Short abstract
A diverse range of clinical infections are on the increase, resulting in part from disruption of the natural microbiome, or even mycobiome, as a result of many different medical interventions. Amphotericin B (AmB) is a leading drug for the treatment of clinical fungal infections. However, AmB is extremely cytotoxic to mammalian cells, making use of the drug problematic. In this work, a drug delivery system made of polymerized cyclodextrin (pCD) allows for the localized administration of AmB, reducing the toxicity to host cells while retaining antifungal activity. A slow, sustained delivery rate of AmB was achieved through exploiting molecular interactions between the CD pockets and the drug. Surface plasmon resonance (SPR) and Fourier transform infrared spectroscopy (FTIR) were used to characterize the interaction between AmB and cyclodextrins (CDs). Through these methods, it was found that amphotericin binds strongly (M−1) to β-cyclodextrin. Release studies showed slow, sustained release of AmB from pCD disks. Antifungal activity was tested against Saccharomyces cerevisiae in assays for zone of inhibition, contact killing, and solution killing. In all assays, AmB-loaded pCD disks were found to exhibit significant antifungal activity. These results indicate that AmB-loaded pCD disks are capable of both the prevention of fungal growth and the elimination of established colonies. Additionally, results suggest that AmB exhibits antifungal activity whether associated with or released from pCD. The usage of pCD as a delivery vehicle also significantly reduces the toxic side effects of AmB, as seen in mammalian cell culture studies. These results show that in addition to reducing side-effects from systemic dosing, local delivery of AmB from pCD disks has the potential to improve its usage both in antifungal efficacy and reduced mammalian cell toxicity.
Impact statement
Amphotericin B (AmB) is an effective and commonly used antifungal agent. However, nephrotoxicity and poor solubility limits its usage. The proposed polymerized cyclodextrin (pCD) system therefore is an attractive method for AmB delivery, as it retains the antifungal activity of AmB while decreasing toxicity, and confining drug release to the local environment. This system could potentially be used for both prevention and treatment of established fungal infections, as AmB is toxic to fungus whether associated or released from pCD.
Keywords: Drug delivery, amphotericin B, antifungal, cyclodextrin, infection, cytotoxicity
Introduction
Invasive fungal infections pose a significant burden upon health care systems due to limitations in both their diagnosis and treatment. One fungal pathogen, Candida spp, causes serious infections in approximately 46,000 patients each year in the United States.1 The proliferation of aggressive antibiotic and chemotherapy regimens, hematopoietic stem cell transplants, organ transplantation, and placement of indwelling devices has led to a disruption of host microbiome (including mycobiome), leaving this population vulnerable to fungal infections.1,2 Critically ill patients in intensive care units are at elevated risk for fungal infections due to a high use of central lines, altered pharmacokinetics, and extreme illness.2,3 The formation of fungal biofilms on in-dwelling devices such as catheters, stents, and shunts can cause bloodstream infections and poses a significant treatment challenge.4,5 Invasive mold infections are also a source of high morbidity and mortality, with stem cell transplant and solid organ transplant patients being particularly vulnerable.2 Additionally, evidence suggests that prophylactic treatment with antifungals and antimicrobials has further increased the incidence of fungal infection.6
Amphotericin B (AmB) is a polyene antifungal compound that has been used to combat fungal infections for over 50 years.7 Despite the use of AmB for decades, resistance remains rare, as the adaptations weaken fungal species such as Candida albicans into non-virulent pathogens.7 AmB is available in two main formulations: free AmB and liposomal AmB.8 Free AmB is poorly soluble and comes with serious nephrotoxicity concerns.9 Liposomal forms of AmB have slightly decreased toxicity and better pharmacokinetic properties,10,11 but some systemic toxicity remains, and their high costs limit utility in developing countries. Lipid-based formulations have also been found to be more effective at shortening length of stay for patients.12 In these formulations, AmB is typically retained within the liposome with the help of a lipid like deoxycholate (e.g. AmBisome), which transfers to the fungal membrane where it ultimately forms a pore leading to cell death.7 This transfer from liposome to fungal cell membrane has been found to be energy dependent.13
Several groups have investigated various methods of delivering AmB from polymers to provide a sustained release14–16 that would be ideally suited for residence in bone marrow where repeated intravenous applications are required. Cyclodextrin (CD) is a ring of 6, 7, or 8 glucose molecules with a hydrophilic exterior and slightly hydrophobic interior, sometimes used pharmacologically to solubilize hydrophobic drugs including AmB.14,17,18 Importantly, liposomes containing AmB complexed with chemically modified β-cyclodextrin (βCD) achieved better area under the curve and maximum serum concentrations than liposomal AmB without βCD complexation.17 In their work, Chakraborty and Naik hypothesized that the βCD–AmB complex slowed transfer of AmB from the liposome to the plasma and host tissues, causing the improved properties that were seen.
Our group takes a different approach, using an insoluble form of pCD as a local drug delivery platform to harness the inclusion complex between CD and drug. The rate of association/dissociation between drug and CD is the rate limiting step for drug release where only drug dissociated from CD is free to diffuse out of the device, compared to conventional delivery polymers where diffusion is the rate limiting step.19 By tailoring the type of CD, we hypothesize that we can control delivery rates while still achieving high drug loading from organic and aqueous solvents. Previous research using pCD has focused on antimicrobial therapies to prevent bacterial device infection.20,21 This work builds upon our extensive antibacterial expertise and extends protection to fungal pathogens.
The main hypothesis of this work is that pCD will load high amounts of AmB and retain that AmB to minimize host toxicity while providing a long-term drug reservoir capable of killing fungal cells through localized delivery. Based on our results, the loading of AmB into pCD disks improves its usage as an antifungal agent, making it potentially useful for prevention and treatment of established fungal infections through drug release and bioactivity on contact. Additionally, the association between AmB and pCD decreases the drug’s cytotoxicity, while local delivery reduces concerns about system side-effects such as nephrotoxicity.
Materials and methods
Chemicals and prepolymers: Lightly cross-linked βCD (2–15 kDa, average 10 CDs per chain) and γ-cyclodextrin as a prepolymer were purchased from CycloLab Ltd (Hungary). Dextran (Dex, 15–20 kDa) was purchased from Polyscience. 1,6-diisocyanatohexane (HMDI, Sigma Aldrich), AmB (GenDEPOT), N,N-dimethylformamide (DMF, extra dry, Applied Biosystems), dimethyl sulfoxide (DMSO, Acros), Tween 80 (Acros Organic), yeast extract (Fisher Scientific), tryptone (Fisher Scientific), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (Sigma Aldrich), and N-hydroxysuccinimide (Sigma Aldrich) were used as received.
Cell cultures: [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium] (MTS) was purchased from Promega (Madison, WI). 3T3 mouse fibroblasts and Saccharomyces cerevisiae (ATCC® 9763™) were purchased from ATCC (Manassas, VA). Dulbecco’s Modification of Eagles Medium (DMEM), fetal bovine serum, and penicillin–streptomycin were purchased from Fisher Scientific and used as received.
Polymerization of an insoluble CD drug delivery system
The lightly cross-linked CD prepolymers were further polymerized as previously published.22–24 Briefly, 1.5 g of CD prepolymer was dissolved in 4.5 mL DMF (33.3% weight/volume) and polymerized with HMDI in a ratio of 1:0.32 (glucose residue:HMDI) for four days at room temperature. Disks (8 mm diameter) were washed with excess DMF, DMF/water, and water to remove unreacted CD and HMDI.
AmB loading into pCD
AmB was dissolved in DMSO (5 wt%) and loaded into pCD disks for three days at room temperature (25°C). Loaded disks were removed from solution, blotted on Kimwipes, and briefly washed in excess water to remove surface DMSO and free AmB prior to drying.
Surface plasmon resonance (SPR)
βCD prepolymer solubilized in HBS-N buffer was immobilized to a CM5 sensor chip via NHS/EDC chemistry (10 µL/min flow; 7 min contact time) on a Biacore X100 system. Coupling was terminated with ethanolamine. Binding was carried out at 25°C. The chip was primed with three cycles of 10 µL 10% DMSO in PBS prior to 180 s binding with AmB (5, 10, 20, and 50 µM). Dissociation proceeded for 540 s prior to two regeneration cycles (30 s each) with 50 mM NaOH, 25% DMSO PBS, or 50% DMSO in PBS. The affinity of AmB to CD was determined from the dissociation constant () calculated by the evaluation software from Biacore X100.
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra were obtained using an Excalibur FTS 3000.23 AmB powder, βCD prepolymer, unloaded pCD-β, and an AmB-loaded pCD-β were pressed into a pellet with KBr (1:100, sample: KBr) and scanned from 5000 to 400.
In vitro AmB release
AmB-loaded pCD disks were incubated with 1 mL of phosphate buffered saline (PBS, pH 7.4). Every 24 ± 5 h release media was removed and replaced with fresh. The concentration of each time point sample was determined spectrophotometrically at 382 nm using a standard curve of AmB in PBS.
Zone of inhibition assay
A modified zone of inhibition assay was used to determine the antifungal efficacy of released AmB in vitro. AmB-loaded pCD-β disks were evaluated against S. cerevisiae. S. cerevisiae was cultured overnight and 70 µL of this culture was then spread on a YM Agar plate. AmB-loaded pCD-β disks were placed on this fresh S. cerevisiae lawn and incubated at 30°C for 72 h. The zone of inhibition around each disk was measured with calipers at three points and recorded. Images were captured for inhibition area analysis using Image J. Each disk was then transferred onto a new S. cerevisiae plate and the process was repeated.
Contact killing assay
S. cerevisiae culture (10,000 or 2000 CFU) was pipetted onto AmB-loaded pCD-β disks (n = 3). Empty pCD disks without any drug were used as a polymer control. The yeast-covered disk was incubated at room temperature (25°C) for 3 h and then was placed into a test tube containing 5 mL of YM broth and incubated at 30°C. After 72 h the medium containing empty pCD disks was diluted 1:10,000. Seventy microliters of the diluted solution (neat for AmB-loaded pCD-β disks) was spread onto YM agar plates. The agar plates were incubated for 72 h and colonies counted to determine CFU and growth.
Solution killing assay
S. cerevisiae was introduced to 5 mL YM broth and allowed to incubate at 30°C for 96 h. During this incubation process, AmB-loaded pCD-β disks were added to specific samples at t = 0, 24, 48, and 72 h. Every 24 h, a sample was taken from each YM broth tubes, diluted 1:10,000, and 70 µL of this diluted solution was spread onto YM agar plates. The agar plates were incubated for 72 h and colonies counted to determine growth. The viable cell count in each sample was used to create a growth curve for S. cerevisiae over time and with varying exposure times to AmB-loaded pCD-β disks.
Cytotoxicity of pCD-released AmB
Cell viability tests were performed using 3T3 mouse fibroblast cells (25,000 cells/well) with DMEM high glucose (HG) in 10% serum in a 24 well plate. 3T3 cells were allowed to adhere for 4 h prior to placing sterile, AmB-loaded pCD disks in Transwell inserts (Costar, 5.0 µm pore size) in the well. Disks were removed after 24 h, media replaced with fresh media containing 1% MTS and incubated for 4 h before reading absorbance at 490 nm (BioTek H1).
To compare free AmB toxicity to that of pCD-released AmB, free AmB was used at a concentration comparable to that released at t = 24 h in the AmB-loaded pCD in vitro release data (10 µg/mL). This was done to ensure that both the pCD-AmB and free AmB conditions resulted in an equal concentration of free/delivered AmB in the well, so that toxicity results were due to formulation differences and not concentration differences. Free AmB is solubilized in 1% DMSO, and a 1% DMSO group was used as a control.
AmB-loaded pCD disks were placed in fresh DMEM-HG with 10% serum and media changed every day to model infinite sink release. On day 6 AmB-loaded pCD disks were placed on 25,000 fresh 3T3s in a 24 well plate as above. After 24 h exposure, disks were removed and metabolic activity assayed with 1% MTS in media as before. For both time points, a standard curve of 3T3s in media only (no drug) and pCD disks with only DMSO treatment (solvent loading control) served as controls.
Statistical analysis
If not otherwise stated, experiments were carried out in triplicate for statistical analysis (n = 3). Data displayed is the mean of each condition, and error bars represent the standard deviation of the triplicate set. One-way ANOVA was done using Prism to determine reported statistical significance. Significance is defined to be a p-value < 0.05.
Results
Determining binding affinity using SPR
SPR was used to characterize the molecular interactions between AmB and pCD. Based on this information regarding binding affinity, we can better understand the binding and release kinetics associated with localized AmB delivery using this system. Increasing the amount of solvent in the regeneration buffer increased the value of (Figure 1). Regeneration with 50 mM NaOH gave a value of M−1 compared to 50% DMSO (5.48 M−1) for a range of 108. In physiological conditions, AmB binds tightly to βCD due to hydrophobic interactions. However, a decrease in these hydrophobic interactions (addition of solvent) increases dissociation.
Figure 1.
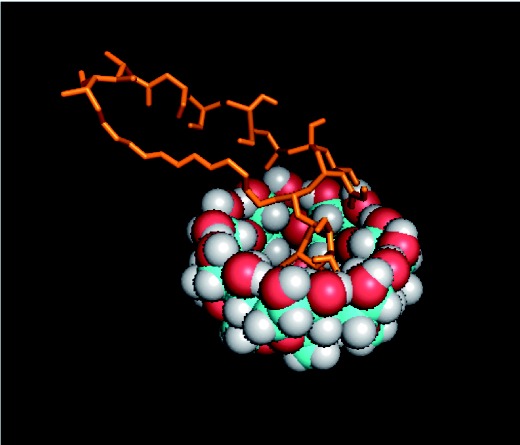
Image shown is a 3D conformer of modeled affinity binding between pCD-beta and AmB. (A color version of this figure is available in the online journal.)
Characterizing AmB–pCD complex using FTIR
By using FTIR, we can characterize the physical association between AmB and pCD and compare it to our initial computer-generated model (Figure 1). Between 2000 and 950 free AmB powder showed peaks at 1670, 1480, and 1242 , which represent C = O, –OH, and C–O stretching of the carboxylic groups, respectively (Figure 2). The peaks at 1670 and 1242 indicate the existence of an ester group. The AmB-loaded pCD spectrum showed a peak at 1670 which corresponds to the C = O indicated in the AmB powder spectrum. This indicates that AmB is associated with the CD pocket and that the ester group on the AmB is outside of the hydrophobic pockets of the pCD.
Figure 2.
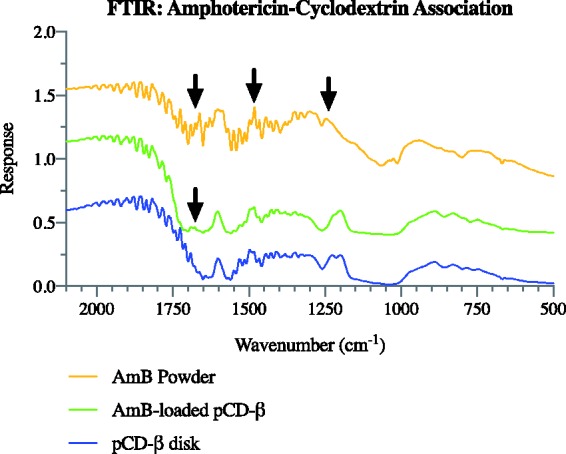
FTIR data for pCD-β disks, AmB-loaded pCD-β disks, and free AmB powder without polymer. Free AmB showed peaks at 1670, 1480, and 1242, which represent C = O, –OH, and C–O stretching of the carboxylic groups, respectively. AmB-loaded pCD showed a peak at 1670 which corresponds to the C = O in free AmB. AmB: amphotericin B; FTIR: Fourier transform infrared spectroscopy; pCD: polymerized cyclodextrin. (A color version of this figure is available in the online journal.)
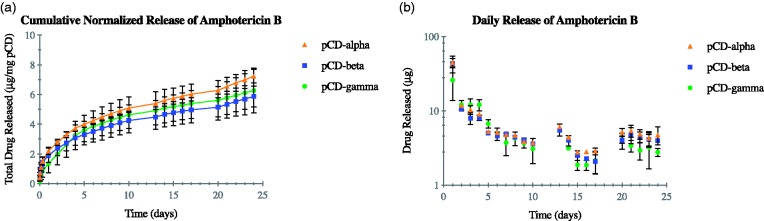
In vitro AmB release profile
SPR results show high affinity between AmB and pCD, indicating a slow and steady release of AmB over time. To confirm this release profile, the results of an in vitro drug release study are reported in Figure 3. Normalized cumulative release can be seen in Figure 3(a) and daily cumulative release is shown in Figure 3(b). All three CD types are shown and have similar release profiles. Release is seen out to at least 24 days. An initial burst release can be seen within the first 24 h (day 1), where an average of 38 µg is released.
Figure 3.
AmB released from all three types of pCD (α, β, and γ at similar rates for more than 24 days (a). Daily release shows a small initial burst followed by steady delivery rates out past day 5 (b). pCD: polymerized cyclodextrin. (A color version of this figure is available in the online journal.)
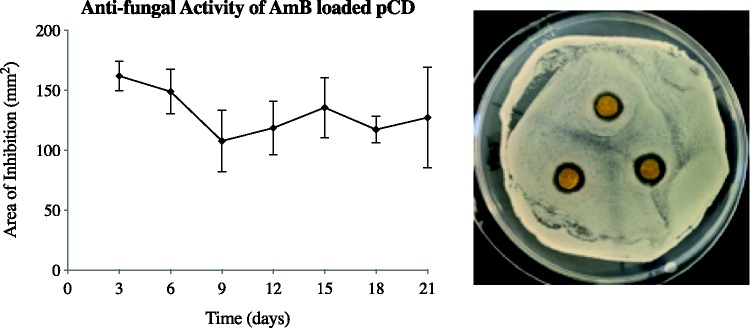
Determining antifungal activity using a zone of inhibition assay
A periodic zone of inhibition assay was used to show sustained, local antifungal effect of drug-loaded polymer implants. Areas cleared on plates replaced every three days are shown in Figure 4. Implants were capable of clearing new zones consistently throughout the three weeks of study, indicating release and antifungal activity capable of preventing fungal growth throughout this period. While the release study (Figure 3) shows an initial burst release of AmB, the zone of inhibition assay does not show significantly higher killing at the first time point. This is likely due to partitioning, as the hydrophobic nature of AmB makes it difficult for the drug to diffuse through the agar. To address this, we also tested the activity of AmB loaded into pCD disks to kill S. cerevisiae in solution (Figure 6).
Figure 4.
AmB-loaded pCD disks show consistent antifungal activity against S. cerevisiae for 21 days. AmB-loaded pCD disks were placed on fresh S. cerevisiae lawns at t = 0 and grown for 72 h at 30°C. Zone of clearance was measured and AmB-loaded disks placed on a fresh lawn every three days. ImageJ analysis was used to calculate the area of clearance; AmB released from pCD showed little variability in the area of inhibition over the course of the assay demonstrating long-term drug stability and efficacy. Image shown is t = day 15. AmB: amphotericin B; pCD: polymerized cyclodextrin. (A color version of this figure is available in the online journal.)
Figure 6.
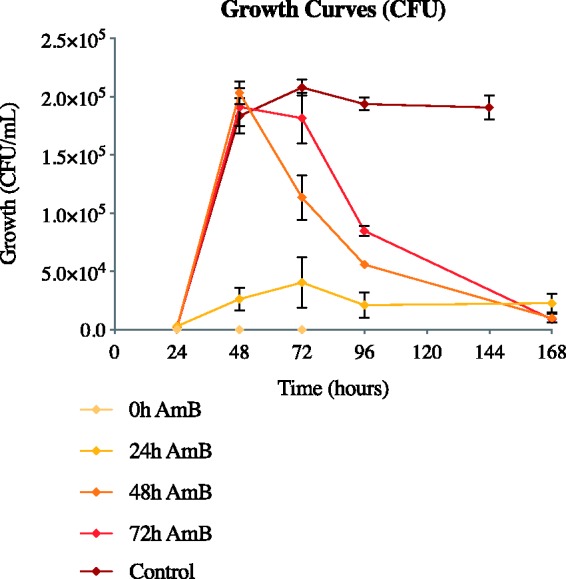
AmB-loaded pCD prevents progression of S. cerevisiae from lag to exponential growth phase and is capable of killing S. cerevisiae once established in late exponential or stationary phase. Growth curves of S. cerevisiae are shown with addition of AmB-loaded pCD-β disks at time = 0, 24, 48, and 72 h postseeding. Control represents S. cerevisiae growth with no antifungal intervention. Addition of AmB-loaded pCD-β disks shows significant killing at all time points. AmB: amphotericin B; CFU: Colony forming units. (A color version of this figure is available in the online journal.)
Determining complexed activity using a contact killing assay
Based on the SPR data, which indicates AmB is more likely to be associated with pCD than released at early time points, we also tested the antifungal activity of AmB-loaded pCD without solution release. AmB-loaded pCD disks showed significant contact killing when challenged with S. cerevisiae on the pCD disk surface, in comparison to disks not loaded with AmB (Figure 5). These data show that AmB dissociating from a CD pocket and diffusing away (i.e. ‘delivery’) is not necessary for antifungal activity, and that AmB retains antifungal abilities when still associated with the CD pockets.
Figure 5.
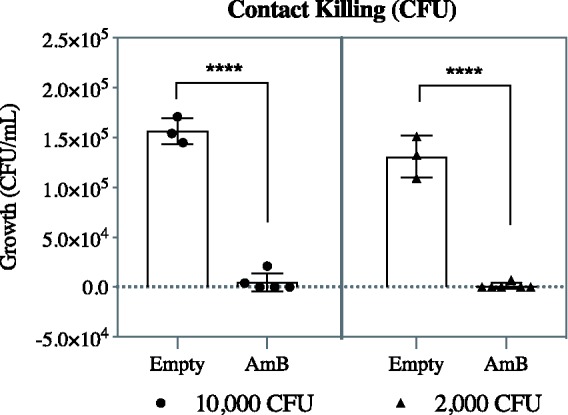
AmB does not need to dissociate from the CD pocket for antifungal activity. S. cerevisiae was cultured on AmB-loaded pCD disks or pCD without drug at two different yeast cell burdens (10,000 or 2000 CFU) for 3 h prior to culturing in sterile YM broth for 72 h, dilution and plating. AmB loaded into pCD significantly reduced viable S. cerevisiae. Statistics: ****represents p < 0.0001. AmB: amphotericin B; CFU: Colony forming units.
Determining activity against established colonies using a solution killing assay
The zone of inhibition assay may give indication of persistence of drug effect, but it does not represent all wound environments, particularly ones with high fluid content. Additionally, while the two previously mentioned assays might give indication as to how this device can prevent new infections, it does not show whether the device is capable of treating existing infections. To address these conditions, antifungal activity of AmB-loaded pCD was also tested in solution with varying time points of device introduction. When AmB-loaded pCD-β disks are added to a S. cerevisiae culture at t = 0 (time of seeding, inhibition), all yeast cells appear eradicated and no future growth is seen (Figure 6). When AmB-loaded pCD-β disks are added to the yeast cultures at t = 24 h, growth is contained at low levels. Treating yeast cultures with AmB-loaded pCD disks at t = 48 h and t = 72 h lines (when growth is established) resulted in a slow, steady killing of yeast cells. After 168 h, all AmB-loaded pCD-β disk curves show significant reduction of microbial load.
Cytotoxicity of pCD-released AmB
AmB-loaded pCD disks show reduced cell toxicity when compared to an equivalent dose of free AmB. Toxicity of free AmB is significant and is due to drug toxicity, not DMSO solvent concentration, which is shown by the relative high cell counts in the 1% DMSO and blank-loaded pCD experimental groups (p < 0.0001 and 0.001, respectively; Figure 7). While free AmB resulted in an average cell survival of 1%, pCD-delivered AmB showed an average survival of 40% after 24 h (day 1), when the small burst release occurs. However, after six days of release, AmB-loaded pCD disks are no longer significantly cytotoxic (Figure 8). This result is consistent with the release observed for AmB from pCD in vitro, where there is a small initial burst followed by a long, steady release after day 5. In addition, the type of pCD shows no impact on cytotoxicity, which is consistent with the release observed for AmB (Figure 3).
Figure 7.
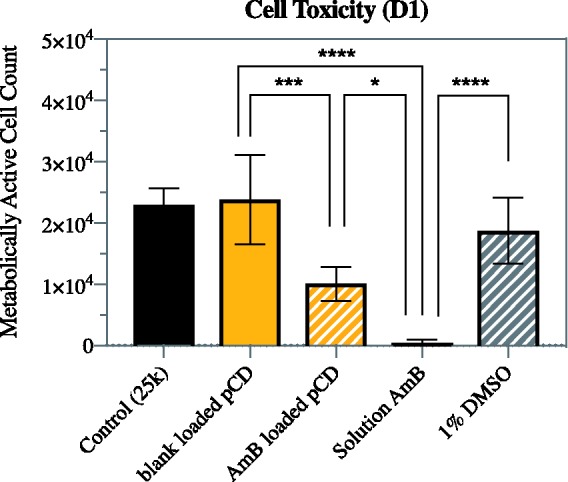
AmB released from pCD shows significantly reduced toxicity compared to free AmB. The cytotoxicity of free drug was confirmed by solvent control and blank-loaded pCD exposed to DMSO solvent but no drug, which both showed minimal toxicity compared to cell only control. All conditions were tested with 3T3 mouse fibroblast cells and 24 h exposure. Statistics: **** represents p < 0.0001, *** represents p < 0.001, and * represents p < 0.03. AmB: amphotericin B; DMSO: dimethyl sulfoxide; pCD: polymerized cyclodextrin. (A color version of this figure is available in the online journal.)
Figure 8.
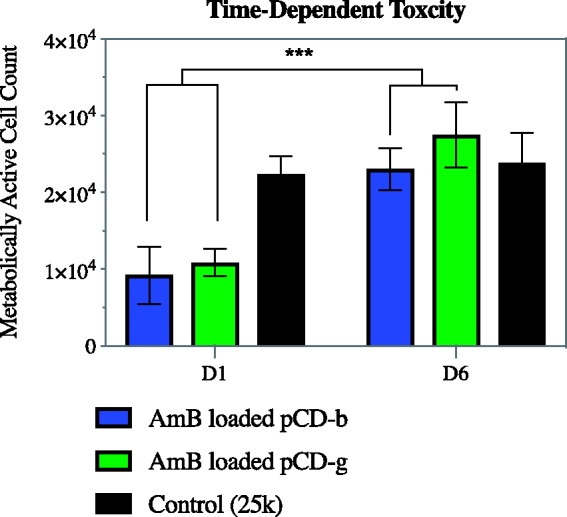
Cell toxicity from AmB-loaded pCD hydrogels is significantly reduced at later time points when a steady amount of AmB is released. AmB-loaded pCD was placed in serum containing media with daily media changes. After one or six days, AmB-loaded pCD disks were placed on 25,000 3T3 cells and after 24 h exposed to MTT for 4 h before reading absorbance. Statistics: *** represents p < 0.001. AmB: amphotericin B; pCD: polymerized cyclodextrin. (A color version of this figure is available in the online journal.)
Discussion
Determining binding affinity using SPR
SPR data indicated a very strong affinity between AmB and βCD ( M−1; Table 1) using aqueous 50 mM NaOH as the regeneration buffer. SPR first allows AmB to flow past immobilized βCD and associate followed by buffer-only dissociation. The regeneration step is designed to interfere with the interaction and cause the drug to dissociate. The addition of DMSO solvent significantly lowered the value by a magnitude of 108 indicating hydrophobic interactions are critical to AmB associating with βCD. Under normal physiological conditions, AmB is favored in the AmB-CD associated state. Adding DMSO to lower the solvent polarity shifts the equilibrium significantly toward dissociation. Understanding the binding strength and mechanism of AmB with βCD is critical to optimizing loading conditions and predicting release kinetics of drugs from pCD.
Table 1.
values of various regenerations determined via SPR.
| Regeneration | KD (M−1) |
|---|---|
| 50 mM NaOH | 5.44 × 10−7 |
| 25% DMSO in PBS | 5.45 × 10−5 |
| 50% DMSO in PBS | 5.48 |
DMSO: dimethyl sulfoxide; PBS: phosphate buffered saline.
Characterizing AmB–pCD complex using FTIR
We used FTIR to confirm that AmB is associated with the CD pocket and begin determining how the drug positioned itself inside the CD pocket. The FTIR spectra of the AmB-loaded pCD showed the 1670 C = O peak (Figure 2), which was not present in the unloaded βCD spectra indicating the presence of AmB. The peak at 1670 corresponds to the ester bond on AmB and also shows that the carboxylic acid group of AmB is outside the hydrophobic CD pocket.
In vitro AmB release profile
The results from the drug release study (Figure 3) confirm the SPR findings that show a very high affinity for AmB to pCD. The small burst-type release seen in the first few days is likely the result of non-specifically bound AmB and the outer shell of CD pockets releasing AmB. After this initial burst, the release shifts into a slow, steady release rate approaching zero-order kinetics (Figure 3(b)). There was no significant difference in the release from pCD-α, -β, and -γ disks, indicating that the difference in the affinity profile of AmB for the three different types of CD is small enough to be overwhelmed by the equilibrium kinetics of dissociation into free solution.
Antifungal activity of pCD complexed and delivered AmB
The zone of inhibition data (Figure 4) shows that the AmB-loaded pCD disks were able to kill yeast out to at least 21 days with no significant reduction in inhibitory area. These results indicate that the AmB-loaded pCD is bioactive and able to kill a large number of yeast on a chronic timescale.
The SPR and release data show that pCD acts as a large sink for AmB meaning that at shorter timescales more drug stays in the pCD polymer than is released. We therefore tested whether AmB associated with pCD is capable of killing yeast through contact rather than direct release. AmB-loaded pCD significantly reduced the number of yeast cells compared to pCD without drug (p < 0.0001; Figure 5). Thus, AmB is cytotoxic to fungus whether associated or released from our pCD polymer.
AmB-loaded pCD was able to prevent S. cerevisiae from entering exponential growth phase (Figure 6) and was able to kill sufficient yeast cells in exponential growth to reduce the burden near initial seeding levels. This solution-based killing assay demonstrates that AmB-loaded pCD can kill even growing populations of fungal cells through a combination of drug release and contact killing. These data indicate further investigation of our AmB–pCD system for both prevention and treatment of fungal infections, up to full (72 h) growth is warranted. As fungal infections are often difficult to diagnose, a system that can be used for both prevention and treatment is a very clinically relevant goal.
Cytotoxicity of pCD-released AmB
The toxicity of free AmB is well known, and liposomal AmB is often used instead in an attempt to minimize the toxicity. Figure 6 shows that AmB-loaded pCD is significantly less toxic than free AmB, where both conditions resulted in a 10 µg/mL concentration of free/delivered AmB in the well (24 h time point). In fact, the AmB-loaded pCD condition could be considered to have more AmB overall, since the 10 µg/mL concentration occurs due to drug that has been released, and there is >100 µg of drug still held within the polymer. The decrease in toxicity seen in the AmB-loaded pCD condition is due to the occurrence of drug release over time, in comparison to the bolus dose used in traditional systemic administration (free AmB condition). While associated with CD, AmB does not appear to exhibit cytotoxic effects, meaning the consistent release of drug into media is what allows for the cytotoxicity that is seen. In this way, cells are likely exposed to fewer active AmB molecules at the beginning of treatment. Additionally, while there is some toxicity associated with the AmB-loaded pCD initially, this toxicity is eliminated once the burst release is over (Figure 8) and slow, steady release of AmB is achieved (Figure 3). This aligns with current usage, as the current burst release of 10 µg/mL is higher than clinically used AmB concentrations (plasma concentrations of 0.5 to 2 µg/mL), while later time points are more consistently within this range. The combination of solution-based killing (Figure 6) with lack of long-term cytotoxicity indicates that AmB-loaded pCD is a potentially attractive option for treating fungal infections in medically fragile populations.
Overall, this work showed that AmB exhibits high affinity for CD and binds tightly to pCD. AmB releases for more than 24 days at near zero-order kinetics following an initial burst. AmB-loaded pCD was capable of clearing S. cerevisiae lawns for > 21 days and cells in contact with the drug-loaded polymer. In addition, AmB-loaded pCD prevented growth of S. cerevisiae in culture from lag into exponential growth. The AmB-loaded pCD also was capable of recovering established colonies of yeast. The toxicity of AmB-loaded pCD is significantly less than free AmB and quickly resolves to non-toxic levels after the initial burst release. Taken together these data indicate that AmB-loaded pCD can both prevent and treat fungal infections, but in vivo testing is required for further evidence.
In conclusion, we provided data demonstrating that AmB-loaded pCD is capable of preventing fungal growth and eliminating established fungal colonies with minimal toxicity to the host cells. This AmB delivery system represents a promising strategy for the treatment of localized invasive fungal infections, where systemic bloodstream administration is not necessary. With the current formulation, we could treat fungal growth at the site of implanted materials such as catheters and shunts, or in the abdominal region, as is the case in intra-abdominal candidiasis (Candida peritonitis). Our lab has also demonstrated the production of our pCD delivery system in a microparticle form.25 This microparticle delivery system could potentially be used to adapt the AmB delivery for use in pulmonary fungal infections. Addressing fungal infections of all types continues to be a growing health care concern as we learn more about the mycobiome and its disruption through various medical interventions.
ACKNOWLEDGMENTS
The authors of this paper would like to thank Sonia Merritt, Lynn Dudash, Sukwoo Xavier Yoon, and CWRU’s Macromolecular Engineering Department’s Research Experience for Undergraduates.
Authors’ contributions
RMH and STZ contributed equally to this paper. RMH and CAG were responsible for data collection and analysis. STZ and JNK worked on experimental design. RMH and STZ wrote and reviewed the manuscript with assistance from HvR.
DECLARATION OF CONFLICTING INTERESTS
STZ and JNK are employees of Affinity Therapeutics and receive salary. HvR is a co-founder of Affinity but does not receive salary.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by an NSF CAREER award, CBET-0954489, and an NIH Research Facilities Construction Grant C06 RR12463-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or National Science Foundation.
References
- 1.Dufour G, Montravers P. Pharmacokinetics of antibiotics or antifungal drugs in intensive care units. Curr Infect Dis Rep 2009; 11:14–20 [DOI] [PubMed] [Google Scholar]
- 2.Suleyman G, Alangaden GJ. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin North Am 2016; 30:1023–52 [DOI] [PubMed] [Google Scholar]
- 3.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013; 13:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raychaudhuri R, Yu W, Hatanpaa K, Cavuoti D, Pride GL, White J. Basilar artery dissection treated by Neuroform stenting: fungal stent infection. Surg Neurol 2009; 71:477–80 [DOI] [PubMed] [Google Scholar]
- 5.Ramage G, Jose A, Sherry L, Lappin DF, Jones B, Williams C. Liposomal amphotericin B displays rapid dose-dependent activity against Candida albicans biofilms. Antimicrob Agents Chemother 2013; 57:2369–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh N. Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clin Infect Dis 2001; 33:1692–6 [DOI] [PubMed] [Google Scholar]
- 7.Vincent BM, Lancaster AK, Scherz-Shouval R, Whitesell L, Lindquist S. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 2013; 11:e1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steimbach LM, Tonin FS, Virtuoso S, Borba HHL, Sanches ACC, Wiens A, Fernandez-Llimós F, Pontarolo R. Efficacy and safety of amphotericin B lipid-based formulations – a systematic review and meta-analysis. Mycoses 2017; 60:146–54 [DOI] [PubMed] [Google Scholar]
- 9.Robledo-Ávila F, Pérez-Tapia M, Limón-Flores A, Pavon L, Hernández-Pando R, Wong-Baeza I, González-González G, Tovar C, Estrada-Parra S, Estrada-García I. Low-dose amphotericin B and murine dialyzable spleen extracts protect against systemic candida infection in mice. Clin Dev Immunol 2013; 2013:194064–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lincopan N, Mamizuka EM, Carmona-Ribeiro AM. Low nephrotoxicity of an effective amphotericin B formulation with cationic bilayer fragments. J Antimicrob Chemother 2005; 55:727–34 [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, Dey A, Singh N, Somvanshi R, Singh S. Evaluation of toxicity & therapeutic efficacy of a new liposomal formulation of amphotericin B in a mouse model. Indian J Med Res 2013; 137:767–76 [PMC free article] [PubMed] [Google Scholar]
- 12.Wade RL, Chaudhari P, Natoli JL, Taylor RJ, Nathanson BH, Horn D. Comparison of adverse events and hospital length of stay associated with various amphotericin B formulations: sequential conventional amphotericin B/lipid versus lipid-only therapy for the treatment of invasive fungal infections in hospitalized patients. P T 2013; 38:278–87 [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu K, Osada M, Takemoto K, Yamamoto Y, Asai T, Oku N. Temperature-dependent transfer of amphotericin B from liposomal membrane of AmBisome to fungal cell membrane. J Control Release 2010; 141:208–15 [DOI] [PubMed] [Google Scholar]
- 14.He J, Chipot C, Shao X, Cai W. Cyclodextrin-mediated recruitment and delivery of amphotericin B. J Phys Chem C 2013; 117:11750–6 [Google Scholar]
- 15.Espuelas MS, Legrand P, Irache JM, Gamazo C, Orecchioni AM, Devissaguet JP.Ygartua P. Poly(ε-caprolacton) nanospheres as an alternative way to reduce amphotericin B toxicity. Int J Pharm 1997; 158:19–27 [Google Scholar]
- 16.Sánchez-Brunete JA, Dea MA, Rama S, Bolás F, Alunda JM, Raposo R, Méndez MT, Torrado-Santiago S, Torrado JJ. Treatment of experimental visceral leishmaniasis with amphotericin B in stable albumin microspheres. Antimicrob Agents Chemother 2004; 48:3246–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborty KK, Naik SR. Pharmacokinetic studies of in-situ liposomal preparation containing amphotericin B complexed with different chemically modified beta-cyclodextrins. J Liposome Res 2001; 11:1–14 [DOI] [PubMed] [Google Scholar]
- 18.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov 2004; 3:1023–35 [DOI] [PubMed] [Google Scholar]
- 19.Fu AS, Thatiparti TR, Saidel GM, Recum von HA. Experimental studies and modeling of drug release from a tunable affinity-based drug delivery platform. Ann Biomed Eng 2011; 39:2466–75 [DOI] [PubMed] [Google Scholar]
- 20.Wang NX, Recum von HA. Affinity-based drug delivery. Macromol Biosci 2011; 11:321–2 [DOI] [PubMed] [Google Scholar]
- 21.Thatiparti TR, Shoffstall AJ, Recum von HA. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials 2010; 31:2335–47 [DOI] [PubMed] [Google Scholar]
- 22.Cyphert EL, Learn GD, Hurley SK, Lu C-Y, Recum von HA. An additive to PMMA bone cement enables postimplantation drug refilling, broadens range of compatible antibiotics, and prolongs antimicrobial therapy. Adv Healthc Mater 2018; 10:e1800812. [DOI] [PubMed] [Google Scholar]
- 23.Thatiparti TR, Recum von HA. Cyclodextrin complexation for affinity-based antibiotic delivery. Macromol Biosci 2010; 10:82–90 [DOI] [PubMed] [Google Scholar]
- 24.Halpern JM, Gormley CA, Keech M, Recum von HA. Thermomechanical properties, antibiotic release, and bioactivity of a sterilized cyclodextrin drug delivery system. J Mater Chem B 2014; 2:2764–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grafmiller KT, Zuckerman ST, Petro C, Liu L, Recum von HA, Rosen MJ, Korley JNJN. Antibiotic-releasing microspheres prevent mesh infection in vivo. J Surg Res 2016; 206:41–7 [DOI] [PMC free article] [PubMed] [Google Scholar]