Short abstract
Inflammatory processes are increasingly being identified at the core of many different disease states (e.g. heart disease, cancer, diabetes). As such, anti-inflammatory strategies available through drug delivery have undergone renewed interest. Due to the systemic side effects of steroidal drugs, non-steroidal anti-inflammatory drugs are often preferred for long-term treatment of inflammation in a variety of applications. While non-steroidal anti-inflammatory drugs are generally safe, there are some serious side effects that can be associated with their usage, particularly when given systemically or orally. Due to the high number of patients taking non-steroidal anti-inflammatory drugs, the reduction or elimination of these side effects, such as is possible through local drug delivery, could have a very powerful effect on patient quality of life. This review comments on a sampling of existing methods for localized or targeted delivery of non-steroidal anti-inflammatory drugs, with the goal of helping future research groups to focus on bettering methods shown to be effective and filling the gaps of knowledge in this field. Additionally, commentary is made on the field as a whole, and the standardization issues that arise from its expansiveness and diversity.
Impact statement
This work provides an overview of research currently being done exploring potential drug delivery device strategies for NSAIDs as an alternative to systemic delivery. Commentary on this field is made in an attempt to aid future experimental design, enabling researchers to determine the drugs and delivery vehicles which are most advantageous for them to pursue, as well as suggestions to standardize the reporting of such future research.
Keywords: Biomedical, drug delivery, inflammation, local delivery, non-steroidal anti-inflammatory drugs, targeted delivery
Introduction
There are a variety of treatments for inflammation, but by far the most common is the administration of non-steroidal anti-inflammatory drugs (NSAIDs). Each day, about 30 million people take NSAIDs around the world.1 They are used for the treatment of both acute and chronic inflammation, and are highly effective in the majority of cases. Over 40 different NSAIDs have been discovered, and they are often organized into multiple different classes based on structure and anticipated risk. The majority of NSAIDs are completely absorbed, have negligible first-pass hepatic metabolism, are tightly bound to serum proteins, and have small volumes of distribution. NSAIDs vary in their elimination half-lives, routes of administration, and tolerability profiles. Even though they are variable as a complete group, NSAIDs within a class tend to have similar characteristics. Some basic information on the NSAIDs mentioned in this review can be seen in Table 1. Diclofenac and ibuprofen are the most widely used NSAIDs in the world. They are followed by naproxen, indomethacin, piroxicam, and ketoprofen. Ibuprofen, naproxen, and aspirin are available over-the-counter, and are used to treat anything from headaches to post-surgical pain. However, the majority of the NSAIDs used across the globe are prescribed in primary healthcare.1
Table 1.
Commonly used NSAIDs and their properties.
| Diclofenac | Ibuprofen | Naproxen | Ketoprofen | Meloxicam | Celecoxib | Piroxicam | Nabumetone | Aceclofenac | Sodium Salicylate | Indomethacin | |
|---|---|---|---|---|---|---|---|---|---|---|---|
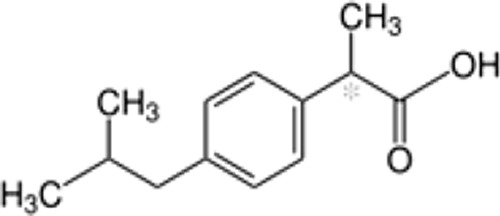
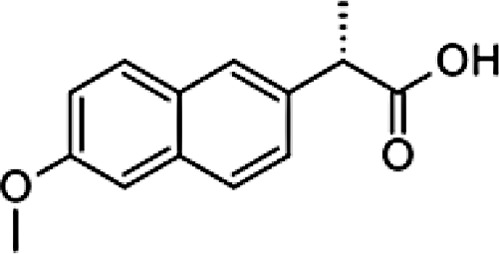
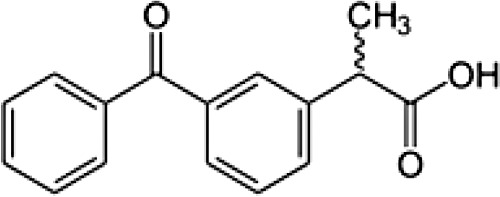
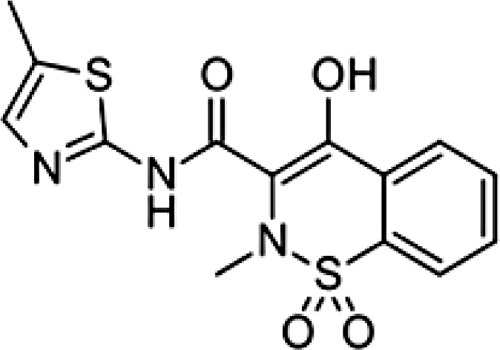
| Chemical formula | C14H11Cl2NO2 | C13H18O2 | C14H14O3 | C16H14O3 | C14H13N3O4S2 | C17H14F3N3O2S | C15H13N3O4S | C15H16O2 | C16H13Cl2NO4 | C7H5NaO3 | C19H16ClNO4 |
| Chemical structure |
|
|
|
|
|
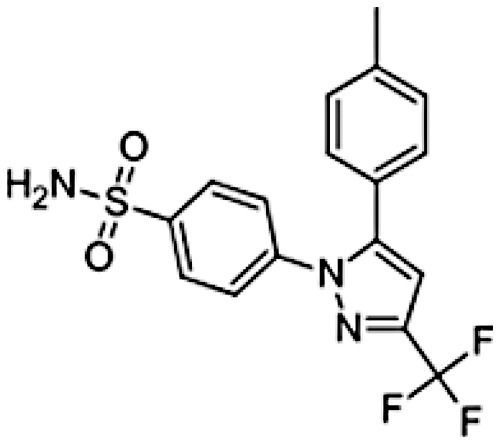
|
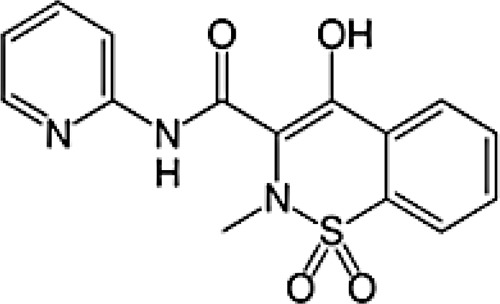
|
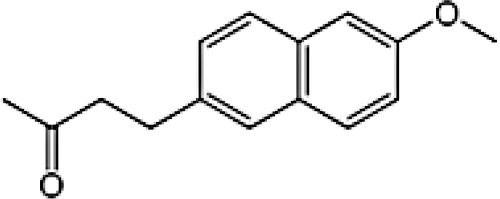
|
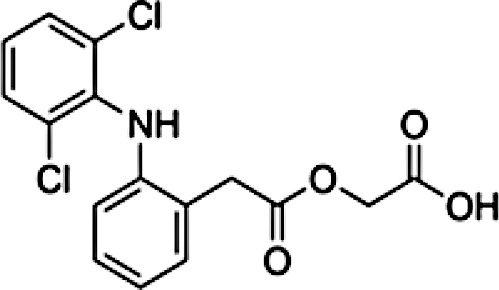
|
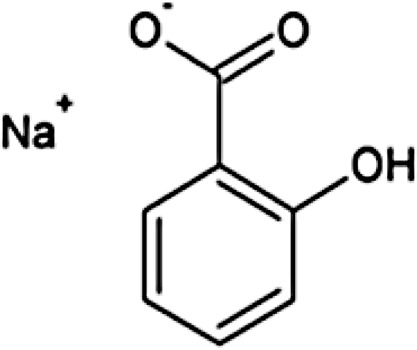
|
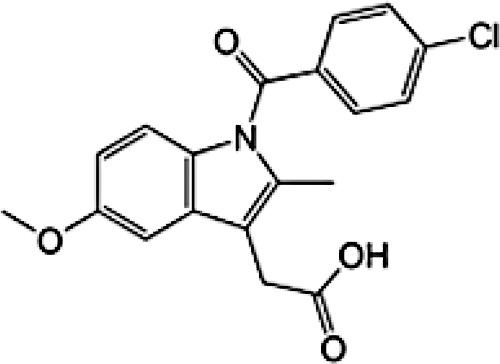
|
| Molecular weight | 296.147 g/mol | 206.285 g/mol | 230.263 g/mol | 254.285 g/mol | 351.395 g/mol | 381.373 g/mol | 331.346 g/mol | 228.291 g/mol | 354.183 g/mol | 160.104 g/mol | 357.79 g/mol |
| Water solubility (at 25°C) | 2.37 mg/L | 21 mg/L | 15.9 mg/L | 51 mg/L | 7.15 mg/L | 3.3 mg/L | 23 mg/L | Insoluble | Insoluble | 0.937 mg/L | |
| Selectivity | COX1 and COX2 | COX1 and COX2 | COX1 and COX2 | COX1 and COX2 | COX1 and COX2 greater affinity for COX-2 | Selective COX2 | COX1 and COX2 | COX1 and COX2 | COX1 and COX2 | COX1 and COX2 | COX1 and COX2 |
| Biological half-life | ∼2 h | ∼2 h | ∼15 h | ∼4 h | 15–20 h | ∼11 h | 30–86 h | ∼23 h | ∼4 h | ∼4.5 h | |
| Protein binding | >99% | >99% | >99% | >99% | >99% | 97% | >99% | >99% | >99% | ||
| Volume of distribution | 1.3 L/kg | 0.3 L/kg | 0.16 L/kg | 10 L | 429 L | 0.14 L/kg | 25 L | 0.34-1.57 L/kg |
NSAID: non-steroidal anti-inflammatory drug; COX: cyclooxygenase.
When tissue experiences injury, due to things like infection or trauma, the body responds by initiating inflammation. In most cases, this inflammation is a good thing, and causes eventual resolution of injury, including clearance of the injurious stimuli and replacement of normal function. Neutrophils are recruited immediately, followed by macrophages and monocytes in the coming days, and finally lymphocytes and plasma cells for tissue remodeling. However, there are certain circumstances when acute inflammation transforms into chronic inflammation, or when even acute inflammation is undesirable (often due to severe pain and loss of function). In these cases, NSAIDs can be used to reduce or halt the inflammatory process, to increase quality of life and/or wound healing.
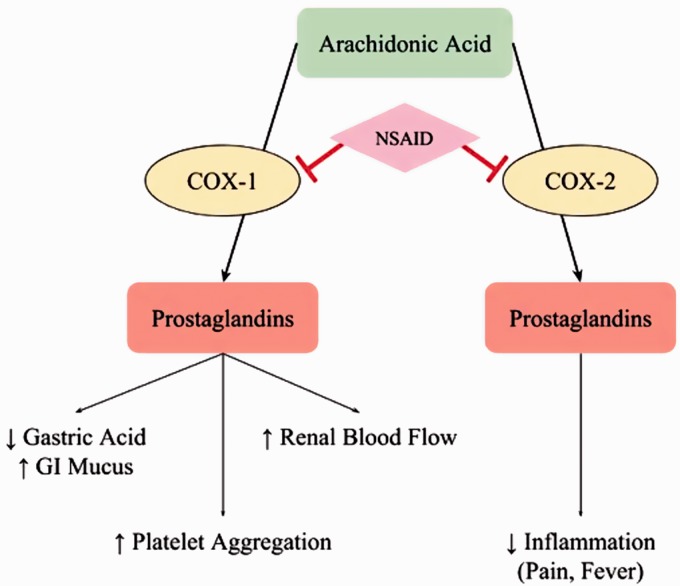
Regardless of their class, all NSAIDs have the same method of action: reduction of prostaglandin levels. Prostaglandins promote inflammation by regulating vasodilation and platelet aggregation, and are found in most every tissue in the human body, as they can be produced by any nucleated cell. There are multiple types of prostaglandins, and their production is catalyzed by two forms of the cyclooxygenase (COX) enzyme: COX-1 and COX-2. NSAIDs act as reversible inhibitors of the COX enzyme, binding to a polar arginine molecule, found in both forms of COX, and inhibiting enzymatic function through steric hindrance.2 This inhibition reduces the level of prostaglandins produced, thereby reducing pain and fever. This process can be seen in Figure 1. Prostaglandins produced by the COX-1 enzyme, which is responsible for baseline prostaglandin levels, have functions to support blood clotting and protect the lining of the stomach from the highly acidic gastric environment. The blocking of COX-1 enzymes, and the loss of these desirable regulatory functions, causes the unsavory side-effects associated with NSAID usage: stomach ulcers and excessive bleeding.1
Figure 1.
Mechanism of action of NSAIDs – the non-selective inhibition of cyclooxygenase (COX) enzymes causes reduction in prostaglandin production, causing both anti-inflammatory therapy and undesirable side-effects. (A color version of this figure is available in the online journal.)
NSAIDs that selectively target only COX-2 have been developed. The active site of COX-2 is slightly larger than that of COX-1, allowing selectivity to be achieved through the use of drugs which are too bulky to access the polar arginine in COX-1.2 However, celecoxib is the only selective drug available in the United States. Previously developed COX-2 selective drugs, such as rofecoxib and valdecoxib, were ultimately withdrawn from public use due to the increased risk of heart attack and stroke which developed with chronic (systemic) use. Therefore, the vast majority of patients receive non-selective NSAIDs, and take them orally. When administered systemically in this way, these drugs can commonly cause a variety of side effects such as nausea, vomiting, abdominal pain, heartburn, dizziness, and headache. The more serious side effects associated with NSAIDs are heart attack, stroke, stomach ulcers, and stomach bleeding. These side effects occur due to the baseline levels of prostaglandin being lowered throughout the entire body, in both tissues that are exhibiting inflammation and those which are not. While the latter, more life-threatening, side effects are far less common, they are of significant concern to patients who already have heart disease or are otherwise in poor health.
Due to these side-effects, there has been a recent movement away from the systemic administration of NSAIDs. Other anti-inflammatory drugs, such as naturally-derived small molecules, are being explored for widespread usage. These alternate therapies have multiple mechanisms through which they mitigate inflammation, and come with their own side-effects. Therefore, it is more advantageous to focus on the improvement of the current NSAID treatments that are available, as they are generally very effective. Localized and targeted delivery are attractive alternate forms of NSAID administration due to their ability to eliminate off-target effects. Drug that is delivered locally reaches therapeutic concentrations only where it is injected or implanted. This eliminates any side effects that are seen in a specific site alternate to that where undesired inflammation is occurring. In the case of NSAIDs, this would include the effects on gastric acid and GI mucus in the stomach, when the delivery vehicle resides elsewhere. Targeted delivery circulates throughout the entire body, but is protected from exhibiting therapeutic effects in locations that are not desired, such as the stomach, resulting in the same off-target protective effects.
If NSAIDs could be delivered in a smarter fashion, either through targeted or localized delivery, their related systemic side effects would be drastically reduced, if not eliminated entirely. There are multiple research groups which have done work to achieve this goal, and this review will outline only a sampling of them, with the intention of quantifying the methods which are commonly done and the ones which stand out as successful. With this information, it is the authors' hope that research groups will tailor their future experiments to fill the gaps of knowledge in this field.
Common NSAIDs in anti-inflammatory treatment
While over-the-counter NSAIDs are commonly known to the general public, it is prescription-grade NSAIDs which are more often used in the United States.3 Due to the widespread usage of prescription-grade NSAIDs such as diclofenac, and the ease of accessibility of over-the-counter drugs such as ibuprofen, extensive research has been done on both drug types. Here we explore 11 commonly used NSAIDs, which are shown in Table 1.
It does not appear that there are specific NSAIDs which necessarily lend themselves to one form of delivery more than another. These 11 NSAIDs have similar molecular weights: between 160 and 390 g/mol. They are all slightly soluble or insoluble in water, and are simple hydrocarbons (composed primarily of carbon, hydrogen, and oxygen). As mentioned previously, celecoxib is the only COX-2 selective drug in this list. Their differences are in distribution and half-life in the body. Distribution can be understood by looking at each drug’s volume of distribution, which represents the theoretical volume that would be necessary to contain the total amount of an administered drug at the same concentration that it is seen in the blood plasma. Low volumes, like those of ibuprofen, naproxen, and piroxicam, indicate that drug is primarily confined to the plasma. High volumes, like celecoxib, indicate much higher distribution and accumulation in body tissue. The more common diclofenac and ibuprofen have limited half-lives, at around 2 h. Ketoprofen, aceclofenac, and indomethacin persist slightly longer – from 4 to 5 h – while naproxen, meloxicam, celecoxib, piroxicam, and nabumetone have longer half-lives, and are typically taken only once per day to maintain therapeutic levels.
When administered orally (systemically), half-lives determine the time between dosages required to achieve and maintain therapeutic concentrations. When these drugs are translated into implanted or otherwise constant drug delivery systems, half-lives determine the release rate required to achieve these same concentrations. The high usage of diclofenac and ibuprofen suggests that a shorter half-life is not a major concern therapeutically. For drug delivery systems which have rapid release profiles, this makes sense, as it allows for quick introduction and clearance of drug. However, as the need for chronic therapy increases, it may be advantageous to more strongly consider NSAIDs which will persist longer. These drugs would require a smaller continuous release dosage, due to increased persistence in the body. By reducing the amount of drug which must be released over a given time, delivery time frame can be prolonged without increasing the original loading amount required. Generally, it seems that NSAIDs should be chosen on a case by case basis. While diclofenac and ibuprofen are very common, they might not always be the best NSAID for the job, due to any one of their pharmacological properties.
Delivery vehicles
Research has been done on the release of NSAIDs from a wide variety of different delivery vehicles. For this review, these have been broken down into six categories. The categories cover a wide range of inflammation causes and diseases, from biomedical implants to osteoarthritis and wound healing. Each vehicle comes with its own advantages and disadvantages, and lends itself to certain medical treatments.
Systemic targeting and/or encapsulation
Systemic delivery is the administration of drug into the circulatory system, so that the entire body is affected. This is the approach which is currently used for most NSAID treatments. This traditional administration via the circulatory system can be improved upon through the introduction of targeting moieties or encapsulation of the drug, in an attempt to only allow drug affects to take place in specific locations, to reduce effects in areas of the body which are off-target. The major advantage of targeting and encapsulation is an added barrier between drug and the gastric mucosa.19 This barrier serves a dual purpose. It protects the drug from degradation in the stomach, and from the full effects of first pass metabolism, increasing its bioavailability. It also protects the stomach from the drug, reducing the effects that it exerts at that site. In the case of NSAIDs, reduced effects at the stomach are especially advantageous as this helps to reduce the stomach ulcers and bleeding which can be caused by NSAID use (Table 2).4–25
Table 2.
| Citation | Vehicle type | Material | NSAID | Delivery time frame | Treating | |
|---|---|---|---|---|---|---|
| Systemic targeting/encapsulation | 4 | Hydrogel | Carrageenan-PAA | Diclofenac | >24 h | |
| Systemic targeting/encapsulation | 5 | Hydrogel | Chitosan | Diclofenac | 1 day | Gastrointestinal delivery |
| Systemic targeting/encapsulation | 6 | Hydrogel | Chitosan/PVA | Diclofenac | 1 day | |
| Systemic targeting/encapsulation | 7 | Aerogel | Pectin-zinc | Diclofenac | 7 h | |
| Systemic targeting/encapsulation | 8 | Nanoparticles | PLGA/chitosan | Diclofenac | 7–9 days | |
| Systemic targeting/encapsulation | 9 | Beads (1 mm) | PVA-g-PAAm and sodium alginate | Diclofenac | 6 h 40 min | Colon-specific |
| Systemic targeting/encapsulation | 10 | Microspheres | PVA/PAA | Diclofenac | ∼h | Deliver to intestine |
| Systemic targeting/encapsulation | 11 | Beads | TSP-alginate | Diclofenac | ||
| Systemic targeting/encapsulation | 12 | Microcapsules, microparticles | Alginate-PLL, PLGA | Ibuprofen | 14 days | |
| Systemic targeting/encapsulation | 13 | Nanoparticles | Chitosan/TiO2 | Ibuprofen | 24–54 h | |
| Systemic targeting/encapsulation | 14 | PDCs – micellar nanostructure | mPEG-PPF | Ibuprofen | 8 days 8 h | Arthritis and cancer |
| Systemic targeting/encapsulation | 15 | Nanoparticles | Solid lipid | Ibuprofen, Ketoprofen, Nabumetone | 6 days | |
| Systemic targeting/encapsulation | 16 | Microparticles | Ethylcellulose | Ketoprofen | 1 day | |
| Systemic targeting/encapsulation | 17 | Electrospun nanofibermat/films | PVA | Ketoprofen | 14 days | |
| Systemic targeting/encapsulation | 18 | Prodrug | Varying saccharide chains (glucose, mannose, galactose, lactose) | Ketoprofen | >10 days | |
| Systemic targeting/encapsulation | 19 | Nanoparticle hydrogel | Poly(mPEGMA-co-MAA) | Meloxicam | >72 h | Rheumatoid arthritis, osteoarthritis |
| Systemic targeting/encapsulation | 20 | Nanosponges | β-cyclodextrin | Meloxicam | >24 h | |
| Systemic targeting/encapsulation | 21 | Lipid-core nano capsules | Meloxicam | Not reported | ||
| Systemic targeting/encapsulation | 22 | Microspheres | Hydroxypropyl cyclosophoraose-pullulan | Naproxen | 3 days | |
| Systemic targeting/encapsulation | 23 | Micelles | mPEG–PCL copolymer | Naproxen | 4 days 4 h | |
| Systemic targeting/encapsulation | 24 | Nanotubes | Silica | Naproxen | 50 m | Chronic inflammation |
| Systemic targeting/encapsulation | 25 | Sol-gel | Zirconium(IV) propoxide/tetraethyl orthosilicate and chitosan (TECN and MC@Z) | Naproxen | >24 h |
NSAID: non-steroidal anti-inflammatory drug.
Even with these barriers, NSAIDs will travel throughout the body, and can affect areas which are not currently experiencing inflammation. While encapsulation and targeting can reduce these off-target effects, delivery vehicles of this type have yet to eliminate the side-effects associated with systemic administration. The methods listed in this review are all improvements on the current standard, and the majority are in the very early stages of development, with primarily characterization experiments and little in vivo experimentation. This category is included for completeness of the field.
Local injection
Delivery vehicles which can release drug in a localized area are advantageous because they eliminate off-target effects entirely, and require smaller dosages to achieve the required local therapeutic concentration. Local delivery vehicles do not need to travel through the circulatory system, and are instead introduced to just the area experiencing inflammation. Local delivery vectors are doubly advantageous when they do not require any excess surgery to implant. In cases where the vehicle is small enough and has a low enough viscosity to pass through a needle, the introduction of this delivery system to the body is facile (Table 3).26–30 Typical systems which have these desired properties are nanoparticles or conjugate systems.26,29,30
Table 3.
| Citation | Vehicle type | Material | NSAID | Delivery time frame | Treating | |
|---|---|---|---|---|---|---|
| Local injections | 26 | Conjugate | PEG | Diclofenac | >100 days | Osteoarthritis |
| Local injections | 27 | Prodrug | Diclofenac | 2 days 7 h | Osteoarthritis, injury | |
| Local injections | 28 | Gel | Poloxamer | Ibuprofen | 2 h 11 min | Epidural injection |
| Local injections | 29 | Viscous polymer | PLG | Ketoprofen | 33 days | |
| Local injections | 30 | Nanoparticles | Alginate/chitosan/ pluronic | Meloxicam | Osteoarthritis |
NSAID: non-steroidal anti-inflammatory drug.
While long-term delivery is not required for this type of delivery, it is advantageous as osteoarthritis is the main research focus for this vehicle type.26,27,30 Injections into affected joints, such as knees, can be done as required, but fewer injections are preferred for higher patient compliance. In this category, there are two papers which show impressively long release profiles and long-term therapeutic efficacy. A PEG diclofenac conjugate produced by Sulistio et al. shows drug release for over 100 days in preliminary in vitro testing, and a viscous polymer loaded with ketoprofen shows release for about three months in similar in vitro conditions.26,29 In these cases, patients need injections only four to five times a year to experience significant pain relief and increased quality of life.
Localized delivery
Localized delivery is one of the largest categories explored in this review due to its loose definition and the high number of different vehicle types that can be applied. Local injection is technically a subcategory of this field, and some vehicles listed here may have the potential for injection with lower gauge needles.32,37–40,45 However, in this review, only vehicles which are specifically formulated for injection were removed from the greater localized delivery category. Hydrogels,31,39 microparticles,32,40 microspheres,37,45 films/membranes,34–36,42,43 and fibers41,44,46 are all vehicle types that can be used to deliver drugs locally (Table 4).31–46 These vehicle types require surgery to implant if too large to inject, unless they are used in periodontal applications, where the mucosa allows for drug penetration at the surface.33,43,45,46 PLGA and chitosan seem to be common materials for this type of delivery, likely due to their ability to biodegrade, eliminating the requirement to remove the vehicle once drug delivery has concluded. There does not seem to be a clear preference to deliver certain NSAIDs over others, and many combinations of NSAID and material/vehicle types have been researched.
Table 4.
| Citation | Vehicle type | Material | NSAID | Delivery time frame | Treating | |
|---|---|---|---|---|---|---|
| Localized delivery | 31 | Hydrogel | PCLA-PEG-PCLA | Celecoxib | 100 days (in vitro), 4–8 weeks (in vivo) | Osteoarthritis |
| Localized delivery | 32 | Microparticles | PLGA | Celecoxib | 60 days | Diabetes in eye |
| Localized delivery | 33 | Nanostructure membrane | poly(N-methacryloyl glycine)/Bacterial nanocellulose | Diclofenac | 4 h | Dermal and oral delivery |
| Localized delivery | 34 | Film | PP | Diclofenac, Ibuprofen | ∼h, 1 day | Graft modification |
| Localized delivery | 35 | Film | PLGA | Ibuprofen | 10 days | |
| Localized delivery | 36 | Fibrous membrane | PLGA | Ibuprofen | 70 days | Tissue anti-adhesion barrier |
| Localized delivery | 37 | Microspheres | PLGA/PVA/Gelatin | Ibuprofen | 63 days | Osteoarthritis |
| Localized delivery | 38 | Microtubes | Polycaprolactone (PCL) | Ibuprofen | 30 days | Peripheral nerve regeneration (nerve guidance conduits) |
| Localized delivery | 39 | Hydrogel | Anionic nanofibrillar cellulose | Ketoprofen | > 72 h | |
| Localized delivery | 40 | Microparticles | PHB/chitosan | Ketoprofen | 2.5 days | |
| Localized delivery | 41 | Electrospun nanofibers | PLA | Ketoprofen | 12.5 days | |
| Localized delivery | 42 | Membrane | Polyurethane matrix | Ketoprofen | 2 h | |
| Localized delivery | 43 | Electrospun fibers/films | Chitosan/PVA/HA | Meloxicam | 1 day | Periodontal disease |
| Localized delivery | 44 | Electrospun nanofibers | PCL | Naproxen | ||
| Localized delivery | 45 | Microspheres | Polyorganophosphazene | Naproxen | 33 days 8 h | Periodontal disease |
| Localized Delivery | 46 | Electrospun fiber mat | Chitosan/HA/PVA | Piroxicam | 5 h | Periodontal disease |
NSAID: non-steroidal anti-inflammatory drug.
Periodontal disease, inflammation of tissue surrounding the teeth, is a good candidate for localized delivery due to the accessibility of the area. Drug-loaded fibers or mats can be placed with precision into the mouth, easily raising the drug concentration at the site of disease.43,45,46 Other specific instances where localized NSAID delivery is desirable are the treatment of diabetic retinopathy in the eye32 and tissue anti-adhesion barriers used in many surgical procedures.36 In these cases, inflammation is highly localized at a site, allowing local delivery to ameliorate all symptoms without affecting off-target areas.
Implant coating and/or incorporation
Inflammation is the body’s natural tissue response to injury and infection, but biomedical implants are a very common perpetuator of undesirable inflammation. When inflammation affects the body long-term at implant sites, it can cause fever and pain, or even necessitate an implant removal. Since adding a drug delivery component to such an implant may be an easy step, such implants have been one of the primary areas developing new delivery strategies (Table 5).47–50 This category contains any localized delivery vehicles that interact with the implant itself, such as coatings48 or alterations to the implant material.47,49,50
Table 5.
| Citation | Vehicle type | Material | NSAID | Delivery time frame | Treating | |
|---|---|---|---|---|---|---|
| Implant coating/incorporation | 47 | Bone tissue engineering scaffold | PLGA/PEG | Diclofenac | 60 days | Bone fracture and defects |
| Implant coating/incorporation | 48 | Aerogel coating | Pectin-Xanthin | Diclofenac, indomethacin | 1 day | Orthopedic implants |
| Implant coating/incorporation | 49 | Nanotubes | PLGA/TiO2 | Ibuprofen | 7 days | Titanium implants |
| Implant coating/incorporation | 50 | Aerogel microspheres | Starch into PCL | Ketoprofen | 3 days | Bone repair |
NSAID: non-steroidal anti-inflammatory drug.
In this application, it is also advantageous to have release profiles on a very long-time scale, as most biomaterial implants are meant to stay in the body long term, and there is usually no opportunity for addition of new or additional drug. While inflammation reduction on a shorter time scale is helpful for things such as wound healing, a chronic time scale must be achieved in order to keep the body from consistently reacting to the implant. Additionally, inflammation may interfere with bone healing and regeneration, a long-term process which is important for common implants such as total hip or knee replacements.
In Table 2, there are four sub-categories listed in the implant category, each with differing NSAIDs, vehicle types, and material. A good example for this category is the successful creation of a PLGA/PEG bone tissue scaffold by Sidney et al.47 which releases diclofenac for a period of greater than eight weeks. This scaffold, which was created to help heal bone fractures and defects, meets both acute and chronic needs. An initial “burst” release of drug helps to reduce any pain that comes with the introduction of the implant, while a smaller dosage long term helps to reduce inflammation at the site of implantation, allowing bone tissue to grow into the scaffold. This is shown via an in vitro osteoblast inflammation model. Experiments in this category which are less successful are those with only short-term release, such as the aerogel coating produced by Horvat et al.48 This coating was developed for total hip replacements, and once again diclofenac was chosen as a delivered drug. However, drug release from this coating lasted only about 24 h in vitro, covering only short-term inflammation, even though hip replacements are meant to last around 20 years.
The papers explored in this review do not seem to indicate a clear path forward in this category in terms of delivery vehicle or material. However, the success of long term delivery indicates that this is a category with promise, that should continue to be a focus for future research, perhaps with NSAIDs that exhibit a longer half-life than diclofenac.
Wound dressings and sutures
Sutures exist to help the body heal after injury, and are a good candidate for localized drug delivery because, like many local delivery vehicles, they already exist at the injury site (Table 6).51–59 The reduction of inflammation at the site of injury is dually advantageous. NSAIDs can help to suppress both pain and infection, which helps with patient quality of life and wound healing.59 During wound healing, the inflammatory phase lasts on the scale of hours to weeks. Therefore, effects on inflammation should last for the same amount of time, in order to be most successful. Delivery vehicles which exhibit only burst release are not useful in this application. Keeping with this understanding, the shortest release curve reported in this sampling of research is a little over 2 days,57 while the longest is up to 70 days.52
Table 6.
| Citation | Vehicle type | Material | NSAID | Delivery time frame | Treating | |
|---|---|---|---|---|---|---|
| Wound dressing/sutures | 51 | Electrospun sheath | PLGA | Aceclofenac | 7–10 days | Suture |
| Wound dressing/sutures | 52 | Melt-spun fibers | PCL and HT | Diclofenac | 50–70 days | Suture |
| Wound dressing/sutures | 53 | Fiber | PLGA | Diclofenac | 7 days | Suture, pain |
| Wound dressing/sutures | 54 | Microgel films | PAH-Dextran, HA | Ibuprofen | 10 days 10 h | Suture, healing |
| Wound dressing/sutures | 55 | Coating | PLA/PCA/PTMC 10/60/30 | Ibuprofen | 20 days | Suture, healing |
| Wound dressing/sutures | 56 | Sheet | PLGA | Ibuprofen | 6 days | Suture, pain |
| Wound dressing/sutures | 57 | Fibrous membrane | Chitosan-poly(ε-caprolactone) | Ketoprofen | 2 days 2 h | Wound healing |
| Wound dressing/sutures | 58 | Membrane | Polycaprolactone (PCL) membranes with tetraethylorthosilicate (TEOS)–chitosan sol–gel | Ketoprofen | 14 days | Wound healing |
| Wound dressing/sutures | 59 | Electrospun nanofibers | Cellulose acetate (CA) | Naproxen | 12 days | Wound healing |
NSAID: non-steroidal anti-inflammatory drug.
Delivery vehicles in this category are either integrated with or coating existing sutures,51,54–58 or novel materials for sutures.52,53,59 Due to the mechanical properties required of surgical sutures, both fabrication types must consider mechanical effects. In order to match the current standard, they must meet the mechanical requirements that come with their usage, and are also preferred to be biodegradable. Because of this, biodegradable polymers such as PLGA and PCL are commonly used.51–53,56,58 The advantages of coating existing sutures include higher baseline mechanical strength, and overall simplicity of fabrication. However, de-lamination and inconsistent coating are potential downsides which do not occur when novel entire sutures are created. Both approaches have merit, and both seem to accommodate all NSAIDs, making them deserving of further research.
Topical and transdermal delivery
The administration of drug through the skin is very simple in concept, and very difficult in reality. There are very few drugs which naturally lend themselves to this delivery method, as molecular weight, hydrophilicity, half-life, and dosage are all factors which can make or break a successful topical delivery.64 However, topical delivery is an attractive option for its ease of use and high patient compliance (Table 7).60–70 Additionally, once drug manages to get past the stratum corneum, the outer barrier of the skin which impedes delivery, it can reach high concentrations locally. This is an attractive goal for both wound healing61 and the treatment of osteoarthritis.63,65
Table 7.
| Citation | Vehicle type | Material | NSAID | Delivery time frame | Treating | |
|---|---|---|---|---|---|---|
| Topical and transdermal delivery | 60 | Film | Polyox and Carrageenan | Diclofenac | ||
| Topical and transdermal delivery | 61 | Membrane | PVA/Chitosan | Ibuprofen | 3 days | Wound healing |
| Topical and transdermal delivery | 62 | Hydrogel | Xanthan | Ibuprofen | >12 h | |
| Topical and transdermal delivery | 63 | Transdermal patch | Drug-in-adhesive (MDIA) | Meloxicam | >24 h | Osteoarthritis |
| Topical and transdermal delivery | 64 | Nanoethosome gel | Ethosomes | Meloxicam | >24 h | Edema |
| Topical and transdermal delivery | 65 | Micro needle patch | Low-molecular weight PVA and polyvinylpyrrolidone | Meloxicam | >24 h | Arthritis |
| Topical and transdermal delivery | 66 | Nanoparticles | Solid lipid | Naproxen | >24 h | |
| Topical and transdermal delivery | 67 | Electrospun mat | TPU | Naproxen | Acute | |
| Topical and transdermal delivery | 68 | Electrospun mat | TPU | Naproxen | ||
| Topical and transdermal delivery | 69 | Electrospun nanofiber mat | PVA | Sodium salicylate, diclofenac, naproxen, indomethacin | 1–25 h | |
| Topical and transdermal delivery | 70 | Electrospun fiber mat | PVA | Sodium salicylate, diclofenac, naproxen, indomethacin | Variable |
NSAID: non-steroidal anti-inflammatory drug.
Meloxicam is one of the few NSAIDs which has consistently been shown to be a very promising candidate for this method, primarily due to its high permeability and low solubility.64 As mentioned prior, most NSAIDs have low solubility, but permeability is a different matter. For example, naproxen, even though it was used in a number of topical delivery experiments,66–70 has poor bioavailability when absorbed through the skin.66 In contrast, there are successful delivery vectors such as patches63,65 and gels64 which release meloxicam to achieve desired concentrations. These formulations were shown to deliver a full day’s treatment in vitro. Due to the ease of use of gels and patches, daily administration is acceptable for control over conditions such as osteoarthritis.
Correlation between NSAIDs and delivery vehicles
While detailed information can be gleaned from looking at each vehicle type individually, it is also helpful to see the usage of certain NSAIDs in certain vehicle categories, regardless of material or release profile. Therefore, the same information from Tables 2 to 7 has been represented in a simpler format in Table 8. From the table, it can be seen that certain drugs are highly prevalent and have been tested across several different platforms and for many different diseases. Other drugs, which might be equally efficacious, seem to be relatively untested in key applications. In this format, it is easy to visualize the NSAID and vehicle combinations which have either been researched extensively or not at all. Diclofenac and ibuprofen have been researched extensively across all vehicle types, regardless of efficacy. In contrast, the latter six NSAIDs have been researched very sparingly. In order to fully explore this field, it may be advantageous to avoid re-doing experiments with diclofenac and ibuprofen which have already been shown to be non-ideal, and instead focus on lesser used NSAIDs which may have as of yet undiscovered advantages when delivered in alternate fashions.
Table 8.
NSAID versus delivery application.
| Diclofenac | Ibuprofen | Ketoprofen | Meloxicam | Naproxen | Celecoxib | Piroxicam | Nabumetone | Aceclofenac | Sodium Salicylate | Indomethacin | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic targeting/encapsulation | 4, 5, 6, 7, 8, 9, 10, 11 | 12, 13, 14, 15 | 15, 16, 17, 18 | 19, 20, 21 | 22, 23, 24, 25 | 15 | |||||
| Local injections | 26, 27 | 28 | 29 | 30 | |||||||
| Localized delivery | 33, 34 | 34, 35, 36, 37, 38 | 39, 40, 41, 42 | 43 | 44, 45 | 31, 32 | 46 | ||||
| Implant coating/incorporation | 47, 48 | 49 | 50 | 48 | |||||||
| Wound dressing/sutures | 52, 53 | 54, 55, 56 | 57, 58 | 59 | 51 | ||||||
| Topical and transdermal delivery | 60, 69, 70 | 61, 62 | 63, 64, 65 | 66, 67, 68, 69, 70 | 69, 70 | 69, 70 |
NSAID: non-steroidal anti-inflammatory drug.
Discussion
Although NSAIDs share similar mechanisms of action and many chemical properties, there are delivery applications in which certain NSAIDs show higher potential for successful and effective anti-inflammatory treatment. While diclofenac and ibuprofen are most commonly used, they may not be the optimal drug for all applications.
In topical and transdermal delivery, the drug must be able to permeate through the skin, a feat which is not easily accomplished by all NSAIDs. While diclofenac and ibuprofen have been used in delivery of this type, meloxicam is more suitable for this approach due to its improved availability through this route. Future research in this area should consider features such as enhanced permeation properties, and not just resort to conventionally used drugs.
In wound dressing and sutures, NSAID compatibility is limited by the impact of the drug on the material's mechanical effects. Any alterations to a surgical suture must not decrease the mechanical properties of the fiber. If the hope is to replace current treatment, drug-loaded sutures must perform at or better than existing technology to be easily implemented by physicians. While mechanical testing was not a particular emphasis of this review, it is important to keep in mind for these applications. Similarly, implant coating or drug incorporation comes with similar concerns. Significantly altering the mechanical properties of an implant can result in mechanical failure or the body's response (e.g. stress shielding) when implanted. In both applications, the interactions between drug and material properties must be taken into account.
In treatments which are intended to act on a chronic time scale, such as the treatment of osteoarthritis, a longer biological half-life, like that of piroxicam, can be advantageous. More commonly used diclofenac and ibuprofen have half-lives of only ∼2 h, placing a higher burden on drug release in order to maintain the same local concentration. Additionally, in long-term drug delivery, poor half-life also leads to a need for larger initial loading. Drug delivery systems which are refillable may have the capacity to overcome these limitations, but as yet there are very few systems with this capability.71,72 Therefore, more common systems must incorporate drugs with longer half-lives to achieve longer total time of release.
For other applications, such as local injection and localized delivery, the requirements placed on NSAID choice are less stringent, at the cost of a higher level of invasiveness. Due to the high variability in terms of delivery vector, adaptations to accommodate specific NSAIDs are more straightforward. In these applications, the high usage of diclofenac and ibuprofen is advantageous, as they are drugs which are more fully understood, and already have successful adaptations and accommodations associated with them.
Commentary on standardization
The field of localized and targeted drug delivery is wide-reaching and varied. This review gives only a taste of the number of different delivery vehicles which exist, and the areas in the body which can be targeted. Therefore, it is unsurprising that there are an equally large number of different experiments and protocols used in this field. While this heterogeneity of experimentation is good for achieving diverse and more complete results, it becomes problematic when it is desired to look at a variety of experiments and compare and contrast them, as it is when writing and researching a literature review such as this. There are three main subjects which seem to lack standardization in this field; perhaps this review can serve as an impetus to help researchers in the field begin such standardization.
The first subject with inconsistent standardization is data representation, specifically the graphing of delivery profiles for various delivery vehicles and drugs. Drug delivery has been represented in terms of both cumulative and daily release, normalized and raw data, and percent and total drug. While each representation has its own value, inconsistency in reporting makes it difficult to compare across platforms. For example, in articles which do not report total drug amounts, and rely solely on percentage or normalized values, it is easy to be misled about the amount of drug which is being released (in some cases, very small amounts of drug). For a release profile to be advantageous for desired therapeutic effect, it must achieve both appropriate release amount and duration.
The second aspect of consistency that would be advantageous to standardize is an official definition of “long-term” vs. “short-term” vs. “burst” release profiles. Currently, these terms seem to have vague meanings, with “long-term” specifically being used to describe continuous release lasting anywhere from 24 h to two to three months. While this information is usually available in the text, it would be useful to standardize time frames associated with them. This way, they could be used to make literature searches or when determining new delivery vehicles that would be appropriate for a certain therapy.
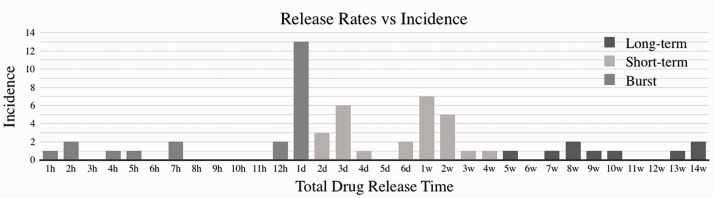
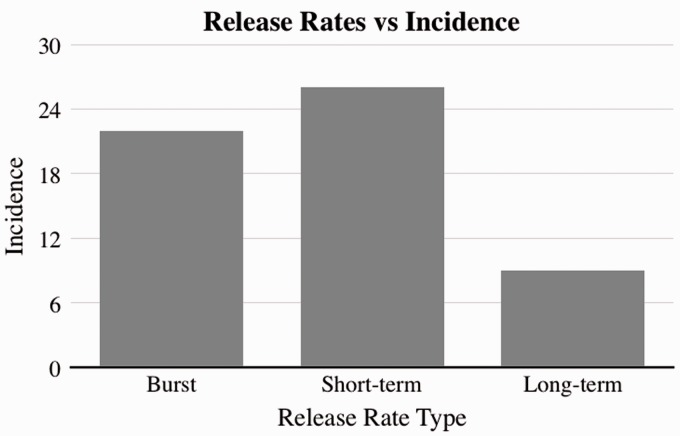
One possible standardization strategy is to use other, previously defined medical terms (e.g. acute and chronic) as the basis for standardization of drug delivery terms. “Short-term” delivery could be defined as a system where a high percentage of the drug (e.g. 80%) is released within the first four weeks of implantation, where high cell infiltration and neutrophil activity dominate the wound healing process. “Long-term” delivery could be defined as a system where the bulk of the drug is released past the four-week window, where macrophage/monocyte activity and tissue remodeling dominate the wound healing processes. “Burst” delivery should be defined as instantaneous or near instantaneous systems (e.g. 80% of drug available in hours to days), with only slight improvement over free drug administration. The distribution of reported release times can be seen in Figure 2, in addition to the proposed categorizing of rates (Figure 3).
Figure 2.
Publication incidence of total drug release times rounded to full hours, days, or weeks. Color indicates categorization into the proposed “Burst,” “Short-term,” and “Long-term” definitions, where burst ≤ 24 h, short-term is ≤ 4 weeks (when cell infiltration and neutrophil activity dominate healing), and long-term > 4 weeks (when macrophage/monocyte activity and tissue remodeling dominate healing).
Figure 3.
Publication incidence of total drug release times categorized into the proposed “Burst,” “Short-term,” and “Long-term” definitions, where burst ≤24 h, short-term is ≤4 weeks (when cell infiltration and neutrophil activity dominate healing), and long-term >4 weeks (when macrophage/monocyte activity and tissue remodeling dominate healing).
The final, and possibly most important, standardization which is currently lacking from the literature is a consistent protocol for in vitro release experiments. Currently, there is high variability in regards to the level of drug sink environment in which in vitro drug release experiments are performed. In this case, the sink is taken to mean the removal of media containing drug, and replacement of this with fresh media. This replacement represents the body’s ability to remove the drug released from a delivery vehicle through transport, metabolism, or elimination. Sinks take on many forms, continuous replacement vs. batch replacement; partial replacement vs. complete replacement; simple media (e.g. phosphate buffered saline) vs. complex and hydrophilic media (e.g. serum, albumin, detergents). While it is well known that drug diffusion follows biological sinks, there is only sparse data linking the effect of the in vivo sink to the nature of each fabricated sink in vitro. Alterations to these sink parameters can have drastic effects on the release profile, making it difficult to both compare against existing data and extrapolate the results to in vivo conditions.
Conclusions
There are a multitude of studies currently being done exploring potential drug delivery device strategies for NSAIDs as an alternative to systemic delivery. Systemic delivery has been shown to cause deleterious side effects, and the mitigation of these side effects is highly desirable, due to the wide-reaching applications of anti-inflammatory drugs. While many of these studies are still in the early stages of development, there is enough data to conclude that the field as a whole could be improved by smarter choices of both vector and NSAID types. These choices will depend heavily on the delivery application. Some, like transdermal delivery, require very specific properties to function correctly. Others, like local delivery, have far fewer considerations. There have been successes in this field, but there have also been avoidable failures, due to a lack of standardization and compilation of resources. As the field continues to expand, literature reviews such as this will become vital to aid future experimental design, enabling researchers to determine the drugs and delivery vehicles which are most advantageous for them to pursue, and eventually to determine optimal NSAID delivery systems for clinical use.
Authors’ contributions
RMH was responsible for literature searches and interpretation and analysis of the literature. Both authors wrote and reviewed the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by UCITE: University Center for Innovation in Teaching and Education.
References
- 1.Badri W, Miladi K, Nazari QA, Greige-Gerges H, Fessi H, Elaissari A. Encapsulation of NSAIDs for inflammation management: overview, progress, challenges and prospects. Int J Pharm 2016; 515:757–73 [DOI] [PubMed] [Google Scholar]
- 2.Hawkey CJ. COX-1 and COX-2 inhibitors. Best Pract Res Clin Gastroenterol 2001; 15:801–20 [DOI] [PubMed] [Google Scholar]
- 3.Jones R. Nonsteroidal anti-inflammatory drug prescribing: past, present, and future. Am J Med 2001; 110:S4–7 [DOI] [PubMed] [Google Scholar]
- 4.Hosseinzadeh H. Controlled release of diclofenac sodium from pH-responsive carrageenan-g-poly(acrylic acid) superabsorbent hydrogel. J Chem Sci 2011; 122:651–9 [Google Scholar]
- 5.Sharma RK, Lalita, Singh AP, Chauhan GS. Grafting of GMA and some comonomers onto chitosan for controlled release of diclofenac sodium. Int J Biol Macromol 2014; 164:368–76 [DOI] [PubMed] [Google Scholar]
- 6.Das S, Subuddhi U. Controlled and targeted delivery of diclofenac sodium to the intestine from pH-Responsive chitosan/poly(vinyl alcohol) interpenetrating polymeric network hydrogels. Polym Sci Ser A 2016; 58:154–66 [Google Scholar]
- 7.Tkalec G, Knez Ž, Novak Z. PH sensitive mesoporous materials for immediate or controlled release of NSAID. Microporous and Mesoporous Mater 2016; 224:190–200 [Google Scholar]
- 8.Khanal S, Adhikari U, Rijal N, Bhattarai S, Sankar J, Bhattarai N. pH-Responsive PLGA nanoparticle for controlled payload delivery of diclofenac sodium. J Funct Biomater 2016; 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Şanlı O, Ay N, Işıklan N. Release characteristics of diclofenac sodium from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate blend beads. Eur J Pharm Biopharm 2007; 65:204–14 [DOI] [PubMed] [Google Scholar]
- 10.Kurkuri MD, Aminabhavi TM. Poly(vinyl alcohol) and poly(acrylic acid) sequential interpenetrating network pH-sensitive microspheres for the delivery of diclofenac sodium to the intestine. J Control Rel 2004; 96:9–20 [DOI] [PubMed] [Google Scholar]
- 11.Nayak AK, Pal D. Development of pH-sensitive tamarind seed polysaccharide–alginate composite beads for controlled diclofenac sodium delivery using response surface methodology. Int J Biol Macromol 2011; 49:784–93 [DOI] [PubMed] [Google Scholar]
- 12.Baruch L, Benny O, Gilert A, Ukobnik M, Ben Itzhak O, Machluf M. Alginate-PLL cell encapsulation system Co-entrapping PLGA-microspheres for the continuous release of anti-inflammatory drugs. Biomed Microdevices 2009; 11:1103–13 [DOI] [PubMed] [Google Scholar]
- 13.Kamari Y, Ghiaci M. Preparation and characterization of ibuprofen/modified chitosan/TiO2 hybrid composite as a controlled drug-delivery system. Microporous Mesoporous Mater 2016; 234:361–9 [Google Scholar]
- 14.Seetharaman G, Kallar AR, Vijayan VM, Muthu J, Selvam S. Design, preparation and characterization of pH-responsive prodrug micelles with hydrolyzable anhydride linkages for controlled drug delivery. J Colloid Interf Sci 2017; 492:61–72 [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Singh A, Garg N, Siril PF. Solid lipid nanoparticles for the controlled delivery of poorly water soluble non-steroidal anti-inflammatory drugs. Ultrason Sonochem 2018; 40:686–96 [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, Onishi H, Machida Y. Sustained release ketoprofen microparticles with ethylcellulose and carboxymethylethylcellulose. J Control Release 2001; 75:271–82 [DOI] [PubMed] [Google Scholar]
- 17.Kenawy E-R, Abdel-Hay FI, El-Newehy MH, Wnek GE. Controlled release of ketoprofen from electrospun poly(vinyl alcohol) nanofibers. Mater Sci Eng 2007; 459:390–6 [Google Scholar]
- 18.Wu C, Quan J, Xie J, Branford-White C, Zhu L, Yu Y, Wang Y. Preparation and controlled release of degradable polymeric ketoprofen–saccharide conjugates. Polym Bull 2010; 67:593–608 [Google Scholar]
- 19.Shi Y, Liu Z, Yang Y, Xu X, Li Y, Li T. Design of poly(mPEGMA-co-MAA) hydrogel-based mPEG-b-PCL nanoparticles for oral meloxicam delivery. Mater Sci Eng C Mater Biol Appl 2017; 76:975–84 [DOI] [PubMed] [Google Scholar]
- 20.Shende PK, Gaud RS, Bakal R, Patil D. Effect of inclusion complexation of meloxicam with β-cyclodextrin- and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surf B 2015; 136:105–10 [DOI] [PubMed] [Google Scholar]
- 21.Villalba BT, Ianiski FR, Vogt AG, Pinz MP, Reis AS, Vaucher RA, Soares MP, Wilhelm EA, Luchese C. Polymeric nanocapsules as a technological alternative to reduce the toxicity caused by meloxicam in mice. Regul Toxicol Pharmacol 2016; 81:316–21 Nov 1 [DOI] [PubMed] [Google Scholar]
- 22.Choi JM, Lee B, Jeong D, Park KH, Choi E-J, Jeon Y-J, Dindulkar SD, Cho E, Do SH, Lee K, Lee I-S, Park S, Jun B-H, Yu J-H, Jung S. Characterization and regulated naproxen release of hydroxypropyl cyclosophoraose-pullulan microspheres. J Ind Eng Chem 2017; 48:108–18 [Google Scholar]
- 23.Karami Z, Sadighian S, Rostamizadeh K, Parsa M, Rezaee S. Naproxen conjugated mPEG–PCL micelles for dual triggered drug delivery. Mater Sci Eng C Mater Biol Appl 2016; 61:665–73 [DOI] [PubMed] [Google Scholar]
- 24.Sousa CT, Nunes C, Proença MP, Leitão DC, Lima Jlfc Reis S, Araújo JP, Lúcio M. pH sensitive silica nanotubes as rationally designed vehicles for NSAIDs delivery. Colloids Surf B 2012; 94:288–95 [DOI] [PubMed] [Google Scholar]
- 25.Ghazaie M, Ghiaci P, Ghiaci M. Study on release of naproxen and metformin encapsulated in biopolymer-inorganic mesoporous matrices as controlled drug-delivery systems. Microporous and Mesoporous Mater 2017; 244:291–300 [Google Scholar]
- 26.Sulistio A, Reyes-Ortega F, D’Souza AM, Ng SMY, Valade D, Quinn JF, Donohue AC, Mansfeld F, Blencowe A, Qiao G, Prankerd R, Quirk S, Whittaker MR, Davis TP, Tait RJ. Precise control of drug loading and release of an NSAID-polymer conjugate for long term osteoarthritis intra-articular drug delivery. J Mater Chem B 2017; 5:6221–6 [DOI] [PubMed] [Google Scholar]
- 27.Thing M, Ågårdh L, Larsen S, Rasmussen R, Pallesen J, Mertz N, Kristensen J, Hansen M, Østergaard J, Larsen CS. A prodrug approach involving in situ depot formation to achieve localized and sustained action of diclofenac after joint injection. J Pharm Sci 2014; 103:4021–9 [DOI] [PubMed] [Google Scholar]
- 28.Paavola A, Bernards CM, Rosenberg PH. Controlled release ibuprofen-poloxamer gel for epidural use – a pharmacokinetic study using microdialysis in pigs. Eur J Pharm Biopharm 2016; 108:180–6 [DOI] [PubMed] [Google Scholar]
- 29.Wang SH, Liang ZH, Zeng S. Monitoring release of ketoprofen enantiomers from biodegradable poly(d,l-lactide-co-glycolide) injectable implants. Int J Pharm 2007; 337:102–8 [DOI] [PubMed] [Google Scholar]
- 30.Fattahpour S, Shamanian M, Tavakoli N, Fathi M, Sheykhi SR, Fattahpour S. Design and optimization of alginate−chitosan−pluronic nanoparticles as a novel meloxicam drug delivery system. J Appl Polym Sci 2015; 132:1–1225866416 [Google Scholar]
- 31.Petit A, Sandker M, Müller B, Meyboom R, van Midwoud P, Bruin P, Redout EM, Versluijs-Helder M, van der Lest CHA, Buwalda SJ, de Leede LGJ, Vermonden T, Kok RJ, Weinans H, Hennink WE. Release behavior and intra-articular biocompatibility of celecoxib-loaded acetyl-capped PCLA-PEG-PCLA thermogels. Biomaterials 2014; 35:7919–28 Sep [DOI] [PubMed] [Google Scholar]
- 32.Amrite AC, Ayalasomayajula SP, Cheruvu NPS, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci 2006; 47:1149–60 Mar [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saïdi L, Vilela C, Oliveira H, Silvestre AJD, Freire CSR. Poly(N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr Polym 2017; 169:357–65 [DOI] [PubMed] [Google Scholar]
- 34.Melendez Ortiz HI, Díaz Rodríguez P, Alvarez-Lorenzo C, Concheiro A, Bucio E. Binary graft modification of polypropylene for anti‐inflammatory drug-device combo products. J Pharm Sci 2014; 103:1269–77 [DOI] [PubMed] [Google Scholar]
- 35.Pang J, Luan Y, Li F, Cai X, Du J, Li Z. Ibuprofen-loaded poly(lactic-co-glycolic acid) films for controlled drug release. Int J Nanomed 2011; 6:659–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Pan G, Liu G, Neves JD, Song S, Chen S, Cheng B, Sun Z, Sarmento B, Cui W, Fan C. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J Control Release 2017; 264:1–13 [DOI] [PubMed] [Google Scholar]
- 37.Park JW, Yun Y-P, Park K, Lee JY, Kim H-J, Kim SE, Song H-R. Ibuprofen-loaded porous microspheres suppressed the progression of monosodium iodoacetate-induced osteoarthritis in a rat model. Colloids and Surf 2016; 147:265–73 [DOI] [PubMed] [Google Scholar]
- 38.Salmoria GV, Paggi RA, Castro F, Roesler CRM, Moterle D, Kanis LA. Development of PCL/Ibuprofen tubes for peripheral nerve regeneration. Procedia CIRP 2016; 49:193–8 [Google Scholar]
- 39.Paukkonen H, Kunnari M, Laurén P, Hakkarainen T, Auvinen V-V, Oksanen T, Koivuniemi R, Yliperttula M, Laaksonen T. Nanofibrillar cellulose hydrogels and reconstructed hydrogels as matrices for controlled drug release. Int J Pharm 2017; 532:269–80 [DOI] [PubMed] [Google Scholar]
- 40.Lins LC, Padoin N, Pires ATN, Soares C. Modeling ketoprofen release from PHB/chitosan composite microparticles. Polym Bull 2015; 73:1515 [Google Scholar]
- 41.Park J-Y, Lee I-H. Controlled release of ketoprofen from electrospun porous polylactic acid (PLA) nanofibers. J Polym Res 2010; 18:1287–91 [Google Scholar]
- 42.Macocinschi D, Filip D, Vlad S, Oprea AM, Gafitanu CA. Characterization of a poly(ether urethane)-based controlled release membrane system for delivery of ketoprofen. Appl Surf Sci 2012; 259:416–23 [Google Scholar]
- 43.Yar M, Farooq A, Shahzadi L, Khan AS, Mahmood N, Rauf A, Chaudhry AA, Ur Rehman I. Novel meloxicam releasing electrospun polymer/ceramic reinforced biodegradable membranes for periodontal regeneration applications. Mater Sci Eng 2016; 64:148–56 [DOI] [PubMed] [Google Scholar]
- 44.Canbolat MF, Celebioglu A, Uyar T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf B 2014; 115:15–21 [DOI] [PubMed] [Google Scholar]
- 45.Veronese FM, Marsilio F, Caliceti P, De Filippis P, Giunchedi P, Lora S. Polyorganophosphazene microspheres for drug release: polymer synthesis, microsphere preparation, in vitro and in vivo naproxen release. J Control Release 1998; 52:227–37 [DOI] [PubMed] [Google Scholar]
- 46.Farooq A, Yar M, Khan AS, Shahzadi L, Siddiqi SA, Mahmood N, Rauf A, Qureshi Z-U-A, Manzoor F, Chaudhry AA, Ur Rehman I. Synthesis of piroxicam loaded novel electrospun biodegradable nanocomposite scaffolds for periodontal regeneration. Mater Sci Eng C Mater Biol Appl 2015; 56:104–13 [DOI] [PubMed] [Google Scholar]
- 47.Sidney LE, Heathman TRJ, Britchford ER, Abed A, Rahman CV, Buttery LDK. Investigation of localized delivery of diclofenac sodium from poly(D,L-lactic acid- co-glycolic acid)/poly(ethylene glycol) scaffolds using an in vitroosteoblast inflammation model. Tissue Eng Part A 2015; 21:362–73 Jan [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horvat G, Xhanari K, Finšgar M, Gradišnik L, Maver U, Knez Ž, Novak Z. Novel ethanol-induced pectin-xanthan aerogel coatings for orthopedic applications. Carbohydr Polym 2017; 166:365–76 [DOI] [PubMed] [Google Scholar]
- 49.Jia H, Kerr LL. Sustained ibuprofen release using composite poly(lactic-co-glycolic acid)/titanium dioxide nanotubes from Ti implant surface. J Pharm Sci 2013; 102:2341–8 [DOI] [PubMed] [Google Scholar]
- 50.Goimil L, Braga MEM, Dias AMA, Gómez-Amoza JL, Concheiro A, Alvarez-Lorenzo C, de Sousa HC, García-González CA. Supercritical processing of starch aerogels and aerogel-loaded poly(ε-caprolactone) scaffolds for sustained release of ketoprofen for bone regeneration. J CO2 Util 2017; 18:237–49 [Google Scholar]
- 51.Padmakumar S, Joseph J, Neppalli MH, Mathew SE, Nair SV, Shankarappa SA, Menon D. Electrospun polymeric core-sheath yarns as drug eluting surgical sutures. ACS Appl Mater Interf 2016; 8:6925–34 [DOI] [PubMed] [Google Scholar]
- 52.Catanzano O, Acierno S, Russo P, Cervasio M, Del Basso De Caro M, Bolognese A, Sammartino G, Califano L, Marenzi G, Calignano A, Acierno D, Quaglia F. Melt-spun bioactive sutures containing nanohybrids for local delivery of anti-inflammatory drugs. Mater Sci Eng 2014; 43:300–9 Oct [DOI] [PubMed] [Google Scholar]
- 53.Huh BK, Kim BH, Kim S-N, Park CG, Lee SH, Kim KR, Heo CY, Choy YB. Surgical suture braided with a diclofenac-loaded strand of poly(lactic- co -glycolic acid) for local, sustained pain mitigation. Mater Sci Eng C Mater Biol Appl 2017; 79:209–15 Oct [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Chen D, Sun J. Layer-by-layer deposition of polymeric microgel films on surgical sutures for loading and release of ibuprofen. Langmuir 2009; 25:7990–4 [DOI] [PubMed] [Google Scholar]
- 55.Zurita R, Puiggalí J, Rodríguez-Galán A. Loading and release of ibuprofen in multi- and monofilament surgical sutures. Macromol Biosci 2006; 6:767. [DOI] [PubMed] [Google Scholar]
- 56.Lee JE, Park S, Park M, Kim MH, Park CG, Lee SH, Choi SY, Kim BH, Park HJ, Park J-H, Heo CY, Choy YB. Surgical suture assembled with polymeric drug-delivery sheet for sustained, local pain relief. Acta Biomater 2013; 9:8318–27 Sep [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Wu H, Liao C, Zhou N, Cheng W, Wan Y. Sustained release of ketoprofen from fibrous chitosan-poly(ɛ-caprolactone) membranes. Carbohydr Polym 2011; 84:624–30 [Google Scholar]
- 58.Mangindaan D, Chen C-T, Wang M-J. Integrating sol–gel with cold plasmas modified porous polycaprolactone membranes for the drug-release of silver-sulfadiazine and ketoprofen. Appl Surf Sci 2012; 262:114–9 [Google Scholar]
- 59.Li Z, Kang H, Che N, Liu Z, Li P, Li W, Zhang C, Cao C, Liu R, Huang Y. Controlled release of liposome-encapsulated Naproxen from core-sheath electrospun nanofibers. Carbohydr Polym 2014; 111:18–24 [DOI] [PubMed] [Google Scholar]
- 60.Boateng JS, Pawar HV, Tetteh J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int J Pharm 2013; 441:181–91 Jan [DOI] [PubMed] [Google Scholar]
- 61.Morgado PI, Miguel SP, Correia IJ, Aguiar-Ricardo A. Ibuprofen loaded PVA/chitosan membranes: a highly efficient strategy towards an improved skin wound healing. Carbohydr Polym 2017; 159:136–45 Mar [DOI] [PubMed] [Google Scholar]
- 62.Djekic L, Martinovic M, Stepanović-Petrović R, Micov A, Tomić M, Primorac M. Formulation of hydrogel-thickened nonionic microemulsions with enhanced percutaneous delivery of ibuprofen assessed in vivo in rats. Eur J Pharm Sci 2016; 92:255–65 [DOI] [PubMed] [Google Scholar]
- 63.Ah Y-C, Choi J-K, Choi Y-K, Ki H-M, Bae J-H. A novel transdermal patch incorporating meloxicam: in vitro and in vivo characterization. Int J Pharm 2010; 385:12–9 [DOI] [PubMed] [Google Scholar]
- 64.Ahad A, Raish M, Al-Mohizea AM, Al-Jenoobi FI, Alam MA. Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int J Biol Macromol 2014; 67:99–104 [DOI] [PubMed] [Google Scholar]
- 65.Amodwala S, Kumar P, Thakkar HP. Statistically optimized fast dissolving microneedle transdermal patch of meloxicam: a patient friendly approach to manage arthritis. Eur J Pharm Sci 2017; 104:114–23 [DOI] [PubMed] [Google Scholar]
- 66.Akbari J, Saeedi M, Morteza-Semnani K, Rostamkalaei SS, Asadi M, Asare-Addo K, Nokhodchi A. The design of naproxen solid lipid nanoparticles to target skin layers. Colloids Surf B Biointerf 2016; 145:626–33 [DOI] [PubMed] [Google Scholar]
- 67.Akduman C, Özgüney I, Akçakoca Kumbasar EP. Electrospun thermoplastic polyurethane mats containing naproxen–cyclodextrin inclusion complex. Autex Res J 2014; 14:239–46 [Google Scholar]
- 68.Akduman C, Özgüney I, Kumbasar EPA. Preparation and characterization of naproxen-loaded electrospun thermoplastic polyurethane nanofibers as a drug delivery system. Mater Sci Eng C Mater Biol Appl 2016; 64:383–90 [DOI] [PubMed] [Google Scholar]
- 69.Taepaiboon P, Rungsardthong U, Supaphol P. Drug-loaded electrospun mats of poly(vinyl alcohol) fibres and their release characteristics of four model drugs. Nanotechnology 2006; 17:2317–29 [Google Scholar]
- 70.Basar AO, Castro S, Torres-Giner S, Lagaron JM, Turkoglu Sasmazel H. Novel poly(ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater Sci Eng C 2017; 81:459–68 [DOI] [PubMed] [Google Scholar]
- 71.Cyphert EL, Zuckerman ST, Korley JN, von Recum HA. Affinity interactions drive post-implantation drug filling, even in the presence of bacterial biofilm. Acta Biomater 2017; 57:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu AS, Thatiparti TR, Saidel GM, von Recum HA. Experimental studies and modeling of drug release from a tunable affinity-based drug delivery platform. . Ann Biomed Eng 2011; 39:2466–75 [DOI] [PubMed] [Google Scholar]