgen.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics. 2006;7:67–80. doi: 10.1007/s10048-006-0032-6. [DOI] [PubMed] [Google Scholar]
- 37.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: Implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 39.Wen D, Deng L, Zhou M, Guo S, Shang L, Xu G, Dong S. A biofuel cell with a single-walled carbon nanohorn-based bioanode operating at physiological condition. Biosens Bioelectron. 2010;25:1544–1547. doi: 10.1016/j.bios.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, Liu D, Trink E, Bojdani E, Ning G, Xing M. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab. 2011;96:E577–E585. doi: 10.1210/jc.2010-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uddin S, Bavi P, Siraj AK, Ahmed M, Al-Rasheed M, Hussain AR, Ahmed M, Amin T, Alzahrani A, Al-Dayel F, et al. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocr Relat Cancer. 2010;17:191–202. doi: 10.1677/ERC-09-0153. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Qin H, Cui Y. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem Biophys Res Commun. 2013;441:958–963. doi: 10.1016/j.bbrc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Cai J, Jin X, Yang J, Shen Q, Ding X, Liang Y. PIG3 plays an oncogenic role in papillary thyroid cancer by activating the PI3K/AKT/PTEN pathway. Oncol Rep. 2015;34:1424–1430. doi: 10.3892/or.2015.4096. [DOI] [PubMed] [Google Scholar]
- 45.Serrano-Nascimento C, da Silva Teixeira S, Nicola JP, Nachbar RT, Masini-Repiso AM, Nunes MT. The acute inhibitory effect of iodide excess on sodium/iodide symporter expression and activity involves the PI3K/AKT signalling pathway. Endocrinology. 2014;155:1145–1156. doi: 10.1210/en.2013-1665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.