Abstract
Acute lipolysis of visceral fat or circulating triglycerides may worsen acute pancreatitis (AP)–associated local and systemic injury. The pancreas expresses pancreatic triacylglycerol lipase (PNLIP), pancreatic lipase-related protein 2 (PNLIPRP2), and carboxyl ester lipase (CEL), which may leak into the visceral fat or systemic circulation during pancreatitis. We, thus, aimed to determine the pancreatic lipase(s) regulating lipotoxicity during AP. For this AP, associated fat necrosis was analyzed using Western blot analysis. Bile acid (using liquid chromatography–tandem mass spectrometry) and fatty acid (using gas chromatography) concentrations were measured in human fat necrosis. The fat necrosis milieu was simulated in vitro using glyceryl trilinoleate because linoleic acid is increased in fat necrosis. Bile acid requirements to effectively hydrolyze glyceryl trilinoleate were studied using exogenous or overexpressed lipases. The renal cell line (HEK 293) was used to study lipotoxic injury. Because dual pancreatic lipase knockouts are lethal, exocrine parotid acini lacking lipases were used to verify the results. PNLIP, PNLIPRP2, and CEL were increased in fat necrosis. Although PNLIP and PNLIPRP2 were equipotent in inducing lipolysis and lipotoxic injury, CEL required bile acid concentrations higher than in human fat necrosis. The high bile acid requirements for effective lipolysis make CEL an unlikely mediator of lipotoxic injury in AP. It remains to be explored whether PNLIP or PNLIPRP2 worsens AP severity in vivo.
Lipolysis of visceral fat,1, 2, 3, 4 circulating triglycerides,5 and s.c. fat6 may contribute to the manifestations of acute pancreatitis (AP) and systemic injury, including acute kidney injury,1, 2, 3, 4 during severe AP (SAP). Elevated long-chain nonesterified fatty acids (NEFAs) have been noted in both the serum7, 8 and necrotic collections1, 3, 9 during clinical SAP. The severity of AP in clinical scenarios is determined by systemic injury, which may be independent of pancreatic parenchymal necrosis.10, 11, 12 Because SAP may occur more often in obese patients in association with fat necrosis or in those with hypertriglyceridemia,13, 14, 15, 16 and lipase inhibition has been shown to improve outcomes in experimental SAP,1, 2, 3, 4 we thought it would be helpful to identify the lipases that mediate the cascade of unregulated lipolysis of visceral or circulating triglyceride that result in SAP.
Fat necrosis is the main part of pancreatic and peripancreatic necrosis in humans,17, 18 and it is included in criteria grading AP severity.12, 19, 20 Fat necrosis has previously been shown to be a part of necrotizing pancreatitis noted at the time of autopsy,21, 22 at the time of surgery,23 and radiologically.20 The lipolysis of visceral fat in SAP-associated fat necrosis was proposed in the late 19th century,24, 25 and its deleterious role is supported by recent mechanistic studies.26 Clues to the pathophysiology of fat necrosis in human SAP include the following: i) fat necrosis in humans composed of free or saponified NEFAs,27 which are unsaturated2, 3, 9; ii) histology showing fatty acid leakage from fat necrosis, causing surrounding parenchymal necrosis1, 2; and iii) immunohistochemistry of AP showing pancreatic lipase, colipase, and carboxyl ester lipase (CEL)28, 29, 30 in fat necrosis.
On the basis of these observations, it is plausible that the lipolytic generation of unsaturated NEFAs during fat necrosis may mediate the worse outcomes noted in SAP. Previous mechanistic studies show that the unsaturated NEFA linoleic acid (LA) or oleic acid can increase cytosolic calcium from an intracellular pool, inhibit mitochondrial complexes I and V,1 cause mitochondrial depolarization,31 cause cytochrome c release,31 and reduce ATP levels,1 eventually increasing annexin V staining (a marker of apoptosis), propidium iodide (PI) uptake,3, 4 and lactate dehydrogenase (LDH) leakage,1, 3, 31 which are consistent with necrosis. Although the exact intermediary steps remain to be figured out, on the basis of published literature, the combination of these upstream steps and end points is consistent with either advanced apoptosis32 or programmed necrosis.33
Clues to the systemic involvement of lipotoxicity in AP include kidney failure5 in hypertriglyceridemic AP, elevated circulating NEFAs in both patients7, 8 and rodents1, 2, 3, 4 with SAP, and the pancreatitis-panniculitis-polyarthritis syndrome, in which distant fat necrosis is noted during AP. Analysis of the composition of s.c. panniculitis during pancreatitis-panniculitis-polyarthritis6 showed the presence of pancreatic triacylglycerol lipase (PNLIP; chromosome 10q24-q26; Enzyme Commission number 3.1.1.3) but not CEL (chromosome 9q34.3; Enzyme Commission number 3.1.1.13). The panniculitis also had extremely high pancreatic lipase activity, free fatty acids >10 mmol/L, and no pancreatic amylase activity. A chronic form of ectopic expression of PNLIP, pancreatic lipase-related protein 2 (PNLIPRP2) is noted during adipose tissue remodeling and after perturbing the peroxisome proliferator-activated receptor-γ–fibroblast growth factor-1 axis.34 Although lipolysis in adipose tissue is normally regulated by adipocyte lipases, PNLIP and PNLIPRP2 (or the PNLIPRP2 gene; Enzyme Commission number 3.1.1.26) have been noted in fat necrosis.4
We, thus, aimed at determining the pancreatic lipase(s) contributing to the lipotoxicity in SAP. Their amounts and activity were measured in the visceral fat of ob/ob mice, which develop lethal SAP.1, 4 The dependence of CEL on bile acids was compared with bile acid concentrations present in human pancreatic necrosis collections. NEFA analysis in these collections also served to guide the substrate used for studying the activity of pancreatic lipases after confirming that these fatty acids caused renal injury.
On the basis of the above, the ability of lipases to mediate lipotoxic injury was studied by adding them exogenously or overexpressing them in cells and studying the effects of the lipase secreted into the medium. These models were used to understand kidney tubular injury in SAP using the widely used cell line HEK 293.35, 36 The models also simulated how lipases leaked into visceral fat during pancreatitis may further worsen injury to the adjacent tissue via lipolysis of the fat.
Deletion of a single lipase gene leaves the other two intact, which may prevent identification of an individual lipase that mediates fat necrosis. PNLIPRP2 and PNLIP consecutively span 104.1 Kb on chromosome 19; therefore dual knockouts of these factors cannot be generated by mating mice that are knockouts for an individual gene. In addition, dual knockouts of PNLIPRP2 and CEL are lethal in utero or shortly after birth.37 We, thus, complimented the studies in HEK 293 cells using parotid acinar cells, which, like pancreatic acinar cells, are polarized exocrine cells present in clusters and contain secretory granules that are released in response to extracellular stimuli.38, 39 Both cell types release calcium from an intracellular pool,40 a phenomenon also noted in response to LA.1 However, because parotid cells lack lipases,41 they are a more suitable cell type to study the effects of recombinant lipases in mediating lipotoxic injury that may result in acinar necrosis. Herein, we present the design and findings regarding the lipase(s) that may contribute to SAP.
Materials and Methods
Reagents
Orlistat was purchased from Cayman Chemical (Ann Arbor, MI). Specific reagents for cell culture, cloning, transfection, and viability assays are described under the specific methods. Glyceryl trilinoleate (GTL), LA, PI, sodium taurocholate (STC), Triton X-100, and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO), as were the rest of the reagents. Just before use, GTL was sonicated into the media in a two-step manner to ensure that the triglyceride stays in solution.
Preparation of GTL
Pure GTL was first directly sonicated into the medium (concentration, 3 mmol/L) using three 10-second pulses. This sonicate was promptly diluted in fresh media to a 0.6 or a 1.2 mmol/L concentration (which is 2× the final concentration of GTL) and resonicated again for two 10-second pulses. A final concentration of 0.3 or 0.6 mmol/L GTL was achieved by directly adding the sonicated 2× GTL solution to an equal volume of medium with the cells being studied. This remained stable in solution, with no evidence of separation of the lipid phase from the aqueous phase over the duration of the studies. Wherever relevant, orlistat was dissolved in dimethyl sulfoxide (50 mmol/L), sonicated briefly for 8 to 10 seconds, and immediately added in the culture media to a final concentration of 50 μmol/L just after the GTL. The 0.1% dimethyl sulfoxide resulting from this does not affect any of the experimental end points in our system, as shown previously.1, 3
Mouse Husbandry
The 8- to 10-week–old male ob/ob (B6.V-lepob/J) mice (Jackson Laboratories, Bar Harbor, ME) or Institute for Cancer Research mice (Charles River Labs, Wilmington, MA) were acclimatized for at least 2 days before use. All animals were housed with a 12-hour light/dark cycle at room temperature, fed normal laboratory chow, and allowed to drink ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (Pittsburgh, PA) and the Mayo Clinic (Scottsdale, AZ).
In Vivo Animal Studies
Acute pancreatitis was induced in obese (ob/ob) mice by hourly i.p. injections of cerulein (50 μg/kg) × 12 doses on day 1 and six injections on day 2 in this study. Mice were sacrificed electively after the 18th injection. With a 12-injection regimen for 2 consecutive days, 90% to 100% of obese mice get moribund before 72 hours and have to be electively sacrificed, as described previously.4 Visceral epididymal adipose tissue was harvested, pancreatic lipase activity was measured in the homogenate, as described below, and the adipose tissue lysate was blotted against anti-PNLIPRP2, anti-PNLIP, anti-CEL, and anti–Na-K-ATPase antibody, as described below.
SDS-PAGE and Western Blot Analysis
Cell lysates were boiled in 1× Laemmle sample buffer, and at least 10 ìg protein was loaded onto 10% to 12% denaturing polyacrylamide gels for protein resolution. Proteins were transferred onto Immobilon polyvinylidene difluoride membranes (EMD Millipore, Burlington, MA) and blocked in Tris-buffered saline (pH 8.0) with 0.1% Tween 20 containing 5% blocking grade blocker (Sigma, St. Louis, MO). Membranes were incubated with primary antibodies [anti-PNLIP and PNLIPRP2, dilution 1:20,00042; anti-PNLIPRP2, dilution 1:1000 for overexpression studies, sc74853 (Santa Cruz Biotechnology, Dallas, TX); anti-CEL, dilution 1:1000, sc34883 (Santa Cruz Biotechnology); anti–adipocyte triglyceride lipase (ATGL), dilution 1:1000, PA5-17436 (Thermo Fisher Scientific, Waltham, MA); and anti-Na+K+ ATPase, dilution 1:4000, a5 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA)], and appropriate horseradish peroxidase–labeled secondary antibodies, at a concentration of 1:10,000, were used to detect the signal using ECL2 Western Blotting substrate (Thermo Fisher Scientific).
Pancreatic Lysate Preparation
For exogenous addition of pancreatic lysate studies, freshly harvested pancreatic tissue was homogenized in a 100× volume of phosphate-buffered saline. The lipase activity was measured and adjusted to 35,000 to 40,000 U/L. This solution (12 μL) was then added to cells for a final volume of 1 mL after the GTL or orlistat, wherever relevant.
Human Pancreatic Fluid Samples
All studies were approved by the institutional review boards of the University of Pittsburgh (Pittsburgh, PA) and the Mayo Clinic Foundation. Clinical residual samples were collected and processed, as described in earlier studies.2, 3 The samples used for the current studies were residual necrotic material from pancreatic necrosis, as per the revised Atlanta criteria.12 These were collected at the time of surgical, endoscopic, or percutaneous debridement. The debridement procedures were clinically indicated, as per the International Association of Pancreatology/American Pancreatic Association's evidence-based guidelines.43 The material did not contain solid tissue. This material was immediately transported to the laboratory, as per regulations approved by the institutional review board. These samples were frozen at –80°C and processed for fatty acid or bile acid analyses, as described below. These samples were characterized as biliary AP (n = 19) versus nonbiliary AP (n = 15) collections, as detailed previously.3
Cloning of Mouse Pancreatic PNLIP, PNLIPRP2, and CEL into pCR 2.1–TOPO and pcDNA 3.1
Using the primer pairs (Table 1), the cDNA sequences corresponding to mouse Pnlip (http://www.ncbi.nlm.nih.gov/nuccore; GenBank accession number NM_026925.4), mouse PNLIPRP2 (GenBank accession number NM_011128.2), and mouse CEL (GenBank accession number U37386.1) were amplified by PCR and cloned into pCR 2.1–TOPO (3.9 kb; Life Technologies, Carlsbad, CA). The presence of insert in correct orientation was confirmed by digestion with corresponding restriction enzymes, as well by sequencing (Genewiz, Inc., South Plainfield, NJ) of the plasmid DNA. Mouse Pnlip, mouse PNLIPRP2, and mouse CEL were gel purified after digestion with corresponding restriction enzymes from pCR 2.1–TOPO/PNLIP, pCR 2.1–TOPO/PNLIPRP2, or pCR 2.1–TOPO/CEL. Purified inserts were ligated into mammalian expression vector pcDNA 3.1 (5.4 kb; Life Technologies) using T4 DNA ligase, as per manufacturer's instruction (Life Technologies), to generate pcDNA 3.1/PNLIP, pcDNA 3.1/PNLIPRP2, and pcDNA 3.1/CEL. The correct orientation of insert in each plasmid was confirmed by digestion with restriction enzymes and further confirmed by sequencing (Genewiz, Inc.) of the insert in plasmid DNA.
Table 1.
Sequences of Primers Used for Cloning of Mouse Pancreatic Lipase in pCR 2.1–TOPO and pcDNA 3.1(+) Vectors
| Primer name | Primer sequence |
|---|---|
| PNLIP-F with HindIII and Kozak sequence | 5′-GTAAAGCTTGCCATGCTAATGCTGTGGACATTTG-3′ |
| PNLIP-R with NotI site | 5′-CATGCGGCCGCCTAACATGGAGACAGTGTGAG-3′ |
| PNLIPRP2-F with HindIII and Kozak sequence | 5′-TATAAGCTTGCCATGCCTATGGATGTCCGTGG-3′ |
| PNLIPRP2-R with XbaI site | 5′-CGTAATTCTAGATTAACAAGGGTACAGAGACTG-3′ |
| CEL-F with HindIII and Kozak sequence | 5′-AATAAGCTTGCCATGGGGCGCCTGGAGGTTC-3′ |
| CEL-R with XbaI site | 5′-AATTCTAGATTAGAAGCCAATGGTGGCAGG-3′ |
| PNLIP-F (S169G) | 5′-GTCCACCTGATTGGCCACGGCCTGGGTTCCCACATTG-3′ |
| PNLIP-R (S169G) | 5′-CAATGTGGGAACCCAGGCCGTGGCCAATCAGGTGGAC-3′ |
| PNLIPRP2-F (S184G) | 5′-CGTGCACCTCATCGGCCACGGCTTGGGCTCACATGTGG-3′ |
| PNLIPRP2-R (S184G) | 5′-CCACATGTGAGCCCAAGCCGTGGCCGATGAGGTGCACG-3′ |
The sites mutated in the serine to glycine mutants of PNLIP and PNLIPRP2 are underlined.
-F, forward; -R, reverse; CEL, carboxyl ester lipase; PNLIP, pancreatic triacylglycerol lipase; PNLIPRP2, pancreatic lipase-related protein 2.
Mutation in Mouse Pancreatic PNLIP and PNLIPRP2 in pcDNA 3.1
Single point mutations in pcDNA 3.1/PNLIP and pcDNA 3.1/PNLIPRP2 were made using plaque-forming unit DNA polymerase (Strategene, La Jolla, CA). Primer pairs (Table 1) were designed to mutate serine to glycine at amino acid residue 169 in pcDNA 3.1/PNLIP and at amino acid residue 184 in pcDNA 3.1/PNLIPRP2 to generate dead mutant of pcDNA 3.1/PNLIP (p.S169G) and pcDNA 3.1/PNLIPRP2 (p.S184G). A mutated base site (Table 1) in the plasmid was verified by sequencing of the plasmid DNA at a molecular biology core laboratory facility (Mayo Clinic, Rochester, MN).
Culture of HEK 293 Cells and Overexpression of Wild-Type and Mutant Mouse Lipases
HEK 293 cells were maintained in a 75-cm2 tissue culture flask in a humidified incubator at 37°C with 5% CO2 in Eagle's minimum essential medium (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (ATCC) and 1% penicillin-streptomycin (Life Technologies). To test the effect of acute lipotoxicity from exogenous addition of pancreatic cell lysates to HEK 293 cells, the lysates were added to cells in HEPES buffer (pH 7.4) with 0.1% albumin,1, 44, 45, 46 in the presence or absence of 600 μmol/L GTL for 2 hours. Overexpression of wild-type and mutant mouse lipase in HEK cells was performed in a 6-well plate using Lipofectamine LTX and Plus Reagent (Life Technologies), per the manufacturer's instructions. Briefly, 2.0 μg plasmid DNA [pcDNA 3.1/PNLIP, pcDNA 3.1/PNLIPRP2, pcDNA 3.1/CEL, pcDNA 3.1/PNLIP (p.S169G), or pcDNA 3.1/PNLIPRP2 (p.S184G)], 4.0 μL Plus Reagent, and 6.0 μL Lipofectamine LTX were mixed in serum-free media and incubated at room temperature for 30 minutes; DNA-reagent complex was used to transfect HEK cells. Control cells were treated with lipofectamine alone. The medium was replaced with fresh serum-free medium 24 hours after transfection. Serum starvation is a well-known method for synchronization of the mammalian cell cycle.47 To determine the effect of overexpressing wild-type and mutant pancreatic lipases on GTL-induced lipotoxicity in transfected HEK cells 48 hours after transfection, overexpression of wild-type or mutant lipase was determined by measuring lipase activity in the media and in the cell lysate by Western blot analysis using anti-PNLIP, anti-PNLIPRP2, and anti-CEL antibodies, as described below; or cells were treated with or without 600 μmol/L GTL for up to 5 hours, and markers of cell injury were measured.
In Vitro Acinar Studies
Freshly prepared mouse parotid acinar cells were prepared by adding 1.25 mg/mL of hyaluronidase from bovine testes (Sigma) to a well-established previously described collagenase digestion recipe1, 44, 45, 46 in HEPES buffer (20 mmol/L HEPES at pH 7.4, 120 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 10 mmol/L glucose, 10 mmol/L sodium pyruvate, and 0.1% bovine serum albumin). After harvesting, the acini were incubated with this buffer minus the collagenase or hyaluronidase, as has been previously described for acinar studies in vitro, including those on lipotoxicity.1, 31, 48, 49 Viability was defined as >95% on Trypan blue exclusion. The pooled acinar cell pellets from parotids of two mice ranged from 100 to 200 μL. The cells were resuspended in 5 mL, and 200 μL acinar suspension was used per milliliter of assay. To ensure homogeneity in dispensing, these cells were constantly shaken manually while being aliquoted. Parotid acini were assayed in 24-well tissue culture plates and shaken at 90 RPM at 37°C in ambient air at a final assay volume of 1 mL/assay per well. Each individual experiment was performed in duplicate. GTL was used as a substrate for pancreatic lipases at a final concentration of 600 μmol/L. Recombinant lipases were added at a final concentration of 1 μg/mL, unless otherwise specified.
Recombinant Lipase Use
To test the effect of exogenous addition of pancreatic lipases, recombinant human PNLIP, PNLIPRP2 (1 μg/mL), or CEL (4 μg/mL) was produced, as described previously,50, 51, 52, 53 and added at the indicated concentrations to parotid acinar cells in HEPES buffer with 0.1% albumin, containing 0.5 μg/mL human colipase (Sino Biological, Wayne, PA) and 300 μmol/L GTL, with STC at the indicated concentrations. In each case, medium was collected for measuring glycerol concentration and LDH content after 4 hours of incubation. The cell pellet was processed for PI uptake, as described below.
Commercial Pancreatic Lipase Assay
A kinetic colorimetric assay kit (Pointe Scientific Inc., Canton, MI; catalog number L-7503) was used to measure pancreatic lipase activity in the media or cell lysate, following the manufacturer's instructions. This kit contains 1,2 diglyceride as a substrate, 5 mmol/L cholic acid as an activator, and 40,000 U/L colipase, a cofactor for pancreatic lipases, but does not contain cofactors for the adipocyte lipase ATGL [eg, comparative gene identification-58 (CGI-58)54]. Glycerol generation is measured by this assay, which ATGL, being a triglyceride lipase, cannot generate.
Glycerol Measurement
Glycerol generation was used as a measure of complete GTL hydrolysis by the pancreatic lipases. This was measured using the free glycerol reagent and the glycerol standard solution (Sigma-Aldrich).
Cell Injury Markers
Caspase-3 Activity and PI Uptake
Caspase-3 activity (NucView 488 Caspase-3 Assay Kit; Biotium, Hayward, CA) and PI uptake (Sigma-Aldrich) were measured in HEK cells in a 6-well tissue culture plate. HEK cells were transfected with 2.0 μg mouse lipase in pcDNA 3.1, as discussed above. GTL (600 μmol/L) was then added for 1.5, 3.0, or 5.0 hours, as discussed above. At the end of treatment, cells were stained with 4 μmol/L NucView 488 caspase substrate, 2 μL/mL PI, and 1 μg/mL Hoechst 33342 (ImmunoChemistry Technologies, LLC, Bloomington, MN) and incubated in the dark for 15 minutes at room temperature. Cell pellet was disrupted in phosphate-buffered saline by sonication, and bright green (NucView 488 Caspase-3 substrate; excitation/emission, 485/515 nm), red (PI; excitation/emission, 535/617 nm), and blue (Hoechst 33342; excitation/emission, 345/460 nm) fluorescence values were measured using the VersaFluor Fluorometer (Bio-Rad, Hercules, CA). Results were expressed as fluorescence unit for caspase-3 activity or PI uptake and normalized, with the fluorescence of Hoechst 33342 as control. Measurement of caspase-3 activity and PI uptake in HEK cells (without overexpression of mouse lipase) after 100 μmol/L LA treatment was done, similar to GTL exposure, as detailed above.
LDH Assay and PI Uptake
Cell death was quantified using a colorimetric cytotoxicity assay for LDH release in the media. Briefly, absorbance at 490 nm and background absorbance at 620 nm were measured in the media using the LDH assay kit (Roche Applied Sciences, Indianapolis, IN) after 4 hours of treatment. Results were expressed as a percentage of total LDH leakage, normalized to that measured in cells lysed with 1% Triton X-100. PI uptake by cells, as a measure of dead necrotic cells, was quantified as previously described1, 55 using a flourimetric method.
ATP Level Determination
An ATP bioluminescent assay kit was used to measure ATP levels (Sigma) following manufacturer's instructions. Briefly, cell pellets were disrupted in trichloroacetic acid and EDTA-containing buffer, followed by appropriate dilution in Tris-EDTA buffer to extract the ATP, as described previously,1 which was followed by the luminescent substrate. Luminescence was measured on a Promega Glomax 20/20 Luminometer (Promega Corporation, Madison, WI), normalized per microgram of protein, and expressed as pmol/μg.
Nonesterified Fatty Acid Analysis
Immediately before analysis, the samples were thawed, spun at 300 × g for 5 minutes, and kept on ice. Long-chain fatty acids were analyzed in the supernatants, as previously described.1, 55 Briefly, lipid was extracted using isopropanol–heptane–hydrochloric acid (1 mol/L; 40:10:1, v/v/v). After mixing and centrifugation, the upper heptane phase was removed, dried, derivatized using the Deoxo-Fluor reagent (Sigma),56 and analyzed by gas chromatography and flame ionization detection using heptadecanoic acid as an internal standard, as described by Kangani et al.56
Bile Acid Levels
Immediately before analysis, the samples were thawed, spun at 300 × g for 5 minutes, and stored on ice. Bile acids were quantified by liquid chromatography–tandem mass spectrometry stable isotope dilution analysis at the Mayo Clinic laboratories (Rochester, MN), as described previously.57 After mixing the sample with isotopically labeled bile acid internal standards, liquid chromatography was performed using mobile phase A (95% water/5% methanol/10 mmol/L ammonium acetate) and mobile phase B (methanol/10 mmol/L ammonium acetate). A reverse-phase C18 column was used to separate free bile acids (lithocholic acid, chenodeoxycholic acid, deoxycholic acid, ursodeoxycholic acid, hyodeoxycholic acid, and cholic acid) and their associated tauro- and glyco- conjugates, along with the internal standards from the bulk of the specimen matrix. The analysis was performed on the ABSciex API 3200 liquid chromatography–tandem mass spectrometry instrument with TurboIonSpray source (Sciex, Framingham, MA). The mass spectrometer was operated in the negative ion mode using multiple reaction monitoring. This was used to follow the precursor to product transitions for the bile acids and their corresponding internal standards. Chromatography was optimized to separate isobaric analytes to reduce interference. The ratios of the extracted peak areas of bile acids/their corresponding internal standards were determined to calculate their respective concentration in the sample. The sum of the individual bile acids (in micrometers) so measured in the sample was used to determine the total bile acids.
Immunohistochemistry and TUNEL Staining
Human pancreatic necrosis samples removed at the time of autopsy (n = 3) were immunostained for pancreatic lipase (anti–human pancreatic triglyceride lipase,42 dilution 1:1000) using the horseradish peroxidase immunohistochemical technique, as described previously.4 Briefly, formalin-fixed, paraffin-embedded pancreatic sections or colon sections from patients with acute diverticulitis (used as a negative control) were retrieved. Deparaffinization and antigen epitope retrieval were performed. Tissues were incubated with the respective antibodies in 5% normal goat serum in Dulbecco's phosphate-buffered saline. These were washed three times and incubated with horseradish peroxidase–conjugated secondary antibody (dilution 1:200; Millipore Corp, Billerica, MA; or Thermo Pierce Scientific, Rockford, IL). Chromogen incubation with an AEC Substrate Kit for Peroxidase and Hematoxylin QS Nuclear Counterstain (Vector Laboratories, Burlingame, CA) was used to complete staining. The slides were examined by a trained morphologist (K.P.). No blinding was done. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed on paraffin sections of the kidneys, as described previously.1, 2, 3, 4
Statistical Analysis
Data depicted are from a minimum of three independent experiments showing means ± SEM for each parameter. Pairwise comparisons were performed using the t-test or a U-test when the distribution was not normal, whereas analysis of variance (Dunnett's method) was performed to make comparison between continuous data from multiple groups. P < 0.05 was considered to be statistically significant.
Results
Pancreatic Lipases Are Present in Fat Necrosis during Severe Acute Pancreatitis
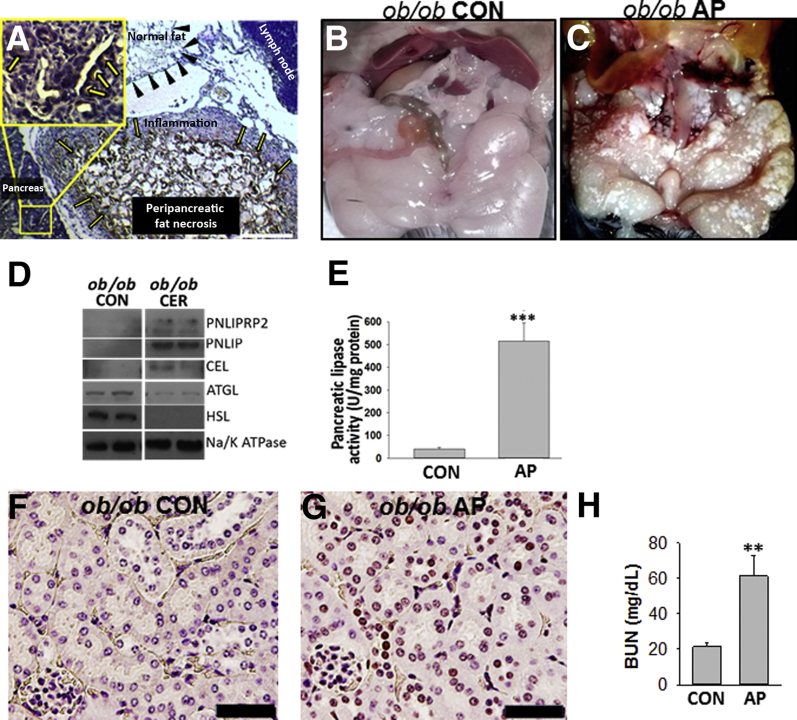
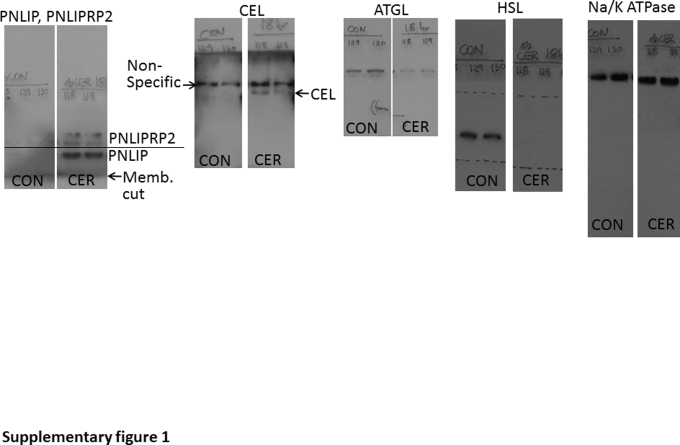
Because SAP commonly occurs in obese patients,58, 59, 60, 61, 62, 63, 64, 65 who develop visceral fat necrosis, which is a cardinal part of SAP,12, 20, 22, 23 the lipases present in autopsy samples of patients with pancreatic necrosis were studied. Previous studies have shown pancreatic enzymes, including CEL, colipase, and chymotrypsin, to be present in human fat necrosis.28, 29, 30 Immunohistochemistry for PNLIP was performed on autopsy samples of patients with pancreatic necrosis. The antibody used binds both PNLIP and PNLIPRP2, as previously shown.42, 66 Positive staining was noted in acinar cells (Figure 1A) and also in the adjacent peripancreatic fat necrosis, surrounded by inflammatory cells, but not in normal fat without inflammation. To study the relevance to severe experimental pancreatitis, a Western blot analysis was performed for pancreatic lipases, which are not normally present in the fat pads (Figure 1, B and C) of control ob/ob mice and ob/ob mice with severe cerulein pancreatitis; these mice have severe fat necrosis and develop multisystem organ failure requiring euthanasia, as shown previously.4 Although control mice had no detectable pancreatic lipase in their fat pads, on the basis of Western blot analysis, all three pancreatic lipases (ie, PNLIP, PNLIPRP2, and CEL) (Figure 1D and Supplemental Figure S1) were increased in the fat pads of mice with pancreatitis, consistent with the fat necrosis observed in their adipose tissue. This was associated with an increase in pancreatic lipase activity over controls in the fat pads with fat necrosis (Figure 1E). The lipase assay measures pancreatic lipase activity and contains colipase and bile acids, but it cannot measure ATGL activity because it contains a diglyceride, which ATGL cannot hydrolyze, and also does not have ATGL's cofactor CGI-58.54 The increase in lipase activity noted in the fat necrosis (Figure 1E) is, therefore, consistent with the Western blot data showing increased pancreatic lipase amounts in the necrosed fat pads. Moreover, there was no detectable increase in the amounts of ATGL or hormone-sensitive lipase on Western blot analysis of the necrosed fat pads from mice with pancreatitis (Figure 1D). Severe cerulein pancreatitis was also associated with renal tubular injury, seen as an increase in TUNEL-positive nuclei (Figure 1G) compared with the control mice (Figure 1F), and renal failure, noted as an elevation in blood urea nitrogen level (Figure 1H). This renal failure is attributable to lipolytic generation of fatty acids because it was prevented by the lipase inhibitor orlistat.1, 2, 4 The lipase activities of the pancreatic lipases were then compared in a milieu relevant to fat necrosis.
Figure 1.
Detection and activity of pancreatic lipases in fat necrosis. A: Immunohistochemistry for pancreatic triacylglycerol lipase (PNLIP) in human pancreatitis, showing positive staining in acinar cells (yellow arrows, inset) and peripancreatic fat necrosis. The inset shows the boxed area in higher magnification. Normal fat (black arrowheads) and lymph node do not stain positive. B and C: Gross images of fat pads from control (CON) ob/ob mice (note smooth glistening appearance; B) and those dying from acute pancreatitis (AP; note fat necrosis seen as numerous chalky white deposits in the fat pads; C). D: Western blot analysis of the fat pads in CON mice and in those with AP. All three pancreatic lipases are increased in AP-associated fat necrosis compared with controls. E: Bar graphs depicting pancreatic lipase activity measured in the fat pads of control mice with cerulein (CER) pancreatitis. F and G: TUNEL staining of mouse kidney in CON (F) mice and in those with AP (G). H: Blood urea nitrogen (BUN) in control mice and in those with AP. Data are expressed as means ± SEM (E and H). n = 6 to 8 mice per group (F–H). ∗∗P < 0.01, ∗∗∗P < 0.001 versus control. Scale bars: 200 μm (A); 100 μm (F and G). ATGL, adipocyte triglyceride lipase; CEL, carboxyl ester lipase; HSL, hormone-sensitive lipase; PNLIPRP2, pancreatic lipase-related protein 2.
Although PNLIP and PNLIPRP2 Efficiently Hydrolyze Triglyceride, CEL Requires Bile Acid Concentrations Higher than Those Present in the Milieu of Pancreatic Necrosis
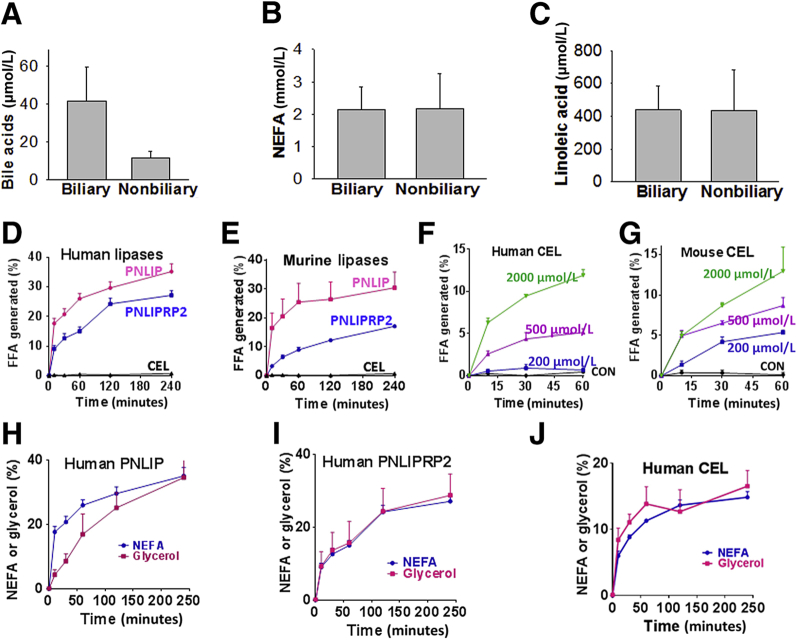
Because the acyl chain length of a lipase substrate67, 68 and the concentrations of bile acids67, 68 in the milieu can affect pancreatic lipase activity, these were first compared in pancreatic fluid collections drained from patients with biliary pancreatitis with those in patients with pancreatitis from nonbiliary causes. Previous studies have shown long-chain NEFAs to be the principal fatty acids generated in fat necrosis.1, 3, 9 Although bile acids tended to be higher (41 ± 17 versus 11 ± 4 μmol/L; P = 0.2) (Figure 2A) in collections from biliary pancreatitis, long-chain NEFAs, including LA (which comprised approximately 20% of the NEFAs in these collections), were similar among the two groups (Figure 2, B and C). Because triglyceride is the major form of lipid in adipocytes, comprising 80% to 90% of their mass,69, 70, 71 the triglyceride of LA (ie, GTL; 300 μmol/L) was chosen to compare the lipolytic activity of pancreatic lipases (1 μg/mL), along with its cofactor colipase (0.5 μg/mL). The sodium salt of taurocholate, STC, was used as a cofactor for CEL, as shown in previous studies68 and its abundance in human bile.72
Figure 2.
Comparison of the lipolysis by pancreatic lipases using conditions relevant to human pancreatitis. A–C: Parameters [bile acid (A), nonesterified fatty acids (NEFAs; B), and linoleic acid (C)] measured in human pancreatic fluid collections of biliary and nonbiliary origin. D and E: Comparison of the ability of 1 μg/mL of human (D) and murine (E) pancreatic lipases to hydrolyze 600 μmol/L glyceryl trilinoleate (GTL) at pH 7.4 in the absence of bile acids. F and G: Effect of different concentrations of sodium taurocholate on human (F) and mouse (G) carboxyl ester lipase (CEL), as measured by the free fatty acid (FFA) generated by GTL (600 μmol/L) lipolysis over 1 hour. H–J: Comparison of the rate of FFA production with glycerol generation by GTL (600 μmol/L) lipolysis among the three pancreatic lipases. Although there is an initial delay in generating glycerol by pancreatic triacylglycerol lipase (PNLIP), by 4 hours, both glycerol and FFA are equivalent. Data are expressed as means ± SEM. CON, control; PNLIPRP2, pancreatic lipase-related protein 2.
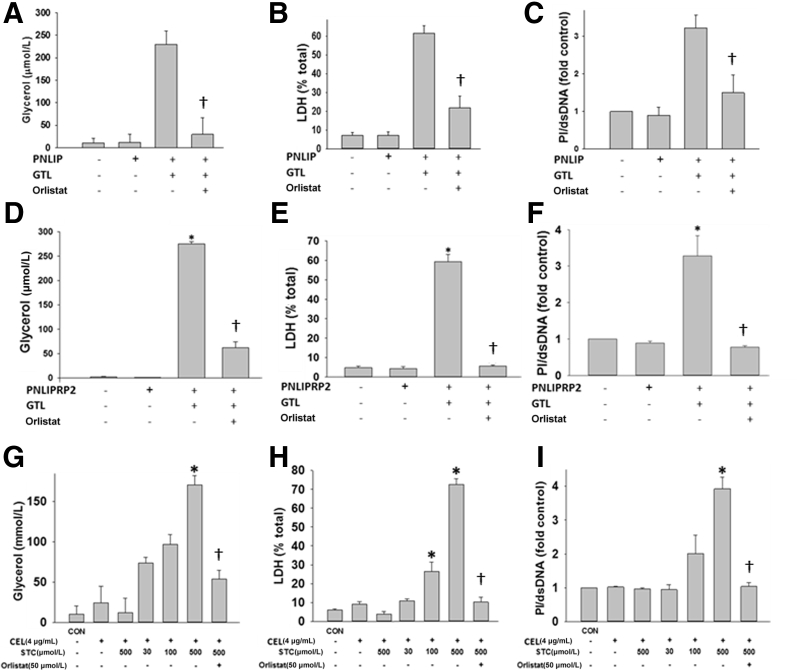
Unlike CEL, both PNLIP and PNLIPRP2 hydrolyzed GTL in the absence of STC (Figure 2, D and E). CEL, however, required concentrations >200 μmol/L to cause a significant increase in lipolysis over controls (Figure 2, F and G). This strong dependence of CEL on STC concentrations is consistent with previous studies showing CEL has little or no detectable activity on long-chain glycerides67 or substrates with ≥16 carbons.68 Although CEL has 10% to 15% of maximal activity at ≤50 μm STC73 on substrates with short six-carbon acyl chains,73 this is not relevant to human fat necrosis during pancreatitis because the fatty acids in this almost exclusively have ≥12 carbons.3, 9 The most rapid increase in GTL lipolysis occurred during the first 10 to 30 minutes. Interestingly, we found that all of the three pancreatic lipases (including murine, data not shown) could release all three acyl chains from GTL and produce fatty acids and glycerol. Although this was delayed for PNLIP (Figure 2H), it was parallel in case of both PNLIPRP2 and CEL (Figure 2, I and J). Overall, on the basis of optimal CEL activity requiring bile acid concentrations much above those present in necrotic collections, it seems that PNLIP and PNLIPRP2 are more likely to contribute to the lipotoxic generation of fatty acids during the progression of pancreatic fat necrosis. The pancreatic lipases were then compared in inducing lipotoxic cell injury in a manner relevant to SAP.
PNLIP and PNLIPRP2 Cause More Lipotoxic Cell Injury than CEL under Conditions Simulating Severe AP
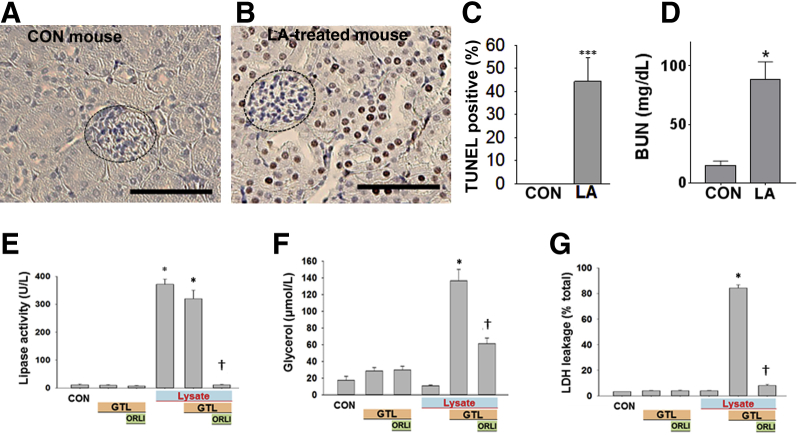
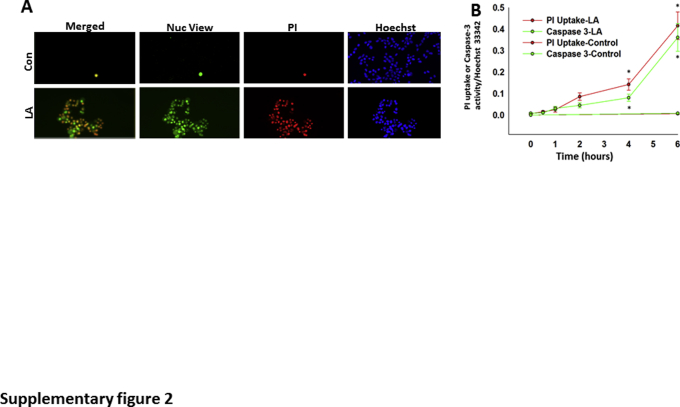
It was first determined whether the renal failure noted in ob/ob mice with extensive fat necrosis (Figure 1) could occur because of the fatty acids generated. LA was administered at 0.2% body weight to lean (CD-1) mice, and renal injury was studied (Figure 3, A–D). This amount is based on LA comprising approximately 20% of fatty acids of visceral fat necrosis in humans1, 3 (Figure 2C), and visceral adipose (composed predominantly of triglyceride69, 70, 71) forms approximately 3% of the body weight of an adult human.74 Thus, assuming that severe fat necrosis in pancreatitis can hydrolyze a third or more of visceral fat, the LA dose (0.2% body weight) is relevant to the pathophysiology being studied. LA caused renal tubular injury, evidenced by an increase in TUNEL-positive renal tubular cells (Figure 3, B and C), whereas glomeruli were not injured. This was associated with an increase in serum blood urea nitrogen (Figure 3D). In addition, LA (100 μmol/L) directly caused injury to the renal cell line (HEK 293), as evidenced by a time-dependent increase in caspase-3 activity and PI uptake (Supplemental Figure S2). This parallel increase is consistent with the multiple cell injury pathways,1 including cytochrome c release (which would trigger effector caspases), along with a decrease in ATP levels31 (which would result in necrosis), associated with increased annexin V staining and PI uptake,3 which are caused by long-chain unsaturated fatty acids, including LA. Therefore, noting that lipotoxicity during AP can cause renal injury as well as injury to HEK 293 cells, this cell line was used to identify the lipase that may mediate the renal component of systemic injury.
Figure 3.
Renal injury induced by the lipolytic product of glyceryl trilinoleate [GTL; ie, linoleic acid (LA)]. A–D:In vivo renal injury induced by linoleic acid. A and B: TUNEL staining in renal tubules of control (CON) mice (A) and those given LA (B). Glomeruli (dashed ovals) were not TUNEL positive. C and D: Quantification of TUNEL-positive cells (C) and serum blood urea nitrogen (BUN) at the time of necropsy (D). E–G: Effect of lipolysis of GTL (600 μmol/L) by pancreatic lysate on injury to the HEK 293 renal cell line. Lipase activity (E), glycerol (F), and lactate dehydrogenase (LDH) leakage (G), measured after the addition of pancreatic lysates (lysate) alone or in the presence of the triglyceride GTL (600 μmol/L). When used, the lipase inhibitor orlistat (ORLI; 50 μmol/L) was added immediately before addition of GTL. All these phenomena, induced in the presence of lysates and GTL, are significantly reduced by orlistat. Lipolysis of GTL is essential to induce injury. Data are expressed as means ± SEM (C–G). n = 6 to 8 mice per group (A–D). ∗P < 0.05, ∗∗∗P < 0.001 versus control; †P < 0.05 versus without orlistat. Scale bars: 100 μm (A and B).
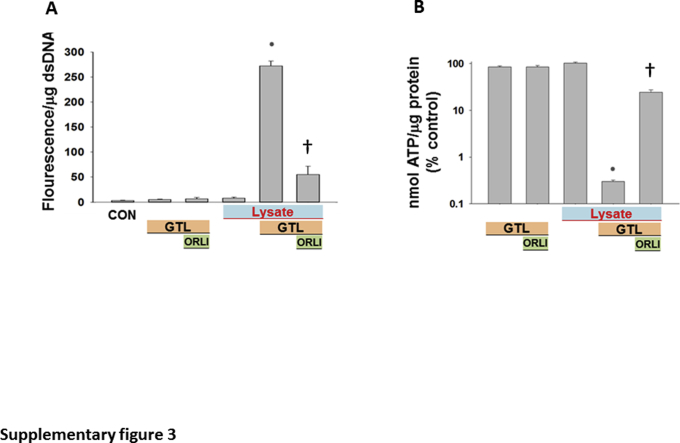
Mouse pancreatic lysate, prepared in HEPES buffer (pH 7.4), was added to HEK 293 cells, alone or along with GTL (600 μmol/L) or lipase inhibitor, orlistat (50 μmol/L) (Figure 3, E–G). As can be seen, there was an appropriate increase in lipase activity in the medium, which was measured at the end of the 2-hour incubation period. This was prevented by orlistat (Figure 3E). Over the incubation period, hydrolysis of GTL by the lysate increased glycerol in the medium (Figure 3F). The lipolysis of GTL resulted in necrosis LDH leakage (Figure 3G), along with an increase in PI fluorescence and a large decrease in cellular ATP levels (Supplemental Figure S3). Orlistat, which inhibited pancreatic lipases, reduced glycerol generation by 70% and significantly reduced the ensuing cell injury (Figure 3, E–G, and Supplemental Figure S3). The partial reduction in glycerol generation is likely because of the early rapid lipolysis (within 10 to 30 minutes) (Figure 2, D–J), whereas orlistat competes with GTL for the active site of the lipases. This, however, was insufficient to cause cell injury, which previously has been shown to be dependent1 on the concentration of fatty acids in this medium.
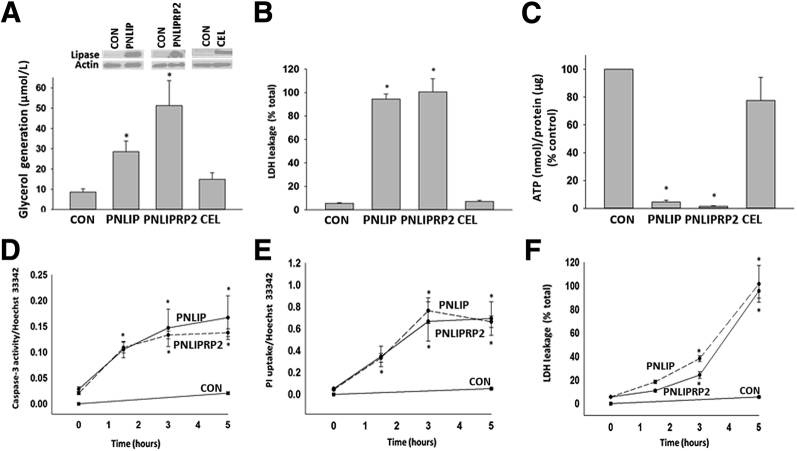
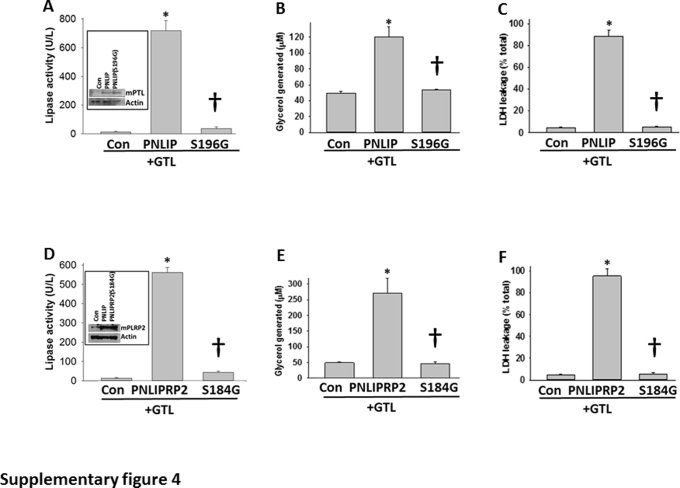
To compare the ability of individual lipases in mediating lipotoxic renal injury, the lipases were overexpressed in HEK 293 cells and the same end points were measured (Figure 4). Lipase expression resulted in 300 to 400 U/L of measurable activity to be present in the medium the next morning. In the absence of bile acids, CEL had only 10% to 15% of the activity of PNLIP (data not shown). Addition of GTL to the medium in the PNLIP and PNLIPRP2 transfected wells resulted in an increase in glycerol generation (29 ± 6 μm in PNLIP versus 51 ± 14 μm in PNLIPRP2; P = 0.14), LDH leakage, and a large decrease in ATP levels (Figure 4, A–C), which were similar to what was noted after the addition of pancreatic lysate and previously in pancreatic acinar cells exposed to GTL.3 This was not observed with CEL. Hydrolysis of triglyceride (as may occur with hypertriglyceridemic pancreatitis) was essential to the injury induced by both PNLIP and PNLIPRP2 because the injury increased in a time-dependent manner after addition of GTL (Figure 4, D–F) and did not occur in the absence of GTL. Moreover, overexpression of the inactive mutants of PNLIP (p.S196G) and PNLIPRP2 (p.S184G) did not result in injury to HEK 293 cells (Supplemental Figure S4). Therefore, although the transfection of lipases or their expression alone did not result in cell injury, lipolysis of the surrounding triglyceride with consequent generation of long-chain unsaturated fatty acids can result in injury to the same cells that produce these lipases. Therefore, in addition to identifying the lipases responsible for renal cell injury, these studies also simulate lipotoxic injury to acinar cells adjacent to ones that leak lipases into fat necrosis, such as the previously shown perifat acinar necrosis during pancreatitis.1, 55
Figure 4.
Comparison of pancreatic lipase overexpression in mediating injury in the kidney cell line HEK 293. A: Mouse lipases were overexpressed in HEK 293 cells and underwent Western blot analysis (bands at top of panel) using actin as a loading control. A–C: The ability to induce triglyceride hydrolysis was measured as glycerol generation (A) from the lipolysis of exogenously added glyceryl trilinoleate (GTL; 600 μmol/L) over 5 hours, and the resulting cell injury was measured as lactate dehydrogenase (LDH) leakage (B) into the medium or ATP decrease in the cell pellet (C). Note the lack of effect of carboxyl ester lipase (CEL). D–F: Effect of adding GTL on the increase in active caspase-3 staining (D), propidium iodide (PI) uptake (E), and LDH leakage (F), induced by mouse pancreatic triacylglycerol lipase (PNLIP) and pancreatic lipase-related protein 2 (PNLIPRP2) versus control (CON), as a function of time. Data are expressed as means ± SEM. ∗P < 0.05 versus control.
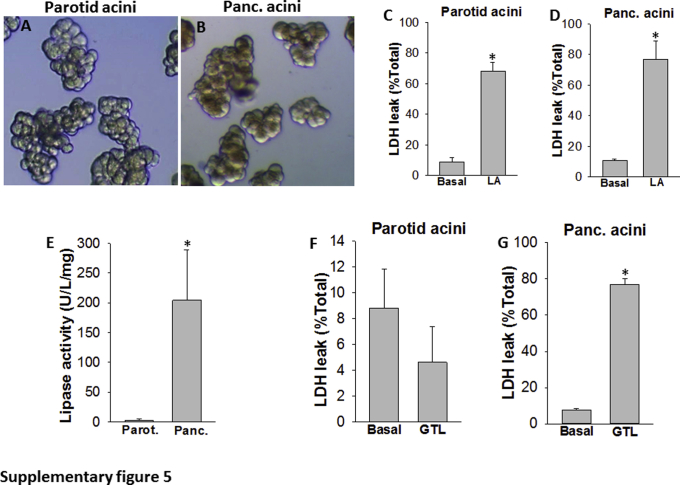
The relevance of these findings was further confirmed by adding recombinant human CEL, PNLIP, or PNLIPRP2 to parotid acini, which model an exocrine cell system lacking lipases (Supplemental Figure S5), and also to HEK 293 cells (data not shown). Parotid acini are morphologically similar to pancreatic acini because the cells are polarized and are normally present in clusters. In addition, these cells exhibited lipotoxic injury similar to pancreatic acini (Supplemental Figure S5, A–D). Because these cells lack enzymes with the activity of pancreatic lipases, and are therefore unlike pancreatic acini and are resistant to lipotoxic injury from the hydrolysis of triglycerides (Supplemental Figure S5, E–G), they can serve as a model to identify lipases that cause exocrine acinar injury.
In both parotid acini (Figure 5) and HEK 293 cells, the hydrolysis of GTL was essential for cell injury (ie, LDH leakage and PI uptake) and the mere presence of the recombinant lipase caused no injury in either of these models. Although both PNLIP and PNLIPRP2 increased lipolysis of GTL and consequent cell injury without STC, exogenous addition of CEL alone was ineffective in hydrolyzing GTL at bile acid concentrations relevant to necrosis collections (Figure 2A and Figure 5, A–F). CEL could induce a significant increase in lipolysis and cell injury only when ≥100 μmol/L STC was present (Figure 5, G–I). These findings again confirmed that CEL, unlike PNLIP or PNLIPRP2, requires bile acid concentrations above those prevalent in local pancreatic necrosis or in the systemic in vivo environment.
Figure 5.
Bar graphs comparing human pancreatic triacylglycerol lipase (hPNLIP; 1 μg/mL), human pancreatic lipase-related protein 2 (hPNLIPRP2; 1 μg/mL), and human carboxyl ester lipase (hCEL; 4 μg/mL) on lipotoxic parotid acinar necrosis. Parotid acini were exposed to exogenous glyceryl trilinoleate (GTL; 300 μmol/L) in the presence of recombinant lipases. In case of hPNLIP and hPNLIPRP2, no bile acids were present in the medium. The concentration of sodium taurocholate (STC) used for CEL is shown under the corresponding bars. The experiments were performed for 4 hours. Lipolysis was measured by glycerol release (A, D, and G), and cell injury in terms of lactate dehydrogenase (LDH) leakage (B, E, and H) and propidium iodide (PI) uptake (C, F, and I) were measured with or without the lipase inhibitor orlistat (50 μmol/L). Note CEL required ≥100 μmol/L STC to induce cell injury. Data are expressed as means ± SEM. n = at least 3 independent experiments. ∗P < 0.05 versus control (CON); †P < 0.05 versus without orlistat. dsDNA, double-stranded DNA.
Discussion
Results show that, although PNLIP and PNLIPRP2 are equipotent in mediating lipotoxicity via triglyceride hydrolysis, CEL is unlikely to have a major role in this phenomenon. This may be relevant to how obesity may worsen local necrosis and AP outcomes58, 59, 60, 61, 62, 63, 64, 65 and also to how hypertriglyceridemia associated with AP75, 76 may worsen systemic injury, resulting in SAP. The role of lipolysis is supported by previous studies showing that lean rodents with AP do not develop fat necrosis and have a mild self-limited course of AP.3, 31 This is unlike rodents that are obese or have increased visceral triglyceride; the adverse outcomes in these rodents are averted by inhibition of visceral fat lipolysis. Several studies show that features of SAP, including renal failure, can be worse in hypertriglyceridemic AP,5, 14, 15, 16, 77, 78 in which the increased circulating triglyceride may undergo unregulated lipolysis.5
The in vitro modeling uses a triglyceride substrate, a cell type commonly injured in SAP (ie, acinar cells or renal tubular cells) and a source of pancreatic lipase (ie, overexpression or exogenous addition of lipase). This provides a reductionist model of local pancreatic and renal injury noted in vivo. The data in HEK 293 cells are relevant to local and kidney injury, which commonly occurs in severe AP,12, 16, 79, 80, 81 whereas the data of parotid acini are relevant to identifying individual lipases mediating exocrine acinar injury because dual pancreatic lipase knockouts are lethal.37 Moreover, because acinar cells for single (eg, PNLIP) lipase knockouts would still contain the other two lipases (ie, PNLIPRP2 and CEL) and would not allow for the identification of individual lipases, a cell type that lacks lipases (ie, parotid acinar cells) was used. Parotid acinar cells replicate the relevant phenotype of pancreatic acinar cells in being susceptible to LA lipotoxicity (Supplemental Figure S5). Because the location of the substrate in vivo can be in the circulation (eg, elevated triglyceride) or in the immediate vicinity (eg, adipocyte triglyceride in or around the pancreas) (Figure 1A), GTL was added to the medium in which the cells were suspended. Although similar modeling has been previously done using adipocytes and acinar cells,1, 55 the use of a pure triglyceride, like GTL, avoids the confounding effects of the type of fatty acid or its location on the glycerol backbone on the degree of lipolysis achieved.
It was first noted that fat necrosis, compared with normal fat tissue, has higher pancreatic lipase amounts on Western blot analysis and activity using a pancreatic lipase activity assay that contains colipase and bile acids, but lacks the ATGL cofactor CGI-58. Conversely, there was a decrease in the amount of hormone-sensitive lipase or ATGL (Figure 1D) in the necrosed fat on the basis of Western blot analysis, perhaps because of proteolysis by pancreatic proteases, the amounts of which also increased in the fat necrosis (data not shown).
To understand the milieu in which individual pancreatic lipases mediate fat necrosis, human pancreatic necrosis collection was analyzed and biliary AP-associated collections were found to have 41 ± 17 μmol/L bile acids and approximately 2 mmol/L fatty acids (Figure 2, A–C). Under these conditions, pancreatic lysate (Figure 3, E–G), PNLIP and PNLIPRP2, can hydrolyze a triglyceride relevant to human pancreatic necrosis (GTL) satisfactorily (Figure 2, D–G) and trigger lipotoxicity (Figures 4 and 5, A–F). However, CEL required bile acid concentrations much higher than those prevalent in the necrotic collections. This is consistent with previous studies showing CEL being less efficacious in hydrolyzing substrates with >16 carbon acyl chains effectively,67, 68 such as would be the precursors of NEFA in human fat necrosis.1, 3 Interestingly, all three pancreatic lipases can hydrolyze all three fatty acids off the glycerol backbone of GTL (Figure 2, H–J).
The presence of GTL was essential for lipotoxicity, which was prevented by orlistat and the inactive lipases, and neither the lysate (despite the presence of other active enzymes) nor the lipases could induce injury on their own. Thus, lipolysis of visceral triglyceride is essential to the lipotoxic manifestations of SAP. The lipolytic product of GTL (ie, LA) can directly cause renal injury (Figure 3, A–C) and has previously been shown to cause pancreatic acinar injury.1, 31 LA has previously shown to be increased in human pancreatic necrotic collections3 in the sera of severe AP8 and hypertriglyceridemic patients,75, 76, 82, 83 who often develop SAP,16, 81 and is therefore a likely mediator of the effects resulting from unregulated lipolysis by pancreatic lipases. Renal tubular injury has previously been noted in autopsies of patients dying with SAP,1 and this damage is routinely seen in the kidneys of rodents with SAP.1, 2, 3, 4 This rationale underlies our choice of GTL in these studies. Lipotoxicity was observed from the lipolytically generated LA at 30 to 50 μmol/L glycerol in serum-free media (Figure 4 and Supplemental Figure S2) and at >100 μmol/L glycerol for those done in the HEPES medium containing 0.1% albumin (Figure 3, E–G, and Figure 5). This is consistent with previous studies on LA toxicity in similar media1, 3, 31 and the ability of albumin to noncovalently interact with multiple molecules of unsaturated fatty acids,84, 85 function as their carrier, and thereby reduce their lipotoxicity.
Although CEL,86 like amylase,87 was reported to be elevated in the sera of patients with SAP more than two decades ago, these findings have not been reproduced. Moreover, the study did not compare PNLIP and PNLIPRP2 levels to those of CEL.86 In addition, elevation of serum markers does not reflect their direct role in mediating fat necrosis in the abdominal cavity. Interestingly, CEL has been shown to play a crucial role in dietary fat absorption in neonatal mice37 and to mediate the formation of fatty acid ethyl esters,88 but it is absent in the panniculitis associated with pancreatitis-panniculitis-polyarthritis syndrome.6 Our findings of low (41 ± 17 μmol/L) bile acid concentrations in necrotic collections suggest that these bile acids do not contribute to the lipotoxicity of AP. These findings also resonate well with the fact that the risk of severe AP is similar in biliary AP and other etiologies, such as alcoholic AP,89, 90, 91 and the clinical observation that procedures to relieve biliary obstruction during AP do not improve outcomes unless there is associated cholangitis.92, 93 Therefore, these low concentrations of bile acids in necrotic collections may be insufficient to contribute to clinical outcomes. In addition, previous studies, showing that millimolar concentrations of bile acids reduce the lipase activity of PNLIP and PNLIPRP2,94, 95 further bring into question the role of bile acids in causing the lipotoxicity found in AP.
The findings that both PNLIP and PNLIPRP2 are present in fat necrosis (Figure 1C) and that their exogenous addition or overexpression can mediate lipotoxic injury in cells support a potentially redundant role of these lipases in acute lipotoxicity if they were present in an equimolar amount. However, because the pancreas makes larger amounts of PNLIP compared with PNLIPRP2, PNLIP likely plays the larger role in fat necrosis.95, 96
There are limitations posed by our lack of knowledge about the mechanisms that may protect against lipotoxicity and how these differ in pancreatic versus parotid acinar cells. For example, pancreatic acinar cells have antiprotease mechanisms, including the pancreatic secreted protease inhibitor and serine protease inhibitor Kazal types 1 and 3, whose mutations increase the risk of hereditary pancreatitis.97, 98 However, because the mechanisms to reduce lipotoxicity or neutralize lipases remain unknown, we cannot comment on their effect on the parotid cell system. However, because the end point of LA-induced cell death is the same in both cell types (Supplemental Figure S5, C and D), this is likely to affect our interpretation only minimally. In addition, although CEL does not increase glycerol, cause LDH leakage, or decrease ATP levels in HEK 293 cells, it remains unknown whether upstream cell death pathways may have been initiated by CEL in these cells. Last, the triglyceride of only a single fatty acid (ie, LA) was used and only a single bile acid (ie, STC) was used, on the basis of their being abundant in fat necrosis and human bile,72 respectively. Whether the triglycerides of other fatty acids or other bile acids would behave differently was not tested. Previous studies showing that CEL poorly hydrolyzes the esters of fatty acids with chains of ≥16 carbons67, 68 suggest that our findings, showing CEL did not significantly increase GTL hydrolysis under conditions relevant to fat necrosis, may also be relevant to other triglycerides whose lipolytic products are enriched in fat necrosis.1, 3 Last, STC (the sodium salt of taurine-conjugated cholic acid) was used because cholic acid forms most of the human bile acid pool.72 Although deoxy cholic acid comprises 30% to 40% of human bile, its sodium salt has been previously shown to be a less efficacious cofactor of CEL73 than STC. Therefore, choosing different bile acids is unlikely to affect the conclusion that CEL plays an unlikely role in human fat necrosis.
In summary, our studies are consistent with a role for PNLIP and PNLIPRP2 in contributing to the lipotoxicity present in SAP. In contrast, CEL likely makes a smaller contribution to lipotoxicity, in part because peripancreatic bile acid concentrations are too low to stimulate maximal activity. The relative contributions of PNLIP and PNLIPRP2 to lipotoxicity remain unclear, although the larger amounts of PNLIP produced in the pancreas support a major role for PNLIP. Additional studies are required to determine whether the genetic deletion or pharmacologic inhibition of PNLIP, alone or in combination with PNLIPR2, will be a viable therapy to treat or prevent SAP.
Footnotes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases grants RO1DK092460 and R01DK100358 and Department of Army PR110417 Award W81XWH-12-1-0327 (V.P.S.).
B.K., R.N.T., and P.N. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2019.02.015.
Supplemental Data
Supplemental Figure S1.
Images of Western blot analyses of the fat pads of control (CON) ob/ob mice and those with 18 hours of cerulein (CER) pancreatitis, showing the film corresponding to the whole image of the membrane (Memb.) blotted for the proteins shown in Figure 1D, above and below the band of interest. The proteins for which the blot analysis was performed are mentioned at the top of the images. The antibody for pancreatic triacylglycerol lipase (PNLIP) and pancreatic lipase-related protein 2 (PNLIPRP2) detects both proteins, as described previously.42 ATGL, adipocyte triglyceride lipase; CEL, carboxyl ester lipase; HSL, hormone-sensitive lipase.
Supplemental Figure S2.
A: Renal HEK cells [untreated control (Con; top panels)] or treated with linoleic acid (LA; 100 μmol/L; bottom panels) in serum-free media for 5 hours and stained with NucView 488 caspase-3 (Nuc), propidium iodide (PI), and Hoechst 33342. B: Quantification of the PI/Hoechst 33342 fluorescence (red lines) and NucView/Hoechst 33342 (green lines) shows a significant increase in caspase-3 activity and PI uptake with LA at 4 and 6 hours. Data are expressed as means ± SEM (B). n = 4 independents experiments (B). ∗P < 0.05 versus control on analysis of variance.
Supplemental Figure S3.
Propidium iodide uptake (A) and ATP levels (B) measured after the addition of pancreatic lysates (Lysate) alone or in the presence of the triglyceride glyceryl trilinoleate (GTL; 600 μmol/L). When used, the lipase inhibitor orlistat (ORLI; 50 μmol/L) was added immediately before addition of GTL. All these phenomena induced in the presence of lysates and GTL are significantly reduced by orlistat. Data are expressed as means ± SEM. ∗P < 0.05 versus control (CON); †P < 0.05 versus control. dsDNA, double-stranded DNA.
Supplemental Figure S4.
Point mutants of mouse pancreatic triacylglycerol lipase (PNLIP) and pancreatic lipase-related protein 2 (PNLIPRP2), lacking lipase activity, do not mediate acute lipotoxicity in HEK 293 cells. A and D: Overexpression of mutant mouse Pnlip and PNLIPRP2 in HEK 293 cells, using pcDNA 3.1/PNLIP (S169G) and pcDNA 3.1/PNLIPRP2 (S184G), shows a significant decrease in pancreatic lipase activity in the medium only in the wild type (WT) and not the mutant mice, which have an equal amount of protein in the lysates (Western blot images; insets). B, C, E, and F: In the presence of glyceryl trilinoleate (GTL; 600 μmol/L), there is a significant increase in glycerol generation (B and E) and lactate dehydrogenase (LDH) leakage (C and F) in the WT, but not the mutant, mice. Data are expressed as means ± SEM. ∗P < 0.05 versus control (Con; increase); †P < 0.05 compared with the wild type (reduction).
Supplemental Figure S5.
A and B: Comparison of parotid (Parot.) and pancreatic (Panc.) acini: Low-power images of parotid (A) and pancreatic (B) acini. C and D: Bar graphs comparing the lactate dehydrogenase (LDH) leakage from parotid and pancreatic acini after 4 hours of exposure to linoleic acid (LA; 300 μmol/L) with untreated (basal) acini. E: Pancreatic lipase activity/mg protein in the homogenates of mouse parotid and pancreas. F and G: LDH leakage from parotid (F) and pancreatic (G) acini after 4 hours of incubation under the basal state or with 300 μmol/L glyceryl trilinoleate (GTL). Data are expressed as means ± SEM. ∗P < 0.05 versus control. Original magnification, ×10 (A and B).
References
- 1.Navina S., Acharya C., DeLany J.P., Orlichenko L.S., Baty C.J., Shiva S.S., Durgampudi C., Karlsson J.M., Lee K., Bae K.T., Furlan A., Behari J., Liu S., McHale T., Nichols L., Papachristou G.I., Yadav D., Singh V.P. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra10. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durgampudi C., Noel P., Patel K., Cline R., Trivedi R.N., DeLany J.P., Yadav D., Papachristou G.I., Lee K., Acharya C., Jaligama D., Navina S., Murad F., Singh V.P. Acute lipotoxicity regulates severity of biliary acute pancreatitis without affecting its initiation. Am J Pathol. 2014;184:1773–1784. doi: 10.1016/j.ajpath.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noel P., Patel K., Durgampudi C., Trivedi R.N., de Oliveira C., Crowell M.D., Pannala R., Lee K., Brand R., Chennat J., Slivka A., Papachristou G.I., Khalid A., Whitcomb D.C., DeLany J.P., Cline R.A., Acharya C., Jaligama D., Murad F.M., Yadav D., Navina S., Singh V.P. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut. 2016;65:100–111. doi: 10.1136/gutjnl-2014-308043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel K., Trivedi R.N., Durgampudi C., Noel P., Cline R.A., DeLany J.P., Navina S., Singh V.P. Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. Am J Pathol. 2015;185:808–819. doi: 10.1016/j.ajpath.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawaz H., Koutroumpakis E., Easler J., Slivka A., Whitcomb D.C., Singh V.P., Yadav D., Papachristou G.I. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110:1497–1503. doi: 10.1038/ajg.2015.261. [DOI] [PubMed] [Google Scholar]
- 6.Loverdos I., Swan M.C., Shekherdimian S., Al-Rasheed A.A., Schneider R., Fish J.S., Ngan B.-Y., Adeli K., Lowe M.E., Singh V.P., Sevilla W.M.A., Langer J.C., Gonska T. A case of pancreatitis, panniculitis and polyarthritis syndrome: elucidating the pathophysiologic mechanisms of a rare condition. J Pediatr Surg Case Rep. 2015;3:223–226. doi: 10.1016/j.epsc.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domschke S., Malfertheiner P., Uhl W., Buchler M., Domschke W. Free fatty acids in serum of patients with acute necrotizing or edematous pancreatitis. Int J Pancreatol. 1993;13:105–110. doi: 10.1007/BF02786078. [DOI] [PubMed] [Google Scholar]
- 8.Sztefko K., Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1:230–236. doi: 10.1159/000055816. [DOI] [PubMed] [Google Scholar]
- 9.Panek J., Sztefko K., Drozdz W. Composition of free fatty acid and triglyceride fractions in human necrotic pancreatic tissue. Med Sci Monit. 2001;7:894–898. [PubMed] [Google Scholar]
- 10.Vege S.S., Gardner T.B., Chari S.T., Munukuti P., Pearson R.K., Clain J.E., Petersen B.T., Baron T.H., Farnell M.B., Sarr M.G. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis.”. Am J Gastroenterol. 2009;104:710–715. doi: 10.1038/ajg.2008.77. [DOI] [PubMed] [Google Scholar]
- 11.Tenner S., Baillie J., Dewitt J., Vege S.S. American College of Gastroenterology guidelines: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 12.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., Tsiotos G.G., Vege S.S. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 13.Buch A., Buch J., Carlsen A., Schmidt A. Hyperlipidemia and pancreatitis. World J Surg. 1980;4:307–314. doi: 10.1007/BF02393387. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez-Munoz J.E., Malfertheiner P., Ditschuneit H.H., Blanco-Chavez J., Uhl W., Buchler M., Ditschuneit H. Hyperlipidemia in acute pancreatitis: relationship with etiology, onset, and severity of the disease. Int J Pancreatol. 1991;10:261–267. [PubMed] [Google Scholar]
- 15.Lloret Linares C., Pelletier A.L., Czernichow S., Vergnaud A.C., Bonnefont-Rousselot D., Levy P., Ruszniewski P., Bruckert E. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas. 2008;37 doi: 10.1097/MPA.0b013e31816074a1. 13–2. [DOI] [PubMed] [Google Scholar]
- 16.Deng L.H., Xue P., Xia Q., Yang X.N., Wan M.H. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558–4561. doi: 10.3748/wjg.14.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker O.J., van Santvoort H., Besselink M.G., Boermeester M.A., van Eijck C., Dejong K., van Goor H., Hofker S., Ahmed Ali U., Gooszen H.G., Bollen T.L. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62:1475–1480. doi: 10.1136/gutjnl-2012-302870. [DOI] [PubMed] [Google Scholar]
- 18.Spanier B.W., Nio Y., van der Hulst R.W., Tuynman H.A., Dijkgraaf M.G., Bruno M.J. Practice and yield of early CT scan in acute pancreatitis: a Dutch Observational Multicenter Study. Pancreatology. 2010;10:222–228. doi: 10.1159/000243731. [DOI] [PubMed] [Google Scholar]
- 19.Schroder T., Kivisaari L., Somer K., Standertskjold-Nordenstam C.G., Kivilaakso E., Lempinen M. Significance of extrapancreatic findings in computed tomography (CT) of acute pancreatitis. Eur J Radiol. 1985;5:273–275. [PubMed] [Google Scholar]
- 20.Balthazar E.J., Robinson D.L., Megibow A.J., Ranson J.H. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 21.Kloppel G., Dreyer T. Oxford; Amsterdam, the Netherlands, New York, NY: 1984. Pathomorphology of Acute Pancreatitis. Analysis of 367 Autopsy Cases and 3 Surgical Specimens. [Google Scholar]
- 22.Schmitz-Moormann P. Comparative radiological and morphological study of the human pancreas, IV: acute necrotizing pancreatitis in man. Pathol Res Pract. 1981;171:325–335. doi: 10.1016/S0344-0338(81)80105-7. [DOI] [PubMed] [Google Scholar]
- 23.Nordback I., Lauslahti K. Clinical pathology of acute necrotising pancreatitis. J Clin Pathol. 1986;39:68–74. doi: 10.1136/jcp.39.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balser W. Ueber Fettnekrose cine zuwcilen todliche Krankheit des Menschen. Arch Pathol Anat Physiol. 1882;90:520–535. [Google Scholar]
- 25.Fitz R.H. Cupples & Hurd; Boston, MA: 1889. Acute Pancreatitis: A Consideration of Pancreatic Hemorrhage, Hemorrhagic, Suppurative, and Gangrenous Pancreatitis, and of Disseminated Fat-Necrosis. [Google Scholar]
- 26.Mossner J., Bodeker H., Kimura W., Meyer F., Bohm S., Fischbach W. Isolated rat pancreatic acini as a model to study the potential role of lipase in the pathogenesis of acinar cell destruction. Int J Pancreatol. 1992;12:285–296. doi: 10.1007/BF02924368. [DOI] [PubMed] [Google Scholar]
- 27.Herbert F. Pancreatic fat necrosis: a chemical study. Br J Exp Pathol. 1928;9:57–63. [Google Scholar]
- 28.Aho H.J., Sternby B., Nevalainen T.J. Fat necrosis in human acute pancreatitis: an immunohistological study. Acta Pathol Microbiol Immunol Scand A. 1986;94:101–105. doi: 10.1111/j.1699-0463.1986.tb02970.x. [DOI] [PubMed] [Google Scholar]
- 29.Aho H.J., Sternby B., Kallajoki M., Nevalainen T.J. Carboxyl ester lipase in human tissues and in acute pancreatitis. Int J Pancreatol. 1989;5:123–134. doi: 10.1007/BF02924413. [DOI] [PubMed] [Google Scholar]
- 30.Kloppel G., Dreyer T., Willemer S., Kern H.F., Adler G. Human acute pancreatitis: its pathogenesis in the light of immunocytochemical and ultrastructural findings in acinar cells. Virchows Arch A Pathol Anat Histopathol. 1986;409:791–803. doi: 10.1007/BF00710764. [DOI] [PubMed] [Google Scholar]
- 31.Patel K., Durgampudi C., Noel P., Trivedi R.N., de Oliveira C., Singh V.P. Fatty acid ethyl esters are less toxic than their parent fatty acids generated during acute pancreatitis. Am J Pathol. 2016;186:874–884. doi: 10.1016/j.ajpath.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva M.T. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 2010;584:4491–4499. doi: 10.1016/j.febslet.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Dorn G.W., 2nd Molecular mechanisms that differentiate apoptosis from programmed necrosis. Toxicol Pathol. 2013;41:227–234. doi: 10.1177/0192623312466961. [DOI] [PubMed] [Google Scholar]
- 34.Jonker J.W., Suh J.M., Atkins A.R., Ahmadian M., Li P., Whyte J., He M., Juguilon H., Yin Y.Q., Phillips C.T., Yu R.T., Olefsky J.M., Henry R.R., Downes M., Evans R.M. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Q., Shao X.M., Kao L., Azimov R., Weinstein A.M., Newman D., Liu W., Kurtz I. Missense mutation T485S alters NBCe1-A electrogenicity causing proximal renal tubular acidosis. Am J Physiol Cell Physiol. 2013;305:C392–C405. doi: 10.1152/ajpcell.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esmail S., Kartner N., Yao Y., Kim J.W., Reithmeier R.A.F., Manolson M.F. Molecular mechanisms of cutis laxa- and distal renal tubular acidosis-causing mutations in V-ATPase a subunits, ATP6V0A2 and ATP6V0A4. J Biol Chem. 2018;293:2787–2800. doi: 10.1074/jbc.M117.818872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller R., Lowe M.E. Carboxyl ester lipase from either mother's milk or the pancreas is required for efficient dietary triglyceride digestion in suckling mice. J Nutr. 2008;138:927–930. doi: 10.1093/jn/138.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messenger S.W., Falkowski M.A., Groblewski G.E. Ca(2)(+)-regulated secretory granule exocytosis in pancreatic and parotid acinar cells. Cell Calcium. 2014;55:369–375. doi: 10.1016/j.ceca.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Warner J.D., Yule D.I., Giovannucci D.R. Spatiotemporal analysis of exocytosis in mouse parotid acinar cells. Am J Physiol Cell Physiol. 2005;289:C1209–C1219. doi: 10.1152/ajpcell.00159.2005. [DOI] [PubMed] [Google Scholar]
- 40.Jo H., Byun H.M., Lee S.I., Shin D.M. Initiation site of Ca(2+) entry evoked by endoplasmic reticulum Ca(2+) depletion in mouse parotid and pancreatic acinar cells. Yonsei Med J. 2007;48:526–530. doi: 10.3349/ymj.2007.48.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson B., Olivecrona G., Ericson T. Lipids in human saliva. Arch Oral Biol. 1996;41:105–110. doi: 10.1016/0003-9969(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 42.Xiao X., Ross L.E., Miller R.A., Lowe M.E. Kinetic properties of mouse pancreatic lipase-related protein-2 suggest the mouse may not model human fat digestion. J Lipid Res. 2011;52:982–990. doi: 10.1194/jlr.M014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Working Group IAP/APA Acute Pancreatitis Guidelines IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 44.Williams J.A., Korc M., Dormer R.L. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol. 1978;235:517–524. doi: 10.1152/ajpendo.1978.235.5.E517. [DOI] [PubMed] [Google Scholar]
- 45.Singh V.P., Bhagat L., Navina S., Sharif R., Dawra R.K., Saluja A.K. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut. 2007;56:958–964. doi: 10.1136/gut.2006.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acharya C., Navina S., Singh V.P. Role of pancreatic fat in the outcomes of pancreatitis. Pancreatology. 2014;14:403–408. doi: 10.1016/j.pan.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosner M., Schipany K., Hengstschlager M. Merging high-quality biochemical fractionation with a refined flow cytometry approach to monitor nucleocytoplasmic protein expression throughout the unperturbed mammalian cell cycle. Nat Protoc. 2013;8:602–626. doi: 10.1038/nprot.2013.011. [DOI] [PubMed] [Google Scholar]
- 48.Singh V.P., Bren G.D., Algeciras-Schimnich A., Schnepple D., Navina S., Rizza S.A., Dawra R.K., Saluja A.K., Chari S.T., Vege S.S., Badley A.D. Nelfinavir/ritonavir reduces acinar injury but not inflammation during mouse caerulein pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1040–G1046. doi: 10.1152/ajpgi.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh V.P., McNiven M.A. Src-mediated cortactin phosphorylation regulates actin localization and injurious blebbing in acinar cells. Mol Biol Cell. 2008;19:2339–2347. doi: 10.1091/mbc.E07-11-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y., Sanchez D., Figarella C., Lowe M.E. Discoordinate expression of pancreatic lipase and two related proteins in the human fetal pancreas. Pediatr Res. 2000;47:184–188. doi: 10.1203/00006450-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Payne R.M., Sims H.F., Jennens M.L., Lowe M.E. Rat pancreatic lipase and two related proteins: enzymatic properties and mRNA expression during development. Am J Physiol. 1994;266:G914–G921. doi: 10.1152/ajpgi.1994.266.5.G914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemeth B.C., Pesei Z.G., Hegyi E., Szucs A., Szentesi A., Hegyi P., Lowe M.E., Sahin-Toth M. The common truncation variant in pancreatic lipase related protein 2 (PNLIPRP2) is expressed poorly and does not alter risk for chronic pancreatitis. PLoS One. 2018;13:e0206869. doi: 10.1371/journal.pone.0206869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X., Lindquist S., Lowe M., Noppa L., Hernell O. Bile salt-stimulated lipase and pancreatic lipase-related protein 2 are the dominating lipases in neonatal fat digestion in mice and rats. Pediatr Res. 2007;62:537–541. doi: 10.1203/PDR.0b013e3181559e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi T., Omatsu N., Morimoto E., Nakashima H., Ueno K., Tanaka T., Satouchi K., Hirose F., Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Acharya C., Cline R.A., Jaligama D., Noel P., Delany J.P., Bae K., Furlan A., Baty C.J., Karlsson J.M., Rosario B.L., Patel K., Mishra V., Dugampudi C., Yadav D., Navina S., Singh V.P. Fibrosis reduces severity of acute-on-chronic pancreatitis in humans. Gastroenterology. 2013;145:466–475. doi: 10.1053/j.gastro.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kangani C.O., Kelley D.E., Delany J.P. New method for GC/FID and GC-C-IRMS analysis of plasma free fatty acid concentration and isotopic enrichment. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:95–101. doi: 10.1016/j.jchromb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perwaiz S., Tuchweber B., Mignault D., Gilat T., Yousef I.M. Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry. J Lipid Res. 2001;42:114–119. [PubMed] [Google Scholar]
- 58.Abu Hilal M., Armstrong T. The impact of obesity on the course and outcome of acute pancreatitis. Obes Surg. 2008;18:326–328. doi: 10.1007/s11695-007-9298-5. [DOI] [PubMed] [Google Scholar]
- 59.Papachristou G.I., Papachristou D.J., Avula H., Slivka A., Whitcomb D.C. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 60.Porter K.A., Banks P.A. Obesity as a predictor of severity in acute pancreatitis. Int J Pancreatol. 1991;10:247–252. doi: 10.1007/BF02924162. [DOI] [PubMed] [Google Scholar]
- 61.Shin K.Y., Lee W.S., Chung D.W., Heo J., Jung M.K., Tak W.Y., Kweon Y.O., Cho C.M. Influence of obesity on the severity and clinical outcome of acute pancreatitis. Gut Liver. 2011;5:335–339. doi: 10.5009/gnl.2011.5.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Leary D.P., O'Neill D., McLaughlin P., O'Neill S., Myers E., Maher M.M., Redmond H.P. Effects of abdominal fat distribution parameters on severity of acute pancreatitis. World J Surg. 2012;36:1679–1685. doi: 10.1007/s00268-011-1414-y. [DOI] [PubMed] [Google Scholar]
- 63.Sempere L., Martinez J., de Madaria E., Lozano B., Sanchez-Paya J., Jover R., Perez-Mateo M. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology. 2008;8:257–264. doi: 10.1159/000134273. [DOI] [PubMed] [Google Scholar]
- 64.Evans A.C., Papachristou G.I., Whitcomb D.C. Obesity and the risk of severe acute pancreatitis. Minerva Gastroenterol Dietol. 2010;56:169–179. [PubMed] [Google Scholar]
- 65.Chen S.M., Xiong G.S., Wu S.M. Is obesity an indicator of complications and mortality in acute pancreatitis? an updated meta-analysis. J Dig Dis. 2012;13:244–251. doi: 10.1111/j.1751-2980.2012.00587.x. [DOI] [PubMed] [Google Scholar]
- 66.Xiao X., Mukherjee A., Ross L.E., Lowe M.E. Pancreatic lipase-related protein-2 (PLRP2) can contribute to dietary fat digestion in human newborns. J Biol Chem. 2011;286:26353–26363. doi: 10.1074/jbc.M111.249813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lombardo D., Fauvel J., Guy O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice, I: action on carboxyl esters, glycerides and phospholipids. Biochim Biophys Acta. 1980;611:136–146. doi: 10.1016/0005-2744(80)90049-2. [DOI] [PubMed] [Google Scholar]
- 68.Fontbonne H., Brisson L., Verine A., Puigserver A., Lombardo D., Ajandouz E.H. Human bile salt-dependent lipase efficiency on medium-chain acyl-containing substrates: control by sodium taurocholate. J Biochem. 2011;149:145–151. doi: 10.1093/jb/mvq132. [DOI] [PubMed] [Google Scholar]
- 69.Garaulet M., Hernandez-Morante J.J., Lujan J., Tebar F.J., Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 2006;30:899–905. doi: 10.1038/sj.ijo.0803219. [DOI] [PubMed] [Google Scholar]
- 70.Ren J., Dimitrov I., Sherry A.D., Malloy C.R. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res. 2008;49:2055–2062. doi: 10.1194/jlr.D800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas L.W. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cogn Med Sci. 1962;47:179–188. doi: 10.1113/expphysiol.1962.sp001589. [DOI] [PubMed] [Google Scholar]
- 72.Lindor K.D., Lacerda M.A., Jorgensen R.A., DeSotel C.K., Batta A.K., Salen G., Dickson E.R., Rossi S.S., Hofmann A.F. Relationship between biliary and serum bile acids and response to ursodeoxycholic acid in patients with primary biliary cirrhosis. Am J Gastroenterol. 1998;93:1498–1504. doi: 10.1111/j.1572-0241.1998.00470.x. [DOI] [PubMed] [Google Scholar]
- 73.Aubert E., Sbarra V., Le Petit-Thevenin J., Valette A., Lombardo D. Site-directed mutagenesis of the basic N-terminal cluster of pancreatic bile salt-dependent lipase: functional significance. J Biol Chem. 2002;277:34987–34996. doi: 10.1074/jbc.M202893200. [DOI] [PubMed] [Google Scholar]
- 74.Camhi S.M., Bray G.A., Bouchard C., Greenway F.L., Johnson W.D., Newton R.L., Ravussin E., Ryan D.H., Smith S.R., Katzmarzyk P.T. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Streja D. Hypertriglyceridemic pancreatitis (HTGP) In: De Groot L.J., Beck-Peccoz P., Chrousos G., Dungan K., Grossman A., Hershman J.M., Koch C., McLachlan R., New M., Rebar R., Singer F., Vinik A., Weickert M.O., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA: 2000. [Google Scholar]
- 76.Valdivielso P., Ramirez-Bueno A., Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689–694. doi: 10.1016/j.ejim.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Li X., Ke L., Dong J., Ye B., Meng L., Mao W., Yang Q., Li W., Li J. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: a retrospective study of 730 patients from a tertiary center. BMC Gastroenterol. 2018;18:89. doi: 10.1186/s12876-018-0821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang Y.X., Jia L., Jiang S.M., Wang S.B., Li M.X., Yang B.H. Incidence and clinical features of hyperlipidemic acute pancreatitis from Guangdong, China: a retrospective multicenter study. Pancreas. 2014;43:548–552. doi: 10.1097/MPA.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 79.Wu B.U., Johannes R.S., Sun X., Conwell D.L., Banks P.A. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology. 2009;137:129–135. doi: 10.1053/j.gastro.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 80.Wu B.U., Johannes R.S., Sun X., Tabak Y., Conwell D.L., Banks P.A. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698–1703. doi: 10.1136/gut.2008.152702. [DOI] [PubMed] [Google Scholar]
- 81.Wu C., Ke L., Tong Z., Li B., Zou L., Li W., Li N., Li J. Hypertriglyceridemia is a risk factor for acute kidney injury in the early phase of acute pancreatitis. Pancreas. 2014;43:1312–1316. doi: 10.1097/MPA.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 82.Smith W.G., Wang J., Dang A.Q., Reeves C., Bibbs D., Faas F.H. Gemfibrozil lowers plasma lipids and increases polyunsaturated fatty acid content and oxidative susceptibility of lipoproteins in hypertriglyceridemia. Clin Chim Acta. 2002;322:77–84. doi: 10.1016/s0009-8981(02)00129-8. [DOI] [PubMed] [Google Scholar]
- 83.Sanders T.A., Sullivan D.R., Reeve J., Thompson G.R. Triglyceride-lowering effect of marine polyunsaturates in patients with hypertriglyceridemia. Arteriosclerosis. 1985;5:459–465. doi: 10.1161/01.atv.5.5.459. [DOI] [PubMed] [Google Scholar]
- 84.Petitpas I., Grune T., Bhattacharya A.A., Curry S. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J Mol Biol. 2001;314:955–960. doi: 10.1006/jmbi.2000.5208. [DOI] [PubMed] [Google Scholar]
- 85.Spector A.A. Fatty acid binding to plasma albumin. J Lipid Res. 1975;16:165–179. [PubMed] [Google Scholar]
- 86.Blind P.J., Buchler M., Blackberg L., Andersson Y., Uhl W., Beger H.G., Hernell O. Carboxylic ester hydrolase: a sensitive serum marker and indicator of severity of acute pancreatitis. Int J Pancreatol. 1991;8:65–73. [PubMed] [Google Scholar]
- 87.Manes G., Rabitti P.G., Laccetti M., Pacelli L., Carraturo I., Uomo G. Early prediction of aetiology and severity of acute pancreatitis by serum amylase and lipase assays. Minerva Gastroenterol Dietol. 1995;41:211–215. [PubMed] [Google Scholar]
- 88.Huang W., Booth D.M., Cane M.C., Chvanov M., Javed M.A., Elliott V.L., Armstrong J.A., Dingsdale H., Cash N., Li Y., Greenhalf W., Mukherjee R., Kaphalia B.S., Jaffar M., Petersen O.H., Tepikin A.V., Sutton R., Criddle D.N. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014;63:1313–1324. doi: 10.1136/gutjnl-2012-304058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isenmann R., Rau B., Beger H.G. Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas. 2001;22:274–278. doi: 10.1097/00006676-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Lankisch P.G., Assmus C., Pflichthofer D., Struckmann K., Lehnick D. Which etiology causes the most severe acute pancreatitis? Int J Pancreatol. 1999;26:55–57. doi: 10.1007/BF02781731. [DOI] [PubMed] [Google Scholar]
- 91.Papachristou G.I., Papachristou D.J., Morinville V.D., Slivka A., Whitcomb D.C. Chronic alcohol consumption is a major risk factor for pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2006;101:2605–2610. doi: 10.1111/j.1572-0241.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 92.van Geenen E.J., van Santvoort H.C., Besselink M.G., van der Peet D.L., van Erpecum K.J., Fockens P., Mulder C.J., Bruno M.J. Lack of consensus on the role of endoscopic retrograde cholangiography in acute biliary pancreatitis in published meta-analyses and guidelines: a systematic review. Pancreas. 2013;42:774–780. doi: 10.1097/MPA.0b013e318287d208. [DOI] [PubMed] [Google Scholar]
- 93.AGA Institute medical position statement on acute pancreatitis. Gastroenterology. 2007;132:2019–2021. doi: 10.1053/j.gastro.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 94.Xiao X., Ross L.E., Sevilla W.A., Wang Y., Lowe M.E. Porcine pancreatic lipase related protein 2 has high triglyceride lipase activity in the absence of colipase. Biochim Biophys Acta. 2013;1831:1435–1441. doi: 10.1016/j.bbalip.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Caro J., Sias B., Grandval P., Ferrato F., Halimi H., Carriere F., De Caro A. Characterization of pancreatic lipase-related protein 2 isolated from human pancreatic juice. Biochim Biophys Acta. 2004;1701:89–99. doi: 10.1016/j.bbapap.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Giller T., Buchwald P., Blum-Kaelin D., Hunziker W. Two novel human pancreatic lipase related proteins, hPLRP1 and hPLRP2: differences in colipase dependence and in lipase activity. J Biol Chem. 1992;267:16509–16516. [PubMed] [Google Scholar]
- 97.Ohmuraya M., Yamamura K. Roles of serine protease inhibitor Kazal type 1 (SPINK1) in pancreatic diseases. Exp Anim. 2011;60:433–444. doi: 10.1538/expanim.60.433. [DOI] [PubMed] [Google Scholar]
- 98.Paju A., Stenman U.H. Biochemistry and clinical role of trypsinogens and pancreatic secretory trypsin inhibitor. Crit Rev Clin Lab Sci. 2006;43:103–142. doi: 10.1080/10408360500523852. [DOI] [PubMed] [Google Scholar]