Short abstract
Microorganisms are widely distributed all over the Earth, inhabiting very diverse natural ecosystems, from the human body to inanimate indoor environments. Until recently, the methods most commonly used to study microbes have been culture-dependent approaches relying on the phenotypic evaluation of isolates that can grow in laboratory conditions. Given the advances in molecular biology and high-throughput DNA sequencing methodologies, scientists could expand their microbiome knowledge to microorganisms that do not grow well in the laboratory or have been considered too difficult and laborious to be cultivated. Culture-independent methods such as direct DNA sequencing can be performed for many samples at once, revealing the entire microbial profile of the samples and making possible the rapid characterization of the whole environmental microbiome. Investigating the microbiome profile of indoor environments such as hospitals, houses, offices and other buildings is of major concern because it could include a number of opportunistic, pathogenic or nosocomial microbes. Additionally, these environments could serve as reservoirs of virulence or antimicrobial resistance, which could be spread by humans or other vectors. High-throughput DNA sequencing has enabled large-scale microbiome screening for multiple indoor areas in a single analysis. Using this approach, we can easily track microorganisms in the environment and monitor microbiome composition related to hygiene processes or environment quality. Gaining such information and resolution regarding indoor microbiome analysis can lend very important assistance for epidemiological surveillance.
Impact statement
Research concerning the microbiome of indoor environments like hospitals, houses or buildings could have several implications for human health. Today, there is an ongoing shift in the paradigm of microbial analysis, from single isolated bacterial samples to entire microbiome profiles using high-throughput DNA sequencing methods. The use of sequencing methods in several studies has revealed an unprecedented microbial diversity in indoor environments, leading to a larger comprehension of the entire microbiome context. Here, we present a review of these microbiome studies using high-throughput DNA sequencing, including some new approaches and ideas that can be broadly applied in microbial tracking and epidemiological surveillance of indoor environments.
Keywords: Microbiome, hospital, bacteria, built environment, high-throughput DNA sequencing
Introduction
Our planet is populated by many complex communities living in different ecosystems. Microorganisms such as bacteria are part of this global structure, constituting the earth microbiota that can live in harmonic or disharmonic association with other organisms. For example, the human body and its inhabiting symbiotic microorganisms can be defined as a holobiont system;1,2 unfortunately, this system can also include some pathogenic bacteria.3,4 All these microorganisms can be identified through their genome sequences or specific marker genes, as has been done in projects like The Earth Microbiome Project5 and The Human Microbiome Project.6 The concept of a microbiome refers to the microorganisms and their respective genomes living in particular habitats,7 including natural or built environments.
Indoor habitats such as buildings, offices, houses or even hospitals have begun to be investigated using new DNA high-throughput sequencing technologies to identify and characterize their microbiome composition.8,9 These approaches are based on culture-independent molecular microbiology methods used to access DNA information directly from a sample. Rapid advances in next-generation sequencing technologies have been increasing the throughput of data and decreasing the experimental costs, transforming the biomedical and biological research fields.10
The application of next-generation sequencing technologies to microbiome characterization in hospitals and built environments is revealing new knowledge regarding indoor microbial colonization and dispersion. These methodologies take less time and are less skewed to specific cultivable microorganisms, allowing large-scale screening of microbes, including those that do not grow well in laboratory conditions. As was reported for one intensive care unit (ICU) in a hospital, only 2.5% of total bacterial diversity was recovered by standard cultivation techniques, in relation to DNA sequencing methodology.11 Nevertheless, new culturomics approaches (microbiological culturing associated with DNA sequencing, mass spectrometry and other methods) are also important in the characterization of novel species.12 The biodiversity of environmental microorganisms revealed by these technologies is constantly increasing, leading researchers to also investigate built habitats in cities and urban environments, such as buildings, hospitals, and cleanrooms.8 Moreover, the study of microbiomes in hospital environments offers some of the strongest implications in the healthcare system in relation to healthcare-associated infections (HAIs) and patient outcomes.
HAI are a subject of high concern in public health, causing morbidity and mortality elevation when patients are infected with a microorganism during a hospital stay.13 Hospital and built surfaces remain overlooked reservoirs for a number of microorganisms that can directly impact human health through contaminations or infections.11,14,15 It is therefore important to better comprehend our closest associations with indoor and environmental microorganisms, along with their implications for human health. In this scenario, molecular microbiology and high-throughput DNA sequencing could be extremely helpful in identifying and characterizing the microbiomes of hospitals and built environments. Microbiome identification through DNA sequencing can be used to detect and track microorganisms in large-scale screenings. These results can be directly applied in microorganism surveillance programs, cleanliness monitoring, and building management to provide more reliable data to aid in health care decisions.
Methods to identify microbes and microbiomes
Microbiological culturing has been the most commonly used method for identifying microorganisms since the beginning of biological studies. It has long been discussed, however, that not all microorganisms possess the ability to grow in laboratorial standard culture media; only a small portion of the total diversity that exists on our planet is readily cultivable by traditional microbiology.12,16,17 Approximately 1% of bacteria found in the environment, representing only half of known bacterial phyla,12 are cultivable using currently available techniques, meaning that there exists a hidden ocean of diversity that has never been seen in previous culturing studies.16 Likewise, clinical studies of HAI have been intensively focused on traditional culture isolates, genotyping of known pathogens, and characterization of potential transmission routes.18 This cultivable/non-cultivable paradox has increased as new high-throughput sequencing technologies have become widely used in research concerning large-scale environment characterization.12,19 Several studies have since been published demonstrating the lower recovery of microbial diversity in laboratory cultivation using conventional microbiological analysis.3,5,20,21 To date, there are two principal approaches to evaluating the microbiome from a sample – culture-dependent and culture-independent methods – each with advantages and disadvantages.
Among culture-dependent methods, microbial identification relies on detection by staining (e.g. Gram or fluorescence), electron microscopy, growth detection on liquid or solid agar medium, morphological or biochemical profiling.12,22 In addition, classical phenotypic characterization can be applied to the cultivable microorganisms, such as biochemical tests, specific substrate degradation, or mass spectrometry profile evaluation. Microbiologists are constantly revitalizing culturing methods, creating new ways of simulating the best environmental conditions favorable to microorganism growth: adding co-cultures with other bacteria from the same environment or mimicking host-associated environments.12,16 In clinical microbiology, culture-dependent methods remain highly used, as the great majority of known human pathogens are readily cultivable and could be present in extremely low amounts to be detected without selective enrichment. These culture-dependent approaches, however, mainly focus on identification of single isolated microorganisms, grown one at a time, requiring several days, weeks, or even months of experimental effort to obtain results that will likely underestimate the true microbiological diversity when considering the whole environment context.22,23
Culture-independent methods have become more frequently used since the rapid improvement of molecular microbiology methods for bacterial identification.9,22 Molecular tools such as whole genome, universal marker gene or transcriptome sequencing, as well as real-time PCR, RT-qPCR and DNA hybridization, are some examples of the many approaches that can be used in bacterial identification and microbiome studies.12,24–26 These molecular approaches can be directly applied with the most diverse environmental samples (natural, built, indoor, hospital, clinical, etc.) to conduct large-scale, cost effective analysis with rapid results. Once these molecular microbiology methods rely on microbe nucleic acid (DNA or RNA) identification, they can be directly applied to samples regardless of microbiome diversity or presence of uncultured microorganisms. Currently, the most commonly used culture-independent method is DNA sequencing directly from an environmental sample. The high-throughput DNA sequencing method is generally applied to DNA recovered from the environment following one of two main approaches: amplicon or shotgun metagenomics. Amplicon sequencing refers to specific sequences that generally target universal marker genes for a microbial group of interest, such as the ribosomal 16S rRNA gene in bacteria or the Internal Transcribed Spacer (ITS) in fungi. In metagenomics, all the DNAs present in a sample are fragmented by shotgun and sequenced; however, this DNA includes not only the microbial DNA, but also hosts and/or environmental DNA. Amplicon approaches are relatively simpler, faster, and less expensive than metagenomics, but both require standardization, careful design, and analysis to achieve reproducible and accurate results.25 Given the diversity of samples being processed and investigated with molecular microbiology methods in microbiome studies, there has been an increase in “microbiological dark matter” – DNA sequences recovered that may not match already known species, requiring additional characterization methods. All experimental methods have vantages, advantages, biases, weaknesses, and strengths that should be considered during experimental design and hypothesis testing. Thus, a combined approach has also emerged – culturomics, which associates both culture-dependent microbiology and culture-independent molecular biology. Culturomics has mostly been applied in human microbiome studies so far, such as in characterizing the vast majority of unknown bacteria in the gut microbiome.12,19,27
With culture-independent methods, there is much concern regarding the microbial cellular viability recovered by DNA sequencing, as the DNA from dead cells could remain in the environment. There are several mechanisms that can be employed to overcome this bias, such as treating the sample with PMA (propidium monoazide) to block the amplification of dead cells’ DNA.28,29 Nevertheless, even the presence of DNA from dead cells can be relevant because nearly all bacteria are capable of horizontal gene transfer and transformation.30 By taking the “dead” DNA from surrounding environments, incorporating and multiplying it, bacteria can spread antimicrobial or virulence genes in hospitals and other built environments, as well as becoming resistant or virulent themselves. This mechanism could be of high importance but requires further investigation; DNA sequencing methods could be extremely useful in this research.
Despite all the methodological singularities, culture-independent methods based on molecular microbiology could bring greater understanding of the microbiomes of hospitals and other built environments. Direct sample analysis, amplicon or metagenomics DNA sequencing, and bioinformatics techniques are revolutionizing microbiological analysis in hospital environments, allowing rapid, efficient, sensitive, and less labor-intensive microbial identification.18,21 With this whole environment sequencing approach, it is possible to track microorganism contamination sources not only based on microorganism presence or absence, but also by phylogenetically comparing microbial DNA in one sample to that in another and thereby inferring functional and ecological traits even for the unknown “dark matter.” Phylogenetic analysis of microbiome data allows the classification of unknown organisms by performing trait and habitat associations to better understand how microbiomes differ and change in response to time or environmental conditions. 31 Additionally, advances in culture-independent molecular biology and nucleic acid sequencing are assisting in resistome studies, characterizing potential antimicrobial resistance genes (AMR) found in microbes and in the environment.4 Using sequencing technologies, it has become possible to shift from identifying already-known pathogenic bacteria to considering the whole environmental ecosystem along with its microbial communities, putting the microbiome into an ecological perspective of interaction with other species,8 including humans in indoor environments.
Discovering indoor microbes using high-throughput DNA sequencing methods
Health-care facilities like hospitals, clinics, and long-term care facilities are not microorganism-free, as bacteria, fungi, viruses and other small organisms are widely distributed over the Earth.5 Nevertheless, the phylogenetic diversity in hospital indoor microbiome has been shown to be lower than that of outdoor environments with a dominance of Betaproteobacteria, whereas the outdoor environment is dominated by more diverse Actinobacteria, Gammaproteobacteria, and Alphaproteobacteria.32 This reduced microbial diversity in indoor environments could have unknown consequences for human health,18 selecting a restricted subset of microorganisms and decreasing exposure to the environmental microbes that coevolved with humans.33 Inanimate surfaces in built environments have been reported as a potential reservoir for microorganisms such as Gram-positive bacteria (Clostridium, Enterococcus, Staphylococcus, Streptococcus), Gram-negative bacteria (Acinetobacter, Escherichia, Klebsiella, Pseudomonas, Proteus, Serratia, Vibrio), fungi (Candida) and viruses, all of which can survive for days or months on dry surfaces.34,35 Commonly, these microorganisms are identified as pathogens implicated in HAI and as important contributors to morbidity and mortality. In general, the hospital microbiome is dominated by a small number of prevalent bacterial taxa, and most of the bacteria identified are human-related commensals or pathogens.11,32 A previous review8 reported that there are multiple sources and reservoirs of microorganisms in the indoor environments of hospitals, including the rooms and patient close surfaces, the personnel that come in contact with the patients, and invasive and non-invasive equipment and devices, as well as textiles from uniforms, bed linen, clothes, and curtains. One ICU investigation compared bacterial profiles of floors, workplaces and devices, which revealed the presence of seven major bacterial phyla: Acidobacteria, Actinobacteria, Bacteroidetes, Cyanobacteria, Firmicutes, Nitrospira and, most abundant of all, Proteobacteria. Hospital devices were the most diverse bacterial reservoirs, followed by workspaces and floor, with Pseudomonas, Propinibacterium, and Burkholderia identified as potential infection sources.11 This potential of the environment to be a microbiological reservoir increases the risk of microorganism dispersion and contamination.35 Additionally, more studies have reported that healthcare workers can contaminate their hands after touching inanimate surfaces and equipment near a contaminated patient,36 making humans one of the most important dispersal vectors for microorganisms in hospital environments.11 Humans as microbiological vectors have also been identified in other built environments, such as our homes, where surfaces and objects can be tracked back to specific human skin, such as a fingerprint.8,17,33,37
Furthermore, studies have found that bacterial diversity varies among different hospital areas, as it has been shown that the halls, living rooms, patient rooms, and rest rooms exhibit more diverse bacterial compositions than that of the ICU.11,38 The ICU generally has stricter sanitation protocols than do other areas in a hospital, which could act selectively for some microorganisms able to live in more challenging environmental conditions.11,38 In fact, ICUs are the most studied areas in hospital microbiome research, given the high rate of ICU-acquired infections related to multidrug-resistant pathogens.8,9,11,36,39,40 More confined habitats, such as the ICU or operating rooms, must be microbiologically monitored and controlled through cleaning and disinfection. These procedures are constantly selecting microorganisms with resistance abilities against physical, chemical and antimicrobial stress, increasing the survival of multi-resistant microorganisms. Multidrug resistant (MDR) Gram-positive microorganisms such as methicillin-resistant Staphyloccoccus aureus and vancomycin-resistant enterococci are commonly identified as part of the hospital microbiome.8 Hospital surveys also frequently identify Gram-negative bacteria such as Enterobacteriaceae (e.g. Klebsiella, Escherichia), demonstrating their major roles in MDR pathways.18 A hospital microbiome can harbor a diverse set of antimicrobial resistance genes that are extremely relevant to human health, reflecting in HAI rates. Some studies have focused mainly on hospital environments and the acquisition of resistance genes by novel evolutionary mechanisms or by lateral gene transfer between microorganisms in the same environment.18 One study also evaluated the presence of antimicrobial resistance genes in the indoor dust microbiome, revealing the bacterial resistome profile related to several antimicrobial chemicals tested.41 Amplicon and whole-genome sequencing methodologies were used to investigate the microbiome and biofilm formations in shower hoses from five different hospitals. That study found several prevalent bacterial genera that can be highly resistant to disinfection procedures, and a diversity profile in the biofilms that was never seen before. Additionally, the authors highlighted the presence of potential genes conferring antimicrobial resistance in the shower hoses.42 These microbe interactions and resistome profiles can be readily tracked and monitored through microbiome investigations using high-throughput DNA sequencing methodologies, adding new perspectives to the microbiology field. Understanding how these microbiomes persist and spread in built environments is essential to elucidating their influences on human health.
A recent study performed a large survey using molecular techniques to investigate the microbiome of a hospital before and after its opening, in order to understand at a community level how microorganisms colonize and spread through the hospital environment, including surfaces, staff, and patients.21 One year after the hospital opened, the researchers found that bacteria in a patient room resembled the skin microbiota of the patient occupying the room and become more similar throughout the patient’s stay. Additionally, they reported the patient acquisition of room microorganisms that were present in the room before the patient’s admission. Using a shotgun metagenomics approach, they found that several antimicrobial resistance genes were more abundant on room surfaces than on the skin of the patients from the investigated rooms.21
The urbanization process has also been investigated by taking swab samples from floors and walls of the indoor environments of houses (kitchens, living rooms, bathrooms and bedrooms), directly extracting microbial DNA and subjecting the DNA to high-throughput sequencing. The microbial diversity analyzed indicates that bacteria from the surfaces of house environments are informative of urbanization levels, as well as being related to specific house areas and the architectural design.33
Indoor microbiomes from our houses can also vary in composition among internal areas of the same built environment, creating multiple microenvironments with specific microbiological characteristics. For example, in the context of built environments, a meta-analysis of several studies revealed that despite the methodological differences among studies, the microbiome of toilets, kitchens, and restrooms were more similar to those same surfaces in different studies than they were among the different environments, even from the same study.9 Even considering different geographical localizations (South Korea or USA), bathrooms showed profiles of skin-associated bacteria (Propionibacterium, Corynebacterium, and Streptococcus) that were more similar to those of other bathrooms than to those of kitchens, which were more populated by environmental-associated Acinetobacter.9 Additionally, another study performed a large-scale investigation using bacterial 16S rRNA gene sequencing of samples from showerheads in houses, apartments, and public buildings. That study found a complex and variable assemblage of microbes in showerheads, as well as a large number of specific opportunistic human pathogens enriched in showerheads biofilms.43
Built environments such as cleanrooms or space stations have also been intensively investigated by swabbing surfaces and subjecting them to high-throughput DNA sequencing to find hidden microbes in these environments. Cleanrooms are intended to have a very low number of microorganisms; however, the application of next-generation sequencing has allowed a better assessment of the total microbiome in confined habitats, showing that microorganisms can survive in these locations and are mainly brought in by human bodies.8
Understanding the microbiome of food-processing built environments is also very important for assessing food product quality. Tracking microbe sources and outcomes is being increasingly investigated by amplicon sequencing, metagenomics, or transcriptomics, in order to understand microbiological contaminations and improve the quality of the food process.44 Shelf-life quality and food deterioration could also be investigated using these microbial sequencing methodologies, leading to improving the final product quality and increasing its shelf-life time.
Overall, the studies discussed above show how microbiome analysis using DNA high-throughput sequencing is widely applicable to many sample types from indoor environments. Nevertheless, there must also be careful experimental design, data collection, analysis and statistical methods to validate the results, making them reproducible. Experimental questions and hypotheses should be addressed and considered regarding kinds of samples, composition, methods of collection, storage, processing, and analysis.25 Following the best practices, we could then achieve significant and reproducible results that would contribute to scientific research, as well as general knowledge and applicability of the microbiome data to improving health and environmental ecosystems.
Microbiome identification and tracking
Identification of the microbiomes of hospitals and other built environments is highly important, not only for investigating and tracking pathogenic microorganisms, but also for understanding the environmental context of microbes and their implications. A number of research studies have contributed to this shift in the microbiology field by using high-throughput DNA sequencing methodologies to uncover hidden microbiomes and reveal their ubiquitous distribution. Given the broad microbial presence discovered, some studies hypothesize a “new hygiene” concept, suggesting that reduced exposure to microorganisms could lead to disease or disorder states in humans.33 Previous studies have also discussed how the high microbial diversity in an environment could prevent pathogenic infections, meaning that a good strategy could involve increasing the beneficial microbes, expecting them to reduce the pathogenic ones.8 More studies are necessary, however, to better characterize the interactions between microbiomes and the environment, and their implications for human health. Until these interactions can be better elucidated, the most accepted and indicated plan is to identify and eliminate potential harmful and pathogenic microorganisms. Improved cleaning procedures are always in development, as cleaning is the best way known to eliminate HAI pathogenic microorganisms.15,45 Thus, understanding the hospital microbiome could be essential to maintaining low levels of HAI infections and helping in the improvement of healthcare assistance.18
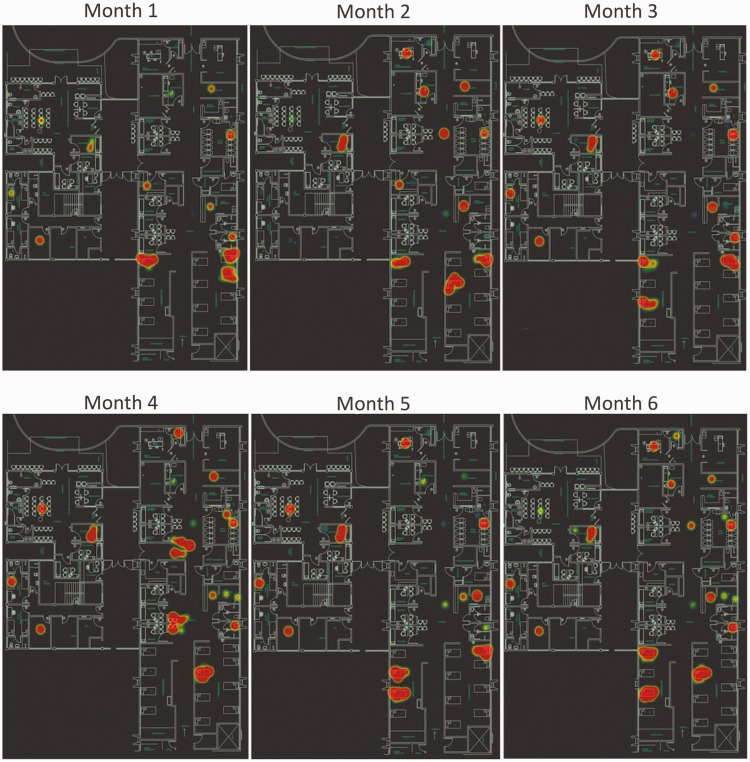
Using large scale methodologies such as microbial amplicon DNA sequencing, hospitals can track microbes to their sink and perform environmental surveillance screening many locations at once to uncover hidden microbiota that will not grow on culture plates or would require much time and effort to achieve the results with conventional microbiology. It is possible to perform large-scale screenings in hospital environments, track down the most contaminated areas, and visualize them in the context of the whole building (Figure 1). These data can be used in microbiome surveillance with diverse purposes, including identification of microbial hotspots, nosocomial pathogens and potential HAI sources, possible contamination pathways and environmental spreading. Monitoring the cleaning process and the seasonal microbiome pattern could also be possible using this large-scale DNA screening. This approach could be applied to any building or facility of interest by performing a large survey with sterile swabs and directly sequencing the microbial DNA recovered from the environment.
Figure 1.
A map assessing hospital microbiome risk. The blue print of the hospital is used to plot the results of DNA sequencing performed over a six-month screening (months 1–6). Red spots represent a heatmap, where larger spots correlate to areas with higher bacterial density. Using this approach, it is possible to perform a large-scale monitoring of indoor areas over time, evaluating cleaning processes, hygienization efficiency and hotspots for bacterial contamination, as well as tracking specific microorganisms and their distribution in hospital areas, contributing to contamination monitoring. (A color version of this figure is available in the online journal.)
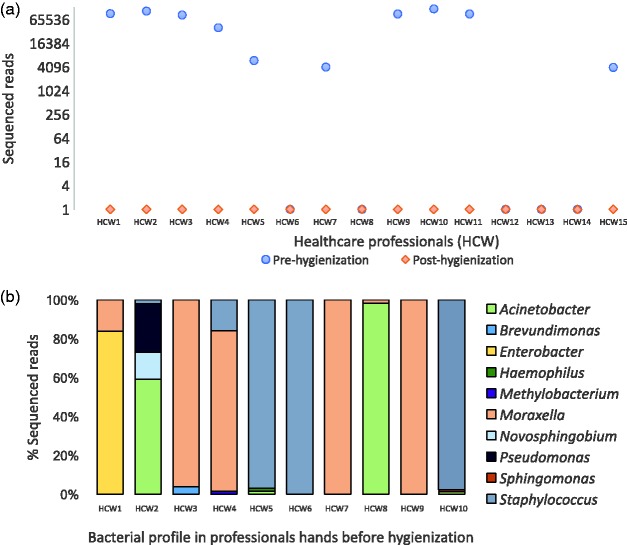
Because the human body is an important microbial carrier interacting with the environment, high-throughput DNA sequencing methodologies can also be employed to monitor and evaluate hand hygiene efficiency (Figure 2). Evaluating the hands of healthcare professionals before and after hygienization demonstrated that high amounts of bacterial sequences are present before sanitation, but these levels drastically decrease after sanitation (Figure 2(a)). Some of the bacteria detected before hygienization belong to known pathogenic and opportunistic genera (Acinetobacter, Enterobacter, Moraxella, Pseudomonas and Staphylococcus; Figure 2(b)), thus reinforcing the importance of adequate hand hygiene by healthcare professionals.46,47
Figure 2.
Tracking hygienization of the hands of healthcare professionals. DNA sequencing was performed directly from swab samples collected from healthcare workers (HCW1 to HCW15) before and after hand hygienization. (a) Pre-hygienization samples have larger numbers of reads for the bacterial 16S rRNA gene. After the hands cleaning protocol, the total number of reads sharply decreases, showing an effective hygienization process. Additionally, one advantage of using high-throughput DNA sequencing is to assess the microbial diversity present in samples and know which microorganisms are present. (b) Identification of bacterial genera sequenced from healthcare professional hands. Generally, species involved in healthcare-associated infections (HAI) and potential pathogens were found (e.g. Staphylococcus, Acinetobacter, Enterobacter, Pseudomonas), highlighting the importance of large-scale monitoring of processes to improve healthcare assistance and decrease HAI. (A color version of this figure is available in the online journal.)
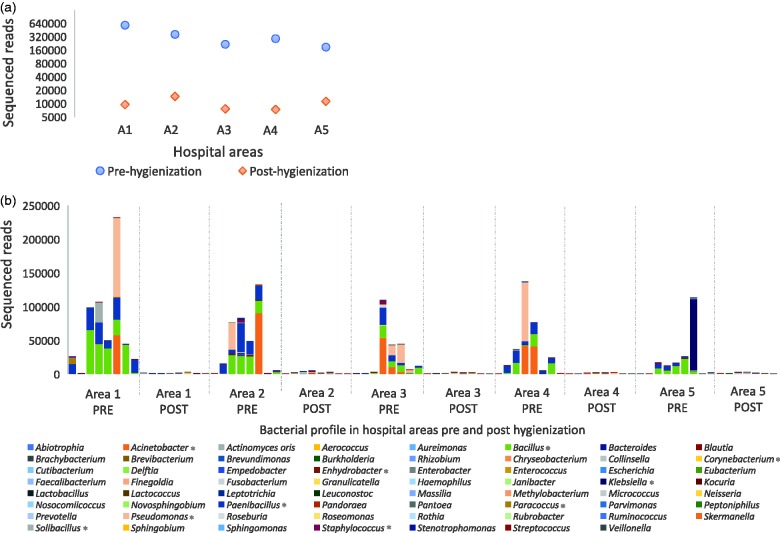
In the same way, microbial presence in the environment can be evaluated before and after hygienization processes (Figure 3). As an example, several hospital areas were sampled with sterile swabs and submitted to direct DNA extraction and high-throughput sequencing. There was a clear decrease in bacterial levels after cleaning processes (Figure 3(a)), showing that the process was efficient. DNA sequencing was an important part of the methods because it permitted bacterial identification, revealing which microbes remained after the cleaning process and if there should have been concern regarding the cleaning process (Figure 3(b)). As discussed above, even if detected DNA was not from a living cell, it must be considered because it could be an environmental reservoir of antimicrobial resistance or virulence genes that could be transferred to other living microbial cells. All experimental data presented above were generated internally (unpublished) by Neoprospecta Microbiome Technologies (Brazil) from an environmental microbiome surveillance using next-generation DNA sequencing (Supplementary material S1) to illustrate some potential applications of the methodology.
Figure 3.
Discovering the hospital microbiome and evaluating hygienization processes. Hospital environment areas (A1–5) were sampled with sterile cotton swabs and submitted to high-throughput DNA sequencing for the bacterial 16S rRNA gene. (a) High numbers of sequenced reads were detected in pre-hygienization areas (blue dots). After the cleaning process, the same areas and locations were re-sampled and exhibited a significant decrease in bacterial reads (post-hygienization). (b) The bacterial profile of each sampled area (A1–5), showing sampling areas before and after hygienization, as well as presenting which bacterial taxon were detected in each location. (*) indicates microorganisms belonging to the most abundant genera detected. This approach could be used in the context of evaluating cleaning processes, but could also investigate environmental microbiome diversity in many areas of the indoor environment. (A color version of this figure is available in the online journal.)
Discussion
Microbiome studies have been the focus of multiple research areas, with increased interest related to human health. The Human Microbiome Project6 is one of the largest surveys being performed to understand human and microbial interactions. The Earth Microbiome Project is another one of the largest collaborative studies evaluating ubiquitous microbial distribution all over the planet. Indoor environments have also been studied in smaller and more isolated studies aiming to characterize the microbiome from these environments. There is great potential for research concerning indoor microbiome environments, such as hospitals, buildings, offices, and homes. Understanding the microbial dynamics of these environments and uncovering their microbial composition can be of great value to improving human health and healthcare assistance. Despite there being some limitations to culture-independent methodologies like amplicon high-throughput sequencing, such methodologies have the incomparable potential of large-scale analysis with lower cost and rapid results, unlike the laborious and specific protocols of culture-dependent methodologies. However, both approaches should be considered as complementary at some extent, such as in studies isolating and characterizing a new microbial species, for example.
High-throughput sequencing of the 16S rRNA gene is very useful in large-scale bacterial screenings. Through approaches like this, it is possible to continuously monitor the environmental microbiome for months or years in multiple indoor areas such as hospital ICUs, Cirurgical Centers, and common areas. Hygienization protocols could also be evaluated, comparing microbial abundance and diversity before and after cleaning procedures. The effectiveness of hygienization can then be verified based on elimination or reduction of HAI pathogens, reflected in a reduced or absent number of sequenced reads, for example. Other complementary methodologies such as shotgun, real-time PCR, mass spectrometry, and microbial cultivation can be additionally performed after the initial amplicon screening to improve microbial characterization for previously unknown microorganisms. The best cost-benefit for evaluating indoor microbiome profiles and discovering hidden microbes, however, is amplicon sequencing of genes such as that of 16S rRNA gene.
High-throughput sequencing methods allow scientists to perform microbial screening in multiple samples at the same time and to obtain rapid results in a single analysis. This type of analysis is extremely important for epidemiological surveillance and microbial tracking in the environment. Using these kinds of data, it is possible to better understand the implications of microbiomes for human health, and to track microorganisms of interest in their surrounding environment using their DNA information. If we can find hidden microbes in large-scale surveys, we should be able to improve healthcare assistance quality, decrease incidence and costs of HAI, and give rapid responses concerning epidemiological surveillance, in addition to generating unprecedented knowledge about indoor microbiomes. In the near future, this methodology may be directly applied to architectonic and engineered indoor environments, perhaps influencing building design or even being manipulated to improve internal environments.
Supplemental Material
Supplemental Material for Uncovering the hidden microbiota in hospital and built environments: New approaches and solutions by Ana P Christoff, Aline FR Sereia, Camila Hernandes and Luiz FV de Oliveira in Experimental Biology and Medicine
Authors’ contributions
APC, AFRS and LFVO conceived and discussed the mini-review ideas. APC wrote the initial draft of the manuscript and every other author (AFRS, LFVO and CH) read, made suggestions for and approved the final version.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Blaser M. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172:1173–7 [DOI] [PubMed] [Google Scholar]
- 2.Van de Guchte M, Blottière H, Doré J. Humans as holobionts: implications for prevention and therapy. Microbiome 2018; 6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshinnekoo E, Meydan C, Chowdhury S, Jaroudi D, Boyer C, Bernstein N, Maritz J, Reeves D, Gandara J, Chhangawala S, Ahsanuddin S, Simmons A, Nessel T, Sundaresh B, Pereira E, Jorgensen E, Kolokotronis S, Kirchberger N, Garcia I, Gandara D, Dhanraj S, Nawrin T, Saletore Y, Alexander N, Vijay P, Hénaff E, Zumbo P, Walsh M, O'Mullan G, Tighe S, Dudley J, Dunaif A, Ennis S, O'Halloran E, Magalhaes T, Boone B, Jones A, Muth T, Paolantonio K, Alter E, Schadt E, Garbarino J, Prill R, Carlton J, Levy S, Mason C. Geospatial resolution of human and bacterial diversity with city-scale metagenomics. Cell Syst 2015; 1:72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crofts T, Gasparrini A, Dantas G. Next-generation approaches to understand and combat the antibiotic resistome. Nat Rev Microbiol 2017; 15:422–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson L, Sanders J, McDonald D, Amir A, Ladau J, Locey K, Prill R, Tripathi A, Gibbons S, Ackermann G, Navas-Molina J, Janssen S, Kopylova E, Vázquez-Baeza Y, González A, Morton J, Mirarab S, Xu Z, Jiang L, Haroon M, Kanbar J, Zhu Q, Song S, Kosciolek T, Bokulich N, Lefler J, Brislawn CJ, Humphrey G, Owens S, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman J, Clauset A, Stevens R, Shade A, Pollard K, Goodwin K, Jansson J, Gilbert J, Knight R. The Earth Microbiome Project Consortium A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017; 551:457–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall A, Brady A, Creasy H, McCracken C, Giglio M, McDonald D, Franzosa E, Knight R, White O, Huttenhower C. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017; 550:61–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchesi J, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome 2015; 3:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora M, Mahnert A, Koskinen K, Pausan M, Oberauner-Wappis L, Krause R, Perras A, Gorkiewicz G, Berg G, Moissl-Eichinger C. Microorganisms in confined habitats: microbial monitoring and control of intensive care units, operating rooms, cleanrooms and the international space station. Front Microbiol 2016; 7:1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams R, Bateman A, Bik H, Meadow J. Microbiota of the indoor environment: a meta-analysis. Microbiome 2015; 3:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardis E. DNA sequencing technologies: 2006-2016. Nat Protoc 2017; 12:213–8 [DOI] [PubMed] [Google Scholar]
- 11.Oberauner L, Zachow C, Lackner S, Högenauer C, Smolle K-H, Berg G. The ignored diversity: complex bacterial communities in intensive care units revealed by 16S pyrosequencing. Sci Rep 2013; 3:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagier J-C, Hugon P, Khelaifia S, Fournier P-E, Scola B, Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev 2015; 28:237–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calfee D. Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med 2012; 63:359–71 [DOI] [PubMed] [Google Scholar]
- 14.Brown Kline J, Mhuireach G, Northcutt D, Stenson J. Making microbiology of the built environment relevant to design. Microbiome 2016; 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dancer S. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev 2014; 27:665–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart E. Growing unculturable bacteria. J Bacteriol 2012; 194:4151–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley S, Gilbert J. Studying the microbiology of the indoor environment. Genome Biol 2013; 14:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lax S, Gilbert J. Hospital-associated microbiota and implications for nosocomial infections. Trends Mol Med 2015; 21:427–32 [DOI] [PubMed] [Google Scholar]
- 19.Lagier J-C, Dubourg G, Million M, Cadoret F, Bilen M, Fenollar F, Levasseur A, Rolain J, Fournier P, Raoult D. Culturing the human microbiota and culturomics. Nat Rev Microbiol 2018; 16:540–50 [DOI] [PubMed] [Google Scholar]
- 20.Knight R, Jansson J, Field D, Fierer N, Desai N, Fuhrman J, Hugenholtz P, van der Lelie D, Meyer F, Stevens R, Bailey M, Gordon J, Kowalchuk G, Gilbert J. Unlocking the potential of metagenomics through replicated experimental design. Nat Biotechnol 2012; 30:513–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lax S, Sangwan N, Smith D, Larsen P, Handley K, Richardson M, Guyton K, Krezalek M, Shogan B, Defazio J, Flemming I, Shakhsheer B, Weber S, Landon E, Garcia-Houchins S, Siegel J, Alverdy J, Knight R, Stephens B, Gilbert JA. Bacterial colonization and succession in a newly opened hospital. Sci Transl Med 2017; 9:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Didelot X, Bowden R, Wilson D, Peto T, Crook D. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet 2013; 13:601–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold C. Rethinking sterile: the hospital microbiome. Environ Health Perspect 2014; 122:A182–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su C, Lei L, Duan Y, Zhang K-Q, Yang J. Culture-independent methods for studying environmental microorganisms: methods, application, and perspective. Appl Microbiol Biotechnol 2012; 93:993–1003 [DOI] [PubMed] [Google Scholar]
- 25.Knight R, Vrbanac A, Taylor B, Aksenov A, Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall L, McDonald D, Melnik A, Morton J, Navas J, Quinn R, Sanders J, Swafford A, Thompson L, Tripathi A, Xu ZZ, Zaneveld J, Zhu Q, Caporaso J, Dorrestein P. Best practices for analysing microbiomes. Nat Rev Microbiol 2018; 16:410–22 [DOI] [PubMed] [Google Scholar]
- 26.Gohl D, Vangay P, Garbe J, MacLean A, Hauge A, Becker A, Gould T, Clayton J, Johnson T, Hunter R, Knights D, Beckman K. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat Biotechnol 2016; 34:942–9 [DOI] [PubMed] [Google Scholar]
- 27.Abdallah R, Beye M, Diop A, Bakour S, Raoult D, Fournier P-E. The impact of culturomics on taxonomy in clinical microbiology. Antonie Van Leeuwenhoek 2017; 110:1327–37 [DOI] [PubMed] [Google Scholar]
- 28.Vaishampayan P, Probst A, Duc M, Bargoma E, Benardini J, Andersen G, Venkateswaran K. New perspectives on viable microbial communities in low-biomass cleanroom environments. ISME J 2012; 7:312–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emerson J, Adams R, Román C, Brooks B, Coil D, Dahlhausen K, Ganz HH, Hartmann E, Hsu T, Justice N, Paulino-Lima I, Luongo J, Lymperopoulou D, Gomez-Silvan C, Rothschild-Mancinelli B, Balk M, Huttenhower C, Nocker A, Vaishampayan P, Rothschild L. Schrödinger’s microbes: tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 2017; 5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soucy S, Huang J, Gogarten J. Horizontal gene transfer: building the web of life. Nat Rev Genet 2015; 16:472–82 [DOI] [PubMed] [Google Scholar]
- 31.Washburne A, Morton J, Sanders J, McDonald D, Zhu Q, Oliverio A, Knight R. Methods for phylogenetic analysis of microbiome data. Nat Microbiol 2018; 3:652–61 [DOI] [PubMed] [Google Scholar]
- 32.Kembel S, Jones E, Kline J, Northcutt D, Stenson J, Womack A, Bohannan B, Brown G, Green J. Architectural design influences the diversity and structure of the built environment microbiome. ISME J 2012; 6:1469–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Calderon J, Cavallin H, Song S, Novoselac A, Pericchi L, Hernandez J, Rios R, Branch O, Pereira H, Paulino L, Blaser M, Knight R, Dominguez-Bello M. Walls talk: microbial biogeography of homes spanning urbanization. Sci Adv 2016; 2:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006; 6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Abreu P, Farias P, Paiva G, Almeida A, Morais P. Persistence of microbial communities including Pseudomonas aeruginosa in a hospital environment: a potential health hazard. BMC Microbiol 2014; 14:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russotto V, Cortegiani A, Raineri S, Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J Intensive Care 2015; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fierer N, Lauber C, Zhou N, McDonald D, Costello E, Knight R. Forensic identification using skin bacterial communities. Proc Natl Acad Sci U S A 2010; 107:6477–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poza M, Gayoso C, Gómez M, Rumbo-Feal S, Tomás M, Aranda J, Fernández A, Bou G. Exploring bacterial diversity in hospital environments by GS-FLX titanium pyrosequencing. PLoS One 2012; 7:e44105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu H, Johani K, Gosbell IB, Jacombs A, Almatroudi A, Whiteley G, Deva A, Jensen S, Vickery K. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J Hosp Infect 2015; 91:35–44 [DOI] [PubMed] [Google Scholar]
- 40.Hewitt K, Mannino F, Gonzalez A, Chase J, Caporaso J, Knight R, Kelley ST. Bacterial diversity in two neonatal intensive care units (NICUs). Plos One 2013; 8:e54703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann E, Hickey R, Hsu T, Román C, Chen J, Schwager R, Kline J, Brown G, Halden R, Huttenhower C, Green J. Antimicrobial chemicals are associated with elevated antibiotic resistance genes in the indoor dust microbiome. Environ Sci Technol 2016; 50:9807–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soto-Giron M, Rodriguez-R L, Luo C, Elk M, Ryu H, Hoelle J, Santo Domingo J, Konstantinidis K. Biofilms on hospital shower hoses: characterization and implications for nosocomial infections. Appl Environ Microbiol 2016; 82:2872–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feazel L, Baumgartner L, Peterson K, Frank D, Harris J, Pace N. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci U S A 2009; 106:16393–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bokulich N, Lewis Z, Boundy-Mills K, Mills D. A new perspective on microbial landscapes within food production. Curr Opin Biotechnol 2016; 37:182–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyce J. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control 2016; 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009; 73:305–15 [DOI] [PubMed] [Google Scholar]
- 47.Allegranzi B, Sax H, Pittet D. Hand hygiene and healthcare system change within multi-modal promotion: a narrative review. J Hosp Infect 2013; 83:S3–S10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Uncovering the hidden microbiota in hospital and built environments: New approaches and solutions by Ana P Christoff, Aline FR Sereia, Camila Hernandes and Luiz FV de Oliveira in Experimental Biology and Medicine