Manganese neurotoxicity, or manganism, was first described by Couper et al. in 1837 [1] and presents as hypophonia, excessive salivation, limb tremor, muscle weakness and a bent posture while walking. The phenotype of manganism can be classified based on the following predominant manifestations: 1) behavioral changes, 2) parkinsonian features, and 3) dystonia with severe gait disturbances, i.e., the “cock walk.” [2] The modern-day phenotype is significantly milder and is most frequently seen in welders who are exposed to manganese fumes [3,4]. A classic MRI shows hyperintensities in the globus pallidus and substantia nigra in the T1 weighted images [4]. Chelating agents, such as trientine, have been reported to improve neurological symptoms and imaging features in patients with manganese toxicity [5].
A 27-year-old man had a 9-year history of occupational welding. He had not utilized respiratory protective measures, and the sites of welding were variable, i.e., indoors, outdoors, etc. He presented with a right upper limb (UL) tremor that had been ongoing for 2 years. The tremor was insidious in onset and was initially noticed while he was holding a welding torch. The tremor only occurred when the elbow was flexed, and he denied the presence of slowness, stiffness, gait difficulty or a tremor elsewhere in the body. He had no other complaints and an unremarkable medical and family history.
Upon examination, his Mini-Mental Status Examination score was 29/30, and there was no speech abnormality, significant dystonia, bradykinesia, rigidity or gait abnormality. A rest tremor of the right UL was elicited by cognitive coactivation (Supplementary Video 1 in the online-only Data Supplement). No tremor was observed with arms outstretched, flexed at the elbow, or abducted at the shoulder. A jerky tremor was observed when the patient flexed the elbow joint with the forearm pronated or supinated and when the arm was internally or externally rotated with the elbow flexed at 90°. Minimal dystonic posturing of the right UL, characterized by wrist flexion and slight ulnar deviation, was also observed. No entrainability or distractibility was observed. Surface electromyography revealed a 4–5 Hz tremor with co-contraction of the right forearm muscles and the right biceps and triceps. An MRI of the brain showed bilateral, symmetric T1-weighted hyperintensities of the globus pallidus and substantia nigra (Figure 1). No calcification was observed on a plain CT scan of the brain (Supplementary Figure 1 in the online-only Data Supplement). Routine blood investigations, serum copper and ceruloplasmin were normal, and a slit lamp examination for a Kayser–Fleischer ring was negative. There was no evidence suggestive of liver dysfunction. The video of the patient was taken after written informed consent.
Figure 1.
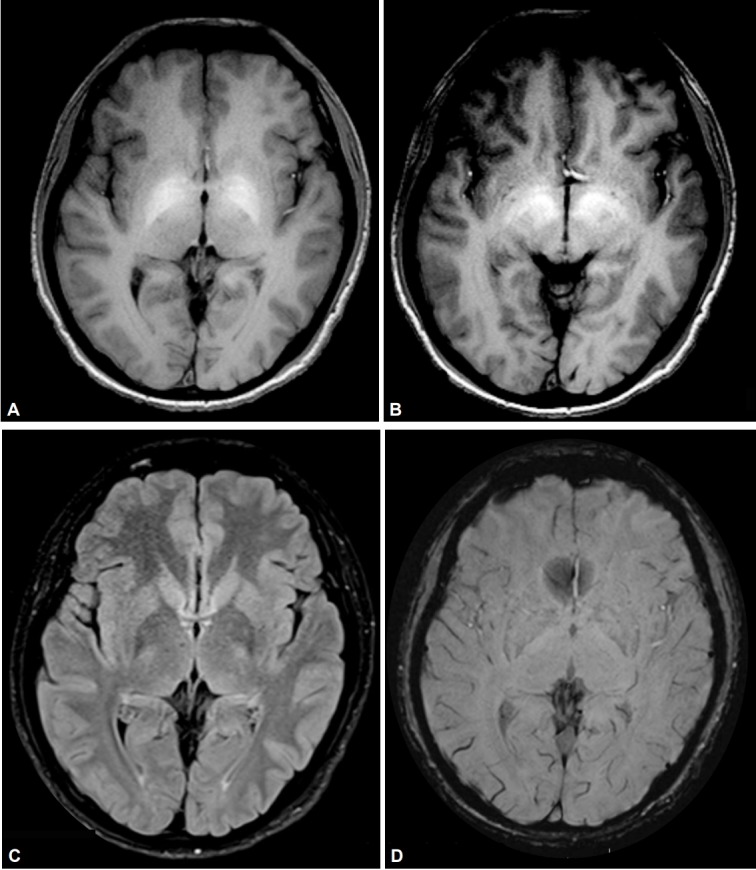
Magnetic resonance images demonstrating features suggestive of manganese toxicity. A and B: Axial T1-weighted images showing bilateral and symmetrical hyperintensities of the globus pallidus and substantia nigra. C: Normal T2 fluid-attenuated inversion recovery imaging. D: Normal susceptibility weighted imaging.
We obtained serum manganese levels based on the imaging findings and occupational history that revealed elevated serum manganese levels of 2.68 ng/mL (0.6–2.3 ng/mL). To rule out the presence of manganese transporter gene defects, we sequenced coding regions for SLC30A10 and SLC39A14, and none of the reported, or novel, pathogenic variants were observed. We prescribed clonazepam to the patient and recommended a change of occupation; however, he was lost to further follow-up.
A diagnosis of manganese neurotoxicity was considered even though the patient had no signs of parkinsonism, which is the hallmark of manganese toxicity. The classical tremor in manganism is a low-amplitude, rapid, postural tremor limited to the ULs [6]. However, a rest tremor may also occur [3]. Reports pertaining to a position specific tremor are sparse and are limited to Wilson’s disease and a few cases with no specific neurological disorder [7,8]. With the presence of an isolated limb tremor with position dependence and minimal dystonic posturing, it is possible to also consider the possibility of a dystonic tremor, which may be observed in scans without evidence of a dopaminergic deficit [9]. However, limb dystonia is a frequent phenotype related to occupational [10] or hereditary manganese exposure [11]. In the present case, although it is uncertain if a direct causal relationship exists between the observed tremor and manganese neurotoxicity, this diagnosis has to be considered in the absence of other abnormal findings.
In conclusion, a unilateral UL tremor, in the absence of overt parkinsonism, may be a presenting symptom in patients with manganese toxicity. This condition should be evaluated and excluded in those who are at risk of manganese exposure.
Acknowledgments
CSIR-IGIB has received funding from the GOMED-TeCh-MLP1802 project.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.18068.
Video 1. Segment 1: A jerky rest tremor was observed with cognitive coactivation. There was no postural tremor when arms were held outstretched, flexed at the elbow or held abducted at the shoulder. No intention tremor was observed. When the patient attempted to flex the elbow joint with the forearm pronated or supinated a jerky tremor was observed. A similar tremor was observed when the arm was internally or externally rotated with the elbow flexed at 90°. Segment 2: Surface electromyography revealed a 4–5 Hz tremor with co-contractions of the right forearm extensor and flexor muscles and of the right biceps and triceps.
Normal CT scan with no evidence of calcification.
REFERENCES
- 1.Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharm Vital Stat Gen Sci. 1837;1:41–42. [Google Scholar]
- 2.Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- 3.Racette BA. Manganism in the 21st century: the Hanninen lecture. Neurotoxicology. 2014;45:201–207. doi: 10.1016/j.neuro.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva CJ, da Rocha AJ, Mendes MF, Braga AP, Jeronymo S. Brain manganese deposition depicted by magnetic resonance imaging in a welder. Arch Neurol. 2008;65:983. doi: 10.1001/archneur.65.7.983. [DOI] [PubMed] [Google Scholar]
- 5.Park HK, Kim SM, Choi CG, Lee MC, Chung SJ. Effect of trientine on manganese intoxication in a patient with acquired hepatocerebral degeneration. Mov Disord. 2008;23:768–770. doi: 10.1002/mds.21957. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer SM, Hallett M, Karp BP, DiCapua DB, Tinaz S. Positional tremor and its treatment. Mov Disord Clin Pract. 2017;4:768–771. doi: 10.1002/mdc3.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frucht S, Sun D, Schiff N, Eidelberg D, Gilliam TC. Arm tremor secondary to Wilson’s disease. Mov Disord. 1998;13:351–353. doi: 10.1002/mds.870130227. [DOI] [PubMed] [Google Scholar]
- 9.Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs) Mov Disord. 2007;22:2210–2215. doi: 10.1002/mds.21685. [DOI] [PubMed] [Google Scholar]
- 10.Kenangil G, Ertan S, Sayilir I, Ozekmekçi S. Progressive motor syndrome in a welder with pallidal T1 hyperintensity on MRI: a two-year follow-up. Mov Disord. 2006;21:2197–2200. doi: 10.1002/mds.21119. [DOI] [PubMed] [Google Scholar]
- 11.Stamelou M, Tuschl K, Chong WK, Burroughs AK, Mills PB, Bhatia KP, et al. Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Mov Disord. 2012;27:1317–1322. doi: 10.1002/mds.25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Segment 1: A jerky rest tremor was observed with cognitive coactivation. There was no postural tremor when arms were held outstretched, flexed at the elbow or held abducted at the shoulder. No intention tremor was observed. When the patient attempted to flex the elbow joint with the forearm pronated or supinated a jerky tremor was observed. A similar tremor was observed when the arm was internally or externally rotated with the elbow flexed at 90°. Segment 2: Surface electromyography revealed a 4–5 Hz tremor with co-contractions of the right forearm extensor and flexor muscles and of the right biceps and triceps.
Normal CT scan with no evidence of calcification.


